Radiomics for the Prediction of Overall Survival in Patients with Bladder Cancer Prior to Radical Cystectomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
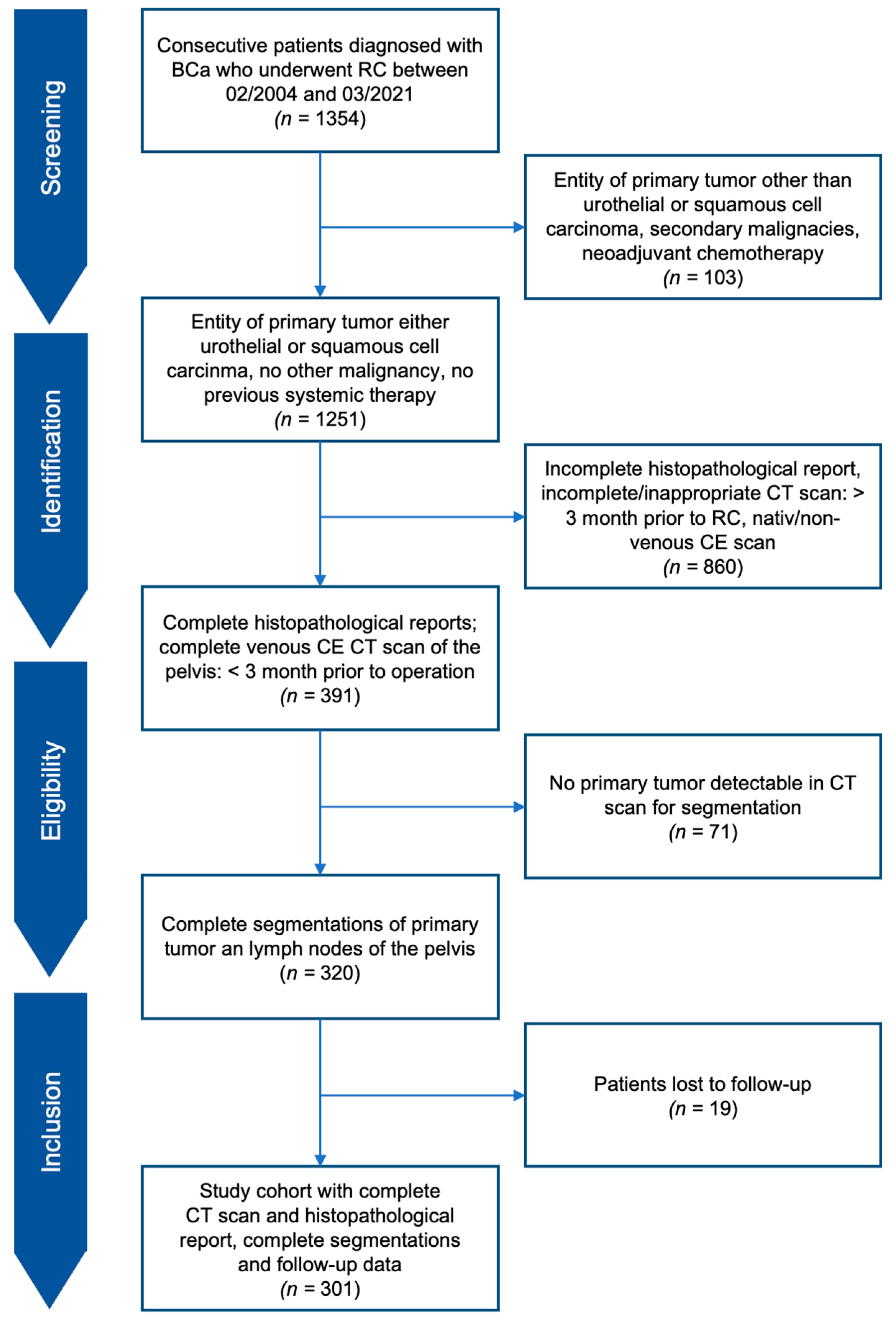
2.1. Study Design and Cohort
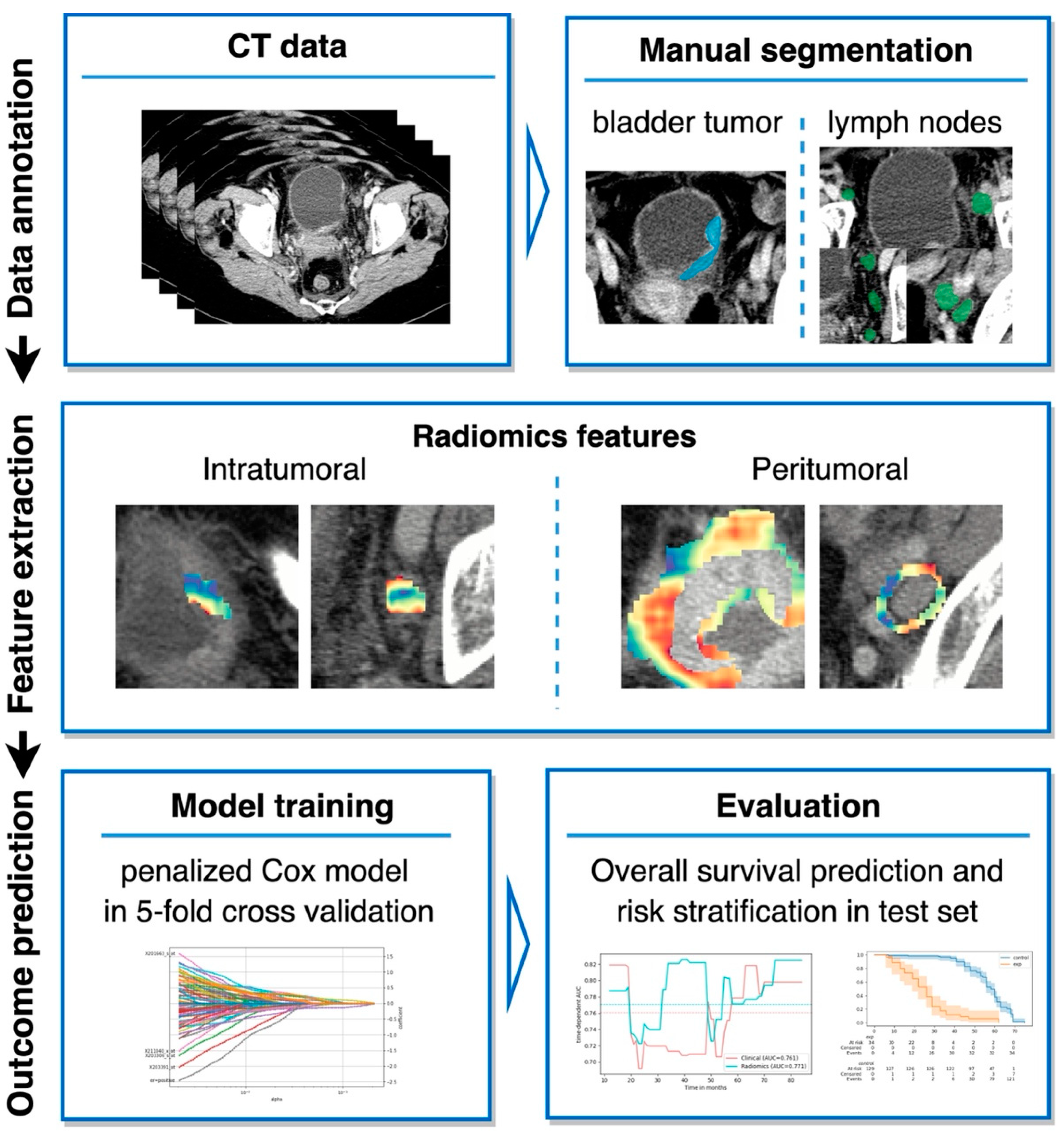
2.2. Segmentation and Radiomics Feature Extraction
2.3. Model Development
2.4. Combining Radiomics and Clinical Parameters
2.5. Evaluation Metrics
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
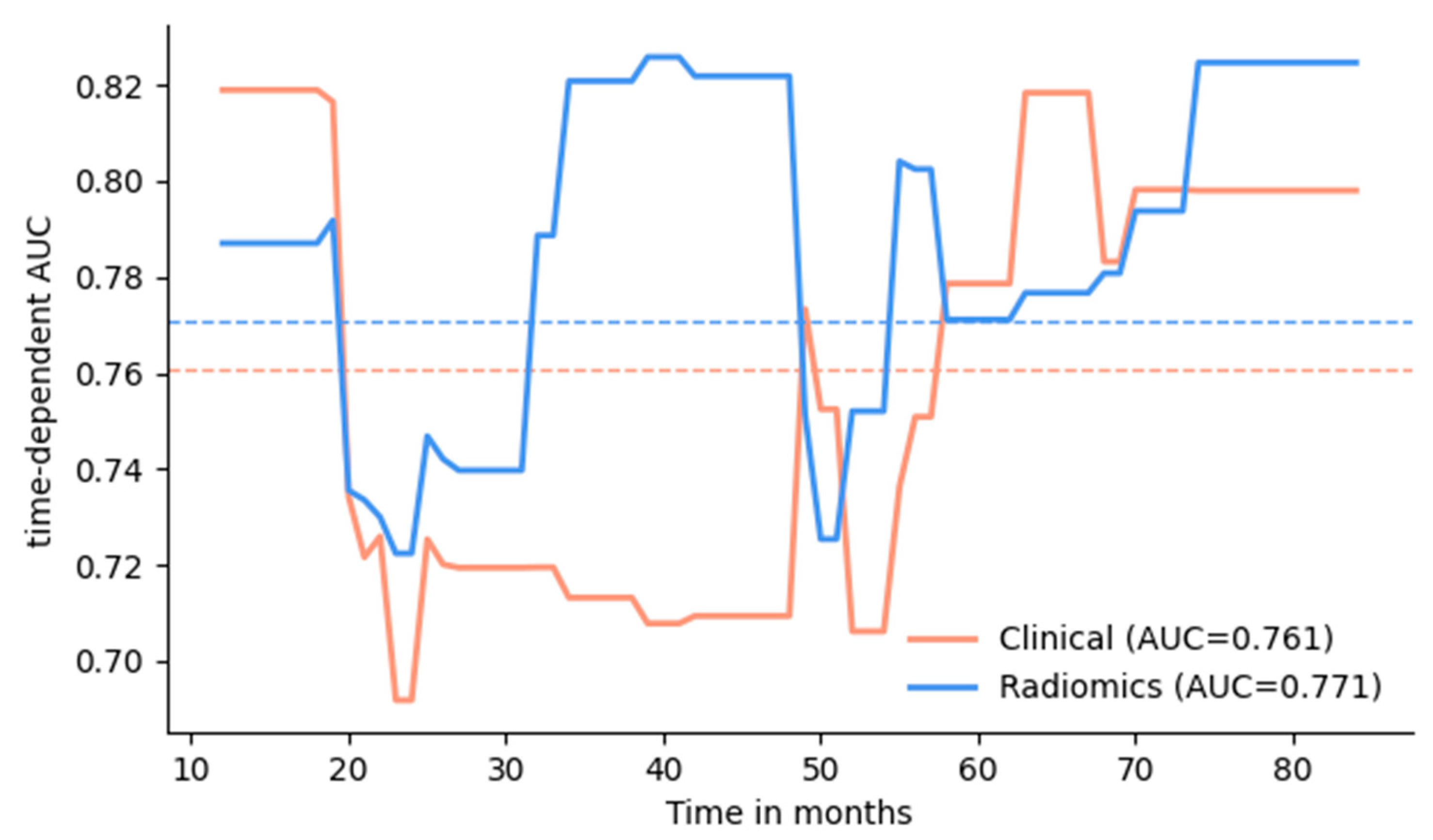
3.2. Survival Prediction
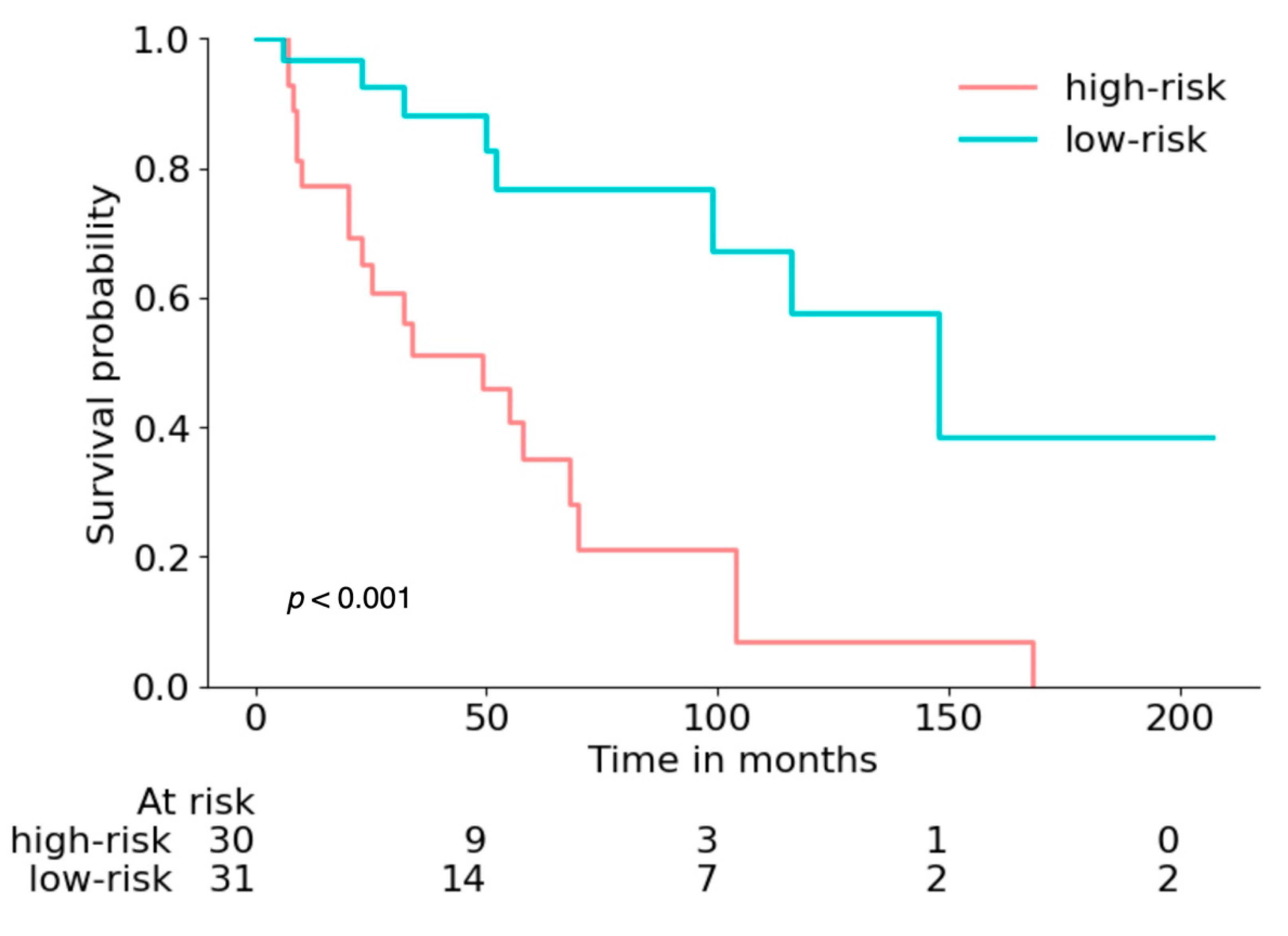
3.3. Prediction Calibration and Interpretability
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The Global Burden of Urinary Bladder Cancer: An Update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Ganeshan, D.; Jensen, C.T.; Devine, C.E. Imaging and Management of Bladder Cancer. Cancers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- ASiegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Rayn, K.N.; Hale, G.R.; Grave, G.P.-L.; Agarwal, P.K. New Therapies in Nonmuscle Invasive Bladder Cancer Treatment. Indian J. Urol. 2018, 34, 11–19. [Google Scholar] [CrossRef]
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder Cancer. Lancet 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Zha, Z.; Qu, W.; Zhao, H.; Yuan, J. Clinicopathological Factors in Bladder Cancer for Cancer-Specific Survival Outcomes Following Radical Cystectomy: A Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 716. [Google Scholar] [CrossRef]
- Borhani, S.; Borhani, R.; Kajdacsy-Balla, A. Artificial Intelligence: A Promising Frontier in Bladder Cancer Diagnosis and Outcome Prediction. Crit. Rev. Oncol. Hematol. 2022, 171, 103601. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kwak, C.; Kim, H.H.; Ku, J.H. Prognostic Significance of Lymphovascular Invasion in Radical Cystectomy on Patients with Bladder Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e89259. [Google Scholar] [CrossRef]
- Aziz, A.; Gierth, M.; Fritsche, H.M.; May, M.; Otto, W.; Denzinger, S.; Wieland, W.F.; Merseburger, A.; Riedmiller, H.; Kocot, A.; et al. Oncological Outcome of Primary versus Secondary Muscle-Invasive Bladder Cancer Is Comparable after Radical Cystectomy. Urol. Int. 2013, 91, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Kim, H.S.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Prognostic Factors for Conditional Survival in Patients with Muscle-Invasive Urothelial Carcinoma of the Bladder Treated with Radical Cystectomy. Sci. Rep. 2015, 5, 12171. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Abdollah, F.; Bianchi, M.; Trinh, Q.-D.; Shariat, S.F.; Jeldres, C.; Tian, Z.; Hansen, J.; Briganti, A.; Graefen, M.; et al. Conditional Survival of Patients with Urothelial Carcinoma of the Urinary Bladder Treated with Radical Cystectomy. Eur. J. Cancer 2012, 48, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Soukup, V.; Čapoun, O.; Cohen, D.; Hernández, V.; Burger, M.; Compérat, E.; Gontero, P.; Lam, T.; Mostafid, A.H.; Palou, J.; et al. Risk Stratification Tools and Prognostic Models in Non-Muscle-Invasive Bladder Cancer: A Critical Assessment from the European Association of Urology Non-Muscle-Invasive Bladder Cancer Guidelines Panel. Eur. Urol. Focus 2020, 6, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Stancanello, J.; El Naqa, I. Beyond Imaging: The Promise of Radiomics. Phys. Med. 2017, 38, 122–139. [Google Scholar] [CrossRef]
- Woznicki, P.; Laqua, F.; Bley, T.; Baeßler, B. AutoRadiomics: A Framework for Reproducible Radiomics Research. Front. Radiol. 2022, 2, 919133. [Google Scholar] [CrossRef]
- Pölsterl, S. Scikit-Survival: A Library for Time-to-Event Analysis Built on Top of Scikit-Learn. J. Mach. Learn. Res. 2020, 21, 1. [Google Scholar]
- Kim, S.P.; Shah, N.D.; Weight, C.J.; Thompson, R.H.; Wang, J.K.; Karnes, R.J.; Han, L.C.; Ziegenfuss, J.Y.; Frank, I.; Tollefson, M.K.; et al. Population-Based Trends in Urinary Diversion among Patients Undergoing Radical Cystectomy for Bladder Cancer. BJU Int. 2013, 112, 478–484. [Google Scholar] [CrossRef]
- Raza, S.J.; Wilson, T.; Peabody, J.O.; Wiklund, P.; Scherr, D.S.; Al-Daghmin, A.; Dibaj, S.; Khan, M.S.; Dasgupta, P.; Mottrie, A.; et al. Long-Term Oncologic Outcomes Following Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. Eur. Urol. 2015, 68, 721–728. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, L.; Zheng, X.; Shao, J.; Wang, T.; Zhang, E.; Gao, F.; Wang, X.; Zheng, J. Clinical Use of Machine Learning-Based Pathomics Signature for Diagnosis and Survival Prediction of Bladder Cancer. Cancer Sci. 2021, 112, 2905–2914. [Google Scholar] [CrossRef]
- Bhambhvani, H.P.; Zamora, A.; Shkolyar, E.; Prado, K.; Greenberg, D.R.; Kasman, A.M.; Liao, J.; Shah, S.; Srinivas, S.; Skinner, E.C.; et al. Development of Robust Artificial Neural Networks for Prediction of 5-Year Survival in Bladder Cancer. Urol. Oncol. 2021, 39, 193.e7–193.e12. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lam, K.-M.; Deng, Z.; Choi, K.-S. Prediction of Mortality after Radical Cystectomy for Bladder Cancer by Machine Learning Techniques. Comput. Biol. Med. 2015, 63, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Gavriel, C.G.; Dimitriou, N.; Brieu, N.; Nearchou, I.P.; Arandjelović, O.; Schmidt, G.; Harrison, D.J.; Caie, P.D. Assessment of Immunological Features in Muscle-Invasive Bladder Cancer Prognosis Using Ensemble Learning. Cancers 2021, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zheng, J.; Li, Y.; Yu, H.; Shi, S.; Xie, W.; Liu, H.; Su, Y.; Huang, J.; Lin, T. A Radiomics Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Bladder Cancer. Clin. Cancer Res. 2017, 23, 6904–6911. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, Z.; Liu, X.; Zhang, X.; Wang, X. CT-Based Radiomics for the Preoperative Prediction of the Muscle-Invasive Status of Bladder Cancer and Comparison to Radiologists’ Assessment. Clin. Radiol. 2022, 77, e473–e482. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, Z.; Zhang, X.; Xu, L.; Mao, L.; Li, X.; Xiao, Y.; Ji, Z.; Sun, H.; Jin, Z. CT-Based Radiomics to Predict Muscle Invasion in Bladder Cancer. Eur. Radiol. 2022, 32, 3260–3268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kong, J.; Wu, S.; Li, Y.; Cai, J.; Yu, H.; Xie, W.; Qin, H.; Wu, Z.; Huang, J.; et al. Development of a Noninvasive Tool to Preoperatively Evaluate the Muscular Invasiveness of Bladder Cancer Using a Radiomics Approach. Cancer 2019, 125, 4388–4398. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Tian, Q.; Li, B.; Wu, Y.; Yang, Z.; Liang, Z.; Liu, Y.; Cui, G.; Lu, H. Radiomics Assessment of Bladder Cancer Grade Using Texture Features from Diffusion-Weighted Imaging. J. Magn. Reson. Imaging 2017, 46, 1281–1288. [Google Scholar] [CrossRef]
- Wang, H.; Hu, D.; Yao, H.; Chen, M.; Li, S.; Chen, H.; Luo, J.; Feng, Y.; Guo, Y. Radiomics Analysis of Multiparametric MRI for the Preoperative Evaluation of Pathological Grade in Bladder Cancer Tumors. Eur. Radiol. 2019, 29, 6182–6190. [Google Scholar] [CrossRef]
- Kozikowski, M.; Suarez-Ibarrola, R.; Osiecki, R.; Bilski, K.; Gratzke, C.; Shariat, S.F.; Miernik, A.; Dobruch, J. Role of Radiomics in the Prediction of Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2022, 8, 728–738. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Du, P.; Zhang, F.; Li, S.; Zhang, Z.; Yuan, J.; Liang, Z.; Zhang, X.; Guo, Y.; et al. A Predictive Nomogram for Individualized Recurrence Stratification of Bladder Cancer Using Multiparametric MRI and Clinical Risk Factors. J. Magn. Reson. Imaging 2019, 50, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Wen, D.-Y.; Chen, L.; Li, X.; Li, S.-H.; Yan, H.-B.; He, R.-Q.; Chen, G.; He, Y.; Yang, H. A Radiogenomics Signature for Predicting the Clinical Outcome of Bladder Urothelial Carcinoma. Eur. Radiol. 2020, 30, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yang, L.; Hu, S.; Gu, S.; Ye, J.; Li, Z.; Du, H.; Shen, H. Feasibility Study on Predicting Recurrence Risk of Bladder Cancer Based on Radiomics Features of Multiphase CT Images. Front. Oncol. 2022, 12, 899897. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhang, J.; Zhang, L.; Wang, S.; Chen, Y. Development of a MRI-Based Radiomics Nomogram for Prediction of Response of Patients with Muscle-Invasive Bladder Cancer to Neoadjuvant Chemotherapy. Front. Oncol. 2022, 12, 878499. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, K.J.; Heo, C.; Park, B.W.; Kim, M.; Kim, J.K. Radiomics-Based Model for Predicting Pathological Complete Response to Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer. Clin. Radiol. 2021, 76, 627.e13–627.e21. [Google Scholar] [CrossRef]
- Rundo, F.; Spampinato, C.; Banna, G.L.; Conoci, S. Advanced Deep Learning Embedded Motion Radiomics Pipeline for Predicting Anti-PD-1/PD-L1 Immunotherapy Response in the Treatment of Bladder Cancer: Preliminary Results. Electronics 2019, 8, 1134. [Google Scholar] [CrossRef]
- Chakiba, C.; Cornelis, F.; Descat, E.; Gross-Goupil, M.; Sargos, P.; Roubaud, G.; Houédé, N. Dynamic Contrast Enhanced MRI-Derived Parameters Are Potential Biomarkers of Therapeutic Response in Bladder Carcinoma. Eur. J. Radiol. 2015, 84, 1023–1028. [Google Scholar] [CrossRef]
- Ge, L.; Chen, Y.; Yan, C.; Zhao, P.; Zhang, P.; Runa, A.; Liu, J. Study Progress of Radiomics With Machine Learning for Precision Medicine in Bladder Cancer Management. Front. Oncol. 2019, 9, 1296. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Nassiri, N.; Varghese, B.; Maas, M.; King, K.G.; Hwang, D.; Abreu, A.; Gill, I.; Duddalwar, V. Radiomics and Bladder Cancer: Current Status. Bladder Cancer 2020, 6, 343–362. [Google Scholar] [CrossRef]
- Park, K.J.; Lee, J.-L.; Yoon, S.-K.; Heo, C.; Park, B.W.; Kim, J.K. Radiomics-Based Prediction Model for Outcomes of PD-1/PD-L1 Immunotherapy in Metastatic Urothelial Carcinoma. Eur. Radiol. 2020, 30, 5392–5403. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lam, H.-M.; Rosser, C.; Theodorescu, D.; Parks, W.C.; Chan, K.S. The Dynamic Roles of the Bladder Tumour Microenvironment. Nat. Rev. Urol. 2022, 19, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Patel, J.; Partovi, S.; Madabhushi, A.; Tiwari, P. Radiomic Features from the Peritumoral Brain Parenchyma on Treatment-Naïve Multi-Parametric MR Imaging Predict Long versus Short-Term Survival in Glioblastoma Multiforme: Preliminary Findings. Eur. Radiol. 2017, 27, 4188–4197. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.-L.; Liu, Q.-P.; Sun, S.-W.; Zhang, J.; Zhu, F.-P.; Yang, G.; Yan, X.; Zhang, Y.-D.; Liu, X.-S. Radiomic Analysis of Contrast-Enhanced CT Predicts Microvascular Invasion and Outcome in Hepatocellular Carcinoma. J. Hepatol. 2019, 70, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-P.; Wang, Y.-C.; Ju, S.; Lu, C.-Q.; Zhong, B.-Y.; Ni, C.-F.; Zhang, Q.; Yu, Q.; Xu, J.; Ji, J.; et al. Radiomics Analysis on Multiphase Contrast-Enhanced CT: A Survival Prediction Tool in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Front. Oncol. 2020, 10, 1196. [Google Scholar] [CrossRef]
- Pérez-Morales, J.; Tunali, I.; Stringfield, O.; Eschrich, S.A.; Balagurunathan, Y.; Gillies, R.J.; Schabath, M.B. Peritumoral and Intratumoral Radiomic Features Predict Survival Outcomes among Patients Diagnosed in Lung Cancer Screening. Sci. Rep. 2020, 10, 10528. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Raghavan, D.; Bawtinhimer, A.; Mahoney, J.; Eckrich, S.; Riggs, S. Adjuvant Chemotherapy for Bladder Cancer—Why Does Level 1 Evidence Not Support It? Ann. Oncol. 2014, 25, 1930–1934. [Google Scholar] [CrossRef]

| Parameter | Training Set (n = 240) | Test Set (n = 61) | p-Value |
|---|---|---|---|
| Sex: | 0.088 | ||
| Male | 168 (70%) | 50 (82%) | |
| Female | 72 (30%) | 11 (18%) | |
| Age | 69 ± 10 | 69 ± 11 | 0.980 |
| T stage: | 0.698 | ||
| pT ≤ 1 | 35 (15%) | 9 (15%) | |
| pT2 | 80 (33%) | 20 (33%) | |
| pT3 | 90 (38%) | 22 (36%) | |
| pT4 | 34 (14%) | 9 (15%) | |
| pTx | 1 (0%) | 1 (1%) | |
| N stage: | 0.244 | ||
| pN0 | 177 (74%) | 50 (82%) | |
| pN1/pN2 | 63 (26%) | 11 (18%) | |
| Tumor volume (mL) | 10.6 [4.6–24.1] | 11.7 [5.5–27.4] | 0.649 |
| Resection status (surgical margins): | 0.338 | ||
| R0 | 207 (86%) | 49 (80%) | |
| R1/R2 | 33 (14%) | 12 (20%) | |
| Lymphovascular invasion present | 71 (30%) | 12 (20%) | 0.260 |
| Time between CT and surgery (days) | 17 [4–35] | 17 [3–35] | 0.891 |
| Time to recurrence (months) | 8 (7–11) | 16 (4–33) | 0.5 |
| Postopertive complications (Clavien-Dindo) | 0.84 | ||
| low-grade (0–2) | 198 | 52 | |
| high-grade (3–5) | 42 | 9 | |
| Deaths | 141 | 29 | 0.06 |
| cancer-specific | 98 (70%) | 14 (48%) | |
| other cause | 38 (36%) | 14 (48%) | |
| unclear/unknown cause | 5 (4%) | 1 (4%) |
| Model | AUC (1–7 y) | p–Val * | Brier (1–7 y) | p–Val * | C–Index |
|---|---|---|---|---|---|
| Clinical model | 0.761 (0.617–0.874) | ref. | 0.185 (0.144–0.232) | ref. | 0.722 (0.609–0.823) |
| Radiomics features: | |||||
| intra (BCa + LN) | 0.706 (0.552–0.837) | 0.90 | 0.221 (0.179–0.263) | 0.50 | 0.676 (0.549–0.791) |
| intra + peri (BCa) | 0.731 (0.626–0.828) | 1.0 | 0.210 (0.169–0.253) | 0.50 | 0.731 (0.626–0.828) |
| intra + peri (BCa + LN) | 0.771 (0.657–0.869) | 0.98 | 0.202 (0.163–0.243) | 0.53 | 0.737 (0.644–0.822) |
| Combined model | 0.785 (0.648–0.891) | 0.69 | 0.175 (0.129–0.224) | 1.0 | 0.740 (0.632–0.836) |
| Model | AUC (1 y) | AUC (2 y) | AUC (3 y) | AUC (5 y) | AUC (7 y) |
| Clinical model | 0.819 (0.647–0.960) | 0.692 (0.482–0.885) | 0.713 (0.545–0.875) | 0.779 (0.622–0.921) | 0.798 (0.638–0.924) |
| Radiomics features: | |||||
| intra (BCa + LN) | 0.758 (0.604–0.897) | 0.712 (0.512–0.925) | 0.784 (0.627–0.925) | 0.802 (0.642–0.928) | 0.820 (0.665–0.945) |
| intra + peri (BCa) | 0.758 (0.545–0.921) | 0.672 (0.456–0.859) | 0.706 (0.524–0.864) | 0.680 (0.493–0.843) | 0.711 (0.517–0.876) |
| intra + peri (BCa + LN) | 0.787 (0.650–0.900) | 0.722 (0.544–0.869) | 0.821 (0.672–0.944) | 0.771 (0.601–0.916) | 0.825 (0.676–0.950) |
| Combined model | 0.845 (0.688–0.968) | 0.769 (0.588–0.918) | 0.790 (0.632–0.915) | 0.749 (0.571–0.907) | 0.803 (0.639–0.907) |
| Model | Brier (1 y) | Brier (2 y) | Brier (3 y) | Brier (5 y) | Brier (7 y) |
| Clinical model | 0.105 (0.067–0.144) | 0.172 (0.120–0.229) | 0.202 (0.145–0.266) | 0.195 (0.131–0.269) | 0.210 (0.136–0.296) |
| Radiomics features: | |||||
| intra (BCa + LN) | 0.130 (0.068–0.178) | 0.195 (0.153–0.242) | 0.214 (0.166–0.267) | 0.214 (0.160–0.275) | 0.252 (0.167–0.358) |
| intra + peri (BCa) | 0.137 (0.087–0.192) | 0.198 (0.151–0.253) | 0.228 (0.181–0.286) | 0.230 (0.176–0.284) | 0.264 (0.184–0.363 |
| intra + peri (BCa + LN) | 0.121 (0.082–0.166) | 0.186 (0.148–0.250) | 0.201 (0.158–0.250) | 0.213 (0.161–0.272) | 0.243 (0.162–0.344) |
| Combined model | 0.098 (0.051–0.156) | 0.160 (0.105–0.226) | 0.174 (0.116–0.245) | 0.197 (0.130–0.275) | 0.205 (0.123–0.294) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźnicki, P.; Laqua, F.C.; Messmer, K.; Kunz, W.G.; Stief, C.; Nörenberg, D.; Schreier, A.; Wójcik, J.; Ruebenthaler, J.; Ingrisch, M.; et al. Radiomics for the Prediction of Overall Survival in Patients with Bladder Cancer Prior to Radical Cystectomy. Cancers 2022, 14, 4449. https://doi.org/10.3390/cancers14184449
Woźnicki P, Laqua FC, Messmer K, Kunz WG, Stief C, Nörenberg D, Schreier A, Wójcik J, Ruebenthaler J, Ingrisch M, et al. Radiomics for the Prediction of Overall Survival in Patients with Bladder Cancer Prior to Radical Cystectomy. Cancers. 2022; 14(18):4449. https://doi.org/10.3390/cancers14184449
Chicago/Turabian StyleWoźnicki, Piotr, Fabian Christopher Laqua, Katharina Messmer, Wolfgang Gerhard Kunz, Christian Stief, Dominik Nörenberg, Andrea Schreier, Jan Wójcik, Johannes Ruebenthaler, Michael Ingrisch, and et al. 2022. "Radiomics for the Prediction of Overall Survival in Patients with Bladder Cancer Prior to Radical Cystectomy" Cancers 14, no. 18: 4449. https://doi.org/10.3390/cancers14184449
APA StyleWoźnicki, P., Laqua, F. C., Messmer, K., Kunz, W. G., Stief, C., Nörenberg, D., Schreier, A., Wójcik, J., Ruebenthaler, J., Ingrisch, M., Ricke, J., Buchner, A., Schulz, G. B., & Gresser, E. (2022). Radiomics for the Prediction of Overall Survival in Patients with Bladder Cancer Prior to Radical Cystectomy. Cancers, 14(18), 4449. https://doi.org/10.3390/cancers14184449







