CRL4CRBN E3 Ligase Complex as a Therapeutic Target in Multiple Myeloma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Immunomodulatory Drugs (IMiDs)
2.1. Mechanism of IMiDs’ Action
2.2. Clinical Efficacy of IMiDs
| Trial | Phase | Regimen | Outcome |
|---|---|---|---|
| Newly-Diagnosed MM with Transplant Intent | |||
| CASSIOPEIA [45] | 3 | Dara-VTD VTD | mPFS: NR vs. NR (HR = 0.47; p < 0.0001) MRD (-): 64% vs. 44% (p < 0.0001) |
| PETHEMA/GEM2012 [46] | 3 | VRD | mPFS: NR; MRD (-): 29% (post induction), 42% (post auto-HSCT) and 45% (post consolidation) |
| GRIFFIN [47] | 3 | Dara-VRD VRD | 2y-PFS: 96% vs. 90% MRD (-): 51% vs. 20% (p < 0.0001) |
| Newly-Diagnosed MM with Non-Transplant Intent | |||
| SWOG S0777 [48] | 3 | VRD RD | mPFS: 43 vs. 30 mo (HR = 0.71; p = 0.0018) mOS: 75 vs. 64 mo (HR = 0.71; p = 0.025) |
| ENDURANCE [49] | 3 | KRD VRD | mPFS: 34.6 vs. 34.4 months (p = 0.74) |
| MAIA [50] | 3 | Dara-RD VRD | mPFS: NR vs. 34.4 mo (HR = 0.53; p < 0.0001) |
| Relapsed/Refractory MM | |||
| Dimopoulos et al. [52] | 3 | RD placebo-D | mTTP, 11.3 vs. 4.7 months (p < 0.001) |
| ASPIRE [54] | 3 | KRD RD | mPFS: 26 vs. 18 mo (HR 0.69; p = 0.0001) mOS: 48 vs. 40 mo (HR = 0.79; p = 0.0045) |
| TOURMALINE [55] | 3 | IRD placebo-RD | mPFS: 20.6 vs. 14.7 mo (HR = 0.74; p = 0.01) |
| POLLUX [56] | 3 | Dara-RD RD | mPFS: 44.5 vs. 17.5 mo (HR = 0.44; p < 0.0001) |
| ELOQUENT-2 [57,58] | 3 | Elo-RD RD | mPFS: 19.4 vs. 14.9 mo (HR = 0.70; p < 0.001) mOS: 48.3 vs. 39.6 mo (HR = 0.82; p = 0.04) |
| CANDOR [59] | 3 | Dara-KD KD | mPFS: 28.6 vs. 15.2 mo (HR = 0.59; p <0.0001) |
| CASTOR [60] | 3 | Dara-VD VD | mPFS: 16.7 vs. 7.1 mo (HR = 0.31; p < 0.0001) |
| ENDEAVOR [61] | 3 | KD VD | mPFS: 18.7 vs. 9.4 mo (HR = 0.53; p < 0.0001) mOS, 47.6 vs. 40 mo (HR = 0.79; p = 0.01) |
| APOLLO [62] | 3 | Dara-PD PD | mPFS: 12.4 vs. 6.9 mo (HR = 0.63; p = 0.0018) |
| ICARIA-MM [63] | 3 | Isa-PD PD | mPFS: 11.5 vs. 6.5 mo (HR = 0.596; p = 0.001) mOS: 24.6 vs. 17.7 mo (HR = 0.76; p = 0.028) |
| ELOQUENT-3 [64] | 2 | Elo-PD PD | mPFS: 10.3 vs. 4.7 mo (HR = 0.54; p = 0.008) mOS: 29.8 vs. 17.4 mo (HR = 0.59; p = 0.0217) |
| OPTIMISMM [65] | 3 | PVD VD | mPFS: 11.2 vs. 7.1 mo (HR = 0.61; p < 0.0001) |
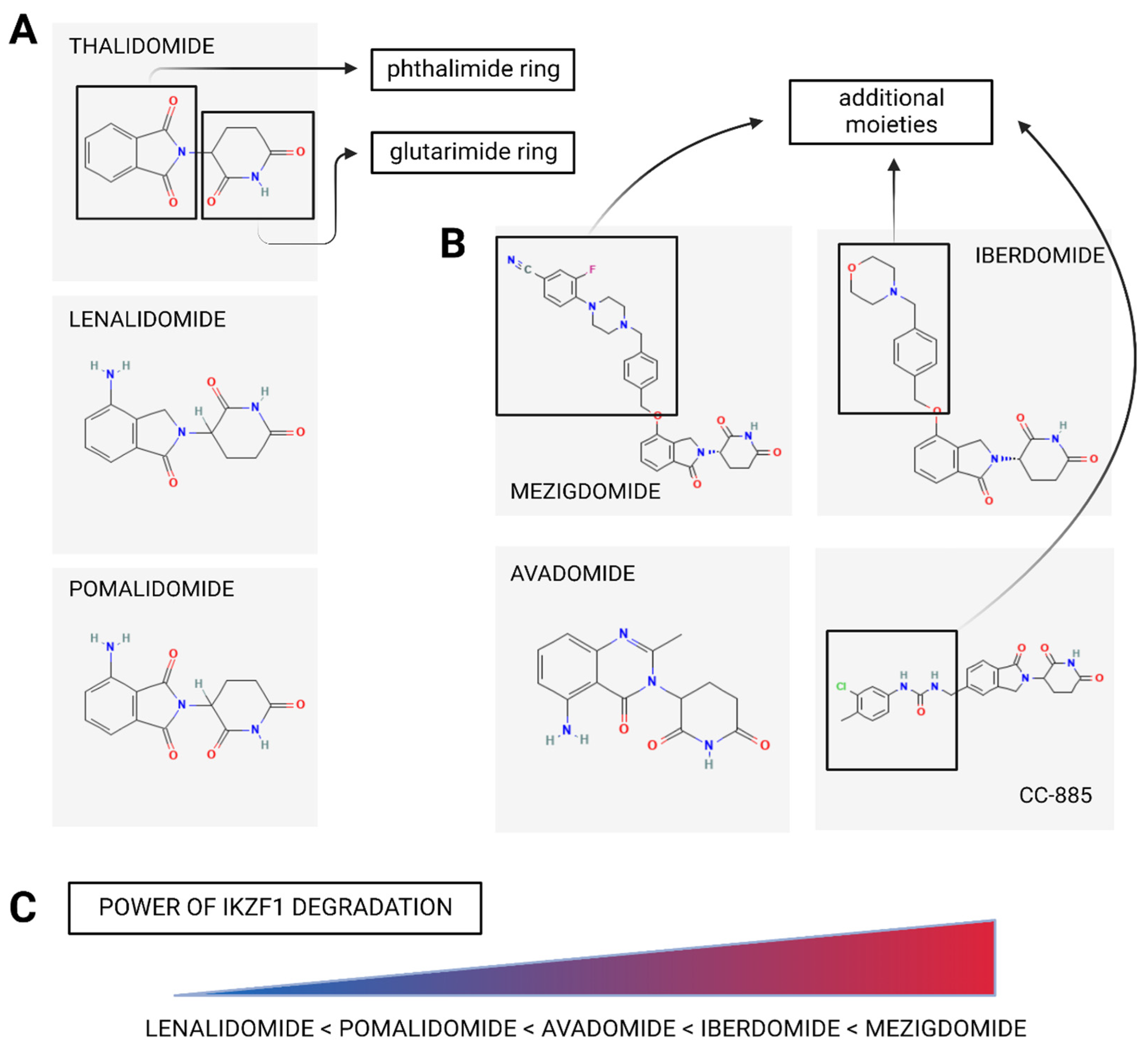
3. Cereblon E3 Ligase Modulators (CELMoDs)
3.1. Mechanism of CELMoDs’ Action
3.2. Clinical Efficacy of CELMoDs
3.2.1. CC-92480 (Mezigdomide)
3.2.2. Iberdomide (CC-220)
3.2.3. Avadomide (CC-122)
3.2.4. CC-885
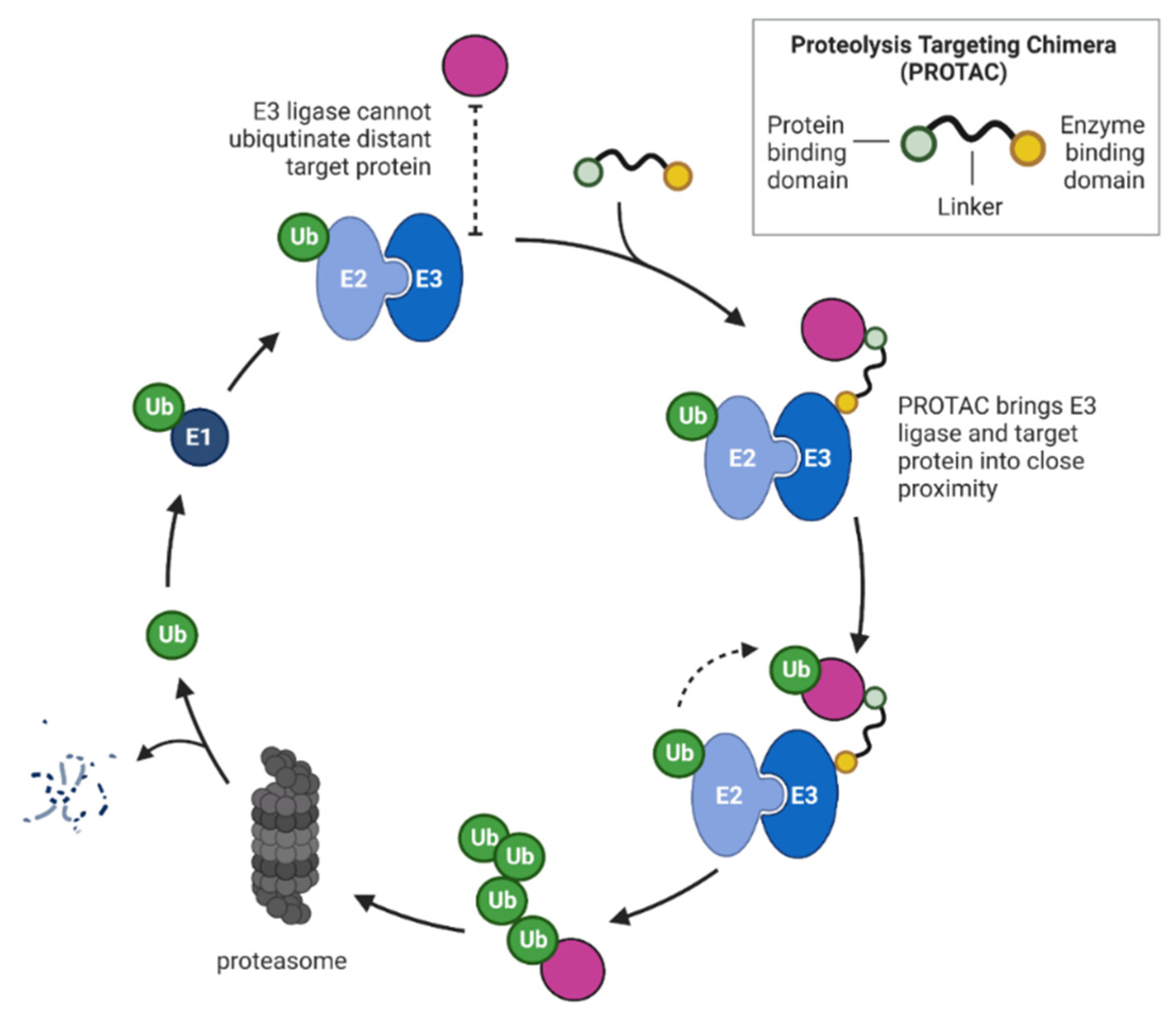
4. Proteolysis Targeting Chimeras (PROTACs)
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Finley, D.; Ciechanover, A.; Varshavsky, A. Ubiquitin as a central cellular regulator. Cell 2004, 116, S29–S34. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Wang, W.; Xiong, Y. Cullin-RING E3 Ubiquitin Ligases: Bridges to Destruction. Macromol. Protein Complexes 2017, 83, 323–347. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Huang, K.-Y.; Weng, J.T.-Y.; Lai, K.R.; Lee, T.-Y. UbiNet: An online resource for exploring the functional associations and regulatory networks of protein ubiquitylation. Database 2016, 2016. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Braggio, E.; Shi, C.-X.; Kortuem, K.M.; Bruins, L.A.; Schmidt, J.E.; Chang, X.-B.; Langlais, P.; Luo, M.; Jedlowski, P.; et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood 2014, 124, 536–545. [Google Scholar] [CrossRef]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.-K.; Bradner, J.E.; Kaelin, W.G., Jr. The Myeloma Drug Lenalidomide Promotes the Cereblon-Dependent Destruction of Ikaros Proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Gandhi, A.K.; Kang, J.; Havens, C.G.; Conklin, T.; Ning, Y.; Wu, L.; Ito, T.; Ando, H.; Waldman, M.F.; Thakurta, A.; et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors I karos and A iolos via modulation of the E 3 ubiquitin ligase complex CRL 4CRBN. Br. J. Haematol. 2013, 164, 811–821. [Google Scholar] [CrossRef]
- Fischer, E.S.; Böhm, K.; Lydeard, J.R.; Yang, H.; Stadler, M.B.; Cavadini, S.; Nagel, J.; Serluca, F.; Acker, V.; Lingaraju, G.M.; et al. Structure of the DDB1–CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [Google Scholar] [CrossRef]
- Krönke, J.; Fink, E.C.; Hollenbach, P.W.; Macbeth, K.J.; Hurst, S.N.; Udeshi, N.D.; Chamberlain, P.P.; Mani, D.R.; Man, H.W.; Gandhi, A.K.; et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 2015, 523, 183–188. [Google Scholar] [CrossRef]
- Petzold, G.; Fischer, E.S.; Thomä, G.P.E.S.F.N.H. Structural basis of lenalidomide-induced CK1α degradation by the CRL4CRBN ubiquitin ligase. Nature 2016, 532, 127–130. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Braggio, E.; Shi, C.-X.; Bruins, L.A.; Schmidt, J.E.; Van Wier, S.; Chang, X.-B.; Bjorklund, C.C.; Fonseca, R.; Bergsagel, P.L.; et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011, 118, 4771–4779. [Google Scholar] [CrossRef]
- Franssen, L.E.; Nijhof, I.S.; Couto, S.; Levin, M.-D.; Bos, G.M.; Broijl, A.; Klein, S.K.; Ren, Y.; Wang, M.; Koene, H.R.; et al. Cereblon loss and up-regulation of c-Myc are associated with lenalidomide resistance in multiple myeloma patients. Haematologica 2018, 103, e368–e371. [Google Scholar] [CrossRef]
- Heider, M.; Eichner, R.; Stroh, J.; Morath, V.; Kuisl, A.; Zecha, J.; Lawatscheck, J.; Baek, K.; Garz, A.-K.; Rudelius, M.; et al. The IMiD target CRBN determines HSP90 activity toward transmembrane proteins essential in multiple myeloma. Mol. Cell 2021, 81, 1170–1186.e10. [Google Scholar] [CrossRef]
- Costacurta, M.; He, J.; Thompson, P.E.; Shortt, J. Molecular Mechanisms of Cereblon-Interacting Small Molecules in Multiple Myeloma Therapy. J. Pers. Med. 2021, 11, 1185. [Google Scholar] [CrossRef]
- Haslett, P.A.; Corral, L.G.; Albert, M.; Kaplan, G. Thalidomide Costimulates Primary Human T Lymphocytes, Preferentially Inducing Proliferation, Cytokine Production, and Cytotoxic Responses in the CD8+ Subset. J. Exp. Med. 1998, 187, 1885–1892. [Google Scholar] [CrossRef]
- Davies, F.; Raje, N.; Hideshima, T.; Lentzsch, S.; Young, G.; Tai, Y.-T.; Lin, B.; Podar, K.; Gupta, D.; Chauhan, D.; et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001, 98, 210–216. [Google Scholar] [CrossRef]
- Hayashi, T.; Hideshima, T.; Akiyama, M.; Podar, K.; Yasui, H.; Raje, N.; Kumar, S.; Chauhan, D.; Treon, S.P.; Richardson, P.; et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: Clinical application. Br. J. Haematol. 2004, 128, 192–203. [Google Scholar] [CrossRef]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar] [CrossRef]
- Charliński, G.; Vesole, D.H.; Jurczyszyn, A. Rapid Progress in the Use of Immunomodulatory Drugs and Cereblon E3 Ligase Modulators in the Treatment of Multiple Myeloma. Cancers 2021, 13, 4666. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a Primary Target of Thalidomide Teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Broyl, A.; Kuiper, R.; Van Duin, M.; Van Der Holt, B.; El Jarari, L.; Bertsch, U.; Zweegman, S.; Buijs, A.; Hose, D.; Lokhorst, H.M.; et al. High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance. Blood 2013, 121, 624–627. [Google Scholar] [CrossRef]
- Barankiewicz, J.; Szumera-Ciećkiewicz, A.; Salomon-Perzyński, A.; Wieszczy, P.; Malenda, A.; Garbicz, F.; Prochorec-Sobieszek, M.; Misiewicz-Krzemińska, I.; Juszczyński, P.; Lech-Marańda, E. The CRBN, CUL4A and DDB1 Expression Predicts the Response to Immunomodulatory Drugs and Survival of Multiple Myeloma Patients. J. Clin. Med. 2021, 10, 2683. [Google Scholar] [CrossRef]
- Heintel, D.; Rocci, A.; Ludwig, H.; Bolomsky, A.; Caltagirone, S.; Schreder, M.; Pfeifer, S.; Gisslinger, H.; Zojer, N.; Jäger, U.; et al. High expression of cereblon (CRBN) is associated with improved clinical response in patients with multiple myeloma treated with lenalidomide and dexamethasone. Br. J. Haematol. 2013, 161, 695–700. [Google Scholar] [CrossRef]
- Misiewicz-Krzeminska, I.; De Ramón, C.; Corchete, L.A.; Krzeminski, P.; Rojas, E.A.; Isidro, I.; García-Sanz, R.; Martínez-López, J.; Oriol, A.; Bladé, J.; et al. Quantitative expression of Ikaros, IRF4, and PSMD10 proteins predicts survival in VRD-treated patients with multiple myeloma. Blood Adv. 2020, 4, 6023–6033. [Google Scholar] [CrossRef]
- Gooding, S.; Ansari-Pour, N.; Towfic, F.; Estévez, M.O.; Chamberlain, P.P.; Tsai, K.-T.; Flynt, E.; Hirst, M.; Rozelle, D.; Dhiman, P.; et al. Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma. Blood 2021, 137, 232–237. [Google Scholar] [CrossRef]
- Jones, J.R.; Barber, A.; Le Bihan, Y.-V.; Weinhold, N.; Ashby, C.; Walker, B.A.; Wardell, C.P.; Wang, H.; Kaiser, M.F.; Jackson, G.H.; et al. Mutations in CRBN and other cereblon pathway genes are infrequently associated with acquired resistance to immunomodulatory drugs. Leukemia 2021, 35, 3017–3020. [Google Scholar] [CrossRef]
- Salomon-Perzyński, A.; Barankiewicz, J.; Machnicki, M.; Misiewicz-Krzemińska, I.; Pawlak, M.; Radomska, S.; Krzywdzińska, A.; Bluszcz, A.; Stawiński, P.; Rydzanicz, M.; et al. Tracking Clonal Evolution of Multiple Myeloma Using Targeted Next-Generation DNA Sequencing. Biomedicines 2022, 10, 1674. [Google Scholar] [CrossRef]
- Shaffer, A.L.; Emre, N.C.T.; Lamy, L.; Ngo, V.N.; Wright, G.; Xiao, W.; Powell, J.; Dave, S.; Yu, X.; Zhao, H.; et al. IRF4 addiction in multiple myeloma. Nature 2008, 454, 226–231. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Heintel, D.; Zhang, L.-H.; Mendy, D.; Gaidarova, S.; Brady, H.; Bartlett, J.B.; Schafer, P.H.; Schreder, M.; Bolomsky, A.; et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br. J. Haematol. 2011, 154, 325–336. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Lu, L.; Kang, J.; Hagner, P.; Havens, C.G.; Amatangelo, M.; Wang, M.; Ren, Y.; Couto, S.S.; Breider, M.; et al. Rate of CRL4CRBN substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-Myc and IRF4. Blood Cancer J. 2015, 5, e354. [Google Scholar] [CrossRef]
- Patil, A.; Manzano, M.; Gottwein, E. CK1α and IRF4 are essential and independent effectors of immunomodulatory drugs in primary effusion lymphoma. Blood 2018, 132, 577–586. [Google Scholar] [CrossRef]
- Manni, S.; Carrino, M.; Manzoni, M.; Gianesin, K.; Nunes, S.C.; Costacurta, M.; Tubi, L.Q.; Macaccaro, P.; Taiana, E.; Cabrelle, A.; et al. Inactivation of CK1α in multiple myeloma empowers drug cytotoxicity by affecting AKT and β-catenin survival signaling pathways. Oncotarget 2017, 8, 14604–14619. [Google Scholar] [CrossRef]
- Carrino, M.; Tubi, L.Q.; Fregnani, A.; Nunes, S.C.; Barilà, G.; Trentin, L.; Zambello, R.; Semenzato, G.; Manni, S.; Piazza, F. Prosurvival autophagy is regulated by protein kinase CK1 alpha in multiple myeloma. Cell Death Discov. 2019, 5, 98. [Google Scholar] [CrossRef]
- Hu, Y.; Song, W.; Cirstea, D.; Lu, D.; Munshi, N.C.; Anderson, K.C. CSNK1α1 mediates malignant plasma cell survival. Leukemia 2014, 29, 474–482. [Google Scholar] [CrossRef]
- Matyskiela, M.E.; Couto, S.; Zheng, X.; Lu, G.; Hui, J.; Stamp, K.; Drew, C.; Ren, Y.; Wang, M.; Carpenter, A.; et al. SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat. Chem. Biol. 2018, 14, 981–987. [Google Scholar] [CrossRef]
- A Donovan, K.; An, J.; Nowak, R.P.; Yuan, J.C.; Fink, E.C.; Berry, B.C.; Ebert, B.L.; Fischer, E.S. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. eLife 2018, 7. [Google Scholar] [CrossRef]
- Asatsuma-Okumura, T.; Ando, H.; De Simone, M.; Yamamoto, J.; Sato, T.; Shimizu, N.; Asakawa, K.; Yamaguchi, Y.; Ito, T.; Guerrini, L.; et al. p63 is a cereblon substrate involved in thalidomide teratogenicity. Nat. Chem. Biol. 2019, 15, 1077–1084. [Google Scholar] [CrossRef]
- Yamanaka, S.; Murai, H.; Saito, D.; Abe, G.; Tokunaga, E.; Iwasaki, T.; Takahashi, H.; Takeda, H.; Suzuki, T.; Shibata, N.; et al. Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF. EMBO J. 2021, 40, e105375. [Google Scholar] [CrossRef]
- An, J.; Ponthier, C.M.; Sack, R.; Seebacher, J.; Stadler, M.B.; Donovan, K.; Fischer, E.S. pSILAC mass spectrometry reveals ZFP91 as IMiD-dependent substrate of the CRL4CRBN ubiquitin ligase. Nat. Commun. 2017, 8, 15398. [Google Scholar] [CrossRef] [Green Version]
- Sievers, Q.L.; Petzold, G.; Bunker, R.D.; Renneville, A.; Słabicki, M.; Liddicoat, B.J.; Abdulrahman, W.; Mikkelsen, T.; Ebert, B.L.; Thomä, N.H. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 2018, 362, eaat0572. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Li, S.; Donnenberg, V.; Fu, J.; Gollin, S.M.; Ma, H.; Lu, C.; Stolz, N.B.; Mapara, M.Y.; Monaghan, S.A.; et al. Immunomodulatory drugs downregulate IKZF1 leading to expansion of hematopoietic progenitors with concomitant block of megakaryocytic maturation. Haematologica 2018, 103, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Monaghan, S.A.; Hassett, A.C.; Mapara, M.Y.; Schafer, P.; Roodman, G.D.; Ragni, M.V.; Moscinski, L.; List, A.; Lentzsch, S. Immunomodulatory derivatives induce PU.1 down-regulation, myeloid maturation arrest, and neutropenia. Blood 2010, 115, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Rosiñol, L.; Oriol, A.; Teruel, A.I.; Hernández, D.; López-Jiménez, J.; de la Rubia, J.; Granell, M.; Besalduch, J.; Palomera, L.; González, Y.; et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: A randomized phase 3 PETHEMA/GEM study. Blood 2012, 120, 1589–1596. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Rosiñol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernández, M.T.; Martínez-Martínez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Kumar, S.K.; Jacobus, S.J.; Cohen, A.D.; Weiss, M.; Callander, N.; Singh, A.K.; Parker, T.L.; Menter, A.; Yang, X.; Parsons, B.; et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 1317–1330. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef]
- Callander, N.S.; Baljevic, M.; Adekola, K.; Anderson, L.D.; Campagnaro, E.; Castillo, J.J.; Costello, C.; Devarakonda, S.; Elsedawy, N.; Faiman, M.; et al. NCCN Guidelines® Insights: Multiple Myeloma, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 8–19. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Spencer, A.; Attal, M.; Prince, H.M.; Harousseau, J.-L.; Dmoszynska, A.; Miguel, J.S.; Hellmann, A.; Facon, T.; Foà, R.; et al. Lenalidomide plus Dexamethasone for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2007, 357, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.S.; Dimopoulos, M.A.; Ludwig, H.; Facon, T.; Goldschmidt, H.; Jakubowiak, A.; Miguel, J.S.; Obreja, M.; Blaedel, J.; Stewart, A.K. Improvement in Overall Survival with Carfilzomib, Lenalidomide, and Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2018, 36, 728–734. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Quach, H.; Mateos, M.-V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Yang, H.; Klippel, Z.; Zahlten-Kumeli, A.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020, 396, 186–197. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Sonneveld, P.; Hungria, V.; Nooka, A.K.; Estell, J.A.; Barreto, W.; Corradini, P.; Min, C.-K.; Medvedova, E.; Weisel, K.; et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients with Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR. Clin. Lymphoma Myeloma Leuk. 2019, 20, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.-J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Delimpasi, S.; Beksac, M.; Katodritou, E.; Moreau, P.; Baldini, L.; Symeonidis, A.; Bila, J.; et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 801–812. [Google Scholar] [CrossRef]
- Richardson, P.G.; Perrot, A.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; Huang, J.S.-Y.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): Follow-up analysis of a randomised, phase 3 study. Lancet Oncol. 2022, 23, 416–427. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; Leblanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Richardson, P.G.; Oriol, A.; Beksac, M.; Liberati, A.M.; Galli, M.; Schjesvold, F.; Lindsay, J.; Weisel, K.; White, D.; Facon, T.; et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): A randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef]
- Shah, J.J.; Stadtmauer, E.A.; Abonour, R.; Cohen, A.D.; Bensinger, W.I.; Gasparetto, C.; Kaufman, J.L.; Lentzsch, S.; Vogl, D.T.; Gomes, C.L.; et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood 2015, 126, 2284–2290. [Google Scholar] [CrossRef]
- Krishnan, A.; Kapoor, P.; Palmer, J.M.; Tsai, N.-C.; Kumar, S.; Lonial, S.; Htut, M.; Karanes, C.; Nathwani, N.; Rosenzweig, M.; et al. Phase I/II trial of the oral regimen ixazomib, pomalidomide, and dexamethasone in relapsed/refractory multiple myeloma. Leukemia 2018, 32, 1567–1574. [Google Scholar] [CrossRef]
- Garderet, L.; Kuhnowski, F.; Berge, B.; Roussel, M.; Escoffre-Barbe, M.; Lafon, I.; Facon, T.; Leleu, X.; Karlin, L.; Perrot, A.; et al. Pomalidomide, cyclophosphamide, and dexamethasone for relapsed multiple myeloma. Blood 2018, 132, 2555–2563. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Ben Yehuda, D.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. N. Engl. J. Med. 2014, 371, 895–905. [Google Scholar] [CrossRef]
- Holstein, S.A.; Jung, S.-H.; Richardson, P.G.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: A randomised, double-blind, phase 3 trial. Lancet Haematol. 2017, 4, e431–e442. [Google Scholar] [CrossRef]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Csg, O.B.O.T.N.H.-O.; Cairns, D.A.; Gregory, W.M.; Collett, C.; Pawlyn, C.; Sigsworth, R.; Striha, A.; Henderson, R.; Kaiser, M.F.; et al. Second malignancies in the context of lenalidomide treatment: An analysis of 2732 myeloma patients enrolled to the Myeloma XI trial. Blood Cancer J. 2016, 6, e506. [Google Scholar] [CrossRef]
- Chavez, B.M.; Barnell, B.E.; Griffith, M.; Skidmore, Z.; Griffith, O.; Tian, L.; Wartman, L.D. B-Cell Acute Lymphoblastic Leukemia Arising in Patients with a Preexisting Diagnosis of Multiple Myeloma Is a Novel Cancer with High Incidence of TP53 Mutations. Blood 2020, 136, 20. [Google Scholar] [CrossRef]
- Germans, S.K.; Kulak, O.; Koduru, P.; Oliver, D.; Gagan, J.; Patel, P.; Anderson, L.D.; Fuda, F.S.; Chen, W.; Jaso, J.M. Lenalidomide-Associated Secondary B-Lymphoblastic Leukemia/Lymphoma—A Unique Entity. Am. J. Clin. Pathol. 2020, 154, 816–827. [Google Scholar] [CrossRef]
- Khan, S.R.; Tariq, M.; Fayyaz, S.M.; Soomar, S.M.; Moosajee, M. Lenalidomide induced secondary Acute Lymphoblastic Leukemia in a Multiple Myeloma patient: A case-report. Leuk. Res. Rep. 2022, 17. [Google Scholar] [CrossRef]
- Sinit, R.; Hwang, D.G.; Vishnu, P.; Peterson, J.F.; Aboulafia, D.M. B-cell acute lymphoblastic leukemia in an elderly man with plasma cell myeloma and long-term exposure to thalidomide and lenalidomide: A case report and literature review. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Matyskiela, M.E.; Zhang, W.; Man, H.-W.; Muller, G.; Khambatta, G.; Baculi, F.; Hickman, M.; Lebrun, L.; Pagarigan, B.; Carmel, G.; et al. A Cereblon Modulator (CC-220) with Improved Degradation of Ikaros and Aiolos. J. Med. Chem. 2017, 61, 535–542. [Google Scholar] [CrossRef]
- Thakurta, A.; Pierceall, W.E.; Amatangelo, M.D.; Flynt, E.; Agarwal, A. Developing next generation immunomodulatory drugs and their combinations in multiple myeloma. Oncotarget 2021, 12, 1555–1563. [Google Scholar] [CrossRef]
- Hansen, J.D.; Correa, M.; Nagy, M.A.; Alexander, M.; Plantevin, V.; Grant, V.; Whitefield, B.; Huang, D.; Kercher, T.; Harris, R.; et al. Discovery of CRBN E3 Ligase Modulator CC-92480 for the Treatment of Relapsed and Refractory Multiple Myeloma. J. Med. Chem. 2020, 63, 6648–6676. [Google Scholar] [CrossRef] [PubMed]
- Matyskiela, M.E.; Lu, G.; Ito, T.; Pagarigan, B.; Lu, C.-C.; Miller, K.; Fang, W.; Wang, N.-Y.; Nguyen, D.; Houston, J.; et al. A novel cereblon modulator recruits GSPT1 to the CRL4CRBN ubiquitin ligase. Nature 2016, 535, 252–257. [Google Scholar] [CrossRef]
- Hao, B.-B.; Li, X.-J.; Jia, X.-L.; Wang, Y.-X.; Zhai, L.-H.; Li, D.-Z.; Liu, J.; Zhang, D.; Chen, Y.-L.; Xu, Y.-H.; et al. The novel cereblon modulator CC-885 inhibits mitophagy via selective degradation of BNIP3L. Acta Pharmacol. Sin. 2020, 41, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hu, M.; Chen, Y.; Liu, H.; Chen, Y.; Liu, B.; Fang, B. Cereblon modulator CC-885 induces CRBN-dependent ubiquitination and degradation of CDK4 in multiple myeloma. Biochem. Biophys. Res. Commun. 2021, 549, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, C.C.; Kang, J.; Lu, L.; Amatangelo, M.; Chiu, H.; Hagner, P.; Gandhi, A.K.; Pourdehnad, M.; Klippel, A.; Thakurta, A. CC-122 Is a Cereblon Modulating Agent That Is Active in Lenalidomide-Resistant and Lenalidomide/Dexamethasone-Double-Resistant Multiple Myeloma Pre-Clinical Models. Blood 2016, 128, 1592. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Kang, J.; Amatangelo, M.; Polonskaia, A.; Katz, M.; Chiu, H.; Couto, S.; Wang, M.; Ren, Y.; Ortiz, M.; et al. Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia 2019, 34, 1197–1201. [Google Scholar] [CrossRef]
- Renneville, A.; Gasser, J.A.; Grinshpun, D.E.; Beltran, P.M.J.; Udeshi, N.D.; Matyskiela, M.E.; Clayton, T.; McConkey, M.; Viswanathan, K.; Tepper, A.; et al. Avadomide Induces Degradation of ZMYM2 Fusion Oncoproteins in Hematologic Malignancies. Blood Cancer Discov. 2021, 2, 250–265. [Google Scholar] [CrossRef]
- Richardson, P.G.; Ocio, E.; Raje, N.S.; Gregory, T.; White, D.; Oriol, A.; Sandhu, I.; Raab, M.-S.; LeBlanc, R.; Rodriguez, C.; et al. CC-92480, a Potent, Novel Cereblon E3 Ligase Modulator (CELMoD) Agent, in Combination with Dexamethasone (DEX) and Bortezomib (BORT) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Preliminary Results from the Phase 1/2 Study CC-92480-MM-002. Blood 2021, 138, 2731. [Google Scholar] [CrossRef]
- Richardson, P.G.; Vangsted, A.J.; Ramasamy, K.; Trudel, S.; Martínez, J.; Mateos, M.-V.; Rodríguez Otero, P.; Lonial, S.; Popat, R.; Oriol, A.; et al. First-in-human phase I study of the novel CELMoD agent CC-92480 combined with dexamethasone (DEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2020, 38, 8500. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Popat, R.; Larsen, J.; Minnema, M.C.; Jagannath, S.; Oriol, A.; Zonder, J.; Richardson, P.G.; Rodriguez-Otero, P.; Badros, A.Z.; et al. First Results of Iberdomide (IBER.;CC-220) in Combination with Dexamethasone (DEX) and Daratumumab (DARA) or Bortezomib (BORT) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 16–17. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Popat, R.; Hulin, C.; Jagannath, S.; Oriol, A.; Richardson, P.G.; Facon, T.; Weisel, K.; Larsen, J.T.; Minnema, M.; et al. P07: RESULTS FROM THE CC-220-MM-001 DOSE-EXPANSION PHASE OF IBERDOMIDE PLUS DEXAMETHASONE IN PATIENTS WITH RELAPSED/REFRACTORY MULTIPLE MYELOMA. HemaSphere 2022, 6, 14–15. [Google Scholar] [CrossRef]
- Rasco, D.W.; Papadopoulos, K.P.; Pourdehnad, M.; Gandhi, A.K.; Hagner, P.R.; Li, Y.; Wei, X.; Chopra, R.; Hege, K.; DiMartino, J.F.; et al. A First-in-Human Study of Novel Cereblon Modulator Avadomide (CC-122) in Advanced Malignancies. Clin. Cancer Res. 2019, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Nastoupil, L.J.; Kuruvilla, J.; Chavez, J.C.; Bijou, F.; Witzig, T.E.; Santoro, A.; Flinn, I.W.; Boccomini, C.; Kenkre, V.P.; Corradini, P.; et al. Phase Ib study of avadomide (CC-122) in combination with rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma and follicular lymphoma. eJHaem 2022, 3, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.-M.; Bouabdallah, R.; Vitolo, U.; Doorduijn, J.K.; Salles, G.; Chiappella, A.; Zinzani, P.L.; Bijou, F.; Kersten, M.J.; Sarmiento, R.; et al. Avadomide plus obinutuzumab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (CC-122-NHL-001): A multicentre, dose escalation and expansion phase 1 study. Lancet Haematol. 2020, 7, e649–e659. [Google Scholar] [CrossRef]
- Li, L.; Xue, W.; Shen, Z.; Liu, J.; Hu, M.; Cheng, Z.; Wang, Y.; Chen, Y.; Chang, H.; Liu, Y.; et al. A Cereblon Modulator CC-885 Induces CRBN- and p97-Dependent PLK1 Degradation and Synergizes with Volasertib to Suppress Lung Cancer. Mol. Ther. Oncolytics 2020, 18, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.E.; Buckley, D.L.; Paulk, J.; Roberts, J.M.; Souza, A.; Dhe-Paganon, S.; Bradner, J.E. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 2015, 348, 1376–1381. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, H.C.; Shirazi, F.; Baladandayuthapani, V.; Lin, H.; Kuiatse, I.; Wang, H.; Jones, R.J.; Berkova, Z.; Singh, R.K.; et al. Protein targeting chimeric molecules specific for bromodomain and extra-terminal motif family proteins are active against pre-clinical models of multiple myeloma. Leukemia 2018, 32, 2224–2239. [Google Scholar] [CrossRef]
- Lim, S.L.; Damnernsawad, A.; Shyamsunder, P.; Chng, W.J.; Han, B.C.; Xu, L.; Pan, J.; Pravin, D.P.; Alkan, S.; Tyner, J.W.; et al. Proteolysis targeting chimeric molecules as therapy for multiple myeloma: Efficacy, biomarker and drug combinations. Haematologica 2019, 104, 1209–1220. [Google Scholar] [CrossRef]
- Steinebach, C.; Ng, Y.L.D.; Sosič, I.; Lee, C.-S.; Chen, S.; Lindner, S.; Vu, L.P.; Bricelj, A.; Haschemi, R.; Monschke, M.; et al. Systematic exploration of different E3 ubiquitin ligases: An approach towards potent and selective CDK6 degraders. Chem. Sci. 2020, 11, 3474–3486. [Google Scholar] [CrossRef]
- Ng, Y.L.D.; Ramberger, E.; Bohl, S.R.; Dolnik, A.; Steinebach, C.; Conrad, T.; Müller, S.; Popp, O.; Kull, M.; Haji, M.; et al. Proteomic profiling reveals CDK6 upregulation as a targetable resistance mechanism for lenalidomide in multiple myeloma. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Papatzimas, J.W.; Gorobets, E.; Maity, R.; Muniyat, M.I.; Maccallum, J.L.; Neri, P.; Bahlis, N.J.; Derksen, D.J. From Inhibition to Degradation: Targeting the Antiapoptotic Protein Myeloid Cell Leukemia 1 (MCL1). J. Med. Chem. 2019, 62, 5522–5540. [Google Scholar] [CrossRef]
- Medvar, B.; Raghuram, V.; Pisitkun, T.; Sarkar, A.; Knepper, M.A. Comprehensive database of human E3 ubiquitin ligases: Application to aquaporin-2 regulation. Physiol. Genom. 2016, 48, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, R.; Matthews, G.M.; Gandolfi, S.; Simoes, R.D.M.; Buckley, D.L.; Vora, J.R.; Sievers, Q.L.; Brüggenthies, J.B.; Dashevsky, O.; Poarch, H.; et al. Functional Genomics Identify Distinct and Overlapping Genes Mediating Resistance to Different Classes of Heterobifunctional Degraders of Oncoproteins. Cell Rep. 2021, 34, 108532. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Khan, S.; Huo, Z.; Lv, D.; Zhang, X.; Liu, X.; Yuan, Y.; Hromas, R.; Xu, M.; Zheng, G.; et al. Proteolysis targeting chimeras (PROTACs) are emerging therapeutics for hematologic malignancies. J. Hematol. Oncol. 2020, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-M.; Dong, J.; Xu, Z.-Y.; Cheng, X.-D.; Zhang, W.-D.; Qin, J.-J. PROTAC: An Effective Targeted Protein Degradation Strategy for Cancer Therapy. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Li, X.; Pu, W.; Zheng, Q.; Ai, M.; Chen, S.; Peng, Y. Proteolysis-targeting chimeras (PROTACs) in cancer therapy. Mol. Cancer 2022, 21, 1–30. [Google Scholar] [CrossRef]
- Gao, X.; Burris, H.A., III; Vuky, J.; Dreicer, R.; Sartor, A.O.; Sternberg, C.N.; Percent, I.J.; Hussain, M.H.A.; Kalebasty, A.R.; Shen, J.; et al. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, 17. [Google Scholar] [CrossRef]
- Hamilton, E.; Vahdat, L.; Han, H.S.; Ranciato, J.; Gedrich, R.; Keung, C.F.; Chirnomas, D.; Hurvitz, S. Abstract PD13-08: First-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2- locally advanced or metastatic breast cancer. Cancer Res. 2022, 82, PD13-08. [Google Scholar] [CrossRef]
- Starodub, A.; Gollerkeri, A.; De Savi, C.; Dey, J.; Agarwal, S.; Donohue, S.; Perea, R.; Klaus, C.; Gollob, J. Phase 1 study of KT-333, a targeted protein degrader, in patients with relapsed or refractory lymphomas, large granular lymphocytic leukemia, and solid tumors. J. Clin. Oncol. 2022, 40, TPS3171. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barankiewicz, J.; Salomon-Perzyński, A.; Misiewicz-Krzemińska, I.; Lech-Marańda, E. CRL4CRBN E3 Ligase Complex as a Therapeutic Target in Multiple Myeloma. Cancers 2022, 14, 4492. https://doi.org/10.3390/cancers14184492
Barankiewicz J, Salomon-Perzyński A, Misiewicz-Krzemińska I, Lech-Marańda E. CRL4CRBN E3 Ligase Complex as a Therapeutic Target in Multiple Myeloma. Cancers. 2022; 14(18):4492. https://doi.org/10.3390/cancers14184492
Chicago/Turabian StyleBarankiewicz, Joanna, Aleksander Salomon-Perzyński, Irena Misiewicz-Krzemińska, and Ewa Lech-Marańda. 2022. "CRL4CRBN E3 Ligase Complex as a Therapeutic Target in Multiple Myeloma" Cancers 14, no. 18: 4492. https://doi.org/10.3390/cancers14184492





