Surgical and Functional Outcome after Resection of 64 Petroclival Meningiomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patient and Tumor Characteristics
2.2. Surgical Approaches and Strategy
2.3. Functional Outcome
2.4. Ethical Declaration and Statistical Analysis
3. Results
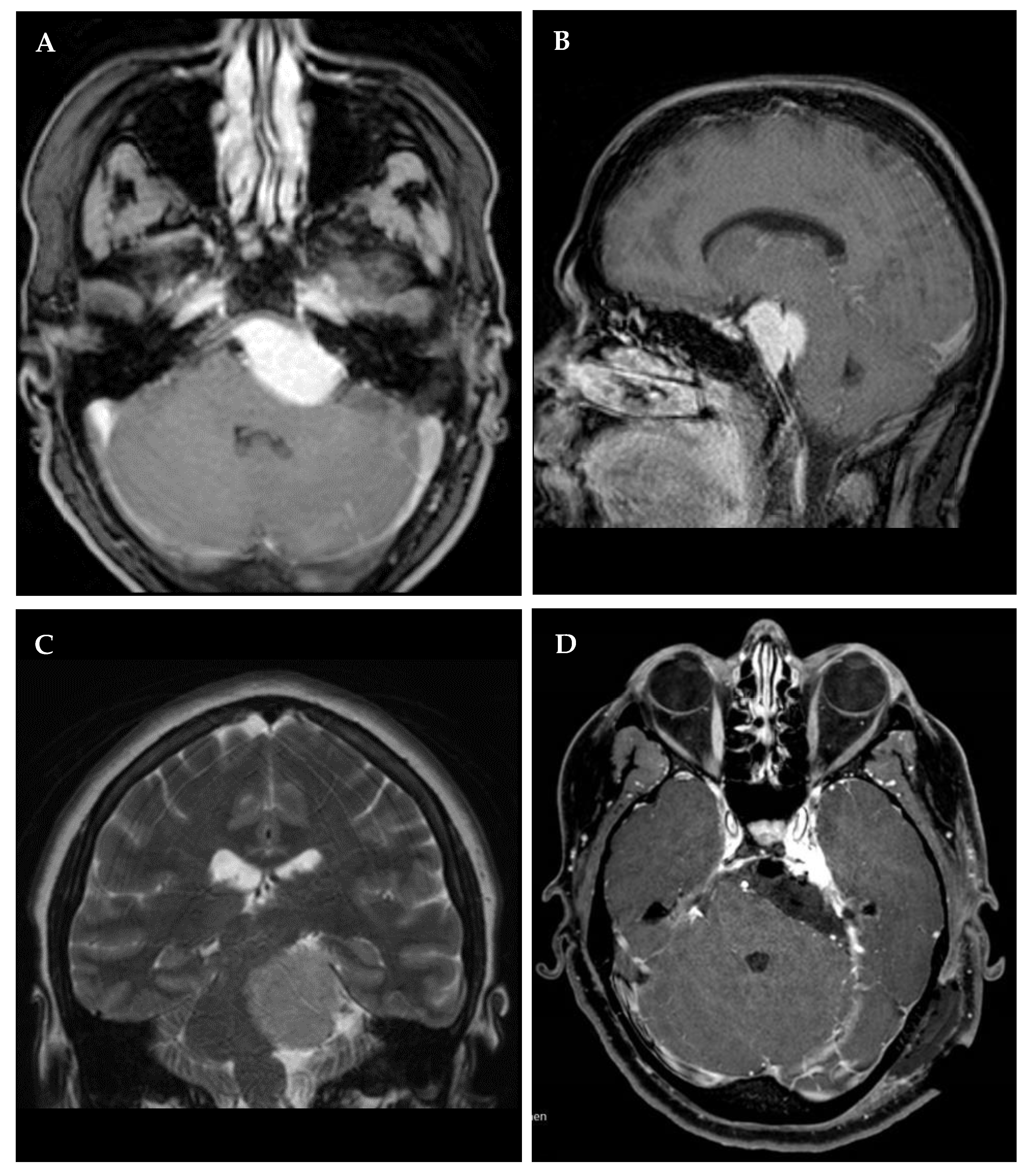
3.1. Cohort and Lesion Characteristics
3.2. Preoperative Complaints and Functional Status
3.3. Surgical Outcome
3.4. Functional and Oncological Outcome
4. Discussion
4.1. Surgery
4.2. Radiotherapy
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, J.T.; Plotkin, S.R. Benign Intracranial Tumors. Neurol. Clin. 2018, 36, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Liu, G. Primary Brain Tumors in Adults: Diagnosis and Treatment. Am. Fam. Physician 2016, 93, 211–217. [Google Scholar] [PubMed]
- Rapalino, O.; Smirniotopoulos, J.G. Extra-axial brain tumors. Handb. Clin. Neurol. 2016, 135, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Subach, B.R.; Lunsford, L.D.; Kondziolka, D.; Maitz, A.H.; Flickinger, J.C. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery 1998, 42, 437–443, discussion 443–435. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Starke, R.M.; Kano, H.; Barnett, G.H.; Mathieu, D.; Chiang, V.; Yu, J.B.; Hess, J.; McBride, H.L.; Honea, N.; et al. Gamma Knife radiosurgery for posterior fossa meningiomas: A multicenter study. J. Neurosurg. 2015, 122, 1479–1489. [Google Scholar] [CrossRef]
- Starke, R.M.; Nguyen, J.H.; Rainey, J.; Williams, B.J.; Sherman, J.H.; Savage, J.; Yen, C.P.; Sheehan, J.P. Gamma Knife surgery of meningiomas located in the posterior fossa: Factors predictive of outcome and remission. J. Neurosurg. 2011, 114, 1399–1409. [Google Scholar] [CrossRef]
- Zentner, J.; Meyer, B.; Vieweg, U.; Herberhold, C.; Schramm, J. Petroclival meningiomas: Is radical resection always the best option? J. Neurol. Neurosurg. Psychiatry 1997, 62, 341–345. [Google Scholar] [CrossRef]
- Samii, M.; Tatagiba, M.; Carvalho, G.A. Resection of large petroclival meningiomas by the simple retrosigmoid route. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 1999, 6, 27–30. [Google Scholar] [CrossRef]
- Tatagiba, M.; Samii, M.; Matthies, C.; Vorkapic, P. Management of Petroclival Meningiomas: A Critical Analysis of Surgical Treatment. Acta Neurochir. Suppl. 1996, 65, 92–94. [Google Scholar] [CrossRef]
- Yaşargil, M.G.; Mortara, R.W.; Curcic, M. Meningiomas of the basal posterior cranial fossa. In Advances and Technical Standards in Neurosurgery; Springer: Berlin/Heidelberg, Germany, 1980; Volume 7, pp. 3–115. [Google Scholar]
- Almefty, R.; Dunn, I.F.; Pravdenkova, S.; Abolfotoh, M.; Al-Mefty, O. True petroclival meningiomas: Results of surgical management. J. Neurosurg. JNS 2014, 120, 40. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Yu, C.; Zhang, H.; Zhang, M.; Qu, Y.; Ren, M.; Gu, C.; Wang, H. Clinical outcomes and survival analysis for petroclival meningioma patients receiving surgical resection: An analysis of 176 cases. Cancer Manag. Res. 2019, 11, 5949–5959. [Google Scholar] [CrossRef]
- Hitselberger, W.E.; House, W.F. A Combined Approach to the Cerebellopontine Angle: A Suboccipital-Petrosal Approach. Arch. Otolaryngol. 1966, 84, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Daspit, C.P.; Pappas, C.T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: Experience with 46 cases. J. Neurosurg. 1992, 76, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Hakuba, A.; Nishimura, S.; Tanaka, K.; Kishi, H.; Nakamura, T. Clivus Meningioma: Six Cases of Total Removal. Neurol. Med.-Chir. 1977, 17 Pt 1, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Quant, E.C.; Wen, P.Y. Response assessment in neuro-oncology. Curr. Oncol. Rep. 2011, 13, 50–56. [Google Scholar] [CrossRef]
- Kaley, T.; Barani, I.; Chamberlain, M.; McDermott, M.; Panageas, K.; Raizer, J.; Rogers, L.; Schiff, D.; Vogelbaum, M.; Weber, D.; et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: A RANO review. Neuro-Oncology 2014, 16, 829–840. [Google Scholar] [CrossRef]
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro-Oncology 2019, 21, 26–36. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncology 2021, 23, 1821–1834. [Google Scholar] [CrossRef]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol.—Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef] [PubMed]
- IBM. IBM SPSS Statistics for Windows, 25th ed.; IBM: Armonk, NY, USA, 2012. [Google Scholar]
- Al-Mefty, O.; Fox, J.L.; Smith, R.R. Petrosal approach for petroclival meningiomas. Neurosurgery 1988, 22, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Tatagiba, M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir. 1992, 118, 27–32. [Google Scholar] [CrossRef]
- Kawase, T.; Shiobara, R.; Toya, S. Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: Surgical method and results in 10 patients. Neurosurgery 1991, 28, 869–875, discussion 875–866. [Google Scholar] [CrossRef]
- Goel, A. Extended middle fossa approach for petroclival lesions. Acta Neurochir. 1995, 135, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ojemann, R.G. Skull-base surgery: A perspective. J. Neurosurg. 1992, 76, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Ammirati, M.; Mahran, A.; Bini, W.; Sepehrnia, A. Surgery of petroclival meningiomas: Report of 24 cases. Neurosurgery 1989, 24, 12–17. [Google Scholar] [CrossRef]
- Bricolo, A.P.; Turazzi, S.; Talacchi, A.; Cristofori, L. Microsurgical removal of petroclival meningiomas: A report of 33 patients. Neurosurgery 1992, 31, 813–828, discussion 828. [Google Scholar] [CrossRef]
- Di Carlo, D.T.; Capo, G.; Fava, A.; Cagnazzo, F.; Margil-Sànchez, M.; Champagne, P.O.; Voormolen, E.H.J.; Morganti, R.; Froelich, S.; Perrini, P. Petroclival meningiomas: The risk of post-operative cranial nerve deficits among different surgical approaches—A systematic review and meta-analysis. Acta Neurochir. 2020, 162, 2135–2143. [Google Scholar] [CrossRef]
- Nanda, A.; Javalkar, V.; Banerjee, A.D. Petroclival meningiomas: Study on outcomes, complications and recurrence rates. J. Neurosurg. 2011, 114, 1268–1277. [Google Scholar] [CrossRef]
- Beniwal, M.; Bhat, D.I.; Rao, N.; Bhagavatula, I.D.; Somanna, S. Surgical management of petroclival meningiomas: Factors affecting early post-operative outcome. Br. J. Neurosurg. 2015, 29, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Yamakami, I.; Higuchi, Y.; Horiguchi, K.; Saeki, N. Treatment policy for petroclival meningioma based on tumor size: Aiming radical removal in small tumors for obtaining cure without morbidity. Neurosurg. Rev. 2011, 34, 327–334, discussion 325–334. [Google Scholar] [CrossRef]
- Ramina, R.; Neto, M.C.; Fernandes, Y.B.; Silva, E.B.; Mattei, T.A.; Aguiar, P.H. Surgical removal of small petroclival meningiomas. Acta Neurochir. 2008, 150, 431–438, discussion 438–439. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Yamanaka, K.; Nakajima, H. Two-staged gamma knife radiosurgery for the treatment of large petroclival and cavernous sinus meningiomas. Surg. Neurol. 2001, 56, 308–314. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Czech, M.; Kessel, K.A.; Habermehl, D.; Weber, D.; Rieken, S.; Bougatf, N.; Jäkel, O.; Debus, J.; Combs, S.E. Clinical outcome after particle therapy for meningiomas of the skull base: Toxicity and local control in patients treated with active rasterscanning. Radiat. Oncol. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Adeberg, S.; Dittmar, J.O.; Welzel, T.; Rieken, S.; Habermehl, D.; Huber, P.E.; Debus, J. Skull base meningiomas: Long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT). Radiother. Oncol. 2013, 106, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Ganswindt, U.; Foote, R.L.; Kondziolka, D.; Tonn, J.C. State-of-the-art treatment alternatives for base of skull meningiomas: Complementing and controversial indications for neurosurgery, stereotactic and robotic based radiosurgery or modern fractionated radiation techniques. Radiat. Oncol. 2012, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Amichetti, M.; Enrici, R.M. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat. Oncol. 2009, 4, 42. [Google Scholar] [CrossRef]
- dos Santos, M.A.; de Salcedo, J.B.; Gutiérrez Diaz, J.A.; Calvo, F.A.; Samblás, J.; Marsiglia, H.; Sallabanda, K. Long-term outcomes of stereotactic radiosurgery for treatment of cavernous sinus meningiomas. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1436–1441. [Google Scholar] [CrossRef]
- Song, C.W.; Kim, M.S.; Cho, L.C.; Dusenbery, K.; Sperduto, P.W. Radiobiological basis of SBRT and SRS. Int. J. Clin. Oncol. 2014, 19, 570–578. [Google Scholar] [CrossRef]
- Joshi, K.C.; Raghavan, A.; Muhsen, B.e.; Hsieh, J.; Borghei-Razavi, H.; Chao, S.T.; Barnett, G.H.; Suh, J.H.; Neyman, G.; Kshettry, V.R.; et al. Fractionated Gamma Knife radiosurgery for skull base meningiomas: A single-institution experience. Neurosurg. Focus FOC 2019, 46, E8. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Starke, R.M.; Kano, H.; Kaufmann, A.M.; Mathieu, D.; Zeiler, F.A.; West, M.; Chao, S.T.; Varma, G.; Chiang, V.L.; et al. Gamma Knife radiosurgery for sellar and parasellar meningiomas: A multicenter study. J. Neurosurg. 2014, 120, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Zachenhofer, I.; Wolfsberger, S.; Aichholzer, M.; Bertalanffy, A.; Roessler, K.; Kitz, K.; Knosp, E. Gamma-knife radiosurgery for cranial base meningiomas: Experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery 2006, 58, 28–36, discussion 28–36. [Google Scholar] [CrossRef]
- Compter, I.; Zaugg, K.; Houben, R.M.; Dings, J.T.; Bosmans, G.; Buescher, C.; Anten, M.M.; Baumert, B.G. High symptom improvement and local tumor control using stereotactic radiotherapy when given early after diagnosis of meningioma. A multicentre study. Strahlenther. Onkol. Organ Dtsch. Rontgenges. 2012, 188, 887–893. [Google Scholar] [CrossRef]
- DeMonte, F.; Smith, H.K.; al-Mefty, O. Outcome of aggressive removal of cavernous sinus meningiomas. J. Neurosurg. 1994, 81, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.W., Jr.; Marcus, R.B., Jr.; Friedman, W.A.; Ballinger, W.E., Jr.; Million, R.R. The meningioma controversy: Postoperative radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 299–304. [Google Scholar] [CrossRef]
- Soyuer, S.; Chang, E.L.; Selek, U.; Shi, W.; Maor, M.H.; DeMonte, F. Radiotherapy after surgery for benign cerebral meningioma. Radiother. Oncol 2004, 71, 85–90. [Google Scholar] [CrossRef]




| N | 64 | ||
|---|---|---|---|
| Mean age at surgery (median; range) | 55 (56; 21–84) | ||
| Female sex (n; %) | 43 (67.2%) | ||
| Mean preoperative tumor diameter in mm (median; range) | 37.3 (37.0; 13–92) | ||
| Localization (n; %) | left | 29 | 45.3% |
| right | 35 | 54.7% | |
| CNS WHO grade | 1 | 61 | 95.3% |
| 2 | 3 | 4.7% | |
| Affection of Cranial Nerves on Imaging | N | % |
|---|---|---|
| II | 16 | 25.0 |
| III | 20 | 31.3 |
| IV | 33 | 51.6 |
| V | 49 | 76.6 |
| VI | 37 | 57.8 |
| VII | 34 | 53.1 |
| VIII | 33 | 51.6 |
| IX | 12 | 18.8 |
| X | 8 | 12.5 |
| XI | 8 | 12.5 |
| XII | 3 | 4.7 |
| Infiltration of | ||
| Internal auditory canal | 27 | 42.2 |
| Cavernous sinus | 34 | 53.1 |
| Meckel’s cave | 35 | 54.7 |
| Nasal sinus | 2 | 3.1 |
| Orbit | 7 | 10.9 |
| Sellar region | 17 | 26.6 |
| Compression of third ventricle | 10 | 15.6 |
| Brain stem affection * | 28 | 43.8 |
| Osteolysis of skull base | 11 | 17.2 |
| Complaint | N | % |
|---|---|---|
| Diplopia | 16 | 25.0 |
| Loss of visual acuity | 12 | 18.8 |
| Visual field deficit | 6 | 9.4 |
| Dysphagia | 3 | 6.3 |
| Hearing loss | 19 | 32.8 |
| Cranial nerve palsy | ||
| III | 9 | 14.1 |
| IV | 5 | 7.8 |
| V | 27 | 42.2 |
| VI | 13 | 20.3 |
| VII | 4 | 6.3 |
| XII | 1 | 1.6 |
| Single-Staged (n = 49) | Two Stages (n = 15) | |
|---|---|---|
| Surgical Approach (N; %) | ||
| Retrosigmoidal | 42 (85.7) | 9 (60.0) * |
| Pterional | 6 (12.2) | 4 (26.7) † |
| Transnasal Transsphenoidal | 1 (2.0) | - |
| Combined Petrosal | - | 2 (13.3) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, A.; Alraun, M.; Kahlig, V.; Dorier, A.-S.; Aftahy, A.K.; Bernhardt, D.; Combs, S.E.; Gempt, J.; Shiban, E.; Meyer, B.; et al. Surgical and Functional Outcome after Resection of 64 Petroclival Meningiomas. Cancers 2022, 14, 4517. https://doi.org/10.3390/cancers14184517
Wagner A, Alraun M, Kahlig V, Dorier A-S, Aftahy AK, Bernhardt D, Combs SE, Gempt J, Shiban E, Meyer B, et al. Surgical and Functional Outcome after Resection of 64 Petroclival Meningiomas. Cancers. 2022; 14(18):4517. https://doi.org/10.3390/cancers14184517
Chicago/Turabian StyleWagner, Arthur, Marie Alraun, Victoria Kahlig, Anne-Sophie Dorier, Amir Kaywan Aftahy, Denise Bernhardt, Stephanie E. Combs, Jens Gempt, Ehab Shiban, Bernhard Meyer, and et al. 2022. "Surgical and Functional Outcome after Resection of 64 Petroclival Meningiomas" Cancers 14, no. 18: 4517. https://doi.org/10.3390/cancers14184517
APA StyleWagner, A., Alraun, M., Kahlig, V., Dorier, A.-S., Aftahy, A. K., Bernhardt, D., Combs, S. E., Gempt, J., Shiban, E., Meyer, B., & Negwer, C. (2022). Surgical and Functional Outcome after Resection of 64 Petroclival Meningiomas. Cancers, 14(18), 4517. https://doi.org/10.3390/cancers14184517





