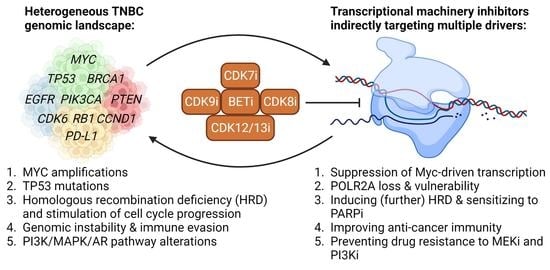
Targeting the Heterogeneous Genomic Landscape in Triple-Negative Breast Cancer through Inhibitors of the Transcriptional Machinery
Abstract
Simple Summary
Abstract
1. Introduction
2. Targeting the TM in TNBC
2.1. Regulation of Transcription by the TM
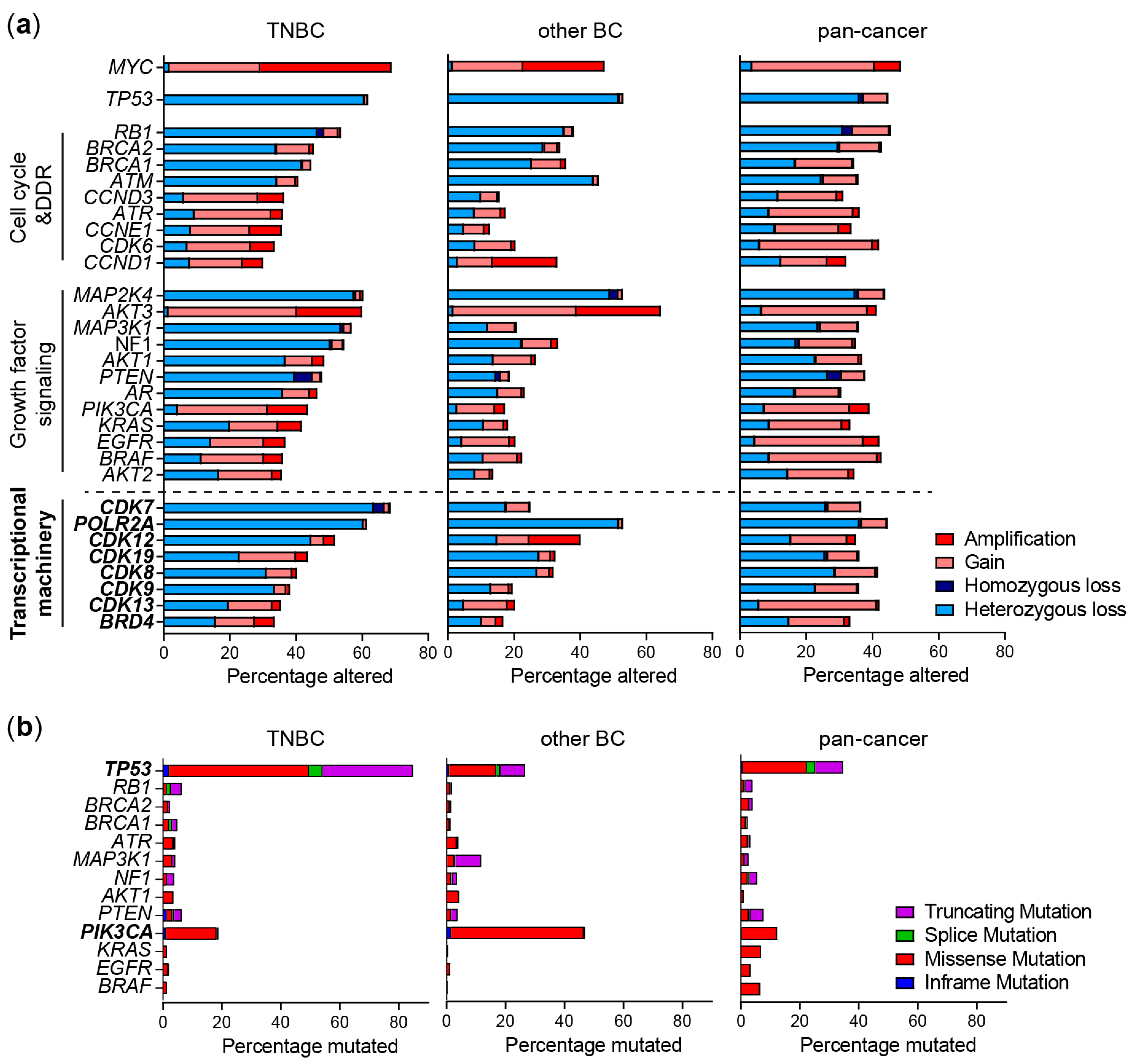
2.2. Genomic Alterations of the TM
2.3. Targeting the TM
3. TM Inhibition to Target MYC and Other Super-Enhancer Driven Oncogenes
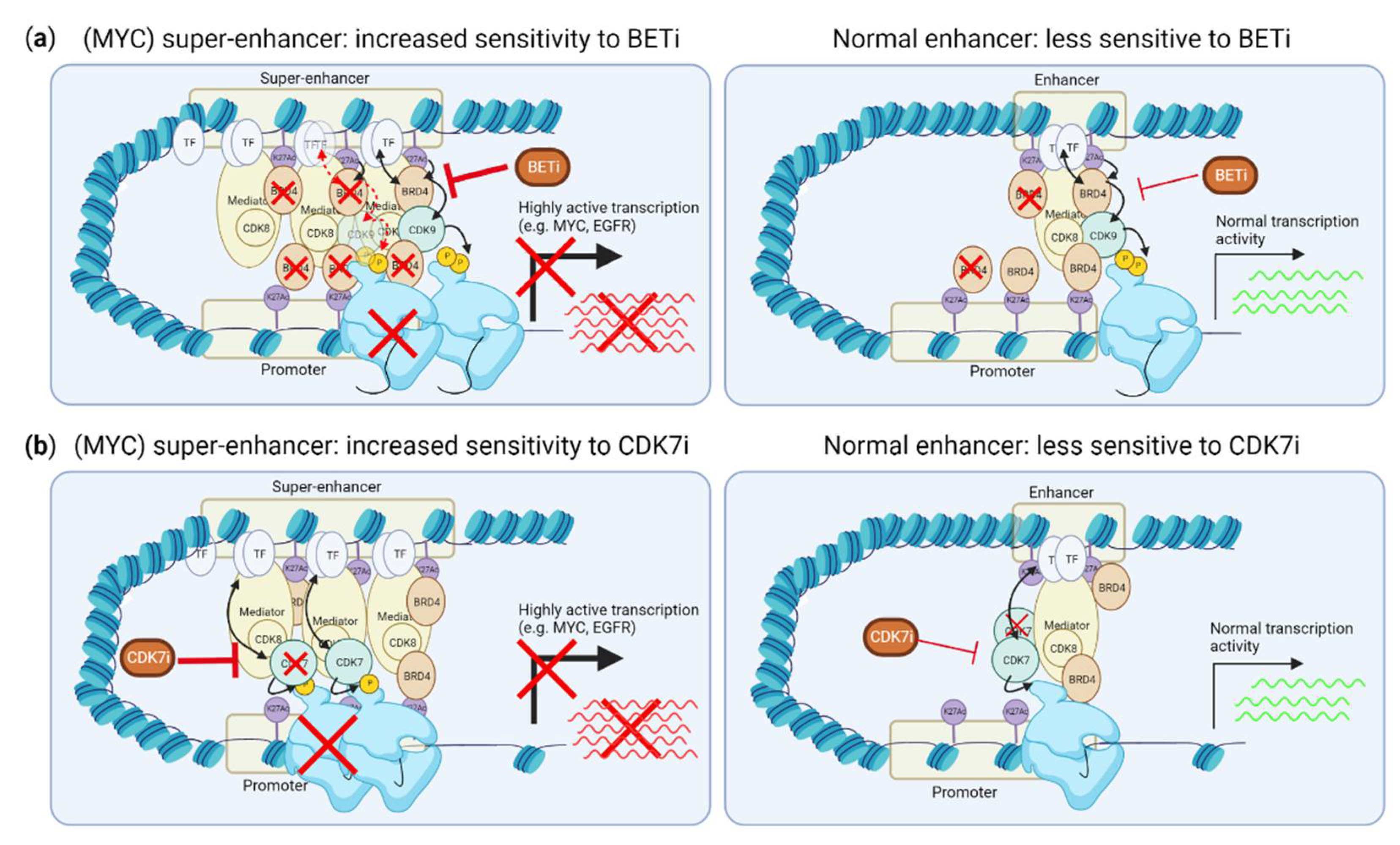
3.1. Inhibiting Super-Enhancer Induced Expression Suppresses MYC-Driven Transcription
3.2. CDK9 and Myc-Dependent Transcription Pause-Release and Gene Expression
4. TP53 Loss in TNBC and Sensitivity to TMi’s
4.1. CDK9 Inhibition Overcomes Negative Regulation of p53 Stability
4.2. BRD4 Interacts with (Mutant) p53 to Induce Gene Transcription
4.3. CDK7, CDK8 and CDK12/13 Inhibitors and Induction of p53 Responses
4.4. Specific Vulnerability Due to Monoallelic P53 Loss Concomitant with POLR2A Loss
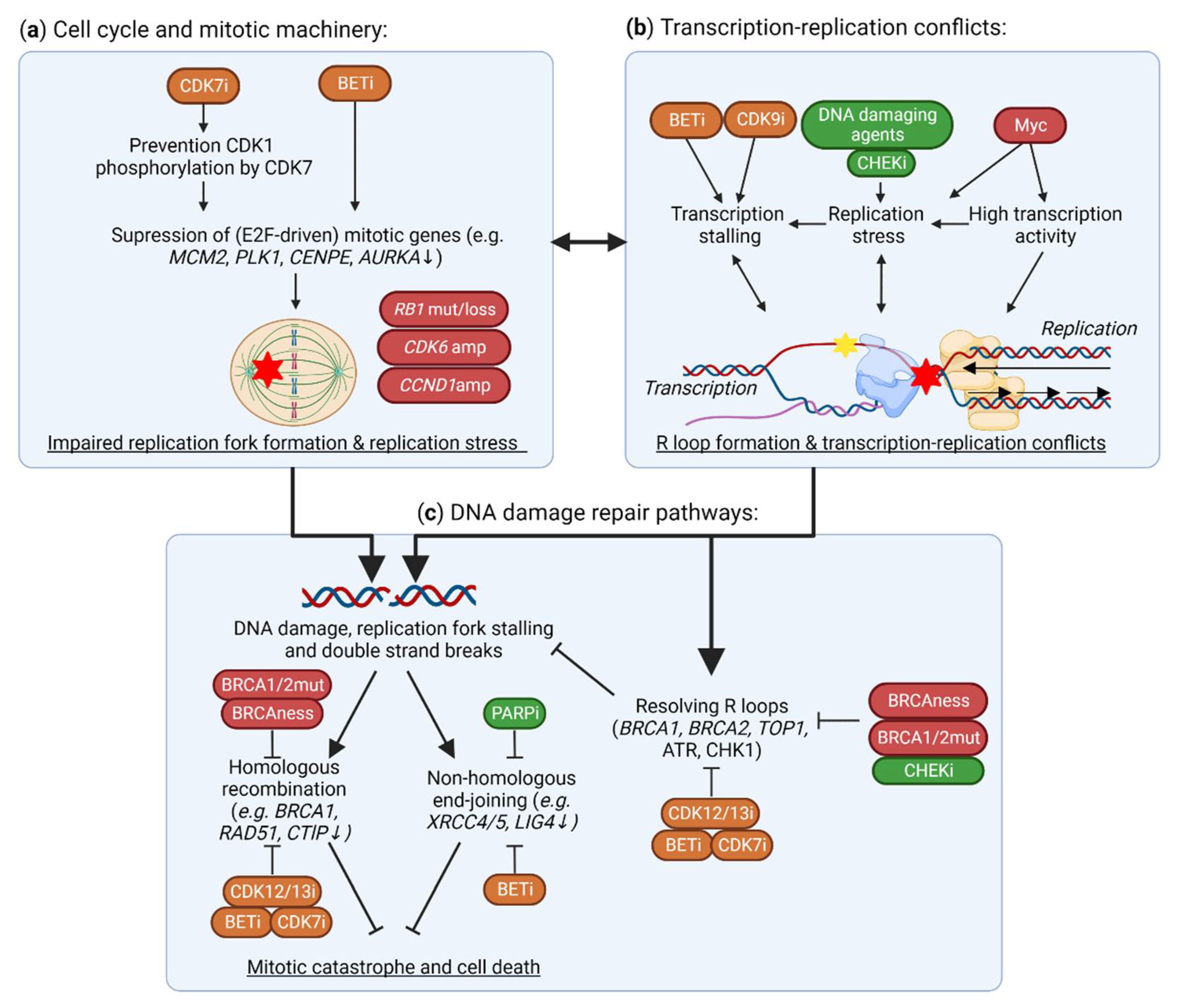
5. TMi’s Interfere with DNA Damage Repair and the Replication Machinery
5.1. Suppression of DNA Damage Repair Genes by TMi’s
5.2. Aberrant Expression and Function of DNA Replication Machinery and Cell Cycle Genes
5.3. BRD4 and CDK9 Inhibition Induce Transcription-Replication Conflicts
6. Targeting the TM to Unleash Anti-Cancer Immunity against the High Mutational Burden in TNBC
6.1. CDK12 Deficiencies Increase Fusion Neoantigens and Immunogenicity
6.2. Induction of Interferon Responses by CDK7 and CDK9 Inhibition
6.3. BRD4 Inhibition Suppresses Immune Escape Mechanisms
6.4. CDK8 Inhibition Potentiates Natural Killer (NK) Cell Activity through STAT1 Inhibition
7. TMi’s Cooperate with Inhibitors of Growth Factor or Hormone Signaling Pathways
7.1. Preventing Kinome Reprogramming upon MAPK/PI3K Pathway Inhibition Using BET Inhibitors
7.2. Targeting Androgen Receptor-Driven Transcription of Luminal TNBC
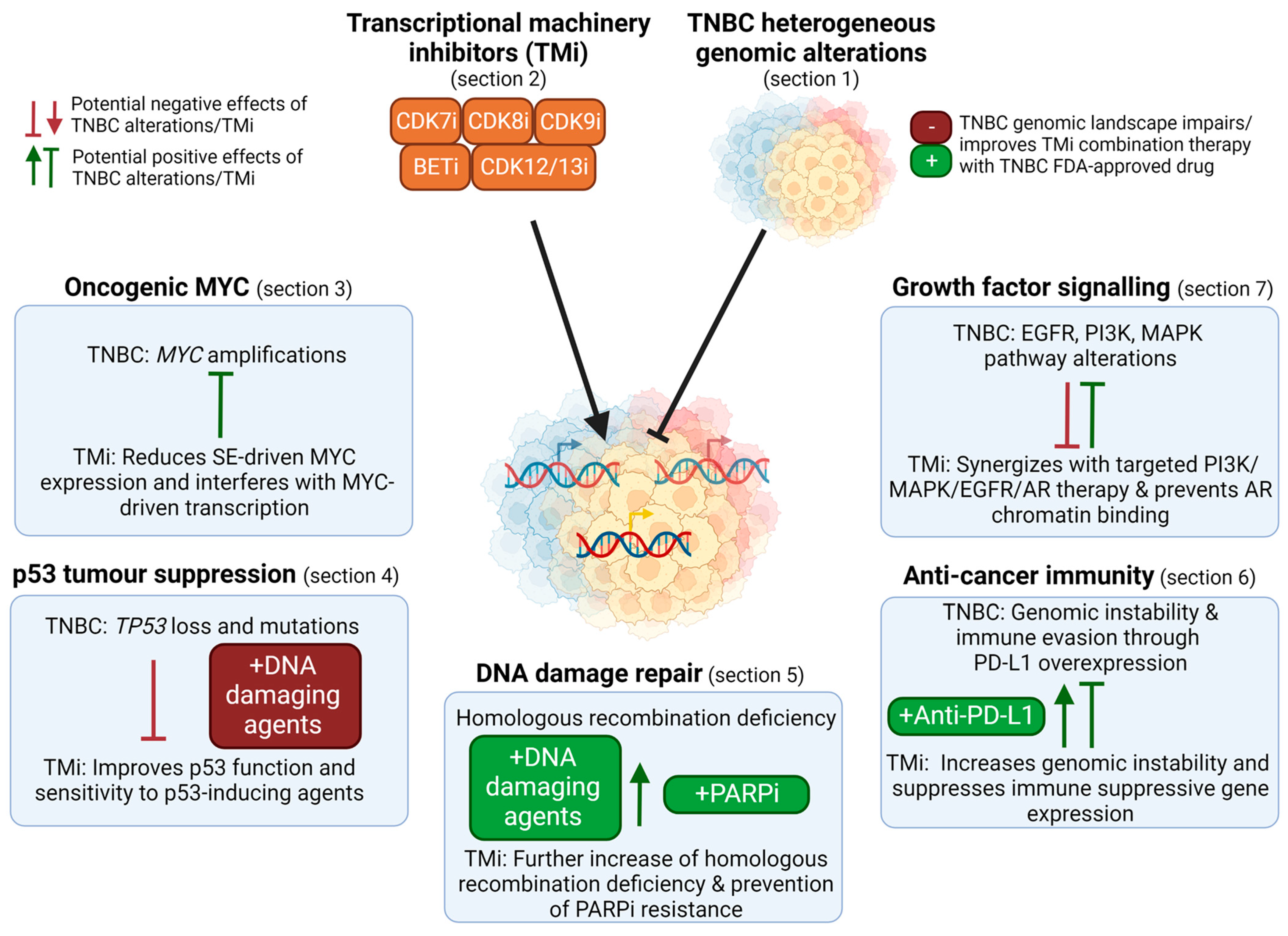
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive Analysis of Estrogen Receptor (ER)-Negative, Progesterone Receptor (PR)-Negative, and HER2-Negative Invasive Breast Cancer, the so-Called Triple-Negative Phenotype: A Population-Based Study from the California Cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.-D.; Liu, Y.-R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of Somatic Mutations in 560 Breast Cancer Whole-Genome Sequences. Nature 2016, 534, 47–54. [Google Scholar] [CrossRef]
- Bertucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic Characterization of Metastatic Breast Cancers. Nature 2019, 569, 560–564. [Google Scholar] [CrossRef]
- Pereira, B.; Chin, S.F.; Rueda, O.M.; Vollan, H.K.M.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.J.; et al. The Somatic Mutation Profiles of 2,433 Breast Cancers Refine Their Genomic and Transcriptomic Landscapes. Nat. Commun. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Bareche, Y.; Venet, D.; Ignatiadis, M.; Aftimos, P.; Piccart, M.; Rothe, F.; Sotiriou, C. Unravelling Triple-Negative Breast Cancer Molecular Heterogeneity Using an Integrative Multiomic Analysis. Ann. Oncol. 2018, 29, 895–902. [Google Scholar] [CrossRef]
- Karaayvaz, M.; Cristea, S.; Gillespie, S.M.; Patel, A.P.; Mylvaganam, R.; Luo, C.C.; Specht, M.C.; Bernstein, B.E.; Michor, F.; Ellisen, L.W. Unravelling Subclonal Heterogeneity and Aggressive Disease States in TNBC through Single-Cell RNA-Seq. Nat. Commun. 2018, 9, 3588. [Google Scholar] [CrossRef]
- Gao, R.; Kim, C.; Sei, E.; Foukakis, T.; Crosetto, N.; Chan, L.-K.; Srinivasan, M.; Zhang, H.; Meric-Bernstam, F.; Navin, N. Nanogrid Single-Nucleus RNA Sequencing Reveals Phenotypic Diversity in Breast Cancer. Nat. Commun. 2017, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Waters, J.; Leung, M.L.; Unruh, A.; Roh, W.; Shi, X.; Chen, K.; Scheet, P.; Vattathil, S.; Liang, H.; et al. Clonal Evolution in Breast Cancer Revealed by Single Nucleus Genome Sequencing. Nature 2014, 512, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-Cell RNA-Seq Enables Comprehensive Tumour and Immune Cell Profiling in Primary Breast Cancer. Nat. Commun. 2017, 8, 15081. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The Clonal and Mutational Evolution Spectrum of Primary Triple-Negative Breast Cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef]
- Koren, S.; Bentires-Alj, M. Breast Tumor Heterogeneity: Source of Fitness, Hurdle for Therapy. Mol. Cell 2015, 60, 537–546. [Google Scholar] [CrossRef]
- Bradner, J.E.; Hnisz, D.; Young, R.A. Transcriptional Addiction in Cancer. Cell 2017, 168, 629–643. [Google Scholar] [CrossRef]
- Schier, A.C.; Taatjes, D.J. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Genes Dev. 2020, 34, 465–488. [Google Scholar] [CrossRef]
- Fant, C.B.; Taatjes, D.J. Regulatory Functions of the Mediator Kinases CDK8 and CDK19. Transcription 2019, 10, 76–90. [Google Scholar] [CrossRef]
- Shi, J.; Vakoc, C.R. The Mechanisms behind the Therapeutic Activity of BET Bromodomain Inhibition. Mol. Cell 2014, 54, 728–736. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Case-Borden, C.; Gegonne, A.; Hsu, C.H.; Chen, Q.; Meerzaman, D.; Dey, A.; Ozato, K.; Singer, D.S. BRD4 Is a Histone Acetyltransferase That Evicts Nucleosomes from Chromatin. Nat. Struct. Mol. Biol. 2016, 23, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Kamikawa, Y.F.; Donohoe, M.E. Brd4′s Bromodomains Mediate Histone H3 Acetylation and Chromatin Remodeling in Pluripotent Cells through P300 and Brg1. Cell Rep. 2018, 25, 1756–1771. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Kim, C.; Zhou, M.M. The Functions of BET Proteins in Gene Transcription of Biology and Diseases. Front. Mol. Biosci. 2021, 8, 787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shilatifard, A. Epigenetic Modifications of Histones in Cancer. Genome Biol. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Calo, E.; Wysocka, J. Modification of Enhancer Chromatin: What, How and Why? Mol. Cell 2013, 49, 825–837. [Google Scholar] [CrossRef]
- Fan, Z.; Devlin, J.R.; Hogg, S.J.; Doyle, M.A.; Harrison, P.F.; Todorovski, I.; Cluse, L.A.; Knight, D.A.; Sandow, J.J.; Gregory, G.; et al. CDK13 Cooperates with CDK12 to Control Global RNA Polymerase II Processivity. Sci. Adv. 2020, 6, AAZ5041. [Google Scholar] [CrossRef]
- Krajewska, M.; Dries, R.; Grassetti, A.v.; Dust, S.; Gao, Y.; Huang, H.; Sharma, B.; Day, D.S.; Kwiatkowski, N.; Pomaville, M.; et al. CDK12 Loss in Cancer Cells Affects DNA Damage Response Genes through Premature Cleavage and Polyadenylation. Nat. Commun. 2019, 10, 1757. [Google Scholar] [CrossRef]
- Liang, K.; Gao, X.; Gilmore, J.M.; Florens, L.; Washburn, M.P.; Smith, E.; Shilatifard, A. Characterization of Human Cyclin-Dependent Kinase 12 (CDK12) and CDK13 Complexes in C-Terminal Domain Phosphorylation, Gene Transcription, and RNA Processing. Mol. Cell Biol. 2015, 35, 928–938. [Google Scholar] [CrossRef]
- Shan, W.; Yuan, J.; Hu, Z.; Jiang, J.; Wang, Y.; Loo, N.; Fan, L.; Tang, Z.; Zhang, T.; Xu, M.; et al. Systematic Characterization of Recurrent Genomic Alterations in Cyclin-Dependent Kinases Reveals Potential Therapeutic Strategies for Cancer Treatment. Cell Rep. 2020, 32, 107884. [Google Scholar] [CrossRef]
- Rescigno, P.; Gurel, B.; Pereira, R.; Crespo, M.; Rekowski, J.; Rediti, M.; Barrero, M.; Mateo, J.; Bianchini, D.; Messina, C.; et al. Characterizing CDK12-Mutated Prostate Cancers. Clin. Cancer Res. 2021, 27, 566–574. [Google Scholar] [CrossRef]
- Joshi, P.M.; Sutor, S.L.; Huntoon, C.J.; Karnitz, L.M. Ovarian Cancer-Associated Mutations Disable Catalytic Activity of CDK12, a Kinase That Promotes Homologous Recombination Repair and Resistance to Cisplatin and Poly(ADP-Ribose) Polymerase Inhibitors. J. Biol. Chem. 2014, 289, 9247–9253. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Wai, P.T.; Maguire, S.L.; Daley, F.; Haider, S.; Kriplani, D.; Campbell, J.; Mirza, H.; Grigoriadis, A.; Tutt, A.; et al. Evaluation of CDK12 Protein Expression as a Potential Novel Biomarker for DNA Damage Response Targeted Therapies in Breast Cancer. Mol. Cancer Ther. 2018, 17, 306. [Google Scholar] [CrossRef] [PubMed]
- Rhyasen, G.W.; Yao, Y.; Zhang, J.; Dulak, A.; Castriotta, L.; Jacques, K.; Zhao, W.; Gharahdaghi, F.; Hattersley, M.M.; Lyne, P.D.; et al. BRD4 Amplification Facilitates an Oncogenic Gene Expression Program in High-Grade Serous Ovarian Cancer and Confers Sensitivity to BET Inhibitors. PLoS ONE 2018, 13, e0200826. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, Y.; Wang, H.; Stewart, S.; van der Jeught, K.; Agarwal, P.; Zhang, Y.; Liu, S.; Zhao, G.; et al. Precise Targeting of POLR2A as a Therapeutic Strategy for Human Triple Negative Breast Cancer. Nat. Nanotechnol. 2019, 14, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kwiatkowski, N.; Olson, C.M.; Dixon-Clarke, S.E.; Abraham, B.J.; Greifenberg, A.K.; Ficarro, S.B.; Elkins, J.M.; Liang, Y.; Hannett, N.M.; et al. Covalent Targeting of Remote Cysteine Residues to Develop CDK12 and CDK13 Inhibitors. Nat. Chem. Biol. 2016, 12, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Quereda, V.; Bayle, S.; Vena, F.; Frydman, S.M.; Monastyrskyi, A.; Roush, W.R.; Duckett, D.R. Therapeutic Targeting of CDK12/CDK13 in Triple-Negative Breast Cancer. Cancer Cell 2019, 36, 545–558.e7. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.M.; Wang, E.S.; Johannessen, L.; Kwiatkowski, N.; Sim, T.; Gray, N.S. Development of Highly Potent and Selective Steroidal Inhibitors and Degraders of CDK8. ACS Med. Chem. Lett. 2018, 9, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Gao, Y.; Che, J.; Lu, W.; Kaltheuner, I.H.; Dries, R.; Kalocsay, M.; Berberich, M.J.; Jiang, J.; You, I.; et al. Discovery and Resistance Mechanism of a Selective CDK12 Degrader. Nat. Chem. Biol. 2021, 17, 675–683. [Google Scholar] [CrossRef]
- Zengerle, M.; Chan, K.H.; Ciulli, A. Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem. Biol. 2015, 10, 1770. [Google Scholar] [CrossRef]
- Gilan, O.; Rioja, I.; Knezevic, K.; Bell, M.J.; Yeung, M.M.; Harker, N.R.; Lam, E.Y.N.; Chung, C.; Bamborough, P.; Petretich, M.; et al. Selective Targeting of BD1 and BD2 of the BET Proteins in Cancer and Immunoinflammation. Science 2020, 368, 387–394. [Google Scholar] [CrossRef]
- Faivre, E.J.; McDaniel, K.F.; Albert, D.H.; Mantena, S.R.; Plotnik, J.P.; Wilcox, D.; Zhang, L.; Bui, M.H.; Sheppard, G.S.; Wang, L.; et al. Selective Inhibition of the BD2 Bromodomain of BET Proteins in Prostate Cancer. Nature 2020, 578, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Vohidov, F.; Andersen, J.N.; Economides, K.D.; Shipitsin, M.v.; Burenkova, O.; Ackley, J.C.; Vangamudi, B.; Nguyen, H.V.T.; Gallagher, N.M.; Shieh, P.; et al. Design of BET Inhibitor Bottlebrush Prodrugs with Superior Efficacy and Devoid of Systemic Toxicities. J. Am. Chem. Soc. 2021, 143, 4714–4724. [Google Scholar] [CrossRef]
- Wan, X.; Sun, R.; Bao, Y.; Zhang, C.; Wu, Y.; Gong, Y. In Vivo Delivery of SiRNAs Targeting EGFR and BRD4 Expression by Peptide-Modified Redox Responsive PEG-PEI Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2021, 18, 3990–3998. [Google Scholar] [CrossRef] [PubMed]
- Llombart, V.; Mansour, M.R. Therapeutic Targeting of “Undruggable” MYC. EBioMedicine 2022, 75, 103756. [Google Scholar] [CrossRef]
- Pott, S.; Lieb, J.D. What Are Super-Enhancers? Nat. Genet. 2014, 47, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET Bromodomain Inhibition as a Therapeutic Strategy to Target C-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Frumm, S.M.; Alexe, G.; Bassil, C.F.; Qi, J.; Chanthery, Y.H.; Nekritz, E.A.; Zeid, R.; Gustafson, W.C.; Greninger, P.; et al. Targeting MYCN in Neuroblastoma by BET Bromodomain Inhibition. Cancer Discov. 2013, 3, 309–323. [Google Scholar] [CrossRef]
- Huang, H.; Hu, J.; Maryam, A.; Huang, Q.; Zhang, Y.; Ramakrishnan, S.; Li, J.; Ma, H.; Ma, V.W.S.; Cheuk, W.; et al. Defining Super-Enhancer Landscape in Triple-Negative Breast Cancer by Multiomic Profiling. Nat. Commun. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Lovén, J.; Hoke, H.A.; Lin, C.Y.; Lau, A.; David, A.; Vakoc, C.R.; Bradner, J.E.; Lee, T.I.; Richard, A. Selective Inhibition of Tumor Oncogenes by Disruption of Super- Enhancers. Cell 2013, 153, 320–334. [Google Scholar] [CrossRef]
- Toyoshima, M.; Howie, H.L.; Imakura, M.; Walsh, R.M.; Annis, J.E.; Chang, A.N.; Frazier, J.; Chau, B.N.; Loboda, A.; Linsley, P.S.; et al. Functional Genomics Identifies Therapeutic Targets for MYC-Driven Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 9545–9550. [Google Scholar] [CrossRef]
- Baratta, M.G.; Schinzel, A.C.; Zwang, Y.; Bandopadhayay, P.; Bowman-Colin, C.; Kutt, J.; Curtis, J.; Piao, H.; Wong, L.C.; Kung, A.L.; et al. An In-Tumor Genetic Screen Reveals That the BET Bromodomain Protein, BRD4, Is a Potential Therapeutic Target in Ovarian Carcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, 232–237. [Google Scholar] [CrossRef]
- Schafer, J.M.; Lehmann, B.D.; Gonzalez-Ericsson, P.I.; Marshall, C.B.; Beeler, J.S.; Redman, L.N.; Jin, H.; Sanchez, V.; Stubbs, M.C.; Scherle, P.; et al. Targeting MYCN-Expressing Triple-Negative Breast Cancer with BET and MEK Inhibitors. Sci. Transl. Med. 2020, 12, aaw8275. [Google Scholar] [CrossRef] [PubMed]
- Zuber, J.; Shi, J.; Wang, E.; Rappaport, A.R.; Herrmann, H.; Sison, E.A.; Magoon, D.; Qi, J.; Blatt, K.; Wunderlich, M.; et al. RNAi Screen Identifies Brd4 as a Therapeutic Target in Acute Myeloid Leukaemia. Nature 2011, 478, 524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Kwiatkowski, N.; Abraham, B.J.; Lee, T.I.; Xie, S.; Yuzugullu, H.; Von, T.; Li, H.; Lin, Z.; et al. CDK7-Dependent Transcriptional Addiction in Triple-Negative Breast Cancer. Cell 2015, 163, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Raisner, R.; Bainer, R.; Haverty, P.M.; Benedetti, K.L.; Gascoigne, K.E. Super-Enhancer Acquisition Drives Oncogene Expression in Triple Negative Breast Cancer. PLoS ONE 2020, 15, e0235343. [Google Scholar] [CrossRef] [PubMed]
- Rathert, P.; Roth, M.; Neumann, T.; Muerdter, F.; Roe, J.S.; Muhar, M.; Deswal, S.; Cerny-Reiterer, S.; Peter, B.; Jude, J.; et al. Transcriptional Plasticity Promotes Primary and Acquired Resistance to BET Inhibition. Nature 2015, 525, 543–547. [Google Scholar] [CrossRef]
- Fong, C.Y.; Gilan, O.; Lam, E.Y.N.; Rubin, A.F.; Ftouni, S.; Tyler, D.; Stanley, K.; Sinha, D.; Yeh, P.; Morison, J.; et al. BET Inhibitor Resistance Emerges from Leukaemia Stem Cells. Nature 2015, 525, 538–542. [Google Scholar] [CrossRef]
- Kuuluvainen, E.; Domènech-Moreno, E.; Niemelä, E.H.; Mäkelä, T.P. Depletion of Mediator Kinase Module Subunits Represses Superenhancer-Associated Genes in Colon Cancer Cells. Mol. Cell Biol. 2018, 38, e00573-17. [Google Scholar] [CrossRef]
- Saenz, D.T.; Fiskus, W.; Mill, C.P.; Perera, D.; Manshouri, T.; Lara, B.H.; Karkhanis, V.; Sharma, S.; Horrigan, S.K.; Bose, P.; et al. Mechanistic Basis and Efficacy of Targeting the β-Catenin-TCF7L2-JMJD6-c-Myc Axis to Overcome Resistance to BET Inhibitors. Blood 2020, 135, 1255–1269. [Google Scholar] [CrossRef]
- Pohl, S.G.; Brook, N.; Agostino, M.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A. Wnt Signaling in Triple-Negative Breast Cancer. Oncogenesis 2017, 6, e310. [Google Scholar] [CrossRef]
- Morris, E.J.; Ji, J.Y.; Yang, F.; di Stefano, L.; Herr, A.; Moon, N.S.; Kwon, E.J.; Haigis, K.M.; Näär, A.M.; Dyson, N.J. E2F1 Represses β-Catenin Transcription and Is Antagonized by Both PRB and CDK8. Nature 2008, 455, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Wang, T.; Wang, L.; Tan, Z.; Wei, W.; Yan, B.; Zhao, J.; Wu, K.; Yang, A.; et al. MicroRNA-26a Is a Key Regulon That Inhibits Progression and Metastasis of c-Myc/EZH2 Double High Advanced Hepatocellular Carcinoma. Cancer Lett. 2018, 426, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.; Kadota, T.; Horie, T.; Tokumura, K.; Terada, R.; Kitaguchi, Y.; Park, G.; Ochiai, S.; Iwahashi, S.; Okayama, Y.; et al. CDK8 Maintains Stemness and Tumorigenicity of Glioma Stem Cells by Regulating the C-MYC Pathway. Oncogene 2021, 40, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Xia, F.; Wang, W.; Lei, Y.; Bo, J.; Li, X. CDK12 Promotes Papillary Thyroid Cancer Progression through Regulating the C-Myc/β-Catenin Pathway. J. Cancer 2020, 11, 4308. [Google Scholar] [CrossRef]
- Pelish, H.E.; Liau, B.B.; Nitulescu, I.I.; Tangpeerachaikul, A.; Poss, Z.C.; da Silva, D.H.; Caruso, B.T.; Arefolov, A.; Fadeyi, O.; Christie, A.L.; et al. Mediator Kinase Inhibition Further Activates Super-Enhancer-Associated Genes in AML. Nature 2015, 526, 273–276. [Google Scholar] [CrossRef]
- Sooraj, D.; Sun, C.; Doan, A.; Garama, D.J.; Dannappel, M.v.; Zhu, D.; Chua, H.K.; Mahara, S.; Wan Hassan, W.A.; Tay, Y.K.; et al. MED12 and BRD4 Cooperate to Sustain Cancer Growth upon Loss of Mediator Kinase. Mol. Cell 2022, 82, 123–139.e7. [Google Scholar] [CrossRef]
- Kwiatkowski, N.; Zhang, T.; Rahl, P.B.; Abraham, B.J.; Reddy, J.; Ficarro, S.B.; Dastur, A.; Amzallag, A.; Ramaswamy, S.; Tesar, B.; et al. Targeting Transcription Regulation in Cancer with a Covalent CDK7 Inhibitor. Nature 2014, 511, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Chipumuro, E.; Marco, E.; Christensen, C.L.; Kwiatkowski, N.; Zhang, T.; Hatheway, C.M.; Abraham, B.J.; Sharma, B.; Yeung, C.; Altabef, A.; et al. CDK7 Inhibition Suppresses Super-Enhancer-Linked Oncogenic Transcription in MYCN-Driven Cancer. Cell 2014, 159, 1126–1139. [Google Scholar] [CrossRef]
- Christensen, C.L.; Kwiatkowski, N.; Abraham, B.J.; Carretero, J.; Al-Shahrour, F.; Zhang, T.; Chipumuro, E.; Herter-Sprie, G.S.; Akbay, E.A.; Altabef, A.; et al. Targeting Transcriptional Addictions in Small Cell Lung Cancer with a Covalent CDK7 Inhibitor. Cancer Cell 2014, 26, 909–922. [Google Scholar] [CrossRef]
- Cayrol, F.; Praditsuktavorn, P.; Fernando, T.M.; Kwiatkowski, N.; Marullo, R.; Calvo-Vidal, M.N.; Phillip, J.; Pera, B.; Yang, S.N.; Takpradit, K.; et al. THZ1 Targeting CDK7 Suppresses STAT Transcriptional Activity and Sensitizes T-Cell Lymphomas to BCL2 Inhibitors. Nat. Commun. 2017, 8, 14290. [Google Scholar] [CrossRef]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Anderson, A.; Harrison, A.; Lange, A.M.; Jin, G.; Watabe, K.; Lo, H.W. Interaction between STAT3 and GLI1/TGLI1 Oncogenic Transcription Factors Promotes the Aggressiveness of Triple-Negative and HER2-Enriched Breast Cancers. Oncogene 2018, 37, 2502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Zhang, Y.; Shang, E.; Shu, C.; Torrini, C.; Zhao, J.; Bianchetti, E.; Mela, A.; Humala, N.; Mahajan, A.; et al. HDAC Inhibitors Elicit Metabolic Reprogramming by Targeting Super-Enhancers in Glioblastoma Models. J. Clin. Investig. 2020, 130, 3699. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.J.; Shanle, E.K.; Khan, A.; Chua, K.F.; Hong, T.; Boxer, L.D.; Allis, C.D.; Josefowicz, S.Z.; Garcia, B.A.; Rothbart, S.B.; et al. HDAC Inhibition Results in Widespread Alteration of the Histone Acetylation Landscape and BRD4 Targeting to Gene Bodies. Cell Rep. 2021, 34, 108638. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.J.; Richmond, P.A.; Bunker, E.N.; Karman, S.S.; Azofeifa, J.; Garnett, A.T.; Xu, Q.; Wheeler, G.E.; Toomey, C.M.; Zhang, Q.; et al. Genome-Wide Dose-Dependent Inhibition of Histone Deacetylases Studies Reveal Their Roles in Enhancer Remodeling and Suppression of Oncogenic Super-Enhancers. Nucleic Acids Res. 2018, 46, 1756. [Google Scholar] [CrossRef]
- Poon, E.; Liang, T.; Jamin, Y.; Walz, S.; Kwok, C.; Hakkert, A.; Barker, K.; Urban, Z.; Thway, K.; Zeid, R.; et al. Orally Bioavailable CDK9/2 Inhibitor Shows Mechanism-Based Therapeutic Potential in MYCN-Driven Neuroblastoma. J. Clin. Investig. 2020, 130, 5875–5892. [Google Scholar] [CrossRef]
- Lu, H.; Xue, Y.; Yu, G.K.; Arias, C.; Lin, J.; Fong, S.; Faure, M.; Weisburd, B.; Ji, X.; Mercier, A.; et al. Compensatory Induction of MYC Expression by Sustained CDK9 Inhibition via a BRD4-Dependent Mechanism. Elife 2015, 4, 06535. [Google Scholar] [CrossRef]
- Dhimolea, E.; de Matos Simoes, R.; Kansara, D.; Al’Khafaji, A.; Bouyssou, J.; Weng, X.; Sharma, S.; Raja, J.; Awate, P.; Shirasaki, R.; et al. An Embryonic Diapause-like Adaptation with Suppressed Myc Activity Enables Tumor Treatment Persistence. Cancer Cell 2021, 39, 240–256.e11. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lujambio, A.; Zuber, J.; Tschaharganeh, D.F.; Doran, M.G.; Evans, M.J.; Kitzing, T.; Zhu, N.; de Stanchina, E.; Sawyers, C.L.; et al. CDK9-Mediated Transcription Elongation Is Required for MYC Addiction in Hepatocellular Carcinoma. Genes Dev. 2014, 28, 1800. [Google Scholar] [CrossRef]
- Rahl, P.B.; Lin, C.Y.; Seila, A.C.; Flynn, R.A.; McCuine, S.; Burge, C.B.; Sharp, P.A.; Young, R.A. C-Myc Regulates Transcriptional Pause Release. Cell 2010, 141, 432–445. [Google Scholar] [CrossRef]
- Patange, S.; Ball, D.A.; Wan, Y.; Karpova, T.S.; Girvan, M.; Levens, D.; Larson, D.R. MYC Amplifies Gene Expression through Global Changes in Transcription Factor Dynamics. Cell Rep. 2022, 38, 110292. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lovén, J.; Rahl, P.B.; Paranal, R.M.; Burge, C.B.; Bradner, J.E.; Lee, T.I.; Young, R.A. Transcriptional Amplification in Tumor Cells with Elevated C-Myc. Cell 2012, 151, 56. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Smith, E.R.; Aoi, Y.; Stoltz, K.L.; Katagi, H.; Woodfin, A.R.; Rendleman, E.J.; Marshall, S.A.; Murray, D.C.; Wang, L.; et al. Targeting Processive Transcription Elongation Via SEC Disruption for Myc Induced Cancer Therapy. Cell 2018, 175, 766. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shi, G.; Cheng, S.; Chen, J.; Wu, S.Y.; Wang, Z.; Xia, N.; Zhai, Y.; Wang, Z.; Peng, Y.; et al. SUMO Suppresses and MYC Amplifies Transcription Globally by Regulating CDK9 Sumoylation. Cell Res. 2018, 28, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, L.A.; Rn Von Eyss, B.; Carstensen, A.; Geyer, M.; Eilers, M.; Correspondence, N.P. Ubiquitin-Dependent Turnover of MYC Antagonizes MYC/PAF1C Complex Accumulation to Drive Transcriptional Elongation. Mol. Cell 2016, 61, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Endres, T.; Solvie, D.; Heidelberger, J.B.; Andrioletti, V.; Baluapuri, A.; Ade, C.P.; Muhar, M.; Eilers, U.; Vos, S.M.; Cramer, P.; et al. Ubiquitylation of MYC Couples Transcription Elongation with Double-Strand Break Repair at Active Promoters. Mol. Cell 2021, 81, 830–844.e13. [Google Scholar] [CrossRef] [PubMed]
- Herold, S.; Kalb, J.; Büchel, G.; Ade, C.P.; Baluapuri, A.; Xu, J.; Koster, J.; Solvie, D.; Carstensen, A.; Klotz, C.; et al. Recruitment of BRCA1 Limits MYCN-Driven Accumulation of Stalled RNA Polymerase. Nature 2019, 567, 545. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Menichini, P.; Speciale, A.; Cutrona, G.; Fais, F.; Taiana, E.; Neri, A.; Bomben, R.; Gentile, M.; Gattei, V.; et al. Heterogeneity of TP53 Mutations and P53 Protein Residual Function in Cancer: Does It Matter? Front. Oncol. 2020, 10, 593383. [Google Scholar] [CrossRef]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting P53 for the Treatment of Cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef]
- Yao, J.; Xu, S.; Sun, Y.; Xu, Y.; Guo, Q.; Wei, L. Novel CDK9 Inhibitor Oroxylin A Promotes Wild-Type P53 Stability and Prevents Hepatocellular Carcinoma Progression by Disrupting Both MDM2 and SIRT1 Signaling. Acta Pharmacol. Sin. 2022, 43, 1033–1045. [Google Scholar] [CrossRef]
- Štětková, M.; Growková, K.; Fojtík, P.; Valčíková, B.; Palušová, V.; Verlande, A.; Jorda, R.; Kryštof, V.; Hejret, V.; Alexiou, P.; et al. CDK9 Activity Is Critical for Maintaining MDM4 Overexpression in Tumor Cells. Cell Death Dis. 2020, 11, 754. [Google Scholar] [CrossRef]
- Wu, J.; Liang, Y.; Tan, Y.; Tang, Y.; Song, H.; Wang, Z.; Li, Y.; Lu, M. CDK9 Inhibitors Reactivate P53 by Downregulating IASPP. Cell Signal 2020, 67, 109508. [Google Scholar] [CrossRef]
- Minzel, W.; Venkatachalam, A.; Fink, A.; Hung, E.; Brachya, G.; Burstain, I.; Shaham, M.; Rivlin, A.; Omer, I.; Zinger, A.; et al. Small Molecules Co-Targeting CKIα and the Transcriptional Kinases CDK7/9 Control AML in Preclinical Models. Cell 2018, 175, 171–185.e25. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, J.; Saha, B.; Powell, C.; Mo, Q.; Perez, B.A.; Chellappan, S. Inhibitors Targeting CDK9 Show High Efficacy against Osimertinib and AMG510 Resistant Lung Adenocarcinoma Cells. Cancers 2021, 13, 3906. [Google Scholar] [CrossRef] [PubMed]
- Bugai, A.; Quaresma, A.J.C.; Friedel, C.C.; Ule, J.; DöLken, L.; Matja, Z.; Barbori, L. P-TEFb Activation by RBM7 Shapes a Pro-Survival Transcriptional Response to Genotoxic Stress. Mol. Cell 2019, 74, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Seelman, S.L.; Qin, L.X.; Schwartz, G.K. The Cyclin-Dependent Kinase Inhibitor Flavopiridol Potentiates the Effects of Topoisomerase I Poisons by Suppressing Rad51 Expression in a P53-Dependent Manner. Cancer Res. 2008, 68, 2312–2320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latif, A.L.; Newcombe, A.; Li, S.; Gilroy, K.; Robertson, N.A.; Lei, X.; Stewart, H.J.S.; Cole, J.; Terradas, M.T.; Rishi, L.; et al. BRD4-Mediated Repression of P53 Is a Target for Combination Therapy in AML. Nat. Commun. 2021, 12, 241. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lee, A.Y.; Lai, H.T.; Zhang, H.; Chiang, C.M. Phospho Switch Triggers Brd4 Chromatin Binding and Activator Recruitment for Gene-Specific Targeting. Mol. Cell 2013, 49, 843–857. [Google Scholar] [CrossRef]
- Webber, L.P.; Yujra, V.Q.; Vargas, P.A.; Martins, M.D.; Squarize, C.H.; Castilho, R.M. Interference with the Bromodomain Epigenome Readers Drives P21 Expression and Tumor Senescence. Cancer Lett. 2019, 461, 10–20. [Google Scholar] [CrossRef]
- Rahnamoun, H.; Lee, J.; Sun, Z.; Lu, H.; Ramsey, K.M.; Komives, E.A.; Lauberth, S.M. RNAs Interact with BRD4 to Promote Enhanced Chromatin Engagement and Transcription Activation. Nat. Struct. Mol. Biol. 2018, 25, 66. [Google Scholar] [CrossRef]
- Rahnamoun, H.; Lu, H.; Duttke, S.H.; Benner, C.; Glass, C.K.; Lauberth, S.M. Mutant P53 Shapes the Enhancer Landscape of Cancer Cells in Response to Chronic Immune Signaling. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Ge, H.; Yao, Y.; Jiang, Y.; Wu, X.; Wang, Y. Pharmacological Inhibition of CDK7 by THZ1 Impairs Tumor Growth in P53-Mutated HNSCC. Oral. Dis. 2022, 28, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Kalan, S.; Amat, R.; Schachter, M.M.; Kwiatkowski, N.; Abraham, B.J.; Liang, Y.; Zhang, T.; Olson, C.M.; Larochelle, S.; Young, R.A.; et al. Activation of the P53 Transcriptional Program Sensitizes Cancer Cells to Cdk7 Inhibitors. Cell Rep. 2017, 21, 467–481. [Google Scholar] [CrossRef]
- Peng, J.; Yang, M.; Bi, R.; Wang, Y.; Wang, C.; Wei, X.; Zhang, Z.; Xie, X.; Wei, W. Targeting Mutated P53 Dependency in Triple-Negative Breast Cancer Cells through CDK7 Inhibition. Front. Oncol. 2021, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Donner, A.J.; Szostek, S.; Hoover, J.M.; Espinosa, J.M. CDK8 Is a Stimulus-Specific Positive Coregulator of P53 Target Genes. Mol. Cell 2007, 27, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Hoshii, T.; Cifani, P.; Feng, Z.; Huang, C.H.; Koche, R.; Chen, C.W.; Delaney, C.D.; Lowe, S.W.; Kentsis, A.; Armstrong, S.A. A Non-Catalytic Function of SETD1A Regulates Cyclin K and the DNA Damage Response. Cell 2018, 172, 1007–1021.e17. [Google Scholar] [CrossRef]
- Dieter, S.M.; Siegl, C.; Codó, P.L.; Huerta, M.; Ostermann-Parucha, A.L.; Schulz, E.; Zowada, M.K.; Martin, S.; Laaber, K.; Nowrouzi, A.; et al. Degradation of CCNK/CDK12 Is a Druggable Vulnerability of Colorectal Cancer. Cell Rep. 2021, 36, 109394. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Kulke, M.; Hechler, T.; van der Jeught, K.; Dong, T.; He, B.; Miller, K.D.; Radovich, M.; Schneider, B.P.; et al. Targeted Immunotherapy ForHER2-Low Breast Cancer with 17p Loss. Sci. Transl. Med. 2021, 13, 6894. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Han, C.; Wan, G.; Huang, X.; Ivan, C.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Rao, P.H.; et al. TP53 Loss Creates Therapeutic Vulnerability in Colorectal Cancer. Nature 2015, 520, 697–701. [Google Scholar] [CrossRef]
- Akashi-Tanaka, S.; Watanabe, C.; Takamaru, T.; Kuwayama, T.; Ikeda, M.; Ohyama, H.; Mori, M.; Yoshida, R.; Hashimoto, R.; Terumasa, S.; et al. BRCAness Predicts Resistance to Taxane-Containing Regimens in Triple Negative Breast Cancer during Neoadjuvant Chemotherapy. Clin. Breast. Cancer 2015, 15, 80–85. [Google Scholar] [CrossRef]
- Lips, E.H.; Mulder, L.; Oonk, A.; van der Kolk, L.E.; Hogervorst, F.B.L.; Imholz, A.L.T.; Wesseling, J.; Rodenhuis, S.; Nederlof, P.M. Triple-Negative Breast Cancer: BRCAness and Concordance of Clinical Features with BRCA1-Mutation Carriers. Br. J. Cancer 2013, 108, 2172–2177. [Google Scholar] [CrossRef]
- Melinda, L.T.; Kirsten, M.T.; Julia, R.; Bryan, H.; Gordon, B.M.; Kristin, C.J.; Zoltan, S.; William, T.B.; Eric, P.W.; Nadine, M.T.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancers. Clin. Cancer Res. 2016, 22, 3764. [Google Scholar] [CrossRef]
- Dubbury, S.J.; Boutz, P.L.; Sharp, P.A. CDK12 Regulates DNA Repair Genes by Suppressing Intronic Polyadenylation. Nature 2018, 564, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Martinez, T.F.; Kim, S.; Donaldson, C.; Shokhirev, M.N.; Saghatelian, A.; Jones, K.A. CDK12 Phosphorylates 4E-BP1 to Enable MTORC1-Dependent Translation and Mitotic Genome Stability. Genes Dev. 2019, 33, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Chirackal Manavalan, A.P.; Pilarova, K.; Kluge, M.; Bartholomeeusen, K.; Rajecky, M.; Oppelt, J.; Khirsariya, P.; Paruch, K.; Krejci, L.; Friedel, C.C.; et al. CDK12 Controls G1/S Progression by Regulating RNAPII Processivity at Core DNA Replication Genes. EMBO Rep. 2019, 20, 47592. [Google Scholar] [CrossRef]
- Shyamsunder, P.; Sridharan, S.P.; Madan, V.; Dakle, P.; Zeya, C.; Kanojia, D.; Chng, W.J.; Ong, S.T.; Koeffler, H.P. THZ531 Induces a State of BRCAness in Multiple Myeloma Cells: Synthetic Lethality with Combination Treatment of THZ 531 with DNA Repair Inhibitors. Int. J. Mol. Sci. 2022, 23, 1207. [Google Scholar] [CrossRef]
- Bajrami, I.; Frankum, J.R.; Konde, A.; Miller, R.E.; Rehman, F.L.; Brough, R.; Campbell, J.; Sims, D.; Rafiq, R.; Hooper, S.; et al. Genome-Wide Profiling of Genetic Synthetic Lethality Identifies CDK12 as a Novel Determinant of PARP1/2 Inhibitor Sensitivity. Cancer Res. 2014, 74, 287–297. [Google Scholar] [CrossRef]
- Niu, T.; Li, K.; Jiang, L.; Zhou, Z.; Hong, J.; Chen, X.; Dong, X.; He, Q.; Cao, J.; Yang, B.; et al. Noncovalent CDK12/13 Dual Inhibitors-Based PROTACs Degrade CDK12-Cyclin K Complex and Induce Synthetic Lethality with PARP Inhibitor. Eur. J. Med. Chem. 2022, 228, 114012. [Google Scholar] [CrossRef]
- Johnson, S.F.; Cruz, C.; Greifenberg, A.K.; Dust, S.; Stover, D.G.; Chi, D.; Primack, B.; Cao, S.; Bernhardy, A.J.; Coulson, R.; et al. CDK12 Inhibition Reverses De Novo and Acquired PARP Inhibitor Resistance in BRCA Wild-Type and Mutated Models of Triple-Negative Breast Cancer. Cell Rep. 2016, 17, 2367–2381. [Google Scholar] [CrossRef]
- Popova, T.; Manie, E.; Boeva, V.; Battistella, A.; Goundiam, O.; Smith, N.K.; Mueller, C.R.; Raynal, V.; Mariani, O.; Sastre-Garau, X.; et al. Ovarian Cancers Harboring Inactivating Mutations in CDK12 Display a Distinct Genomic Instability Pattern Characterized by Large Tandem Duplications. Cancer Res. 2016, 76, 1882–1891. [Google Scholar] [CrossRef]
- Wu, Y.M.; Cieślik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; Ning, Y.; Wang, L.; Kunju, L.P.; de Sarkar, N.; et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018, 173, 1770–1782.e14. [Google Scholar] [CrossRef]
- Menghi, F.; Barthel, F.P.; Yadav, V.; Verhaak, R.G.W.; Jonkers, J.; Liu Correspondence, E.T.; Tang, M.; Ji, B.; Tang, Z.; Carter, G.W.; et al. The Tandem Duplicator Phenotype Is a Prevalent Genome-Wide Cancer Configuration Driven by Distinct Gene Mutations Cancer Cell The Tandem Duplicator Phenotype Is a Prevalent Genome-Wide Cancer Configuration Driven by Distinct Gene Mutations. Cancer Cell 2018, 34, 197–210.e5. [Google Scholar] [CrossRef] [PubMed]
- He, D.D.; Shang, X.Y.; Wang, N.; Wang, G.X.; He, K.Y.; Wang, L.; Han, Z.G. BRD4 Inhibition Induces Synthetic Lethality in ARID2-Deficient Hepatocellular Carcinoma by Increasing DNA Damage. Oncogene 2022, 41, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Shan, W.; Hu, Z.; Yuan, J.; Pi, J.; Wang, Y.; Fan, L.; Tang, Z.; Li, C.; et al. Repression of BET Activity Sensitizes Homologous Recombination-Proficient Cancers to PARP Inhibition. Sci. Transl. Med. 2017, 9, 1645. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yin, J.; Fang, Y.; Chen, J.; Jeong, K.J.; Chen, X.; Vellano, C.P.; Ju, Z.; Zhao, W.; Zhang, D.; et al. BRD4 Inhibition Is Synthetic Lethal with PARP Inhibitors through the Induction of Homologous Recombination Deficiency. Cancer Cell 2018, 33, 401–416.e8. [Google Scholar] [CrossRef]
- Li, X.; Baek, G.H.; Ramanand, S.G.; Sharp, A.; Gao, Y.; Yuan, W.; Welti, J.; Rodrigues, D.N.; Dolling, D.; Figueiredo, I.; et al. BRD4 Promotes DNA Repair and Mediates the Formation of TMPRSS2-ERG Gene Rearrangements in Prostate Cancer. Cell Rep. 2018, 22, 796. [Google Scholar] [CrossRef]
- Miller, A.L.; Fehling, S.C.; Garcia, P.L.; Gamblin, T.L.; Council, L.N.; van Waardenburg, R.C.A.M.; Yang, E.S.; Bradner, J.E.; Yoon, K.J. The BET Inhibitor JQ1 Attenuates Double-Strand Break Repair and Sensitizes Models of Pancreatic Ductal Adenocarcinoma to PARP Inhibitors. EBioMedicine 2019, 44, 419–430. [Google Scholar] [CrossRef]
- Takashima, Y.; Kikuchi, E.; Kikuchi, J.; Suzuki, M.; Kikuchi, H.; Maeda, M.; Shoji, T.; Furuta, M.; Kinoshita, I.; Dosaka-Akita, H.; et al. Bromodomain and Extraterminal Domain Inhibition Synergizes with WEE1-Inhibitor AZD1775 Effect by Impairing Nonhomologous End Joining and Enhancing DNA Damage in Nonsmall Cell Lung Cancer. Int. J. Cancer 2020, 146, 1114–1124. [Google Scholar] [CrossRef]
- Zhang, B.; Lyu, J.; Liu, Y.; Wu, C.; Yang, E.J.; Pardeshi, L.; Tan, K.; Wong, K.H.; Chen, Q.; Xu, X.; et al. BRCA1 Deficiency Sensitizes Breast Cancer Cells to Bromodomain and Extra-Terminal Domain (BET) Inhibition. Oncogene 2018, 37, 6341–6356. [Google Scholar] [CrossRef]
- Wilson, A.J.; Stubbs, M.; Liu, P.; Ruggeri, B.; Khabele, D. The BET Inhibitor INCB054329 Reduces Homologous Recombination Efficiency and Augments PARP Inhibitor Activity in Ovarian Cancer. Gynecol. Oncol. 2018, 149, 575–584. [Google Scholar] [CrossRef]
- Fehling, S.C.; Miller, A.L.; Garcia, P.L.; Vance, R.B.; Yoon, K.J. The Combination of BET and PARP Inhibitors Is Synergistic in Models of Cholangiocarcinoma. Cancer Lett. 2020, 468, 48–58. [Google Scholar] [CrossRef]
- Karakashev, S.; Zhu, H.; Yokoyama, Y.; Zhao, B.; Fatkhutdinov, N.; Kossenkov, A.v.; Wilson, A.J.; Simpkins, F.; Speicher, D.; Khabele, D.; et al. BET Bromodomain Inhibition Synergizes with PARP Inhibitor in Epithelial Ovarian Cancer. Cell Rep. 2017, 21, 3398–3405. [Google Scholar] [CrossRef]
- Ni, M.; Li, J.; Zhao, H.; Xu, F.; Cheng, J.; Yu, M.; Ke, G.; Wu, X. BRD4 Inhibition Sensitizes Cervical Cancer to Radiotherapy by Attenuating DNA Repair. Oncogene 2021, 40, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Pongas, G.; Kim, M.K.; Min, D.J.; House, C.D.; Jordan, E.; Caplen, N.; Chakka, S.; Ohiri, J.; Kruhlak, M.J.; Annunziata, C.M. BRD4 Facilitates DNA Damage Response and Represses CBX5/Heterochromatin Protein 1 (HP1). Oncotarget 2017, 8, 51402–51415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dulak, A.M.; Hattersley, M.M.; Willis, B.S.; Nikkilä, J.; Wang, A.; Lau, A.; Reimer, C.; Zinda, M.; Fawell, S.E.; et al. BRD4 Facilitates Replication Stress-Induced DNA Damage Response. Oncogene 2018, 37, 3763. [Google Scholar] [CrossRef]
- Veo, B.; Danis, E.; Pierce, A.; Wang, D.; Fosmire, S.; Sullivan, K.D.; Joshi, M.; Khanal, S.; Dahl, N.; Karam, S.; et al. Transcriptional Control of DNA Repair Networks by CDK7 Regulates Sensitivity to Radiation in MYC-Driven Medulloblastoma. Cell Rep. 2021, 35, 109013. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, Y.; Qin, T.; You, L.; Lu, F.; Hu, D.; Xiao, R.; Qin, X.; Guo, E.; Yang, B.; et al. AZD5153 Reverses Palbociclib Resistance in Ovarian Cancer by Inhibiting Cell Cycle-Related Proteins and the MAPK/PI3K-AKT Pathway. Cancer Lett. 2022, 528, 31–44. [Google Scholar] [CrossRef]
- Sun, B.; Fiskus, W.; Qian, Y.; Rajapakshe, K.; Raina, K.; Coleman, K.G.; Crew, A.P.; Shen, A.; Saenz, D.T.; Mill, C.P.; et al. BET Protein Proteolysis Targeting Chimera (PROTAC) Exerts Potent Lethal Activity against Mantle Cell Lymphoma Cells. Leukemia 2018, 32, 343–352. [Google Scholar] [CrossRef]
- Liao, S.; Maertens, O.; Cichowski, K.; Elledge, S.J. Genetic Modifiers of the BRD4-NUT Dependency of NUT Midline Carcinoma Uncovers a Synergism between BETis and CDK4/6is. Genes Dev. 2018, 32, 1188–1200. [Google Scholar] [CrossRef]
- Ge, J.Y.; Shu, S.; Kwon, M.; Jovanović, B.; Murphy, K.; Gulvady, A.; Fassl, A.; Trinh, A.; Kuang, Y.; Heavey, G.A.; et al. Acquired Resistance to Combined BET and CDK4/6 Inhibition in Triple-Negative Breast Cancer. Nat. Commun. 2020, 11, 14. [Google Scholar] [CrossRef]
- Sahni, J.M.; Gayle, S.S.; Webb, B.M.; Weber-Bonk, K.L.; Seachrist, D.D.; Singh, S.; Sizemore, S.T.; Restrepo, N.A.; Bebek, G.; Scacheri, P.C.; et al. Mitotic Vulnerability in Triple-Negative Breast Cancer Associated with LIN9 Is Targetable with BET Inhibitors. Cancer Res. 2017, 77, 5395–5408. [Google Scholar] [CrossRef]
- Nieto-Jimenez, C.; Galan-Moya, E.M.; Corrales-Sanchez, V.; Noblejas-Lopez, M.d.M.; Burgos, M.; Domingo, B.; Montero, J.C.; Gomez-Juarez, M.; Picazo-Martinez, M.G.; Esparis-Ogando, A.; et al. Inhibition of the Mitotic Kinase PLK1 Overcomes Therapeutic Resistance to BET Inhibitors in Triple Negative Breast Cancer. Cancer Lett. 2020, 491, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.M.; Liang, Y.; Leggett, A.; Park, W.D.; Li, L.; Mills, C.E.; Elsarrag, S.Z.; Ficarro, S.B.; Zhang, T.; Düster, R.; et al. Development of a Selective CDK7 Covalent Inhibitor Reveals Predominant Cell-Cycle Phenotype. Cell Chem. Biol. 2019, 26, 792–803.e10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Christensen, C.L.; Dries, R.; Oser, M.G.; Deng, J.; Diskin, B.; Li, F.; Pan, Y.; Zhang, X.; Yin, Y.; et al. CDK7 Inhibition Potentiates Genome Instability Triggering Anti-Tumor Immunity in Small Cell Lung Cancer. Cancer Cell 2020, 37, 37–54.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, H.; Wang, X.; Yin, X.; Ma, P.; Jing, Y.; Cai, M.-C.; Liu, J.; Zhang, M.; Zhang, S.; et al. Preclinical Efficacy and Molecular Mechanism of Targeting CDK7-Dependent Transcriptional Addiction in Ovarian Cancer. Mol. Cancer Ther. 2017, 16, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, S.; Ribas, R.; Simigdala, N.; Schuster, E.; Nikitorowicz-Buniak, J.; Ressa, A.; Gao, Q.; Leal, M.F.; Bhamra, A.; Thornhill, A.; et al. Tumour Kinome Re-Wiring Governs Resistance to Palbociclib in Oestrogen Receptor Positive Breast Cancers, Highlighting New Therapeutic Modalities. Oncogene 2020, 39, 4781–4797. [Google Scholar] [CrossRef]
- Albero, R.; Enjuanes, A.; Demajo, S.; Castellano, G.; Pinyol, M.; García, N.; Capdevila, C.; Clot, G.; Suárez-Cisneros, H.; Shimada, M.; et al. Cyclin D1 Overexpression Induces Global Transcriptional Downregulation in Lymphoid Neoplasms. J. Clin. Investig. 2018, 128, 4132–4147. [Google Scholar] [CrossRef]
- Warner, E.; Herberts, C.; Fu, S.; Yip, S.; Wong, A.; Wang, G.; Ritch, E.; Murtha, A.J.; Vandekerkhove, G.; Fonseca, N.M.; et al. BRCA2, ATM, and CDK12 Defects Differentially Shape Prostate Tumor Driver Genomics and Clinical Aggression. Clin. Cancer Res. 2021, 27, 1650–1662. [Google Scholar] [CrossRef]
- García-Muse, T.; Aguilera, A. R Loops: From Physiological to Pathological Roles. Cell 2019, 179, 604–618. [Google Scholar] [CrossRef]
- Marnef, A.; Cohen, S.; Legube, G. Transcription-Coupled DNA Double-Strand Break Repair: Active Genes Need Special Care. J. Mol. Biol. 2017, 429, 1277–1288. [Google Scholar] [CrossRef]
- Kotsantis, P.; Silva, L.M.; Irmscher, S.; Jones, R.M.; Folkes, L.; Gromak, N.; Petermann, E. Increased Global Transcription Activity as a Mechanism of Replication Stress in Cancer. Nat. Commun. 2016, 7, 13087. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, S.Y.; Gong, F.; Battenhouse, A.M.; Boutz, D.R.; Bashyal, A.; Refvik, S.T.; Chiang, C.-M.; Xhemalce, B.; Paull, T.T.; et al. Systematic Bromodomain Protein Screens Identify Homologous Recombination and R-Loop Suppression Pathways Involved in Genome Integrity. Genes Dev. 2019, 33, 1751–1774. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.S.; Maganti, R.; Tanksley, J.P.; Luo, J.; Park, J.J.H.; Balkanska-Sinclair, E.; Ling, J.; Floyd, S.R. BRD4 Prevents R-Loop Formation and Transcription-Replication Conflicts by Ensuring Efficient Transcription Elongation. Cell Rep. 2020, 32, 108166. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Kong, Y.W.; Huang, Q.; Vu Han, T.L.; Maffa, A.D.; Kasper, E.M.; Yaffe, M.B. BRD4 Prevents the Accumulation of R-Loops and Protects against Transcription-Replication Collision Events and DNA Damage. Nat. Commun. 2020, 11, 4083. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.Y.; Zhang, X.; Gu, Y.; Xiao, R.; Shao, C.; Tang, P.; Qian, H.; Luo, D.; Li, H.; et al. R-ChIP Using Inactive RNase H Reveals Dynamic Coupling of R-Loops with Transcriptional Pausing at Gene Promoters. Mol. Cell 2017, 68, 745. [Google Scholar] [CrossRef] [PubMed]
- Petermann, E.; Lan, L.; Zou, L. Sources, Resolution and Physiological Relevance of R-Loops and RNA–DNA Hybrids. Nat. Rev. Mol. Cell Biol. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Baranello, L.; Wojtowicz, D.; Lewis, B.A.; Zhao, K.; Levens, D. RNA Polymerase II Regulates Topoisomerase 1 Activity to Favor Efficient Transcription the N-Term Domain of TOP1 Mediates Interaction and Stimulation by RNAPII d BRD4 Inhibitors and TOP1 Inhibitors Synergize in Killing Cells in Brief the Transcription Machinery Directly Controls Topoisomerase 1 Activity to Adjust DNA Topology throughout the Transcription Cycle. Cell 2016, 165, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular Alterations in Triple-Negative Breast Cancer—the Road to New Treatment Strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Barrett, M.T.; Anderson, K.S.; Lenkiewicz, E.; Andreozzi, M.; Cunliffe, H.E.; Klassen, C.L.; Dueck, A.C.; McCullough, A.E.; Reddy, S.K.; Ramanathan, R.K.; et al. Genomic Amplification of 9p24.1 Targeting JAK2, PD-L1, and PD-L2 Is Enriched in High-Risk Triple Negative Breast Cancer. Oncotarget 2015, 6, 26483–26493. [Google Scholar] [CrossRef] [PubMed]
- García-Teijido, P.; Cabal, M.L.; Fernández, I.P.; Pérez, Y.F. Tumor-Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin. Med. Insights Oncol. 2016, 10, 31. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Ha, G.; Gulati, R.; Brown, L.C.; McKay, R.R.; Dorff, T.; Hoge, A.C.H.; Reichel, J.; Vats, P.; Kilari, D.; et al. CDK12-Mutated Prostate Cancer: Clinical Outcomes With Standard Therapies and Immune Checkpoint Blockade. JCO Precis Oncol. 2020, 4, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Li, Q.; Zou, P.; Huang, X.; Wu, C.; Tan, L. CDK12/13 Inhibition Induces Immunogenic Cell Death and Enhances Anti-PD-1 Anticancer Activity in Breast Cancer. Cancer Lett. 2020, 495, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, M.C.; Bludau, I.; Stafford, C.A.; Hornung, V.; Mann, M. Phosphoproteome Profiling Uncovers a Key Role for CDKs in TNF Signaling. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.L.; Kellner, D.; Bajrami, B.; Anderson, J.E.; Beyna, M.; Bhisetti, G.; Cameron, T.; Capacci, A.G.; Bertolotti-Ciarlet, A.; Feng, J.; et al. CDK12-Mediated Transcriptional Regulation of Noncanonical NF-KB Components Is Essential for Signaling. Sci. Signal. 2018, 11, 8216. [Google Scholar] [CrossRef]
- Zhang, H.; Pandey, S.; Travers, M.; Sun, H.; Morton, G.; Madzo, J.; Chung, W.; Khowsathit, J.; Perez-Leal, O.; Barrero, C.A.; et al. Targeting CDK9 Reactivates Epigenetically Silenced Genes in Cancer. Cell 2018, 175, 1244. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, L.; Shen, J.Z.; Liang, Z.; Wu, Q.; Yang, K.; Min, L.; Gimple, R.C.; Yang, Q.; Bhargava, S.; et al. Transcription Elongation Machinery Is a Druggable Dependency and Potentiates Immunotherapy in Glioblastoma Stem Cells. Cancer Discov. 2022, 12, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Tian, F.; Zhan, Y.; Kong, D. Cyclin-Dependent Kinase 9 Expression and Its Association with CD8+ T Cell Infiltration in Microsatellite-Stable Colorectal Cancer. Oncol. Lett. 2019, 18, 6046–6056. [Google Scholar] [CrossRef]
- Modur, V.; Singh, N.; Mohanty, V.; Chung, E.; Muhammad, B.; Choi, K.; Chen, X.; Chetal, K.; Ratner, N.; Salomonis, N.; et al. Defective Transcription Elongation in a Subset of Cancers Confers Immunotherapy Resistance. Nat. Commun. 2018, 9, 4410. [Google Scholar] [CrossRef]
- Chen, E.W.; Tay, N.Q.; Brzostek, J.; Gascoigne, N.R.J.; Rybakin, V. A Dual Inhibitor of Cdc7/Cdk9 Potently Suppresses T Cell Activation. Front. Immunol. 2019, 10, 1718. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Lin, Z.; Zhang, S.; Chen, Y.; Tang, J.; Hong, J.; Zhou, X.; Zong, Y.; Xu, Y.; et al. CDK7 Inhibitor THZ1 Enhances AntiPD-1 Therapy Efficacy via the P38α/MYC/PD-L1 Signaling in Non-Small Cell Lung Cancer. J. Hematol. Oncol. 2020, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zheng, Z.; Liu, J.; Chen, Y.; Ding, J.; Hu, G.; Hu, Y.; Liu, S.; Luo, W.; Xia, N.; et al. Targeting Triple-Negative Breast Cancer with Combination Therapy of EGFR CAR T Cells and CDK7 Inhibition. Cancer Immunol. Res. 2021, 9, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, C.; Bian, H.; Qian, W.; Jin, K.; Xu, T.; Guo, X.; Lu, X.; Su, F. Targeting CDK7 Suppresses Super Enhancer-Linked Inflammatory Genes and Alleviates CAR T Cell-Induced Cytokine Release Syndrome. Mol. Cancer 2021, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bengsch, F.; Svoronos, N.; Rutkowski, M.R.; Bitler, B.G.; Allegrezza, M.J.; Yokoyama, Y.; Kossenkov, A.v.; Bradner, J.E.; Conejo-Garcia, J.R.; et al. BET Bromodomain Inhibition Promotes Anti-Tumor Immunity by Suppressing PD-L1 Expression. Cell Rep. 2016, 16, 2829–2837. [Google Scholar] [CrossRef]
- Hogg, S.J.; Vervoort, S.J.; Deswal, S.; Ott, C.J.; Li, J.; Cluse, L.A.; Beavis, P.A.; Darcy, P.K.; Martin, B.P.; Spencer, A.; et al. BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1. Cell Rep. 2017, 18, 2162–2174. [Google Scholar] [CrossRef]
- Ebine, K.; Kumar, K.; Pham, T.N.; Shields, M.A.; Collier, K.A.; Shang, M.; DeCant, B.T.; Urrutia, R.; Hwang, R.F.; Grimaldo, S.; et al. Interplay between Interferon Regulatory Factor 1 and BRD4 in the Regulation of PD-L1 in Pancreatic Stellate Cells. Sci Rep 2018, 8, 13225. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Rao, X.; Zhang, R.; Tang, J.; Zhang, D.; Jie, X.; Zhu, K.; Wang, X.; Xu, Y.; et al. BRD4-IRF1 Axis Regulates Chemoradiotherapy-Induced PD-L1 Expression and Immune Evasion in Non-Small Cell Lung Cancer. Clin. Transl. Med. 2022, 12, e718. [Google Scholar] [CrossRef]
- Jing, X.; Shao, S.; Zhang, Y.; Luo, A.; Zhao, L.; Zhang, L.; Gu, S.; Zhao, X. BRD4 Inhibition Suppresses PD-L1 Expression in Triple-Negative Breast Cancer. Exp. Cell Res. 2020, 392, 112034. [Google Scholar] [CrossRef]
- Yin, M.; Guo, Y.; Hu, R.; Cai, W.L.; Li, Y.; Pei, S.; Sun, H.; Peng, C.; Li, J.; Ye, R.; et al. Potent BRD4 Inhibitor Suppresses Cancer Cell-Macrophage Interaction. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Riganti, C.; Lingua, M.F.; Salaroglio, I.C.; Falcomatà, C.; Righi, L.; Morena, D.; Picca, F.; Oddo, D.; Kopecka, J.; Pradotto, M.; et al. Bromodomain Inhibition Exerts Its Therapeutic Potential in Malignant Pleural Mesothelioma by Promoting Immunogenic Cell Death and Changing the Tumor Immune-Environment. Oncoimmunology 2017, 7, 1398874. [Google Scholar] [CrossRef]
- Liu, A.; Fan, D.; Wang, Y. The BET Bromodomain Inhibitor I-BET151 Impairs Ovarian Cancer Metastasis and Improves Antitumor Immunity. Cell Tissue Res. 2018, 374, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Tan, X.; Risnik, D.; Gao, M.; Song, X.; Ermine, K.; Shen, L.; Wang, S.; Yu, J.; Zhang, L. BET Protein Degradation Triggers DR5-Mediated Immunogenic Cell Death to Suppress Colorectal Cancer and Potentiate Immune Checkpoint Blockade. Oncogene 2021, 40, 6566–6578. [Google Scholar] [CrossRef]
- Wellinger, L.C.; Hogg, S.J.; Newman, D.M.; Friess, T.; Geiss, D.; Michie, J.; Ramsbottom, K.M.; Bacac, M.; Fauti, T.; Marbach, D.; et al. BET Inhibition Enhances TNF-Mediated Antitumor Immunity. Cancer Immunol. Res. 2022, 10, 87–107. [Google Scholar] [CrossRef]
- Zou, Z.; Huang, B.; Wu, X.; Zhang, H.; Qi, J.; Bradner, J.; Nair, S.; Chen, L.F. Brd4 Maintains Constitutively Active NF-ΚB in Cancer Cells by Binding to Acetylated RelA. Oncogene 2014, 33, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, X.; Zhao, S.; Liao, X.; Younis, M.R.; Wang, S.; Zhang, C.; Lu, G. JQ1-Loaded Polydopamine Nanoplatform Inhibits c-MYC/Programmed Cell Death Ligand 1 to Enhance Photothermal Therapy for Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2019, 11, 46626–46636. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, D.; Yu, H.; Feng, B.; Zhou, F.; Zhang, H.; Zhou, L.; Jiao, S.; Li, Y. A Cancer Vaccine-Mediated Postoperative Immunotherapy for Recurrent and Metastatic Tumors. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kagoya, Y.; Nakatsugawa, M.; Yamashita, Y.; Ochi, T.; Guo, T.; Anczurowski, M.; Saso, K.; Butler, M.O.; Arrowsmith, C.H.; Hirano, N. BET Bromodomain Inhibition Enhances T Cell Persistence and Function in Adoptive Immunotherapy Models. J. Clin. Investig. 2016, 126, 3479–3494. [Google Scholar] [CrossRef]
- Kong, W.; Dimitri, A.; Wang, W.; Jung, I.Y.; Ott, C.J.; Fasolino, M.; Wang, Y.; Kulikovskaya, I.; Gupta, M.; Yoder, T.; et al. BET Bromodomain Protein Inhibition Reverses Chimeric Antigen Receptor Extinction and Reinvigorates Exhausted T Cells in Chronic Lymphocytic Leukemia. J. Clin. Investig. 2021, 131, 1–16. [Google Scholar] [CrossRef]
- Andrieu, G.P.; Shafran, J.S.; Smith, C.L.; Belkina, A.C.; Casey, A.N.; Jafari, N.; Denis, G.V. BET Protein Targeting Suppresses the PD-1/PD-L1 Pathway in Triple-Negative Breast Cancer and Elicits Anti-Tumor Immune Response. Cancer Lett. 2019, 465, 45–58. [Google Scholar] [CrossRef]
- Leal, A.S.; Liu, P.; Krieger-Burke, T.; Ruggeri, B.; Liby, K.T. The Bromodomain Inhibitor, INCB057643, Targets Both Cancer Cells and the Tumor Microenvironment in Two Preclinical Models of Pancreatic Cancer. Cancers 2020, 13, 96. [Google Scholar] [CrossRef]
- Abruzzese, M.P.; Bilotta, M.T.; Fionda, C.; Zingoni, A.; Soriani, A.; Vulpis, E.; Borrelli, C.; Zitti, B.; Petrucci, M.T.; Ricciardi, M.R.; et al. Inhibition of Bromodomain and Extra-Terminal (BET) Proteins Increases NKG2D Ligand MICA Expression and Sensitivity to NK Cell-Mediated Cytotoxicity in Multiple Myeloma Cells: Role of CMYC-IRF4-MiR-125b Interplay. J. Hematol. Oncol. 2016, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Singh, A.R.; Liu, K.X.; Pham, T.v.; Zulcic, M.; Skola, D.; Chun, H.B.; Glass, C.K.; Morales, G.A.; Garlich, J.R.; et al. SF2523: Dual PI3K/BRD4 Inhibitor Blocks Tumor Immunosuppression and Promotes Adaptive Immune Responses in Cancer. Mol. Cancer Ther. 2019, 18, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, Y.; Yang, B.; Guo, E.; Wu, Y.; Huang, J.; Zhang, X.; Xiao, R.; Li, K.; Wang, B.; et al. BRD4 Inhibition by AZD5153 Promotes Antitumor Immunity via Depolarizing M2 Macrophages. Front. Immunol. 2020, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, N.; Banito, A.; Roe, J.S.; Alonso-Curbelo, D.; Camiolo, M.; Tschaharganeh, D.F.; Huang, C.H.; Aksoy, O.; Bolden, J.E.; Chen, C.C.; et al. BRD4 Connects Enhancer Remodeling to Senescence Immune Surveillance. Cancer Discov. 2016, 6, 613–629. [Google Scholar] [CrossRef]
- Hofmann, M.H.; Mani, R.; Engelhardt, H.; Impagnatiello, M.A.; Carotta, S.; Kerenyi, M.; Lorenzo-Herrero, S.; Böttcher, J.; Scharn, D.; Arnhof, H.; et al. Selective and Potent CDK8/19 Inhibitors Enhance NK-Cell Activity and Promote Tumor Surveillance. Mol. Cancer Ther. 2020, 19, 1018–1030. [Google Scholar] [CrossRef]
- Knab, V.M.; Gotthardt, D.; Klein, K.; Grausenburger, R.; Heller, G.; Menzl, I.; Prinz, D.; Trifinopoulos, J.; List, J.; Fux, D.; et al. Triple-Negative Breast Cancer Cells Rely on Kinase-Independent Functions of CDK8 to Evade NK-Cell-Mediated Tumor Surveillance. Cell Death Dis. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Witalisz-Siepracka, A.; Gotthardt, D.; Prchal-Murphy, M.; Didara, Z.; Menzl, I.; Prinz, D.; Edlinger, L.; Putz, E.M.; Sexl, V. NK Cell-Specific CDK8 Deletion Enhances Antitumor Responses. Cancer Immunol. Res. 2018, 6, 458–466. [Google Scholar] [CrossRef]
- Steinparzer, I.; Sedlyarov, V.; Rubin, J.D.; Eislmayr, K.; Galbraith, M.D.; Levandowski, C.B.; Vcelkova, T.; Sneezum, L.; Wascher, F.; Amman, F.; et al. Transcriptional Responses to IFN-γ Require Mediator Kinase-Dependent Pause Release and Mechanistically Distinct CDK8 and CDK19 Functions. Mol. Cell 2019, 76, 485–499.e8. [Google Scholar] [CrossRef]
- Lee, C.-K.; Rao, D.T.; Gertner, R.; Gimeno, R.; Frey, A.B.; Levy, D.E. Distinct Requirements for IFNs and STAT1 in NK Cell Function. J. Immunol. 2000, 165, 3571–3577. [Google Scholar] [CrossRef]
- Zhang, P.; He, F.; Bai, J.; Yamamoto, S.; Chen, S.; Zhang, L.; Sheng, M.; Zhang, L.; Guo, Y.; Man, N.; et al. Chromatin Regulator Asxl1 Loss and Nf1 Haploinsufficiency Cooperate to Accelerate Myeloid Malignancy. J. Clin. Investig. 2018, 128, 5383–5398. [Google Scholar] [CrossRef]
- Echevarría-Vargas, I.M.; Reyes-Uribe, P.I.; Guterres, A.N.; Yin, X.; Kossenkov, A.V.; Liu, Q.; Zhang, G.; Krepler, C.; Cheng, C.; Wei, Z.; et al. Co-Targeting BET and MEK as Salvage Therapy for MAPK and Checkpoint Inhibitor-Resistant Melanoma. EMBO Mol. Med. 2018, 10, e8446. [Google Scholar] [CrossRef] [PubMed]
- de Raedt, T.; Beert, E.; Pasmant, E.; Luscan, A.; Brems, H.; Ortonne, N.; Helin, K.; Hornick, J.L.; Mautner, V.; Kehrer-Sawatzki, H.; et al. PRC2 Loss Amplifies Ras-Driven Transcription and Confers Sensitivity to BRD4-Based Therapies. Nature 2014, 514, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.; Gong, K.; Song, K.; He, Y.; Shi, J. Enhanced JunD/RSK3 Signalling Due to Loss of BRD4/FOXD3/MiR-548d-3p Axis Determines BET Inhibition Resistance. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zawistowski, J.S.; Bevill, S.M.; Goulet, D.R.; Stuhlmiller, T.J.; Beltran, A.S.; Olivares-Quintero, J.F.; Singh, D.; Sciaky, N.; Parker, J.S.; Rashid, N.U.; et al. Enhancer Remodeling during Adaptive Bypass to MEK Inhibition Is Attenuated by Pharmacological Targeting of the P-TEFb Complex. Cancer Discov. 2017, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Kurimchak, A.M.; Shelton, C.; Herrera-Montavez, C.; Duncan, K.E.; Chernoff, J.; Duncan, J.S. Intrinsic Resistance to MEK Inhibition through BET Protein-Mediated Kinome Reprogramming in NF1-Deficient Ovarian Cancer. Mol. Cancer Res. 2019, 17, 1721–1734. [Google Scholar] [CrossRef]
- Duncan, J.S.; Whittle, M.C.; Nakamura, K.; Abell, A.N.; Midland, A.A.; Zawistowski, J.S.; Johnson, N.L.; Granger, D.A.; Jordan, N.V.; Darr, D.B.; et al. Dynamic Reprogramming of the Kinome in Response to Targeted MEK Inhibition in Triple-Negative Breast Cancer. Cell 2012, 149, 307–321. [Google Scholar] [CrossRef]
- Kitajima, S.; Asahina, H.; Chen, T.; Guo, S.; Quiceno, L.G.; Cavanaugh, J.D.; Merlino, A.A.; Tange, S.; Terai, H.; Kim, J.W.; et al. Overcoming Resistance to Dual Innate Immune and MEK Inhibition Downstream of KRAS. Cancer Cell 2018, 34, 439–452.e6. [Google Scholar] [CrossRef]
- Wyce, A.; Matteo, J.J.; Foley, S.W.; Felitsky, D.J.; Rajapurkar, S.R.; Zhang, X.P.; Musso, M.C.; Korenchuk, S.; Karpinich, N.O.; Keenan, K.M.; et al. MEK Inhibitors Overcome Resistance to BET Inhibition across a Number of Solid and Hematologic Cancers. Oncogenesis 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Yin, Y.; Sun, M.; Zhan, X.; Wu, C.; Geng, P.; Sun, X.; Wu, Y.; Zhang, S.; Qin, J.; Zhuang, Z.; et al. EGFR Signaling Confers Resistance to BET Inhibition in Hepatocellular Carcinoma through Stabilizing Oncogenic MYC. J. Exp. Clin. Cancer Res. 2019, 38, 1–15. [Google Scholar] [CrossRef]
- Wu, D.; Yan, Y.; Wei, T.; Ye, Z.; Xiao, Y.; Pan, Y.; Orme, J.J.; Wang, D.; Wang, L.; Ren, S.; et al. An Acetyl-Histone Vulnerability in PI3K/AKT Inhibition-Resistant Cancers Is Targetable by Both BET and HDAC Inhibitors. Cell Rep. 2021, 34, 108744. [Google Scholar] [CrossRef]
- Risom, T.; Langer, E.M.; Chapman, M.P.; Rantala, J.; Fields, A.J.; Boniface, C.; Alvarez, M.J.; Kendsersky, N.D.; Pelz, C.R.; Johnson-Camacho, K.; et al. Differentiation-State Plasticity Is a Targetable Resistance Mechanism in Basal-like Breast Cancer. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, R.; Sayad, A.; Brown, K.R.; Sanchez-Garcia, F.; Reimand, J.; Haider, M.; Virtanen, C.; Bradner, J.E.; Bader, G.D.; Mills, G.B.; et al. Functional Genomic Landscape of Human Breast Cancer Drivers, Vulnerabilities, and Resistance. Cell 2016, 164, 293. [Google Scholar] [CrossRef] [PubMed]
- Stratikopoulos, E.E.; Dendy, M.; Szabolcs, M.; Khaykin, A.J.; Lefebvre, C.; Zhou, M.M.; Parsons, R. Kinase and BET Inhibitors Together Clamp Inhibition of PI3K Signaling and Overcome Resistance to Therapy. Cancer Cell 2015, 27, 837. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, N.; Hasson, D.; Mathur, D.; Stratikopoulos, E.E.; Sachidanandam, R.; Bernstein, E.; Parsons, R.E. PTEN Interacts with the Transcription Machinery on Chromatin and Regulates RNA Polymerase II-Mediated Transcription. Nucleic Acids Res. 2019, 47, 5573. [Google Scholar] [CrossRef]
- Ghezzi, C.; Wong, A.; Chen, B.Y.; Ribalet, B.; Damoiseaux, R.; Clark, P.M. A High-Throughput Screen Identifies That CDK7 Activates Glucose Consumption in Lung Cancer Cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, T.; Terai, H.; Ficarro, S.B.; Kwiatkowski, N.; Hao, M.-F.F.; Sharma, B.; Christensen, C.L.; Chipumuro, E.; Wong, K.-k.; et al. Overcoming Resistance to the THZ Series of Covalent Transcriptional CDK Inhibitors. Cell Chem. Biol. 2018, 25, 135–142. [Google Scholar] [CrossRef]
- Krchniakova, M.; Skoda, J.; Neradil, J.; Chlapek, P.; Veselska, R. Repurposing Tyrosine Kinase Inhibitors to Overcome Multidrug Resistance in Cancer: A Focus on Transporters and Lysosomal Sequestration. Int. J. Mol. Sci. 2020, 21, 3157. [Google Scholar] [CrossRef]
- Urbanucci, A.; Barfeld, S.J.; Kytölä, V.; Itkonen, H.M.; Coleman, I.M.; Vodák, D.; Sjöblom, L.; Sheng, X.; Tolonen, T.; Minner, S.; et al. Androgen Receptor Deregulation Drives Bromodomain-Mediated Chromatin Alterations in Prostate Cancer. Cell Rep. 2017, 19, 2045–2059. [Google Scholar] [CrossRef]
- Asangani, I.A.; Dommeti, V.L.; Wang, X.; Malik, R.; Cieslik, M.; Yang, R.; Escara-Wilke, J.; Wilder-Romans, K.; Dhanireddy, S.; Engelke, C.; et al. Therapeutic Targeting of BET Bromodomain Proteins in Castration-Resistant Prostate Cancer. Nature 2014, 510, 278–282. [Google Scholar] [CrossRef]
- Wen, S.; He, Y.; Wang, L.; Zhang, J.; Quan, C.; Niu, Y.; Huang, H. Aberrant Activation of Super Enhancer and Choline Metabolism Drive Antiandrogen Therapy Resistance in Prostate Cancer. Oncogene 2020, 39, 6556–6571. [Google Scholar] [CrossRef]
- Richters, A.; Doyle, S.K.; Freeman, D.B.; Lee, C.; Leifer, B.S.; Jagannathan, S.; Kabinger, F.; Koren, J.V.; Struntz, N.B.; Urgiles, J.; et al. Modulating Androgen Receptor-Driven Transcription in Prostate Cancer with Selective CDK9 Inhibitors. Cell Chem. Biol. 2021, 28, 134–147.e14. [Google Scholar] [CrossRef]
- Gao, X.T.; Liang, J.; Wang, L.Y.; Zhang, Z.; Yuan, P.; Wang, J.; Gao, Y.; Ma, F.; Calagua, C.; Ye, H.; et al. Phosphorylation of the Androgen Receptor at Ser81 Is Co-Sustained by CDK1 and CDK9 and Leads to AR-Mediated Transactivation in Prostate Cancer. Mol. Oncol. 2021, 15, 1901–1920. [Google Scholar] [CrossRef]
- Chymkowitch, P.; le May, N.; Charneau, P.; Compe, E.; Egly, J.M. The Phosphorylation of the Androgen Receptor by TFIIH Directs the Ubiquitin/Proteasome Process. EMBO J. 2011, 30, 468. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Gollavilli, P.N.; Wang, S.; Asangani, I.A. Resistance to BET Inhibitor Leads to Alternative Therapeutic Vulnerabilities in Castration-Resistant Prostate Cancer. Cell Rep. 2018, 22, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Ur Rasool, R.; Natesan, R.; Deng, Q.; Aras, S.; Lal, P.; Effron, S.S.; Mitchell-Velasquez, E.; Posimo, J.M.; Carskadon, S.; Baca, S.C.; et al. CDK7 Inhibition Suppresses Castration-Resistant Prostate Cancer through MED1 Inactivation. Cancer Discov. 2019, 9, 1538. [Google Scholar] [CrossRef]
- Park, I.H.; Yang, H.N.; Jeon, S.Y.; Hwang, J.A.; Kim, M.K.; Kong, S.Y.; Shim, S.H.; Lee, K.S. Anti-Tumor Activity of BET Inhibitors in Androgen-Receptor-Expressing Triple-Negative Breast Cancer. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Menzl, I.; Witalisz-Siepracka, A.; Sexl, V. CDK8-Novel Therapeutic Opportunities. Pharmaceuticals 2019, 12, 92. [Google Scholar] [CrossRef]

| Target | Selection Most Selective Inhibitors a | Tested in TNBC In Vivo (PMID) b | Proposed Specific Effect In Vivo Model c | Combination Therapy or Formulation c |
|---|---|---|---|---|
| RNA pol II | α-amanitin, Oncrasin-1 | α-amanitin (33568521) | Inhibition of global transcription | Formulated as HER2-conjugated drug |
| CDK7 | BS-181, THZ1, THZ2, LDC4297, LY3405105, ICEC0942 (Samuraciclib), IV-361, YKL-5-124, SY-1365 (Mevociclib) | THZ1/THZ2 (26406377), | Suppression of super-enhancer gene transcription | - |
| THZ1 (33875483) | Suppression of immunosuppressive genes | EGFR CAR T cells | ||
| CDK8/ CDK19 | AS2863619, BI-1347, BRD6989, CCT251545, Corticostatin A, MSC2530818, SEL120, Senexin A/B, JH-XI-10-02 (CDK8i only), JH-XVI-178 | BI-1347 (32024684) | Activation of NK cells | SMAC mimetic |
| CDK8 shRNA (34689158) | Suppressing metastatic genes and activation of NK cells, effect on re-growth and metastasis | - | ||
| CDK9 | NVP2, AZ5576, LDC000067, AZD4573, BAY-1143572, BAY-1251152 (VIP152), LY2857785, (THAL-)SNS-032 | Compound 45 (34538051) | Downregulation key oncogenes, e.g., MYC and Mcl-1 | - |
| Complex 1 (35530158) | Especially anti-metastatic, also MYC/Mcl-1 levels reduced | - | ||
| NVP2 (+CDK1i, 33417832) | Inhibition of persister cells with reduced MYC expression | Docetaxel | ||
| 4ab (+CDK2i, 29144137) | Not described | - | ||
| CDK12/ CDK13 | THZ531, SR4835, BSJ-4-116 (CDK12i only) | SR4835 (31668947) | Suppression DNA damage response genes | DNA damaging agent & PARP inhibition |
| SR4835 (32941949) | Immunogenic cell death | Anti-PD1 therapy | ||
| BET/BRD4 | A1874, ABBV-075 (Mivebresib), ARV-771, ARV-825, BI-2536, Bromosporine, CPI-0610 (Pelabresib), CPI-203, dBET6, I-BET151 (GSK1210151A), I-BET726 (GSK1324726A), JQ1, MS417, MS436, MZ1, OTX015 (Birabresib), PF-6405761 (PFI-1) | JQ1 (27292261) | Suppression of hypoxia-induced genes and angiogenesis | - |
| JQ1 (32735909) | Expression of mitotic genes confers resistance to BETi | PLK inhibition | ||
| JQ1 (27650498) | Suppression of aurora kinases | - | ||
| JQ1 (26735014) | Basal to luminal dedifferentiation | - | ||
| JQ1 (32339606) | Suppression of PD-L1 expression induced by IFN-y | - | ||
| JQ1 & MS417 (24525235) | Suppression WNT5a expression and stem cell properties | - | ||
| JQ1 & dBET-6 (32416067) | Multiple factors synthetically lethal in JQ-1 resistant cells | JAK2, BCL2/BCL-XL, CDK4/6 inhibition | ||
| JQ1 & INCB054329 (32161105) | Suppression of N-MYC | MEK inhibition | ||
| BETd-246 (28209615) | Suppression of Mcl-1 | BCL-XL inhibition | ||
| MZ1 (31470872) | NA (G2/M arrest and apoptosis) | - | ||
| i-BET151 (28108460) | Suppression of chromatin remodeling upon MEKi | MEK inhibition | ||
| OTX-015 pro-drug (33739832) | Improved pharmacokinetics and reduced toxicity | Bottlebrush pro-drug formulation |
| Inhibitors a | Cancer Type b | Mono- or Combination Therapy | Trial ID, Phase and Status c | |
|---|---|---|---|---|
| CDK7i | SY5609 | HR+ BC, SCLC, PanCa | Fluvestrant, Gemcitabine or Nab-paclitaxel | NCT04247126 (Phase I) |
| SY1365 | OvCa, HR+ BC | Carboplatin, Fulvestrant | NCT03134638 (Phase I, terminated due to management decision) | |
| XL102 | OvCa, TNBC, HR+ BC, CRPC | Mono (TNBC) or Fulvestrant, Abiraterone, Prednisone | NCT04726332 (Phase I) | |
| CT7001 | TNBC, CRPC, HR+ breast cancer | Mono (TNBC) or Fulvestrant | NCT03363893 + NCT04802759 (Phase I/II) | |
| CDK9i | AZD4573 | Hematologic | Mono | NCT03263637 (Completed, Phase I), NCT05140382 + NCT04630756 (Phase I) |
| PRT2527 | Sarcomas, CRPC, HR+ BC, TNBC, tumors with MYC amplification | Mono | NCT05159518 (Phase I) | |
| GFH009 | Hematologic | Mono | NCT04588922 (Phase I) | |
| Fadraciclib/CYC065 | Various solid and lymphoma, including TNBC | Mono or Venetoclax | NCT02552953+ NCT05168904+ NCT04017546 (Phase I), NCT04983810 (Phase I solid tumors, Phase II lymphoma) | |
| KB-0742 | Solid tumors and non-Hodgkin lymphoma | Mono | NCT04718675 (Phase I) | |
| BAY 1251152 | Solid and hematologic | Mono or Pembrolizumab | NCT04978779+ NCT02635672 (Phase I), NCT02745743 (Phase I, completed) | |
| BAY1143572 | Various solid and hematologic | Mono or G-CSF | NCT01938638+ NCT02345382 (Phase I, completed) | |
| TP-1287 | Solid tumors and sarcoma | Mono | NCT03604783 (Phase I) | |
| CDK8i | TSN084 (also other targets) | Various solid and hematologic | Mono | NCT05300438 (Phase I) |
| SEL120 | AML, myelodysplastic syndrome | Mono | NCT04021368 (Phase I) | |
| BCD-115 | ER+HER2- BC | Endocrine therapy | NCT03065010 (Phase I, completed) | |
| BET/BRD4i | ZEN-3694 | Solid/lymphomas | Talazoparib, Ipilimumab/Nivolumab, Enzalutamide/Pembrolizumab, Talazoparib, Binimetinib, Entinostat, Etoposide+Cisplatin | NCT05327010 + NCT04471974 + NCT05071937 (Phase II), NCT04840589 + NCT05111561 (Phase I) NCT05053971 + NCT05019716 (Phase I/II), NCT02711956 (Phase I/II, completed), NCT02705469 (Phase I, completed) |
| FT-1101 | Hematologic | Azacitidine | NCT02543879 (Phase I, completed) | |
| RO6870810 | Multiple myeloma | Daratumumab | NCT02543879 (Phase I, completed) | |
| TQB3617 | Malignant tumors | Mono | NCT05110807 (Phase I) | |
| CPI-0610 | Multiple myeloma, lymphoma | Mono | NCT02157636 + NCT01949883 (Phase I, completed, NCT02158858 (Phase 1/2) | |
| BMS-986158 or BMS-986378 | Pedriatric solid tumors/brain tumors and lymphoma | Mono or Ruxolointib | NCT03936465 (Phase I) | |
| BMS-986158 | Various solid and hematologic | Mono or Nivolumab | NCT02419417 (phase I/II) | |
| GSK525762 | Various hematologic/solid, incl. TNBC | Mono | NCT01943851 (Phase II, completed), NCT01587703 (Phase I, completed) | |
| NUV868 | Solid cancers | Mono or Olaparib, Enzalutamide | NCT05252390 (Phase I/II) | |
| INCB054329 | Various solid/hematologic | Mono | NCT02431260 (Phase 1/2: Terminated due to PK variability) | |
| CC-95775 | Solid, non-Hodgkin lymphomas | Mono | NCT04089527 (Completed, Phase I) | |
| OTX015 | NUT-midline carcinoma, TNBC, NSCLC, CRPC, PDAC, AML, Lymphoma | Mono | NCT02259114 + NCT01713582 (Phase I, completed), NCT02698176 + NCT02698189 (Phase I, terminated: limited efficacy), NCT02296476 (Phase II, terminated: lack of activity) | |
| CC-90010 | Solid, non-Hodgkin lymphomas | Mono | NCT03220347 (Phase I) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Noord, V.E.; van de Water, B.; Le Dévédec, S.E. Targeting the Heterogeneous Genomic Landscape in Triple-Negative Breast Cancer through Inhibitors of the Transcriptional Machinery. Cancers 2022, 14, 4353. https://doi.org/10.3390/cancers14184353
van der Noord VE, van de Water B, Le Dévédec SE. Targeting the Heterogeneous Genomic Landscape in Triple-Negative Breast Cancer through Inhibitors of the Transcriptional Machinery. Cancers. 2022; 14(18):4353. https://doi.org/10.3390/cancers14184353
Chicago/Turabian Stylevan der Noord, Vera E., Bob van de Water, and Sylvia E. Le Dévédec. 2022. "Targeting the Heterogeneous Genomic Landscape in Triple-Negative Breast Cancer through Inhibitors of the Transcriptional Machinery" Cancers 14, no. 18: 4353. https://doi.org/10.3390/cancers14184353
APA Stylevan der Noord, V. E., van de Water, B., & Le Dévédec, S. E. (2022). Targeting the Heterogeneous Genomic Landscape in Triple-Negative Breast Cancer through Inhibitors of the Transcriptional Machinery. Cancers, 14(18), 4353. https://doi.org/10.3390/cancers14184353