Simple Summary
Hepatocellular carcinoma (HCC) is the most common primary liver cancer with expected increasing frequency in the next few decades. The Barcelona Clinic Liver Cancer (BCLC) Staging System is a widely adopted tool for guiding the therapeutic algorithms of patients with HCC. This classification has been guiding clinical practice for the last two decades. However, emerging data demonstrate that patients beyond the traditional criteria of operability or resectability can benefit from surgical treatment. We present the Greek multicentre experience of treating HCC within and beyond BCLC guidelines.
Abstract
Background: Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the third leading cause of death worldwide. The management of HCC is complex, with surgical treatment providing long-term survival in eligible patients. This study aims to present the experience of aggressive surgical management of HCC in Greece. Methods: This is a retrospective multicentre clinical study with 242 patients. Results: Most patients were male (79%) and had a median age of 71 yrs. According to the most recent BCLC criteria, 172 patients (71.1%) were classified as BCLC 0-A stage, 33 patients (13.6%) were classified as BCLC B, and 37 (15.3%) were classified as BCLC C. A total of 54% of the patients underwent major hepatectomy. Major postoperative morbidity was 15.6%, and the 90-day postoperative mortality rate was 4.5%. The median follow-up was 33.5 months. Three- and five-year overall survival was 65% and 48%, respectively. The median overall survival was 55 months. Significantly, five-year survival was 55% for BCLC A, and 34% and 21% for BCLC B and C, respectively. In univariate analysis, cirrhosis, type of resection (R status), and BCLC stage were associated with overall survival. Multivariate analysis indicated that R1 and R2 resections compared to R0, and BCLC C compared to BCLC 0-A, were independently associated with increased mortality. Conclusions: Aggressive surgical treatment of HCC offers satisfactory long-term survival prospects. A significant percentage (29%) of HCCs that underwent liver resection were of the intermediate and advanced BCLC stage. The management of patients with HCC should be discussed in multidisciplinary tumour board meetings on a case-by-case basis to be more effective.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver tumour (90% of tumours). It is also the 5th most common cancer worldwide and the second cause of mortality for men [1,2,3]. HCC usually occurs in the territory of pre-existing liver pathology [3,4]. Risk factors are hepatitis C virus (HCV) and hepatitis B virus (HBV), infection, alcoholic liver disease, aflatoxins, non-alcoholic fatty liver disease (NAFLD), and steatohepatitis (NASH) [5]. Hepatocarcinogenesis is a long process in which multiple molecular mechanisms are implicated [4,6,7,8,9,10]. Although effective antiviral agents for HBV and HCV and effective preventive measures, such as vaccination at birth against HBV infection, are available, the incidence of HCC is increasing [11]. The main reason for this is the increase in obesity and metabolic syndrome, closely related to NAFLD and NASH. NAFLD and NASH are expected to become the first cause of liver cirrhosis and hepatocellular carcinoma in the following decades [12,13].
During the last three decades, numerous staging systems have been proposed for the prognostication and decision-making guidance in HCC. Even though there is no consensus regarding the implementation of one universal staging system, since all of the proposed classifications have deficiencies, the Barcelona Clinic Liver Cancer (BCLC) Staging System remains the most widely used classification system for HCC management guidelines [14]. The main concern around the BCLC Classification is that it groups highly heterogeneous patients under the same stage, recommending treatment modalities that cannot be equally beneficial to all patients [15]. Thus, there is an emerging body of literature identifying patients who are not considered eligible for any surgical intervention with curative intent according to current BCLC recommendations, but can benefit from surgical treatment [16,17,18,19,20,21,22].
Furthermore, there have been significant advances over the last five years in the systemic treatment of HCC, as along with the first-line (Sorafenib, Lenvatinib) and second-line therapies (Regorafenib, cabozantinib, or ramucirumab), the use of immunotherapy and anti-angiogenetic therapy have improved overall survival [23,24,25]. Moreover, metronomic therapy has shown promising results [26,27].
This study aims to present the Greek national experience in the surgical management of HCC using a multicentre database of consecutive patients undergoing resection with curative intent over the last 14 years in five hepatobiliary (HPB) centres. This analysis captures the modifications made to BCLC guidelines, how these changes affected the surgical practice, and the current practice and outcomes of surgical management of HCC within and beyond BCLC guidelines.
2. Materials and Methods
This is a multicentre retrospective study on patients with HCC who underwent liver resection from January 2007 to December 2020. The study retrospectively analysed prospectively entered data using the Greek National HPB database. The study included 242 cases from five HPB Centres in Greece, which are, in alphabetical order: Hepatobiliary Centre, Department of Surgery, “Hygeia” Hospital Athens; HPB Unit, Department of Surgery, University Hospital of Ioannina, Ioannina; 1st Surgical Department, Laiko University Hospital, Athens; 2nd Propaedeutic Department of Surgery, Laiko University Hospital, Athens; Department of Surgical Oncology, St. Savvas Oncological Centre, Athens. The primary endpoint was the overall survival (OS) of patients undergoing hepatectomy for HCC in any BCLC stage. Secondary endpoints were postoperative morbidity and mortality. The type of liver resection was classified according to the Brisbane classification [28,29,30]. Postoperative complications were defined using the Dindo–Clavien classification [31]. Tumour burden score (TBS) was defined as the distance from the origin of a Cartesian plane, and calculated by using the maximum tumour diameter and number of tumours from the histopathology report: maximum tumour size (x-axis) and number of tumours (y-axis), so that TBS2 = (maximum tumour diameter)2 + (number of tumours)2 [32,33].
Patients with a radiological diagnosis of HCC were discussed at the multidisciplinary institutional meetings in the presence of a hepatobiliary and transplant-trained surgeon, medical oncologist, radiation oncologist, radiologist, and pathologist. Preoperative staging included computerized tomography (CT) of the chest and abdomen, and liver magnetic resonance imaging (MRI) when indicated. The preoperative screening was performed to determine the pathogenesis of the disease, the preoperative levels of tumour marker (AFP), and the Child-Pugh stage. When a major liver resection (resection of more than three liver segments) [34] was required, liver volumetry was performed, and the risk of postoperative hepatic insufficiency was assessed. In general, less than 40% of residual liver remnant was regarded as high risk for postoperative liver failure, and a right portal vein embolization was performed [35]. In addition, the performance status (PS) score was assessed in all patients.
The BCLC staging system was used to guide the included patients’ decision-making in our analysis. Thus, factors such as the patient’s general condition, the extent, and characteristics of the tumour and liver function were considered. The patients with HCC are classified in five stages: very early-stage disease [stage 0, tumour ≤ 2 cm, preserved liver function, good general condition (PS0)], early-stage disease (stage A, a solitary nodule, or up to 3 each one ≤ 3 cm, preserved liver function, PS 0), intermediate stage patients (stage B, more than two nodules with diameter > 3 cm, preserved liver function, PS 0), advanced stage HCC (C, patients with portal invasion or extrahepatic spread, preserved liver function, PS1–2), and terminal stage (D, end-stage liver function, PS 3–4). The classification was performed according to recent guidelines [36,37,38] and with each period’s existing guidelines [14,39]. Subsequently, early, intermediate, and selected advanced stage patients (mainly with portal vein invasion) underwent hepatectomy with an individualized decision. Intraoperative ultrasound was performed on all patients. The duration of hospitalization, immediate postoperative complications, and 90 days mortality was recorded. Upon histological examination, patients were reassessed on a regular external basis, based on a follow-up protocol by the HPB surgical team of each unit. The follow-up included history, clinical examination, liver function tests, AFP levels, chest, abdomen, and pelvis CT every four months for the first two years, and then every six months. Recurrence of the disease was diagnosed and treated accordingly (hepatic resection, liver transplantation, embolization, ablation, systemic therapy) considering the extent of the disease and the patient’s performance status.
Statistical Analysis
Patients’ demographic, clinical, and pathological characteristics are described overall and stratified according to the BCLC Classification as frequency rates and percentages. Overall survival rates were evaluated by the Kaplan–Meier method and were compared using the log-rank test. Univariate analysis was performed to estimate the association of age, sex, cirrhosis, hepatitis B virus, hepatitis C virus, alcohol, non-alcoholic fatty liver disease, R0 resection vs. R1-R2 resection, tumour burden score, and BCLC Classification with mortality. All the significant variables were introduced into a multivariate Cox proportional hazards model; p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the STATA 17 (StataCorp LP, College Station, TX, USA) software.
3. Results
3.1. Patients’ Demographic, Clinical, and Pathological Characteristics
Two hundred and forty-two patients were included in the study, with 79% of them being male. The median age was 71 years (range 21–89). In 52% of the patients, HCC was developed in the setting of a cirrhotic liver. Regarding the aetiology of HCC, 34.7% of the patients had HBV infection, 15.3% had HCV infection, while alcoholic liver disease (ALD) history was present in 46% of the study’s patients. Finally, NAFLD was found in 33% of cases. In 4% of the patients with HCC, the etiopathogenesis was due to other factors. The majority of the patients were Child–Pugh A (73%), while the remaining 27% were Child B. Table 1 presents the demographic, clinical, and pathological characteristics of patients stratified according to the BCLC Classification.

Table 1.
Demographic, clinical, and pathological characteristics of patients stratified according to the BCLC classification.
Regarding the BCLC Classification, the most recent criteria were used. Of the patients, 172 (71.1%) were BCLC A, 33 patients (13.6%) were BCLC B, and 37 patients (15.3%) were BCLC C (Table 1). If we used the BCLC criteria according to the period they were implemented, then the classification is different: 106 patients would be classified as BCLC A (43.8%), 99 patients as BCLC B (41%), and 37 as BCLC C (15.2%).
In summary, the majority of the patients were male (79%), HBV infection and NAFLD were the main causes of chronic liver disease, and 70% of the patients were BCLC A according to the most recent criteria.
3.2. Type of Liver Resections and Histopathology Results
A total of 131 patients (54%) underwent major hepatectomy while 111 patients (46%) underwent minor hepatectomy. Laparoscopic liver resection was performed in 13 patients (5.4% of liver resections) (Table 2).

Table 2.
Type of resection.
Based on the pathological examination of the liver tissue, 126 patients (52%) had pre-existing cirrhosis, and 73% were R0 resections. The median tumour diameter was 7.37 cm (range 1–25 cm) (Table 3).

Table 3.
Histopathology results.
In summary, the majority of patients (54%) underwent major liver resection, while liver cirrhosis was found in 52% of them.
3.3. Postoperative Outcomes and Survival
The median length of hospitalization was 11 days (range 3–100 days). Forty per cent of the patients developed postoperative complications, whereas 24.4% had a minor complication according to the Clavien–Dindo score, while 15.6% had a major complication (Dindo–Clavien class III. IV). The 90-day mortality was 4.5%. The remaining patients underwent regular follow-ups. The median follow-up was 33.5 months (range 1–146 months). The median overall survival time was 55 months (95% confidence interval: 46–70 months). The 1-, 3-, and 5-year overall survival rates were 89%, 65%, and 48%, respectively (Figure 1). Concerning BCLC Classification, there was a significant survival difference between the three categories (Figure 2; p < 0.001). The overall survival at 1, 3, and 5 years was 95%, 77%, and 55%, respectively for BCLC 0-A; 74%, 42%, and 34%, respectively for BCLC B; 70%, 25%, and 21%, respectively for BCLC C (Table 2). There was also a significant survival difference based on cirrhosis status (Figure 3; p = 0.034). Specifically, for cirrhotic patients, the 1-, 3-, and 5-year overall survival were 86%, 61%, and 43%, respectively and for non-cirrhotic were 92%, 70%, and 53%, respectively (Table 4). The recurrence rate was 42% in 3 years and 56% in 5 years. The recurrence rate in 5 years was 47% for BCLC A, 59% for BCLC B, and 89% for BCLC C.
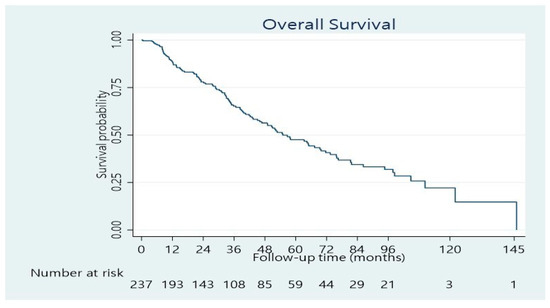
Figure 1.
Overall survival distribution of patients resected for HCC.
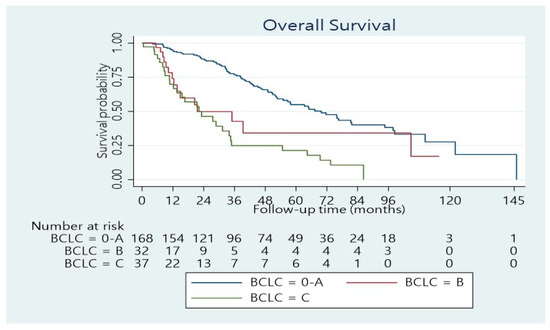
Figure 2.
Overall survival distribution stratified according to the BCLC stages (p < 0.001).
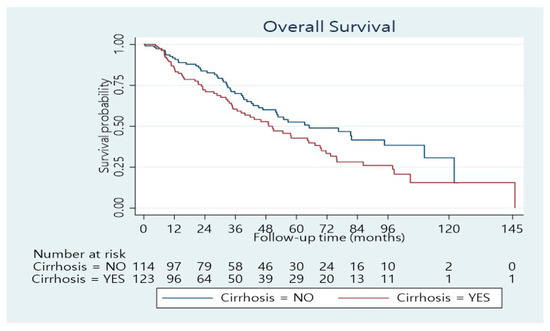
Figure 3.
Overall survival distribution stratified according to Cirrhosis status (p = 0.034).

Table 4.
Univariate and multivariate analysis of overall survival.
In summary, major complications were noted in 16% of patients and the 90-day mortality was 4.5%. The median survival was 55 months, and the 5-year overall survival was 48%. BCLC B and C stage and the presence of cirrhosis had a negative effect on overall survival.
3.4. Univariate and Multivariate Analysis
In univariate analysis, cirrhosis, R type of resection, and BCLC stage were associated with overall survival. Multivariate Cox model indicated that R1-R2 resection compared to R0 (HR: 2.43, 95% CI: 1.63 3.64, p = 1.5 × 10−5) and BCLC C compared to BCLC 0-A (HR: 2.54, 95%CI: 1.53–4.21, p = 3.3 × 10−4) were independently associated with increased mortality (Table 4).
4. Discussion
This study presents long-term outcomes of patients with HCC undergoing liver resection within and beyond BCLC guidelines. Our data support the role of surgical resection with curative intent in selected patients with BCLC B achieving a 5-year survival rate of 34% and a median survival of 32 months. The current BCLC recommendations suggest mainly embolization (TACE) for these patients, achieving a median survival of 18–27 months [36,40]. A recent phase II clinical trial showed that TACE in patients with intermediate-stage HCC has an OS of 26 months and 3- and 5-year survival of 36% and 2.7%, respectively [41].
The BCLC staging system is the most popular for prognosis and therapeutic guidance in patients with HCC [42]. However, it is considered to be very restrictive regarding indications for surgical management, including resection and transplantation, and for this reason, it has been heavily questioned by the HPB community [20,43,44,45,46,47,48].
The current study has used more liberal selection criteria in the surgical management of patients with HCC. Liver resection has been applied at the early stage as Greece is a country where organ availability for liver transplantation is limited [49,50]. It has also been applied for HCCs in intermediate and advanced stages, in 29% of the cohort of patients, providing satisfactory long-term survival and good quality of life. Significantly, when we adjusted our analysis by applying the contemporary BCLC criteria (BCLC criteria used at the time of liver resection), we found that the majority of patients (56%) were in the intermediate (41%) and advanced stage (15%). Advanced stage (BCLC-C) includes a very heterogeneous group of patients: portal invasion, lymph node or distal metastases, and decreased performance status (PS 1–2). According to BCLC criteria, systemic targeted therapy is the standard of care with expected progression-free survival of 6.8 months, and overall survival of <20 months [23,51]. However, patients with limited portal vein invasion (pV1 or pV2) or responding well to neo-adjuvant treatment could benefit from surgical resection [22].
More importantly, the present study shows that the Greek HPB centres have an aggressive approach in patients with HCC, as it is reflected by the median diameter of the tumours resected (7.37 cm), the high percentage of major hepatectomies (54%), and the fact that 27% of these patients were Child B. Furthermore, the indications were outside the BCLC criteria in more than 50% of the liver resections for HCC, if the existing criteria were applied at the time of operation. Our R0 resection rate is relatively low compared to other studies [20,22], and the high percentage of patients with advanced HCCs (tumours > 10 cm, BCLC B and C) could partially explain this finding. Despite these unfavourable parameters, a good 5-year OS of 48% was achieved. The selection criteria used and the long-term results achieved are in accordance with the results of large multicentre studies [20,52,53,54], and comparable with the results of recent systematic reviews [21,22]. A recent systematic review and meta-analysis showed that anatomic liver resections offer better overall and disease-free survival [55]. This finding could partially explain our long-term results, as most patients underwent major anatomic resections.
Surrogates of HCC behaviour, such as tumour burden score (TBS), and patient clinical performance, such as Model for End-Stage Liver Disease (MELD) score, can identify patients who benefit from surgical management beyond traditional criteria. These nuances have not been captured in the updated BCLC guidelines published in 2022 [38]. More specifically, recent multicentre data support a beneficial role of surgery in patients with BCLC B/C; despite the fact that they have a higher risk for early (<2 years) or multiple intrahepatic recurrences compared to BCLC 0/A (p = 0.011), and shorter 5-year OS (51.6% for BCLC B/C versus 76.9% for BCLC 0/A, p = 0.003), half of these patients can survive for five years after resection, a finding that is above any expectation from other recommended treatment (TACE and sorafenib) [56]. Similarly, in patients with multinodular HCC undergoing resection, those with low TBS achieved a 73.7% 5-year OS survival, whereas patients with high TBS had only 13.1% (p < 0.001). This highlights how tumour burden can refine the management of patients with presumably unresectable disease [57]. In our study, low TBS was associated with better survival. However, in the univariate analysis, there were no statistically significant differences between the three groups. This finding could be due to the relatively small number of patients in the present study.
Finally, a machine-learning analysis showed that factors such as comorbidities and high pre-resection AFP, as well as post-resection factors such as TBS and lymphovascular invasion, could be the best predictors of OS in patients with BCLC-A, and TBS was the single best predictor of outcomes in patients with BCLC-B undergoing resection for HCC [58].
A recent randomized phase II trial showed that the perioperative use of immunotherapy in resectable HCCs is safe and feasible. Immunotherapy as neo-adjuvant therapy in advanced HCC may contribute, in combination with surgery, to better long-term survival [59].
The present study, along with emerging literature, supports an individualized approach for the surgical management of patients with HCC based on clinical performance status, satisfactory baseline liver function (Child A or B, total bilirubin < 2 mg /dL, INR < 2, platelets > 80,000), adequate future live remnant (>40% in the presence of cirrhosis), in BCLC 0-A patients, as well as selected cases of BCLC B and C. The decision should be taken at the multidisciplinary tumour board level. We are happy to see that the recent update to BCLC guidelines proposes a personalized HCC treatment approach where the tumour board should choose the option which provides the best survival [38].
Another interesting finding of our study is that liver resection is safe for elderly patients (>75 yrs) and leads to long-term survival rates similar to those of younger patients [60,61].
In the near future, there is a need to identify reliable prognostic markers to help us choose the best treatment option for each patient [62]. Furthermore, novel treatments such as immunotherapy and anti-angiogenetic therapy should have a role in the neo-adjuvant setting in choosing a radical surgical treatment (resection or transplantation) for the patients who respond well to the neo-adjuvant therapies.
The present study has several strengths and weaknesses that should be mentioned. First, the study captures more than ten years of HPB practice in both academic and private practice settings. Moreover, the patient population was homogeneous, thus limiting the potential bias related to different racial backgrounds. Moreover, Greek HPB surgeons have demonstrated adaptability to novel technologies that facilitate the safe, bloodless, and efficient performance of major hepatectomies, including ablation devices [63,64,65] and novel techniques [66,67] despite the limited resources, such as ICU availability [68,69]. As far as weaknesses are concerned, this is a retrospective study, and as such it is subject to bias. The number of patients included is relatively low, in order to have a clear conclusion regarding predictive markers such as TBS or selection criteria for liver resection in BCLC B and C patients. Moreover, the percentage of laparoscopic liver resections in this study was low, and this is an area which requires improvement in the near future, as significant advances have taken place in the era of laparoscopic and robotic surgery in recent years and patients with HCC have a significant benefit in postoperative morbidity with these approaches [62,70,71,72,73,74].
5. Conclusions
In conclusion, this study shows that a significant percentage of patients with HCC managed surgically in Greece are of intermediate and advanced stage, or Child B, and require major liver resections. Through an individualized approach, good long-term results have been achieved. Further prospective studies are required to clarify the subgroups of patients with intermediate or advanced stage HCC who will benefit from liver resection.
Author Contributions
Conceptualization, G.K.G. and E.F.; methodology, G.K.G., G.C.S., A.P. (Athanasios Paliouras), A.-G.A. and E.F.; software, A.K., A.P. (Athanasios Paliouras), S.D. and A.P. (Alexandros Papalampros); validation, G.T., A.-G.A., S.D. and A.P. (Alexandros Papalampros); formal analysis, A.K., A.P. (Athanasios Paliouras) and A.-G.A.; investigation, A.K., A.P. (Athanasios Paliouras) and S.D.; resources, G.K.G., D.K., G.C.S., G.T. and E.F.; data curation, D.K., G.C.S., G.T., A.K., A.P. (Athanasios Paliouras) and S.D. writing—original draft preparation, G.K.G.; writing—review and editing, D.K., G.C.S., G.T., A.-G.A., D.M. and E.F.; visualization, A.K., A.P. (Athanasios Paliouras), S.D. and D.M.; supervision, G.K.G. and E.F.; project administration, A.K., A.P. (Athanasios Paliouras), S.D. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional or Ethics Committee of “Hygeia” Hospital Athens, University Hospital of Ioannina, Laiko University Hospital, and St. Savvas Oncological Centre (AK-1345 and 14 July 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study as part of the national database.
Data Availability Statement
The data can be shared up on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Tumin, D.; Kabir, A.; Moris, D.; Zhang, X.F.; Chakedis, J.; Washburn, K.; Black, S.; Schmidt, C.M.; Pawlik, T.M. Trends in the Mortality of Hepatocellular Carcinoma in the United States. J. Gastrointest. Surg. 2017, 21, 2033–2038. [Google Scholar] [CrossRef] [PubMed]
- Veracruz, N.; Gish, R.G.; Cheung, R.; Chitnis, A.S.; Wong, R.J. Global incidence and mortality of hepatitis B and hepatitis C acute infections, cirrhosis and hepatocellular carcinoma from 2010 to 2019. J. Viral Hepat. 2022, 29, 352–365. [Google Scholar] [CrossRef]
- Karampa, A.D.; Goussia, A.C.; Glantzounis, G.K.; Mastoridou, E.M.; Anastasopoulos, N.T.; Charchanti, A.V. The Role of Macroautophagy and Chaperone-Mediated Autophagy in the Pathogenesis and Management of Hepatocellular Carcinoma. Cancers 2022, 14, 760. [Google Scholar] [CrossRef]
- Argyrou, C.; Moris, D.; Vernadakis, S. Hepatocellular carcinoma development in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Is it going to be the “Plague” of the 21st century? A literature review focusing on pathogenesis, prevention and treatment. J. Balk. Union Oncol. 2017, 22, 6–20. [Google Scholar]
- Moris, D.; Rahnemai-Azar, A.A.; Zhang, X.; Ntanasis-Stathopoulos, I.; Tsilimigras, D.I.; Chakedis, J.; Argyrou, C.; Fung, J.J.; Pawlik, T.M. Program death-1 immune checkpoint and tumor microenvironment in malignant liver tumors. Surg. Oncol. 2017, 26, 423–430. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Moris, D.; Pawlik, T.M. Liver Tumor Microenvironment. In Tumor Microenvironments in Organs; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology 1296; Springer: Cham, Switzerland, 2020; pp. 227–241. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Moris, D.; Spartalis, E.; Pawlik, T.M. Histone deacetylase inhibitors in hepatocellular carcinoma: A therapeutic perspective. Surg. Oncol. 2018, 27, 611–618. [Google Scholar] [CrossRef]
- Cucarull, B.; Tutusaus, A.; Rider, P.; Hernaez-Alsina, T.; Cuno, C.; de Frutos, P.G.; Colell, A.; Mari, M.; Morales, A. Hepatocellular Carcinoma: Molecular Pathogenesis and Therapeutic Advances. Cancers 2022, 14, 621. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Beal, E.W.; Tumin, D.; Kabir, A.; Moris, D.; Zhang, X.F.; Chakedis, J.; Washburn, K.; Black, S.; Schmidt, C.M.; Pawlik, T.M. Cohort Contributions to Race- and Gender-Specific Trends in the Incidence of Hepatocellular Carcinoma in the USA. World J. Surg. 2018, 42, 835–840. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef] [PubMed]
- Anastasopoulos, N.T.; Lianos, G.D.; Tatsi, V.; Karampa, A.; Goussia, A.; Glantzounis, G.K. Clinical heterogenity in patients with non-alcoholic fatty liver disease-associated hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1025–1033. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Moris, D. A farewell to Barcelona Clinic Liver Cancer (BCLC) classification for hepatocellular carcinoma. J. Balk. Union Oncol. 2021, 26, 298–302. [Google Scholar]
- Tsilimigras, D.I.; Bagante, F.; Sahara, K.; Moris, D.; Hyer, J.M.; Wu, L.; Ratti, F.; Marques, H.P.; Soubrane, O.; Paredes, A.Z.; et al. Prognosis After Resection of Barcelona Clinic Liver Cancer (BCLC) Stage 0, A, and B Hepatocellular Carcinoma: A Comprehensive Assessment of the Current BCLC Classification. Ann. Surg. Oncol. 2019, 26, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, T.; Lu, Q.; Li, M.; Guo, J.Y.; Shen, Y.; Wu, Z.; Nan, K.J.; Lv, Y.; Zhang, X.F. Surgical resection improves long-term survival of patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages. Cancer Manag. Res. 2018, 10, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Sinn, D.H.; Yu, S.J.; Gwak, G.Y.; Kim, J.H.; Yoo, Y.J.; Jun, D.W.; Kim, T.Y.; Lee, H.Y.; Cho, E.J.; et al. Survival Analysis of Single Large (>5 cm) Hepatocellular Carcinoma Patients: BCLC A versus B. PLoS ONE 2016, 11, e0165722. [Google Scholar] [CrossRef]
- Ng, K.K.; Vauthey, J.N.; Pawlik, T.M.; Lauwers, G.Y.; Regimbeau, J.M.; Belghiti, J.; Ikai, I.; Yamaoka, Y.; Curley, S.A.; Nagorney, D.M.; et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann. Surg. Oncol. 2005, 12, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations?: An observational study of the HCC East-West study group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef]
- Zhong, J.H.; Rodriguez, A.C.; Ke, Y.; Wang, Y.Y.; Wang, L.; Li, L.Q. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine 2015, 94, e396. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Paliouras, A.; Stylianidi, M.C.; Milionis, H.; Tzimas, P.; Roukos, D.; Pentheroudakis, G.; Felekouras, E. The role of liver resection in the management of intermediate and advanced stage hepatocellular carcinoma. A systematic review. Eur. J. Surg. Oncol. 2018, 44, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D. PD-L1, TMB and other potential predictiors of repsonse to immunotherapy for hepatocellular carcinoma: How can they assist drug clinical trials? Expert Opin. Investig. Drugs 2022, 31, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, S.; Tovoli, F.; Barbera, M.A.; Garuti, F.; Palloni, A.; Frega, G.; Garajovà, I.; Rizzo, A.; Trevisani, F.; Brandi, G. Metronomic capeciatbine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: A proof of concept. Sci. Rep. 2018, 8, 9997. [Google Scholar] [CrossRef]
- Peristeri, D.V.; Tepelenis, K.; Karamba, A.; Kapodistrias, N.; Goussia, A.C.; Pappas-Gogos, G.; Glantzounis, G.K. Metronomic chemotherapy with cyclophosphamide for the treatment of advanced hepatocellyular cancer: A case report. Ann. Med. Surg. 2021, 72, 103043. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Phillips, C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann. Surg. 2013, 257, 377–382. [Google Scholar] [CrossRef]
- Celinski, S.A.; Gamblin, T.C. Hepatic resection nomenclature and techniques. Surg. Clin. N. Am. 2010, 90, 737–748. [Google Scholar] [CrossRef]
- Moris, D.; Rahnemai-Azar, A.A.; Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Marques, H.P.; Spartalis, E.; Felekouras, E.; Pawlik, T.M. Updates and Critical Insights on Glissonian Approach in Liver Surgery. J. Gastrointest. Surg. 2018, 22, 154–163. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The tumor burden score: A new ‘metroticket’ prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Moris, D.; Hyer, J.M.; Bagante, F.; Sahara, K.; Moro, A.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; et al. Hepatocellular carcinoma tumor burden score to stratify prognosis after resection. J. Br. Surg. 2020, 107, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Barbas, A.S.; Turley, R.S.; Steel, J.L.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Clary, B.M. A standard definition of major hepatectomy: Resection of four or more liver segments. HPB 2011, 13, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Tokidis, E.; Basourakos, S.P.; Ntzani, E.E.; Lianos, G.D.; Pentheroudakis, G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur. J. Surg. Oncol. 2017, 43, 32–41. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practise Guidelines:Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.E.; de Lope, C.R.; Bruix, J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef]
- Malagari, K.; Moschouris, H.; Kiakidis, T.; Harward, S.; Kelekis, A.; Vrakas, S.; Koundouras, D.; Filipiadis, D.; Glantzounis, G.; Emmanouil, E.; et al. Five-Years Outcome Analysis of 142 Consecutive Hepatocellular Carcinoma Patients Treated with Doxorubicin Eluting Microspheres 30–60 mum: Results from a Single-Centre Prospective Phase II Trial. Cardiovasc. Interv. Radiol. 2019, 42, 1551–1562. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Vernadakis, S.; Papalampros, A.; Petrou, A.; Dimitroulis, D.; Spartalis, E.; Felekouras, E.; Fung, J.J. The effect of Guidelines in surgical decision making: The paradigm of hepatocellular carcinoma. J. Balk. Union Oncol. 2016, 21, 1332–1336. [Google Scholar]
- Chen, L.T.; Martinelli, E.; Cheng, A.L.; Pentheroudakis, G.; Qin, S.; Bhattacharyya, G.S.; Ikeda, M.; Lim, H.Y.; Ho, G.F.; Choo, S.P.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: A TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann. Oncol. 2020, 31, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Felekouras, E. Ignore reality but not the consequences of its ignorance: Broaden guidelines in surgery of hepatocellular carcinoma. Hepatology 2017, 65, 1772–1773. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Cabibbo, G.; Colombo, M. Adjusting BCLC staging system to the evolving landscape of Hepatocellular Carcinoma. A look to the future. Gastroenterology 2022, 162, 2106–2108. [Google Scholar] [CrossRef]
- Famularo, S.; Donadon, M.; Cipriani, F.; Giuliante, F.; Ferri, S.; Celsa, C.; Ferrero, A.; Foschi, F.G.; Baiocchi, G.L.; Biasini, E.; et al. Hepatectomy Versus Sorafenib in Advanced Nonmetastatic Hepatocellular Carcinoma: A Real-life Multicentric Weighted Comparison. Ann. Surg. 2022, 275, 743–752. [Google Scholar] [CrossRef]
- Ciria, R.; Lopez-Cillero, P.; Gallardo, A.B.; Cabrera, J.; Pleguezuelo, M.; Ayllon, M.D.; Luque, A.; Zurera, L.; Espejo, J.J.; Rodriguez-Peralvarez, M.; et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur. J. Surg. Oncol. 2015, 41, 1153–1161. [Google Scholar] [CrossRef]
- Moris, D.; Menoudakou, G.; Zavos, G. Organ Transplantation in Greece. Transplantation 2016, 100, 1589–1591. [Google Scholar] [CrossRef]
- Moris, D.; Zavos, G.; Menoudakou, G.; Karampinis, A.; Boletis, J. Organ donation during the financial crisis in Greece. Lancet 2016, 387, 1511–1512. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Moris, D. Atezolizumab plus bevacizumab for advanced, unresectable hepatocellular carcinoma. J. Balk. Union Oncol. 2021, 26, 637. [Google Scholar]
- Pinna, A.D.; Yang, T.; Mazzaferro, V.; De Carlis, L.; Zhou, J.; Roayaie, S.; Shen, F.; Sposito, C.; Cescon, M.; Di Sandro, S.; et al. Liver Transplantation and Hepatic Resection can Achieve Cure for Hepatocellular Carcinoma. Ann. Surg. 2018, 268, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Zhong, J.; Berhane, S.; Toyoda, H.; Shi, K.; Tada, T.; Chong, C.C.N.; Xiang, B.D.; Li, L.Q.; Lai, P.B.S.; et al. The chances of hepatic resection curing hepatocellular carcinoma. J. Hepatol. 2020, 72, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Takemura, N.; Aoki, T.; Hasegawa, K.; Kaneko, J.; Arita, J.; Akamatsu, N.; Makuuchi, M.; Kokudo, N. Hepatectomy for hepatocellular carcinoma after perioperative management of portal hypertension. Br. J. Surg. 2019, 106, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Tsilimigras, D.I.; Kostakis, I.D.; Ntanasis-Stathopoulos, I.; Shah, K.N.; Felekouras, E.; Pawlik, T.M. Anatomic versus non-anatomic resection for hepatocellular carcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2018, 44, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Bagante, F.; Moris, D.; Hyer, J.M.; Sahara, K.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; Soubrane, O.; et al. Recurrence Patterns and Outcomes after Resection of Hepatocellular Carcinoma within and beyond the Barcelona Clinic Liver Cancer Criteria. Ann. Surg. Oncol. 2020, 27, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Mehta, R.; Paredes, A.Z.; Moris, D.; Sahara, K.; Bagante, F.; Ratti, F.; Marques, H.P.; Silva, S.; Soubrane, O.; et al. Overall Tumor Burden Dictates Outcomes for Patients Undergoing Resection of Multinodular Hepatocellular Carcinoma Beyond the Milan Criteria. Ann. Surg. 2020, 272, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Mehta, R.; Moris, D.; Sahara, K.; Bagante, F.; Paredes, A.Z.; Farooq, A.; Ratti, F.; Marques, H.P.; Silva, S.; et al. Utilizing Machine Learning for Pre- and Postoperative Assessment of Patients Undergoing Resection for BCLC-0, A and B Hepatocellular Carcinoma: Implications for Resection Beyond the BCLC Guidelines. Ann. Surg. Oncol. 2020, 27, 866–874. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Hasanov, E.; Cao, H.S.T.; Xiao, L.; Vauthey, J.N.; Lee, S.S.; Yavuz, B.G.; Mohamed, Y.I.; Qayyum, A.; Jindal, S.; et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: A randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 208–218. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, C.W.; Liang, L.; Wu, H.; Zhang, W.G.; Zeng, Y.Y.; Gu, W.M.; Chen, T.H.; Li, J.; Zhang, Y.M.; et al. Major hepatectomy in Elderly Patients with Large Hepatocellular carcinoma: A Multicenter Retrospective Observational study. Cancer Manag. Res. 2020, 12, 5607–5618. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Xu, S.; Hu, M.-G.; Zhao, Z.-M.; Wang, Z.-H.; Zhao, G.-D.; Li, C.-G.; Tan, X.-L.; Liu, R. Short and long-term outcomes after robotic and open liver resection for elderly patients with hepatocellular carcinoma: A propensity-score matched study. Surg. Endosc. 2022. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Karampa, A.; Peristeri, D.V.; Pappas-Gogos, G.; Tepelenis, K.; Tzimas, P.; Cyrochristos, D.J. Recent advances in the surgical management of hepatocellular carcinoma. Ann. Gastroenterol. 2021, 34, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.; Neofytou, K.; Mihas, C.; Bagenal, J.; Kontos, M.; Griniatsos, J.; Felekouras, E. Radiofrequency ablation-assisted liver resection: A step toward bloodless liver resection. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Felekouras, E.; Petrou, A.; Neofytou, K.; Giakoustidis, A.; Bagenal, J.; Cananzi, F.; Pikoulis, E.; Mudan, S. Combined ultrasonic aspiration and saline-linked radiofrequency precoagulation: A step toward bloodless liver resection without the need of liver inflow occlusion: Analysis of 313 consecutive patients. World J. Surg. Oncol. 2014, 12, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prassas, E.; Petrou, A.; Kontos, M.; Rizos, D.; Neofytou, K.; Pikoulis, E.; Diamantis, T.; Felekouras, E. Radiofrequency ablation assisted resection for hepatocellular carcinoma: Morbidity, mortality and long term survival. J. Balk. Union Oncol. 2014, 19, 256–262. [Google Scholar]
- Baili, E.; Tsilimigras, D.I.; Filippou, D.; Ioannidis, A.; Bakopoulos, A.; Machairas, N.; Papalampros, A.; Petrou, A.; Schizas, D.; Moris, D. Associating liver partition and portal vein ligation for staged hepatectomy in patients with primary liver malignancies: A systematic review of the literature. J. Balk. Union Oncol. 2019, 24, 1371–1381. [Google Scholar]
- Moris, D.; Dimitroulis, D.; Papalampros, A.; Petrou, A.; Felekouras, E. ALPPS Procedure for Hepatocellular Carcinoma in Patients With Chronic Liver Disease: Revealing a Terra Incognita. Ann. Surg. 2017, 266, e106–e107. [Google Scholar] [CrossRef]
- Moris, D.; Schizas, D.; Papalampros, A.; Felekouras, E.; Liakakos, T. The struggle for intensive care coverage of patients with hepatobiliary malignancies in Greece: Patients are not numbers. J. Balk. Union Oncol. 2017, 22, 1363–1364. [Google Scholar]
- Sotiropoulos, G.C.; Machairas, N.; Kostakis, I.D.; Kouraklis, G. The struggle for intensive care coverage after hepatic resections: The Greek reality. Lancet 2017, 389, 364–365. [Google Scholar] [CrossRef]
- Cho, J.Y.; Han, H.S.; Wakabayashi, G.; Soubrane, O.; Geller, D.; O’Rourke, N.; Buell, J.; Cherqui, D. Practical guidelines for performing laparoscopic liver resection based on the second international laparoscopic liver consensus conference. Surg. Oncol. 2018, 27, A5–A9. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Prodromidou, A.; Kostakis, I.D.; Machairas, N. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg. 2017, 69, 291–311. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Machairas, N.; Stamopoulos, P.; Kostakis, I.D.; Dimitroulis, D.; Mantas, D.; Kouraklis, G. Laparoscpic versus open liver resection for hepatocellular carcinoma: Initial epxeprience in greece. Ann. Gastroenterol. 2016, 29, 521–529. [Google Scholar] [PubMed]
- Tsilimigras, D.I.; Moris, D.; Vagios, S.; Merath, K.; Pawlik, T.M. Safety and oncologic outcomes of robotic liver resections: A systematic review. J. Surg. Oncol. 2018, 117, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Vernadakis, S. Laparoscopic Hepatectomy for Hepatocellular Carcinoma: The Opportunities, the Challenges, and the Limitations. Ann. Surg. 2018, 268, e16. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).