Body Composition as a Predictor of the Survival in Anal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
- Histologically proven squamous cell cancer of anal canal;
- Stage I–III (union for international cancer control “UICC” 8th edition);
- The treatment was delivered as intensity-modulated radiotherapy (IMRT).
- Non-squamous cell cancer histology;
- Stage IV disease (UICC 8th edition);
- Radiation was applied as conventional or conformal three-dimensional radiotherapy (3DRT);
- Radiation was applied as local palliative treatment.
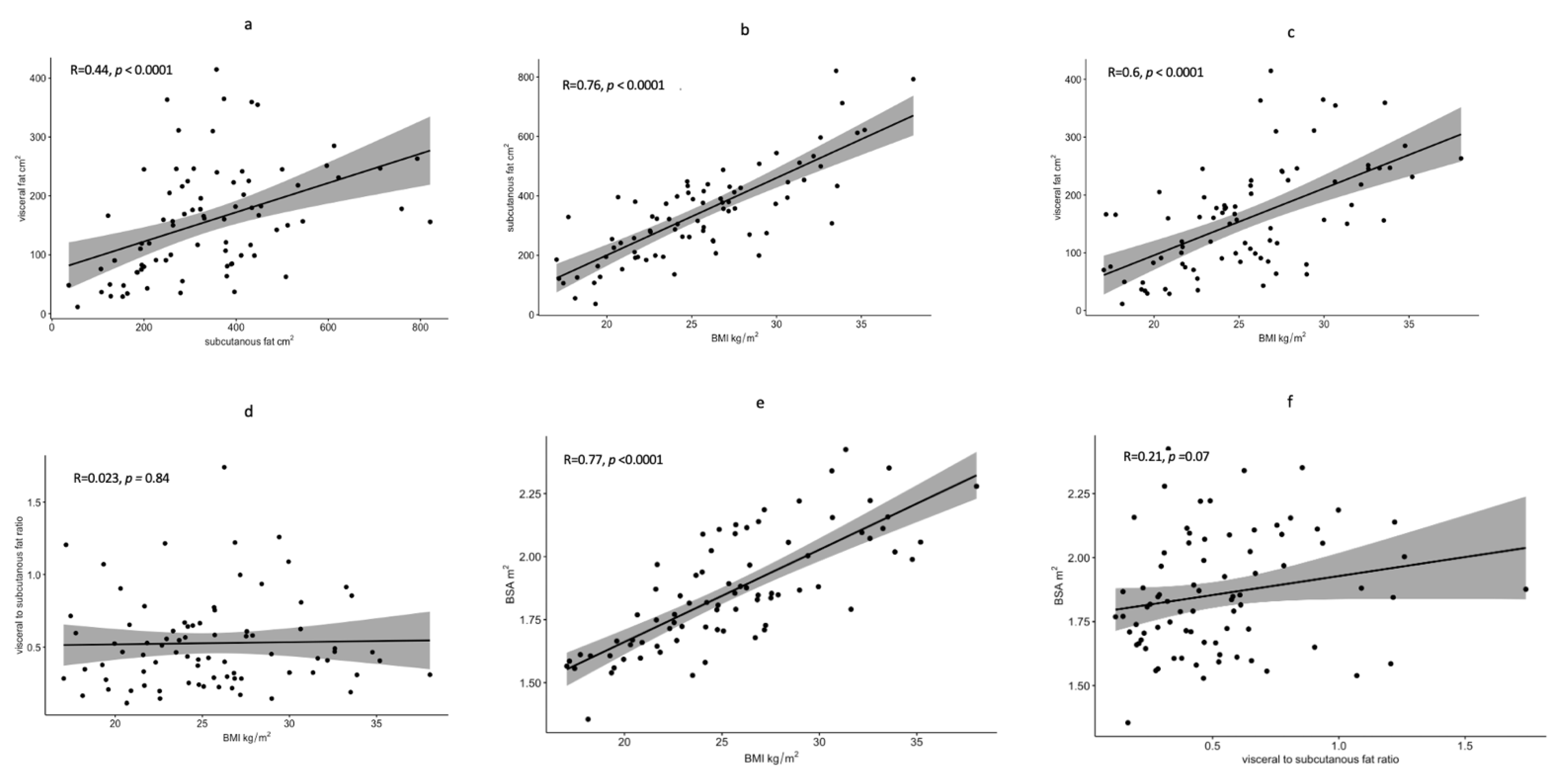
2.1. Image Analysis
2.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
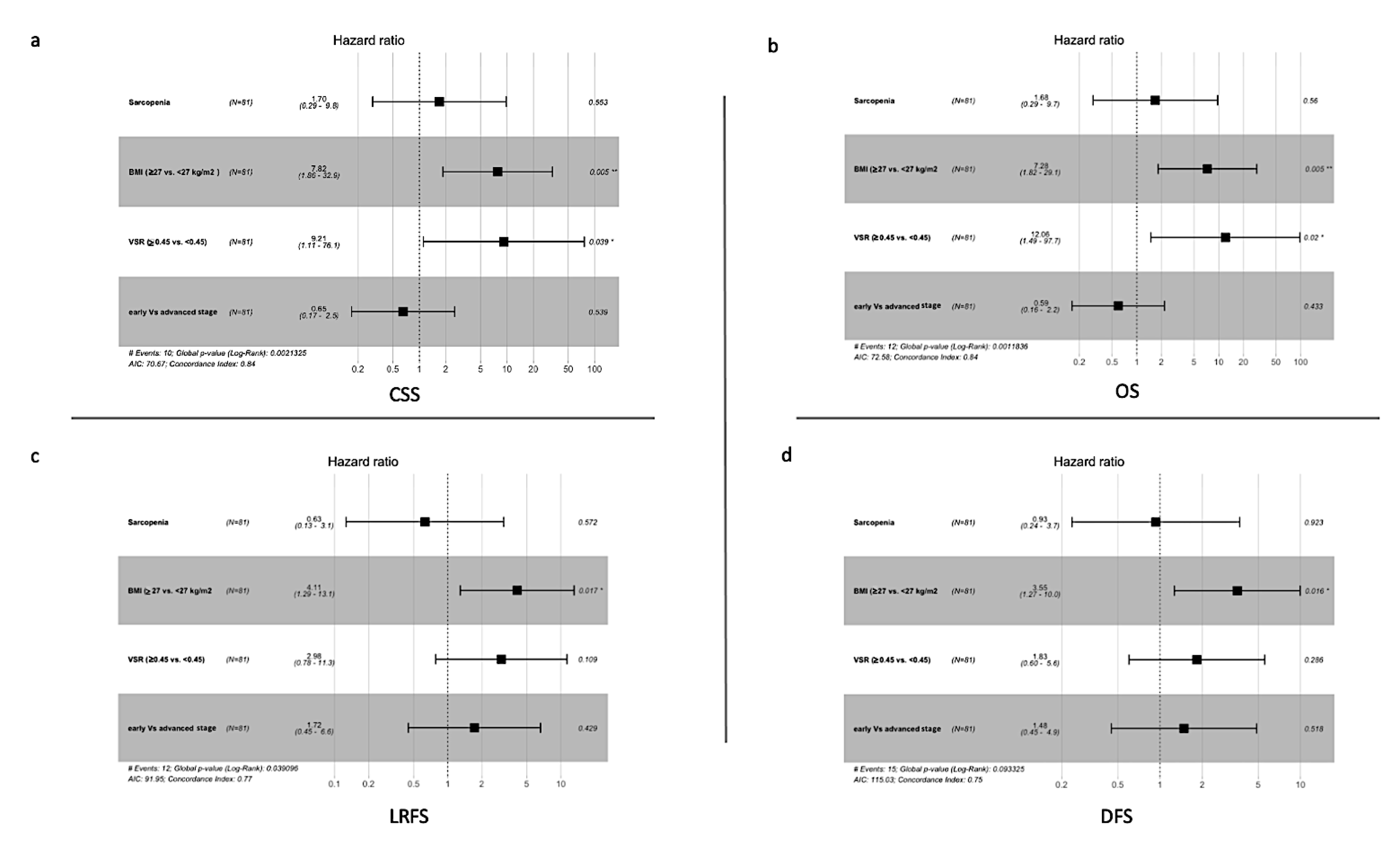
3.2. The Univariate Analysis
3.3. Multivariate Analysis
3.4. Toxicity Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deshmukh, A.A.; Suk, R.; Shiels, M.S.; Sonawane, K.; Nyitray, A.G.; Liu, Y.; Gaisa, M.M.; Palefsky, J.M.; Sigel, K. Recent Trends in Squamous Cell Carcinoma of the Anus Incidence and Mortality in the United States, 2001–2015. JNCI J. Natl. Cancer Inst. 2020, 112, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.H.; Reinert, C.P.; Zips, D.; Nikolaou, K.; Pfannenberg, C.; Gani, C. Treatment outcome after radiochemotherapy in anal cancer patients staged with 18F-FDG-PET-CT. Clin. Transl. Radiat. Oncol. 2020, 24, 83–87. Available online: http://www.ctro.science/article/S2405630820300574/fulltext (accessed on 1 August 2022). [CrossRef] [PubMed]
- Kachnic, L.A.; Winter, K.; Myerson, R.J.; Goodyear, M.D.; Abitbol, A.A.; Koenig, J.M.; Augspurger, M.; Schefter, T.; Katz, A.W.; Fisher, B.; et al. NRG Oncology/RTOG 0529: Long-Term Outcomes of Dose-Painted Intensity Modulated Radiation Therapy, 5-Fluorouracil, and Mitomycin-C in Anal Canal Cancer. Int. J. Radiat. Oncol. 2017, 99, S64–S65. [Google Scholar] [CrossRef]
- Shakir, R.; Adams, R.; Cooper, R.; Downing, A.; Geh, I.; Gilbert, D.; Jacobs, C.; Jones, C.; Lorimer, C.; Namelo, W.C.; et al. Patterns and Predictors of Relapse Following Radical Chemoradiation Therapy Delivered Using Intensity Modulated Radiation Therapy with a Simultaneous Integrated Boost in Anal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Kataria, T.; Gupta, D.; Basu, T.; Bisht, S.; Goyal, S.; Karrthick, K.; Rosati, L.; Hsu, C.; Hacker-Prietz, A.; et al. The Development of an Umbrella Trial (PLATO) to Address Radiation Therapy Dose Questions in the Locoregional Management of Squamous Cell Carcinoma of the Anus. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E164–E165. Available online: http://www.redjournal.org/article/S0360301616313323/fulltext (accessed on 10 August 2022). [CrossRef]
- Mettu, N.B.; Twohy, E.; Ou, F.S.; Halfdanarson, T.R.; Lenz, H.J.; Breakstone, R.; Boland, P.M.; Crysler, O.; Wu, C.; Grothey, A.; et al. Pre-specified pilot analysis of a randomised pilot/phase II/III trial comparing standard dose vs dose-escalated concurrent chemoradiotherapy (CRT) in anal cancer (PLATO-ACT5). Ann. Oncol. 2019, 30, v203–v204. Available online: http://www.annalsofoncology.org/article/S0923753419587567/fulltext (accessed on 10 August 2022). [CrossRef]
- Martin, D.; Balermpas, P.; Gollrad, J.; Weiß, C.; Valentini, C.; Stuschke, M.; Schäfer, H.; Henckenberens, C.; Debus, J.; Krug, D.; et al. RADIANCE—Radiochemotherapy with or without Durvalumab in the treatment of anal squamous cell carcinoma: A randomized multicenter phase II trial. Clin. Transl. Radiat. Oncol. 2020, 23, 43–49. Available online: http://www.ctro.science/article/S2405630820300343/fulltext (accessed on 1 August 2022). [CrossRef] [PubMed]
- Dahl, O.; Myklebust, M.P.; Dale, J.E.; Leon, O.; Serup-Hansen, E.; Jakobsen, A.; Pfeiffer, P.; Løes, I.M.; Pfeffer, F.; Spindler, K.L.G.; et al. Evaluation of the stage classification of anal cancer by the TNM 8th version versus the TNM 7th version. Acta Oncol. 2020, 59, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Theophanous, S.; Samuel, R.; Lilley, J.; Henry, A.; Sebag-Montefiore, D.; Gilbert, A.; Appelt, A.L. Prognostic factors for patients with anal cancer treated with conformal radiotherapy—A systematic review. BMC Cancer 2022, 22, 607. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-022-09729-4 (accessed on 29 June 2022). [CrossRef]
- Wada, M.; Yamaguchi, K.; Yamakage, H.; Inoue, T.; Kusakabe, T.; Abiko, K.; Takakura, K.; Konishi, I.; Satoh-Asahara, N. Visceral-to-subcutaneous fat ratio is a possible prognostic factor for type 1 endometrial cancer. Int. J. Clin. Oncol. 2021, 27, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. Available online: https://pubmed.ncbi.nlm.nih.gov/26882087/ (accessed on 3 July 2022). [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Medici, F.; Bazzocchi, A.; Buwenge, M.; Zamagni, A.; Macchia, G.; Deodato, F.; Cilla, S.; De Iaco, P.; Perrone, A.M.; Strigari, L.; et al. Impact and Treatment of Sarcopenia in Patients Undergoing Radiotherapy: A Multidisciplinary, AMSTAR-2 Compliant Review of Systematic Reviews and Metanalyses. Front. Oncol. 2022, 12, 2315. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2022.887156/full (accessed on 29 June 2022). [CrossRef] [PubMed]
- Del Grande, M.; Rizzo, S.; Nicolino, G.M.; Colombo, I.; Rossi, L.; Manganaro, L.; Del Grande, F. Computed Tomography–Based Body Composition in Patients With Ovarian Cancer: Association With Chemotoxicity and Prognosis. Front. Oncol. 2021, 11, 4759. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, Z.; Yang, L.; Wang, X.; Yi, Z.; Zhou, L.; Chen, Y.; Yang, L.; Zhuo, H.; Bao, Y.; et al. Body Composition Parameters May Be Prognostic Factors in Upper Urinary Tract Urothelial Carcinoma Treated by Radical Nephroureterectomy. Front. Oncol. 2021, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323. [Google Scholar] [CrossRef]
- Steele, S.; Lin, F.; Le, T.L.; Medline, A.; Higgins, M.; Sandberg, A.; Evans, S.; Hong, G.; Williams, M.A.; Bilen, M.A.; et al. Segmentation and Linear Measurement for Body Composition Analysis using Slice-O-Matic and Horos. J. Vis. Exp. 2021, 2021, e61674. Available online: https://pubmed.ncbi.nlm.nih.gov/33818558/ (accessed on 6 July 2022). [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Goulson, R.L.; Ross, B.E.; Wang, N.C.; Sullivan, J.A.; Su, G.L.; Wang, S.C. Quantifying Sarcopenia Reference Values Using Lumbar and Thoracic Muscle Areas in a Healthy Population. J. Nutr. Health Aging 2018, 22, 180–185. Available online: https://link.springer.com/article/10.1007/s12603-017-0983-3 (accessed on 29 June 2022). [CrossRef] [PubMed]
- Diefenhardt, M.; Ludmir, E.B.; Hofheinz, R.D.; Ghadimi, M.; Minsky, B.D.; Fleischmann, M.; Fokas, E.; Rödel, C. Impact of body-mass index on treatment and outcome in locally advanced rectal cancer: A secondary, post-hoc analysis of the CAO/ARO/AIO-04 randomized phase III trial. Radiother. Oncol. 2021, 164, 223–231. Available online: https://pubmed.ncbi.nlm.nih.gov/34619239/ (accessed on 24 July 2022). [PubMed]
- Nattenmüller, J.; Wochner, R.; Muley, T.; Steins, M.; Hummler, S.; Teucher, B.; Wiskemann, J.; Kauczor, H.U.; Wielputz, M.O.; Heussel, C.P. Prognostic Impact of CT-Quantified Muscle and Fat Distribution before and after First-Line-Chemotherapy in Lung Cancer Patients. PLoS ONE 2017, 12, e0169136. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0169136 (accessed on 24 July 2022). [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. Available online: https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-9-88 (accessed on 3 July 2022). [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity With Survival Outcomes in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e213520. Available online: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2777839 (accessed on 3 July 2022). [CrossRef] [PubMed]
- De Schutter, A.; Lavie, C.J.; Arce, K.; Menendez, S.G.; Milani, R.V. Correlation and discrepancies between obesity by body mass index and body fat in patients with coronary heart disease. J. Cardiopulm. Rehabil. Prev. 2013, 33, 77–83. Available online: https://journals.lww.com/jcrjournal/Fulltext/2013/03000/Correlation_and_Discrepancies_Between_Obesity_by.3.aspx (accessed on 4 July 2022). [CrossRef]
- Silveira, E.A.; Kliemann, N.; Noll, M.; Sarrafzadegan, N.; De Oliveira, C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obes. Rev. 2020, 22, e13088. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. Available online: https://pubmed.ncbi.nlm.nih.gov/22051112/ (accessed on 4 July 2022). [CrossRef]
- Ye, J. Emerging Role of Adipose Tissue Hypoxia in Obesity and Insulin Resistance. Int. J. Obes. 2009, 33, 54. [Google Scholar] [CrossRef]
- Norouzirad, R.; González-Muniesa, P.; Ghasemi, A. Hypoxia in Obesity and Diabetes: Potential Therapeutic Effects of Hyperoxia and Nitrate. Oxid. Med. Cell. Longev. 2017, 2017, 5350267. [Google Scholar] [CrossRef]
- Tokunaga, R.; Nakagawa, S.; Miyamoto, Y.; Ohuchi, M.; Izumi, D.; Kosumi, K.; Taki, K.; Higashi, T.; Miyata, T.; Yoshida, N.; et al. The clinical impact of preoperative body composition differs between male and female colorectal cancer patients. Color. Dis. 2020, 22, 62–70. [Google Scholar] [CrossRef]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; García-Cañaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e26. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Schlenter, M.; Heinzel, A.; Kintsler, S.; Eble, M. Intensity-modulated radiotherapy associated with improved survival outcome in anal cancer. Front. Oncol. 2022, 12, 2566. [Google Scholar] [CrossRef]
- Beumer, J.H.; Chu, E.; Salamone, S.J. Body-surface area-based chemotherapy dosing: Appropriate in the 21st century? J. Clin. Oncol. 2012, 30, 3896–3897. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Cardaci, T.D.; McDonald, S.J.; Madero, S.S.; Unger, C.A.; Bullard, B.M.; Enos, R.T.; Velázquez, K.T.; Kubinak, J.L.; Fan, D.; et al. Obesity reduced survival with 5-fluorouracil and did not protect against chemotherapy-induced cachexia or immune cell cytotoxicity in mice. Cancer Biol. Ther. 2022, 23, 1–15. Available online: https://www.tandfonline.com/doi/abs/10.1080/15384047.2022.2108306 (accessed on 11 September 2022). [CrossRef] [PubMed]
- Nilsson, M.P.; Johnsson, A.; Scherman, J. Sarcopenia and dosimetric parameters in relation to treatment-related leukopenia and survival in anal cancer. Radiat. Oncol. 2021, 16, 152. Available online: https://pubmed.ncbi.nlm.nih.gov/34399812/ (accessed on 3 July 2022). [CrossRef]
- Bingmer, K.; Kondray, V.; Ofshteyn, A.; Bliggenstorfer, J.T.; Dietz, D.W.; Charles, R.; Stein, S.L.; Paspulati, R.; Steinhagen, E. Sarcopenia is associated with worse overall survival in patients with anal squamous cell cancer. J. Surg. Oncol. 2020, 121, 1148–1153. Available online: https://pubmed.ncbi.nlm.nih.gov/32133665/ (accessed on 3 July 2022). [CrossRef] [PubMed]
- Martin, D.; von der Grün, J.; Rödel, C.; Fokas, E. Sarcopenia Is Associated with Hematologic Toxicity during Chemoradiotherapy in Patients with Anal Carcinoma. Front. Oncol. 2020, 10, 1576. Available online: https://pubmed.ncbi.nlm.nih.gov/32903529/ (accessed on 3 July 2022). [CrossRef] [PubMed]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. JNCI J. Natl. Cancer Inst. 2015, 107, 86. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Goh, V.; Sebag-Montefiore, D.; Gilbert, D.C. Biomarkers in anal cancer: From biological understanding to stratified treatment. Br. J. Cancer 2017, 116, 156. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | |
|---|---|
| Sex | |
| Female | 48 (59.3%) |
| Male | 33 (40.7%) |
| Age: median/range | 58 (35–86) |
| BMI: median/range | 25 kg/m2 (17.2–38 kg/m2) |
| BSA: median/range | 1.83 m2 (1.35–2.4 m2) |
| SAT: median/ range | 323 cm2 (36.6–821 cm2) |
| VAT: median/range | 157 cm2 (11.5–414.6 cm2) |
| VSR: median/range | 0.45 (0.11–1.74) |
| Sarcopenia: | |
| Yes | 14 |
| No | 65 |
| undetermined | 2 |
| cT-Status | |
| 1 | 15 |
| 2 | 34 |
| 3 | 22 |
| 4 | 9 |
| Undetermined | 1 |
| cN-status | |
| 0 | 40 |
| 1 | 41 |
| Stage (UICC TNM 8th, 2017) | |
| I | 10 |
| IIA | 21 |
| IIB | 7 |
| IIIA | 20 |
| IIIB | 3 |
| IIIC | 20 |
| P-16 status | |
| Positive | 8 |
| Negative | 3 |
| Undetermined | 70 |
| Concurrent chemotherapy | |
| Yes | 74 |
| No | 7 |
| Parameter | LRFS | DFS | OS | CSS |
|---|---|---|---|---|
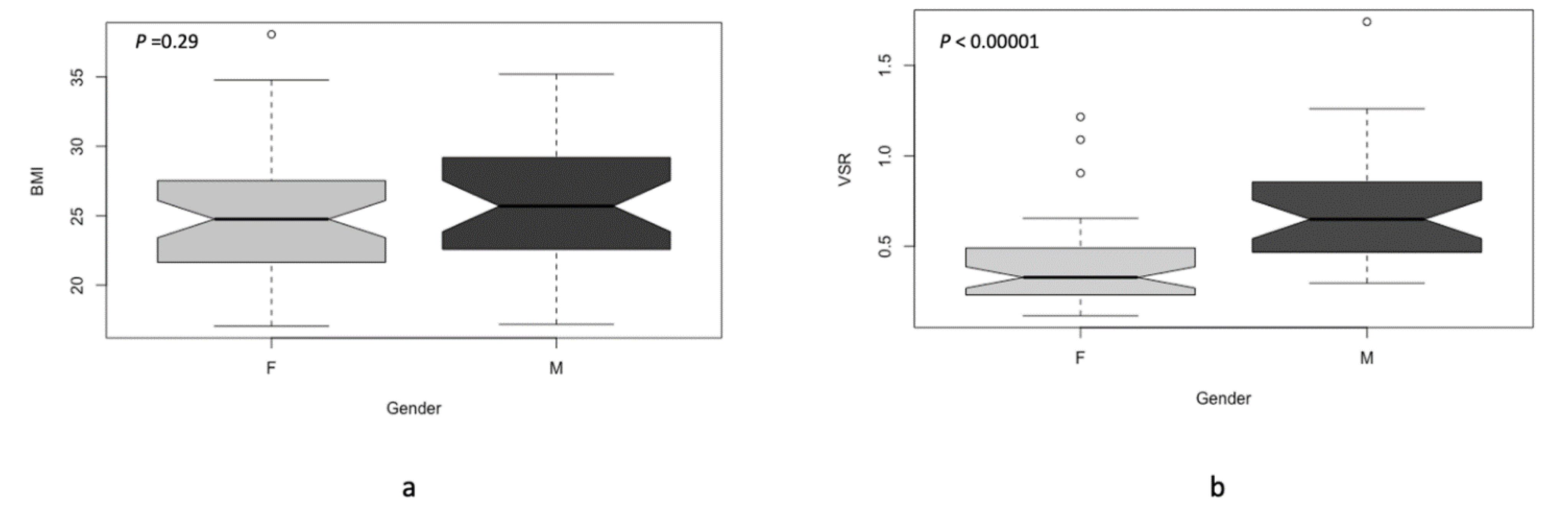
| Sex (Female vs. Male) | 0.079 | 0.129 | 0.016 * | 0.008 * |
| T1 + 2 vs. T3 + 4 | 0.089 | 0.124 | 0.403 | 0.403 |
| Nodal involvement (N0 vs. N1) | 0.26 | 0.243 | 0.66 | 0.885 |
| early (T1-2,N0) vs. advanced (T3,4 orN1) stage | 0.43 | 0.47 | 0.57 | 0.79 |
| Sarcopenia (No vs. Yes) | 0.81 | 0.92 | 0.84 | 0.84 |
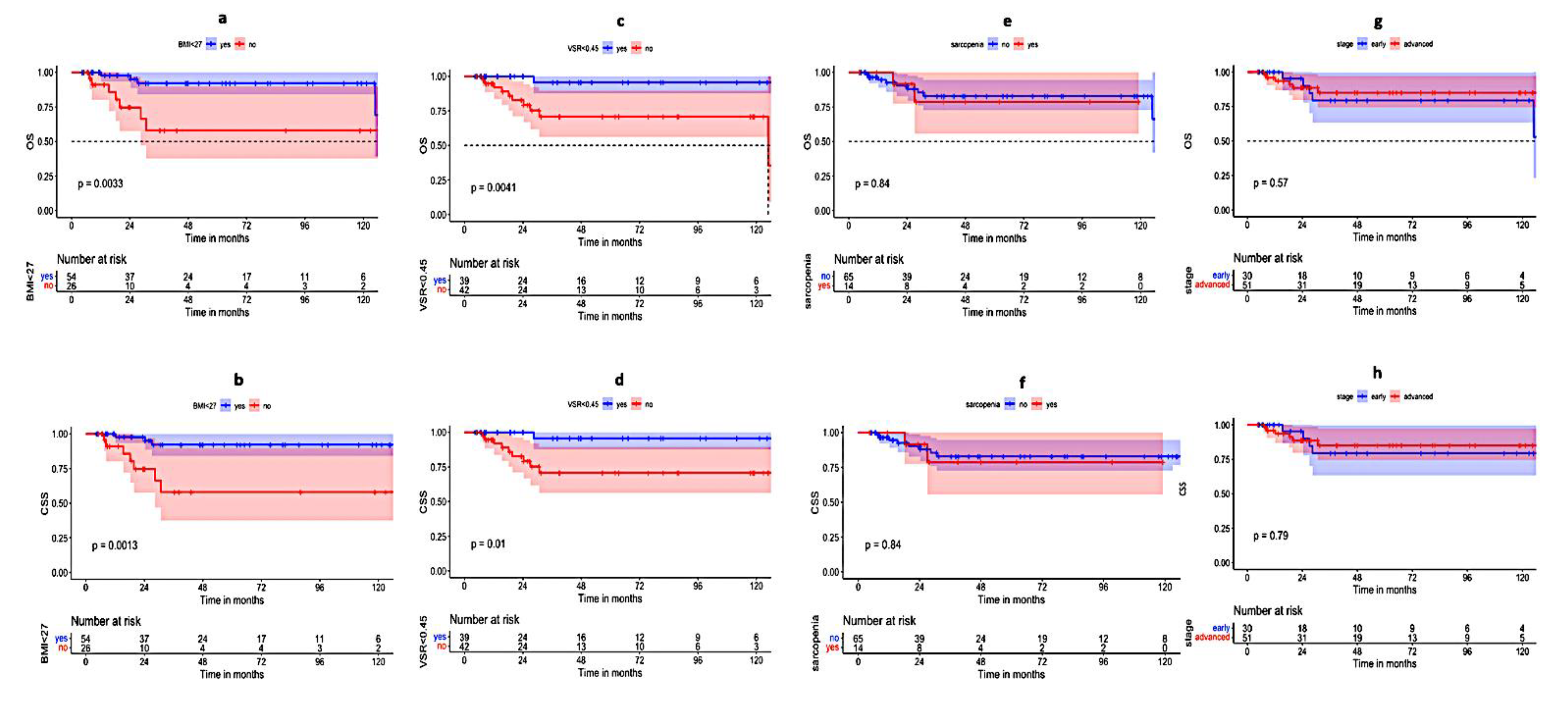
| BMI (<27 vs. ≥27 kg/m2) | 0.0024 * | 0.003 * | 0.0013 * | 0.0033 * |
| VSR (<0.45 vs. ≥0.45) | 0.11 | 0.23 | 0.0041 * | 0.001 * |
| Treatment duration (≤44 days vs. >44 days) | 0.169 | 0.142 | 0.132 | 0.047 * |
| Parameter No. of Patients | T1-2 | T3-4 | p-Value $ | N0 | N1 | p-Value $ |
|---|---|---|---|---|---|---|
| Sarcopenia (14 patients) | 3 | 11 | 0.002 | 5 | 9 | 0.172 |
| BMI ≥ 27 kg/m2 (26 patients) | 16 | 10 | 0.58 | 13 | 13 | 0.59 |
| VSR ≥ 0.45) (42 patients) | 18 | 24 | <0.001 * | 20 | 22 | 0.46 |
| Parameter | LRFS | DFS | OS | CSS |
|---|---|---|---|---|
| Early (T1,2 N0) vs. advanced (T3,4 or N1) stage | 0.429 | 0.518 | 0.433 | 0.539 |
| Sarcopenia (No vs. Yes) | 0.572 | 0.923 | 0.56 | 0.553 |
| VSR (<0.45 vs. ≥0.45) | 0.109 | 0.286 | 0.02 * | 0.039 * |
| BMI (<27 vs. ≥27 kg/m2) | 0.017 * | 0.016 * | 0.005 * | 0.005 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.A.; Risse, K.; Stock, J.; Heinzel, A.; Mottaghy, F.M.; Bruners, P.; Eble, M.J. Body Composition as a Predictor of the Survival in Anal Cancer. Cancers 2022, 14, 4521. https://doi.org/10.3390/cancers14184521
Mohamed AA, Risse K, Stock J, Heinzel A, Mottaghy FM, Bruners P, Eble MJ. Body Composition as a Predictor of the Survival in Anal Cancer. Cancers. 2022; 14(18):4521. https://doi.org/10.3390/cancers14184521
Chicago/Turabian StyleMohamed, Ahmed Allam, Kathrin Risse, Jennifer Stock, Alexander Heinzel, Felix M. Mottaghy, Philipp Bruners, and Michael J. Eble. 2022. "Body Composition as a Predictor of the Survival in Anal Cancer" Cancers 14, no. 18: 4521. https://doi.org/10.3390/cancers14184521
APA StyleMohamed, A. A., Risse, K., Stock, J., Heinzel, A., Mottaghy, F. M., Bruners, P., & Eble, M. J. (2022). Body Composition as a Predictor of the Survival in Anal Cancer. Cancers, 14(18), 4521. https://doi.org/10.3390/cancers14184521






