A Review on Annona muricata and Its Anticancer Activity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Botanical Description and Distribution
3. Bioactive Metabolites Responsible for Various Pharmacological Activities in A. muricata
3.1. Alkaloids
3.2. Phenolic Compounds
3.3. Other Compounds
3.4. Annonaceous Acetogenins
4. Ethnomedicinal Uses
5. Role of Annona muricata against Various Types of Cancer
5.1. Pancreatic Cancer
5.2. Lung Carcinoma
5.3. Prostate Carcinoma
5.4. Breast Cancer
5.5. Colon Carcinoma
5.6. Head and Neck Cancers
5.7. Hematological Malignancies
5.8. Liver Cancer
5.9. Cervical Cancer
6. Potential Role of A. muricata as a Modulator of Epithelial-Mesenchymal Transitions (EMTs)
7. Toxicological Studies
8. In Vivo Studies
9. Clinical Studies
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 31 August 2022).
- Global Cancer Facts & Figures|American Cancer Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/global.html (accessed on 31 August 2022).
- Minkler, S.; Lucien, F.; Kimber, M.J.; Sahoo, D.K.; Bourgois-Mochel, A.; Musser, M.; Johannes, C.; Frank, I.; Cheville, J.; Allenspach, K.; et al. Emerging Roles of Urine-Derived Components for the Management of Bladder Cancer: One Man’s Trash Is Another Man’s Treasure. Cancers 2021, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Borcherding, D.C.; Chandra, L.; Jergens, A.E.; Atherly, T.; Bourgois-Mochel, A.; Ellinwood, N.M.; Snella, E.; Severin, A.J.; Martin, M.; et al. Differential Transcriptomic Profiles Following Stimulation with Lipopolysaccharide in Intestinal Organoids from Dogs with Inflammatory Bowel Disease and Intestinal Mast Cell Tumor. Cancers 2022, 14, 3525. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Mosichuk, A.P.; Lucien, F.; Frank, I.; Cheville, J.C.; Allenspach, K.; Mochel, J.P. Abstract 3092: Urine-Derived Urinary Carcinoma Organoids: A Novel Tool for Providing New Insights into Human and Canine Bladder Cancer Treatment. Cancer Res. 2022, 82, 3092. [Google Scholar] [CrossRef]
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural Products to Prevent Drug Resistance in Cancer Chemotherapy: A Review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gullett, N.P.; Ruhul Amin, A.R.M.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.J.; Kucuk, O. Cancer Prevention with Natural Compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef]
- Borchardt, J.K. Natural Product Chemistry for Drug Discovery. Drug News Perspect. 2002, 15, 187–192. [Google Scholar] [CrossRef]
- WHO Establishes the Global Centre for Traditional Medicine in India. Available online: https://www.who.int/news/item/25-03-2022-who-establishes-the-global-centre-for-traditional-medicine-in-india (accessed on 5 September 2022).
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Farah, I.; Siddiqui, S.; Sahu, K. Role of Natural Products in Drug Discovery Process. Int. J. Drug Dev. Res. 2014, 6, 172–204. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Parekh, J.; Chanda, S. In Vitro Antibacterial Activity of the Crude Methanol Extract of Woodfordia Fruticosa Kurz. Flower (Lythraceae). Braz. J. Microbiol. 2007, 38, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.M.; Palthur, S.; Chitta, S.K. Umar Nutraceuticals: Concept and Regulatory Scenario. Int. J. Pharm. Pharm. Sci. 2010, 2, 14–20. [Google Scholar]
- Urbi, Z.; Hossain, M.S.; Rahman, K.M.H.; Zayed, T.M. Grape: A Medicinal Fruit Species in the Holy Qur’an and Its Ethnomedicinal Importance. World Appl. Sci. J. 2014, 30, 253–265. [Google Scholar] [CrossRef]
- Dafni, A.; Böck, B. Medicinal Plants of the Bible-Revisited. J. Ethnobiol. Ethnomed. 2019, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial Activity of Some Ethnomedical Plants Used by Paliyar Tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35. [Google Scholar] [CrossRef]
- Syed Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Abd Rahman, N.M.A.N. Anti-Cancer Effect of Annona muricata Linn Leaves Crude Extract (AMCE) on Breast Cancer Cell Line. BMC Complement. Altern. Med. 2016, 16, 311. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of Survival, Proliferation, Invasion, Angiogenesis, and Metastasis of Tumor Cells through Modulation of Inflammatory Pathways by Nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef]
- Rady, I.; Bloch, M.B.; Chamcheu, R.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxid. Med. Cell Longev. 2018, 2018, 1826170. [Google Scholar] [CrossRef]
- Chan, W.J.J.; McLachlan, A.J.; Hanrahan, J.R.; Harnett, J.E. The Safety and Tolerability of Annona muricata Leaf Extract: A Systematic Review. J. Pharm. Pharmacol. 2020, 72, 1–16. [Google Scholar] [CrossRef]
- Foster, K.; Younger, N.; Aiken, W.; Brady-West, D.; Delgoda, R. Reliance on Medicinal Plant Therapy among Cancer Patients in Jamaica. Cancer Causes Control 2017, 28, 1349–1356. [Google Scholar] [CrossRef]
- Clement, Y.N.; Mahase, V.; Jagroop, A.; Kissoon, K.; Maharaj, A.; Mathura, P.; Quan, C.M.; Ramadhin, D.; Mohammed, C. Herbal Remedies and Functional Foods Used by Cancer Patients Attending Specialty Oncology Clinics in Trinidad. BMC Complement. Altern. Med. 2016, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Rojas Rojas, T.; Bourdy, G.; Ruiz, E.; Cerapio, J.P.; Pineau, P.; Gardon, J.; Doimi, F.; Deparis, X.; Deharo, E.; Bertani, S. Herbal Medicine Practices of Patients With Liver Cancer in Peru: A Comprehensive Study Toward Integrative Cancer Management. Integr. Cancer Ther. 2018, 17, 52–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atawodi, S. Nigerian Foodstuffs with Prostate Cancer Chemopreventive Polyphenols. Infect. Agents Cancer 2011, 6, S9. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.G.; Kim, Y.D. Quantitative Ethnobotanical S.Study of the Medicinal Plants Used by the Ati Negrito Indigenous Group in Guimaras Island, Philippines. J. Ethnopharmacol. 2014, 157, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Badrie, N.; Schauss, A.G. Soursop (Annona muricata L.): Composition, Nutritional Value, Medicinal Uses, and Toxicology. In Bioactive Foods in Promoting Health: Fruits and Vegetables; Academic Press: Oxford, UK, 2010; pp. 621–643. [Google Scholar]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican Medicinal Plants Used for Cancer Treatment: Pharmacological, Phytochemical and Ethnobotanical Studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef]
- Mishra, S.; Ahmad, S.; Kumar, N.; Sharma, B.K. Annona muricata (the Cancer Killer): A Review. Glob. J. Pharma. Res. 2013, 2, 1613–1618. [Google Scholar]
- Leboeuf, M.; Cavé, A.; Bhaumik, P.; Mukherjee, B.; Mukherjee, R. The Phytochemistry of the Annonaceae. Phytochemistry 1980, 21, 2783–2813. [Google Scholar] [CrossRef]
- Adewole, S.O.; Caxton-Martins, E.A. Morphological Changes and Hypoglycemic Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Pancreatic B-Cells of Streptozotocin-Treated Diabetic Rats. Afr. J. Biomed. Res. 2006, 9, 173–187. [Google Scholar] [CrossRef]
- de Souza, R.; Benassi, E.; da Silva, R.R.; Afonso, S.; Scarminio, I.S. Enhanced Extraction Yields and Mobile Phase Separations by Solvent Mixtures for the Analysis of Metabolites in Annona muricata L. Leaves. J. Sep. Sci. 2009, 32, 4176–4185. [Google Scholar] [CrossRef]
- Errayes, A.O.; Abdussalam-Mohammed, W.; Darwish, M.O. Review of Phytochemical and Medical Applications of Annona muricata Fruits. J. Chem. Rev. 2020, 2, 70–79. [Google Scholar]
- Nugraha, A.S.; Haritakun, R.; Lambert, J.M.; Dillon, C.T.; Keller, P.A. Alkaloids from the Root of Indonesian Annona muricata L. Nat. Prod. Res. 2021, 35, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Nguyen, V.T.; Minh, L.V.; Trieu, L.H.; Cang, M.H.; Bui, L.B. Determination of the Phytochemical Screening, Total Polyphenols, Flavonoids Content, and Antioxidant Activity of Soursop Leaves (Annona muricata Linn.). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062011. [Google Scholar] [CrossRef]
- Moghadamtousi, S.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.; Kadir, H. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities. Molecules 2019, 24, 4419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Hernández, G.; Zepeda-Vallejo, L.G.; García-Magaña, M.D.L.; Vivar-Vera, M.D.L.Á.; Pérez-Larios, A.; Girón-Pérez, M.I.; Coria-Tellez, A.V.; Rodríguez-Aguayo, C.; Montalvo-González, E. Extraction of Alkaloids Using Ultrasound from Pulp and By-Products of Soursop Fruit (Annona muricata L.). Appl. Sci. 2020, 10, 4869. [Google Scholar] [CrossRef]
- Taiwo, F.O.; Oyedeji, O.; Osundahunsi, M.T. Antimicrobial and Antioxidant Properties of Kaempferol-3-O-Glucoside and 1-(4-Hydroxyphenyl)-3-Phenylpropan-1-One Isolated from the Leaves of Annona muricata (Linn.). J. Pharm. Res. Int. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Yathzamiry, V.G.D.; Cecilia, E.G.S.; Antonio, M.C.J.; Daniel, N.F.S.; Carolina, F.G.A.; Alberto, A.V.J.; Raúl, R.H. Isolation of Polyphenols from Soursop (Annona muricata L.) Leaves Using Green Chemistry Techniques and Their Anticancer Effect. Braz. Arch. Biol. Technol. 2021, 64. [Google Scholar] [CrossRef]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; da Silva, N.M.; Espindola, F.S. Annona muricata Linn. Leaf as a Source of Antioxidant Compounds with in Vitro Antidiabetic and Inhibitory Potential against α-Amylase, α-Glucosidase, Lipase, Non-Enzymatic Glycation and Lipid Peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar] [CrossRef]
- Correa-Gordillo, J.; Ortiz, J.; Sánchez-Mejía, M.; Pachón, H. Antioxidant Activity in Guanabana (Annona muricata L.): A Literature Review. Lat. Am. Caribb. Bull. Med. Aromat. Plants 2012, 11, 111–126. [Google Scholar]
- Vijayameena, C.; Subhashini, G.; Loganayagi, M.; Ramesh, B. Phytochemical Screening and Assessment of Antibacterial Activity for the Bioactive Compounds in Annona muricata. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 1–8. [Google Scholar]
- Wélé, A.; Ndoye, I.; Badiane, M. Fatty Acid and Essential Oil Compositions of the Seed Oil of Five Annona Species. Niger. J. Nat. Prod. Med. 2005, 8, 62–65. [Google Scholar] [CrossRef]
- Cheong, K.; Tan, C.; Mirhosseini, H.; Chin, S. Optimization of equilibrium headspace analysis of volatile flavor compounds of Malaysian soursop (Annona muricata): Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC × GC-TOFMS). Food Chem. 2011, 125, 1481–1489. [Google Scholar] [CrossRef]
- Kossouoh, C.; Moudachirou, M.; Adjakidje, V.; Chalchat, J.C.; Figuérédo, G. Essential Oil Chemical Composition of Annona muricata L. Leaves from Benin. J. Essent. Oil Res. 2007, 19, 307–309. [Google Scholar] [CrossRef]
- Thang, T.D.; Dai, D.N.; Hoi, T.M.; Ogunwande, I.A. Study on the Volatile Oil Contents of Annona Glabra L., Annona Squamosa L., Annona muricata L. and Annona reticulata L., from Vietnam. Nat. Prod. Res. 2013, 27, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.S.; Ogundajo, A.L.; Dosoky, N.S.; Setzer, W.N. The Cytotoxic Activity of Annona muricata Leaf Oil from Badagary, Nigeria. Am. J. Essent. Oil Nat. Prod. 2013, 1, 1–3. [Google Scholar]
- Gavamukulya, Y.; Wamunyokoli, F.; El-Shemy, H.A. Annona muricata: Is the Natural Therapy to Most Disease Conditions Including Cancer Growing in Our Backyard? A Systematic Review of Its Research History and Future Prospects. Asian Pac. J. Trop. Med. 2017, 10, 835–848. [Google Scholar] [CrossRef]
- Prasad, S.K.; Pradeep, S.; Shimavallu, C.; Kollur, S.P.; Syed, A.; Marraiki, N.; Egbuna, C.; Gaman, M.A.; Kosakowska, O.; Cho, W.C.; et al. Evaluation of Annona muricata Acetogenins as Potential Anti-SARS-CoV-2 Agents Through Computational Approaches. Front. Chem. 2021, 8, 1281. [Google Scholar] [CrossRef]
- Agu, K.C.; Okolie, N.P.; Falodun, A.; Engel-Lutz, N. In Vitro Anticancer Assessments of Annona muricata Fractions and in Vitro Antioxidant Profile of Fractions and Isolated Acetogenin (15-Acetyl Guanacone). J. Cancer Res. Pract. 2018, 5, 53–66. [Google Scholar] [CrossRef]
- Md Roduan, M.R.; Hamid, R.A.; Cheah, Y.K.; Mohtarrudin, N. Cytotoxicity, Antitumor-Promoting and Antioxidant Activities of Annona muricata in Vitro. J. Herb. Med. 2019, 15, 100219. [Google Scholar] [CrossRef]
- Naik, A.V.; Sellappan, K. Assessment of Genotoxic Potential of Annonacin and Annona muricata L. Extracts on Human Breast Cancer (MCF-7) Cells. Adv. Tradit. Med. 2021, 21, 779–789. [Google Scholar] [CrossRef]
- Han, B.; Cao, Y.X.; Li, Z.M.; Wu, Z.X.; Mao, Y.Q.; Chen, H.L.; Yao, Z.J.; Wang, L.S. Annonaceous Acetogenin Mimic AA005 Suppresses Human Colon Cancer Cell Growth in vivo through Downregulation of Mcl-1. Acta Pharm. Sin. 2019, 40, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Jeevitha Priya, M.; Vidyalakshmi, S.; Rajeswari, M. Study on Reversal of ABCB1 Mediated Multidrug Resistance in Colon Cancer by Acetogenins: An in-Silico Approach. J. Biomol. Struct. Dyn. 2020, 40, 4273–4284. [Google Scholar] [CrossRef] [PubMed]
- Antony, P.; Vijayan, R. Acetogenins from Annona muricata as Potential Inhibitors of Antiapoptotic Proteins: A Molecular Modeling Study. Drug Des. Devel. 2016, 10, 1399. [Google Scholar] [CrossRef]
- Yajid, A.I.; Ab Rahman, H.S.; Wong, M.P.K.; Zain, W.Z.W. Potential Benefits of Annona muricata in Combating Cancer: A Review. Malays. J. Med. Sci. 2018, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Los, M.; Wesselborg, S.; Schulze-Osthoff, K. The Role of Caspases in Development, Immunity, and Apoptotic Signal Transduction: Lessons from Knockout Mice. Immunity 1999, 10, 629–639. [Google Scholar] [CrossRef]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.Q.; Schulze-Osthoff, K. Activation and Caspase-Mediated Inhibition of PARP: A Molecular Switch between Fibroblast Necrosis and Apoptosis in Death Receptor Signaling. Mol. Biol. Cell 2002, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Alali, F.Q.; Liu, X.X.; McLaughlin, J.L. Annonaceous Acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef]
- Hadisaputri, Y.E.; Habibah, U.; Abdullah, F.F.; Halimah, E.; Mutakin, M.; Megantara, S.; Abdulah, R.; Diantini, A. Antiproliferation Activity and Apoptotic Mechanism of Soursop (Annona muricata L.) Leaves Extract and Fractions on MCF7 Breast Cancer Cells. Breast Cancer Targets Ther. 2021, 13, 447. [Google Scholar] [CrossRef]
- Kojima, N.; Abe, M.; Suga, Y.; Ohtsuki, K.; Tanaka, T.; Iwasaki, H.; Yamashita, M.; Miyoshi, H. Critical Role of a Methyl Group on the γ-Lactone Ring of Annonaceous Acetogenins in the Potent Inhibition of Mitochondrial Complex I. Bioorg. Med. Chem. Lett. 2013, 23, 1217–1219. [Google Scholar] [CrossRef]
- Martinou, J.C.; Youle, R.J. Mitochondria in Apoptosis: Bcl-2 Family Members and Mitochondrial Dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.S.F.; Chang, H.L.; Chen, H.W.; Kuo, F.C.; Liaw, C.C.; Su, J.H.; Wu, Y.C. Selective Cytotoxicity of Squamocin on T24 Bladder Cancer Cells at the S-Phase via a Bax-, Bad-, and Caspase-3-Related Pathways. Life Sci. 2006, 78, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Rouhollahi, E.; Karimian, H.; Fadaeinasab, M.; Firoozinia, M.; Abdulla, M.A.; Kadir, H.A. The Chemopotential Effect of Annona muricata Leaves against Azoxymethane-Induced Colonic Aberrant Crypt Foci in Rats and the Apoptotic Effect of Acetogenin Annomuricin E in HT-29 Cells: A Bioassay-Guided Approach. PLoS ONE 2015, 10, e0122288. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complementary Altern. Med. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Deewatthanawong, R.; Kongchinda, P.; Deewatthanawong, P.; Pumnuan, J.; Insung, A. GC-MS Analysis and Biopesticide Properties of Different Crude Extracts of Annona Squamosa and Annona muricata. Int. J. Agric. Technol. 2019, 15, 859–868. [Google Scholar]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A Comprehensive Review on Its Traditional Medicinal Uses, Phytochemicals, Pharmacological Activities, Mechanisms of Action and Toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef]
- Bikomo, E.O.; Magbagbeola, O.A.; Ebuehi, O.A. Antidepressant Activity of Ethanol Leaf Extract of Annona muricata L. In Sprague-Dawley Rats; Scientific & Academic Publishing: Rosemead, CA, USA, 2017; Volume 7, pp. 1–5. [Google Scholar]
- Parthiban, E.; Arokiyaraj, C.; Ramanibai, R. Annona muricata: An Alternate Mosquito Control Agent with Special Reference to Inhibition of Detoxifying Enzymes in Aedes Aegypti. Ecotoxicol. Environ. Saf. 2020, 189, 11005. [Google Scholar] [CrossRef]
- Ezemuoka, L.C.; Nwankwo, E.N.; Ogbonna, C.U. Toxicity of the Aqueous Leaf and Stem-Bark Extracts of Annona muricata to the 4th Instar Larvae of Aedes Aegypti. J. Entomol. Zool. Stud. 2019, 7, 1047–1052. [Google Scholar]
- Hemalatha, G.; Sivakumari, K.; Rajesh, S.; Shyamala Devi, K. Phytochemical Profiling, Anticancer and Apoptotic Activity of Graviola (Annona muricata) Fruit Extract against Human Hepatocellular Carcinoma (HepG-2) Cells. Int. J. Zool. Appl. Biosci. 2020, 5, 32–47. [Google Scholar]
- Ojowu, J.O.; Onwuchukwu, C.N.; Daramola, M.E.; Ebhohon, S.O. Annona Muricata (L.): Investigating the Ameliorative Effect of Leaves Extract on Liver and Kidney Function in Carbon Tetrachloride (CCl4) Induced Rats. J. Biomed. Sci. Res. 2020, 2, 2. [Google Scholar]
- Priya, M.R.K.; Iyer, P.R. Antiproliferative Effects on Tumor Cells of the Synthesized Gold Nanoparticles against Hep2 Liver Cancer Cell Line. Egypt. Liver J. 2020, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Abdul Wahab, S.M.; Jantan, I.; Haque, M.; Arshad, L. Exploring the Leaves of Annona muricata L. as a Source of Potential Anti-Inflammatory and Anticancer Agents. Front. Pharm. 2018, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Patel, J.K. A Review on a Miracle Fruits of Annona muricata. J. Pharm. Phytochem. 2016, 5, 137. [Google Scholar]
- Bobadilla, M.; Zavala, F.; Sisniegas, M.; Zavaleta, G.; Mostacero, J.; Taramona, L. Evaluación larvicida de suspensiones acuosas de Annona muricata Linnaeus «guanábana» sobre Aedes aegypti Linnaeus (Diptera, Culicidae). Rev. Peru. Biol. 2005, 12, 145–152. [Google Scholar] [CrossRef]
- Trindade, R.C.P.; Luna, J.d.; de Lima, M.R.F.; da Silva, P.P.; Sant’ana, A.A.E.G. Larvicidal Activity and Seasonal Variation of Annona muricata (Annonaceae) Extract on Plutella Xylostella (Lepidoptera: Plutellidae). Rev. Colomb. Entomol. 2011, 37, 223–227. [Google Scholar] [CrossRef]
- Langenberger, G.; Prigge, V.; Martin, K.; Belonias, B.; Sauerborn, J. Ethnobotanical Knowledge of Philippine Lowland Farmers and Its Application in Agroforestry. Agrofor. Syst. 2009, 76, 173–194. [Google Scholar] [CrossRef]
- Miranda, N.C.; Araujo, E.C.B.; Justino, A.B.; Cariaco, Y.; Mota, C.M.; Costa-Nascimento, L.A.; Espindola, F.S.; Silva, N.M. Anti-Parasitic Activity of Annona muricata L. Leaf Ethanolic Extract and Its Fractions against Toxoplasma Gondii in Vitro and in Vivo. J. Ethnopharmacol. 2021, 273, 114019. [Google Scholar] [CrossRef]
- Bories, C.; Loiseau, P.; Cortes, D.; Myint, S.H.; Hocquemiller, R.; Gayral, P.; Cave, A.; Laurens, A. Antiparasitic Activity of Annona muricata and Annona Cherimolia Seeds. Planta Med. 1991, 57, 434–436. [Google Scholar] [CrossRef]
- Osorio, E.; Arango, G.J.; Jiménez, N.; Alzate, F.; Ruiz, G.; Gutiérrez, D.; Paco, M.A.; Giménez, A.; Robledo, S. Antiprotozoal and Cytotoxic Activities in Vitro of Colombian Annonaceae. J. Ethnopharmacol. 2007, 111, 630–635. [Google Scholar] [CrossRef]
- Nwokocha, C.R.; Owu, D.U.; Gordon, A.; Thaxter, K.; Mccalla, G.; Ozolua, R.I.; Young, L. Possible Mechanisms of Action of the Hypotensive Effect of Annona muricata (Soursop) in Normotensive Sprague–Dawley Rats. Pharm. Biol. 2012, 50, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Banne, Y.; Barung, E.N.; Juyta; Dumanauw, J.M. Antipyretic Effect of Soursop Leaves Extract (Annona muricata L.) on Rats. Nat. Prod. Chem. Res. 2017, 5, 5. [Google Scholar]
- Souza, D.O.; Dos Santos Sales, V.; de Souza Rodrigues, C.K.; de Oliveira, L.R.; Santiago Lemos, I.C.; de Araújo Delmondes, G.; Monteiro, Á.B.; do Nascimento, E.P.; Sobreira Dantas Nóbrega de Figuêiredo, F.R.; Martins da Costa, J.G.; et al. Phytochemical Analysis and Central Effects of Annona muricata Linnaeus: Possible Involvement of the Gabaergic and Monoaminergic Systems. Iran. J. Pharm. Res. 2018, 17, 1306–1317. [Google Scholar] [PubMed]
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and Cytotoxic Activities of Ethnopharmacologically Selected Medicinal Plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Rouhollahi, E.; Karimian, H.; Fadaeinasab, M.; Abdulla, M.A.; Kadir, H.A. Gastroprotective Activity of Annona muricata Leaves against Ethanol-Induced Gastric Injury in Rats via Hsp70/Bax Involvement. Drug Des. Dev. Ther. 2014, 8, 2099. [Google Scholar] [CrossRef]
- Padma, P.; Chansouria, J.P.; Khosa, R.L. Hepatoprotective Activity of Annona muricata Linn. and Polyalthia Cerasoides Bedd. Anc. Sci. Life 1999, 19, 7–10. [Google Scholar] [PubMed]
- Adewole, S.O.; Ojewole, J.A.O. Protective Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Serum Lipid Profiles and Oxidative Stress in Hepatocytes of Streptozotocin-Treated Diabetic Rats. Afr. J. Tradit. Complementary Altern. Med. 2009, 6, 30–41. [Google Scholar] [CrossRef]
- Shukry, M.; El-Shehawi, A.M.; El-Kholy, W.M.; Elsisy, R.A.; Hamoda, H.S.; Tohamy, H.G.; Abumandour, M.M.; Farrag, F.A. Ameliorative Effect of Graviola (Annona muricata) on Mono Sodium Glutamate-Induced Hepatic Injury in Rats: Antioxidant, Apoptotic, Anti-Inflammatory, Lipogenesis Markers, and Histopathological Studies. Animals 2020, 10, 1996. [Google Scholar] [CrossRef]
- Ola-Davies, O.E.; Oyagbemi, A.A.; Omobowale, T.O.; Akande, I.; Ashafa, A. Ameliorative Effects of Annona muricata Linn. (Annonaceae) against Potassium Dichromate-Induced Hypertension in Vivo: Involvement of Kim-1/P38 MAPK/Nrf2 Signaling. J. Basic Clin. Physiol. Pharm. 2019, 30, 20180172. [Google Scholar] [CrossRef]
- Son, Y.; Lee, H.; Son, S.Y.; Lee, C.H.; Kim, S.Y.; Lim, Y. Ameliorative Effect of Annona muricata (Graviola) Extract on Hyperglycemia Induced Hepatic Damage in Type 2 Diabetic Mice. Antioxidants 2021, 10, 1546. [Google Scholar] [CrossRef]
- Florence, N.T.; Benoit, M.Z.; Jonas, K.; Alexandra, T.; Désiré, D.D.P.; Pierre, K.; Théophile, D. Antidiabetic and Antioxidant Effects of Annona muricata (Annonaceae), Aqueous Extract on Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2014, 151, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kang, Y.R.; Zu, H.D.; Lim, I.S.; Jung, S.K.; Chang, Y.H. Effects of Time on Phenolics and in Vitro Bioactivity in Autoclave Extraction of Graviola (Annona muricata) Leaf. Biotechnol. Bioprocess. Eng. 2020, 25, 9–15. [Google Scholar] [CrossRef]
- Olugbuyiro, J.A.O.; Omotosho, O.E.; Taiwo, O.S.; Ononiwu, F.O.; Banwo, A.S.; Akintokun, O.A.; Obaseki, O.S.; Ogunleye, O.M. Antimicrobial activities and phytochemical properties of Annona muricata leaf. Covenant J. Phys. Life Sci. 2018, 5, 2. [Google Scholar]
- Orak, H.H.; Bahrisefit, I.S.; Sabudak, T. Antioxidant Activity of Extracts of Soursop (Annona muricata L.) Leaves, Fruit Pulps, Peels and Seeds. Pol. J. Food Nutr. Sci. 2019, 69, 4. [Google Scholar] [CrossRef]
- Mansour, H.H.; Elkady, A.A.; Elrefaei, A.H.; Hafez, H.F. Radioprotective, Antioxidant and Antitumor Efficacy of Annona muricata L. Leaf Extract. Indian J. Biochem. Biophys. 2018, 55, 205–214. [Google Scholar]
- Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Gálvez-Ruiz, J.C.; Ruí-Cruz, S.; Chaidez-Quiroz, C.; Morán-Palacio, E.F. Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extra. Plants 2020, 9, 1650. [Google Scholar] [CrossRef]
- Kuete, V.; Dzotam, J.K.; Voukeng, I.K.; Fankam, A.G.; Efferth, T. Cytotoxicity of Methanol Extracts of Annona muricata, Passiflora Edulis and Nine Other Cameroonian Medicinal Plants towards Multi-Factorial Drug-Resistant Cancer Cell Lines. Springerplus 2016, 5, 1666. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; Madivoli, E.S.; El-Shemy, H.A.; Wamunyokoli, F.; Magoma, G. Green Synthesis and Characterization of Highly Stable Silver Nanoparticles from Ethanolic Extracts of Fruits of Annona muricata. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1231–1242. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Oladipo, T.D.; Akanni, O.O.; Abiola, O.J. Hexane Fraction of Annona muricata (Sour Sop) Seed Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Biomed. Pharmacother. 2019, 111, 403–413. [Google Scholar] [CrossRef]
- Agu, K.C.; Okolie, P.N. Proximate Composition, Phytochemical Analysis, and in Vitro Antioxidant Potentials of Extracts of Annona muricata (Soursop). Food Sci. Nutr. 2017, 5, 1029–1036. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Silva, A.A.S.; Pinto, C.C.C.; Santos, D.L.D.; Freitas, J.C.C.D.; Martins, V.E.P.; Morais, S.M.D. Larvicidal and Enzymatic Inhibition Effects of Annona muricata Seed Extract and Main Constituent Annonacin against Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae). Pharmaceuticals 2019, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, Characterization and Antimicrobial Activity of Gold Nanoparticles from Leaf Extracts of Annona muricata. J. Nanostruct. Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- Oridupa, O.A.; Falade, F.B.; Oyagbemi, A.A.; Abegunde, B.A.; Ekwem, P.C.; Badmus, A.; Omobowale, T.O. Annona muricata Linn Leaves or Curcuma Longa Linn Rhizomes Ameliorates Oxidative Stress Associated with Hypertension in Uninephrectomized Wistar Rats Daily Loaded with Sodium Chloride. Eur. J. Med. Plants 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Ong, H.C.; Norzalina, J. Malay Herbal Medicine in Gemencheh, Negri Sembilan, Malaysia. Fitoterapia 1999, 70, 10–14. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Karimian, H.; Rouhollahi, E.; Paydar, M.; Fadaeinasab, M.; Kadir, H.A. Annona muricata Leaves Induce G1 Cell Cycle Arrest and Apoptosis through Mitochondria-Mediated Pathway in Human HCT-116 and HT-29 Colon Cancer Cells. J. Ethnopharmacol. 2014, 156, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Stan, S.D.; Singh, S.V.; Brand, R.E. Chemoprevention Strategies for Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 347–356. [Google Scholar] [CrossRef]
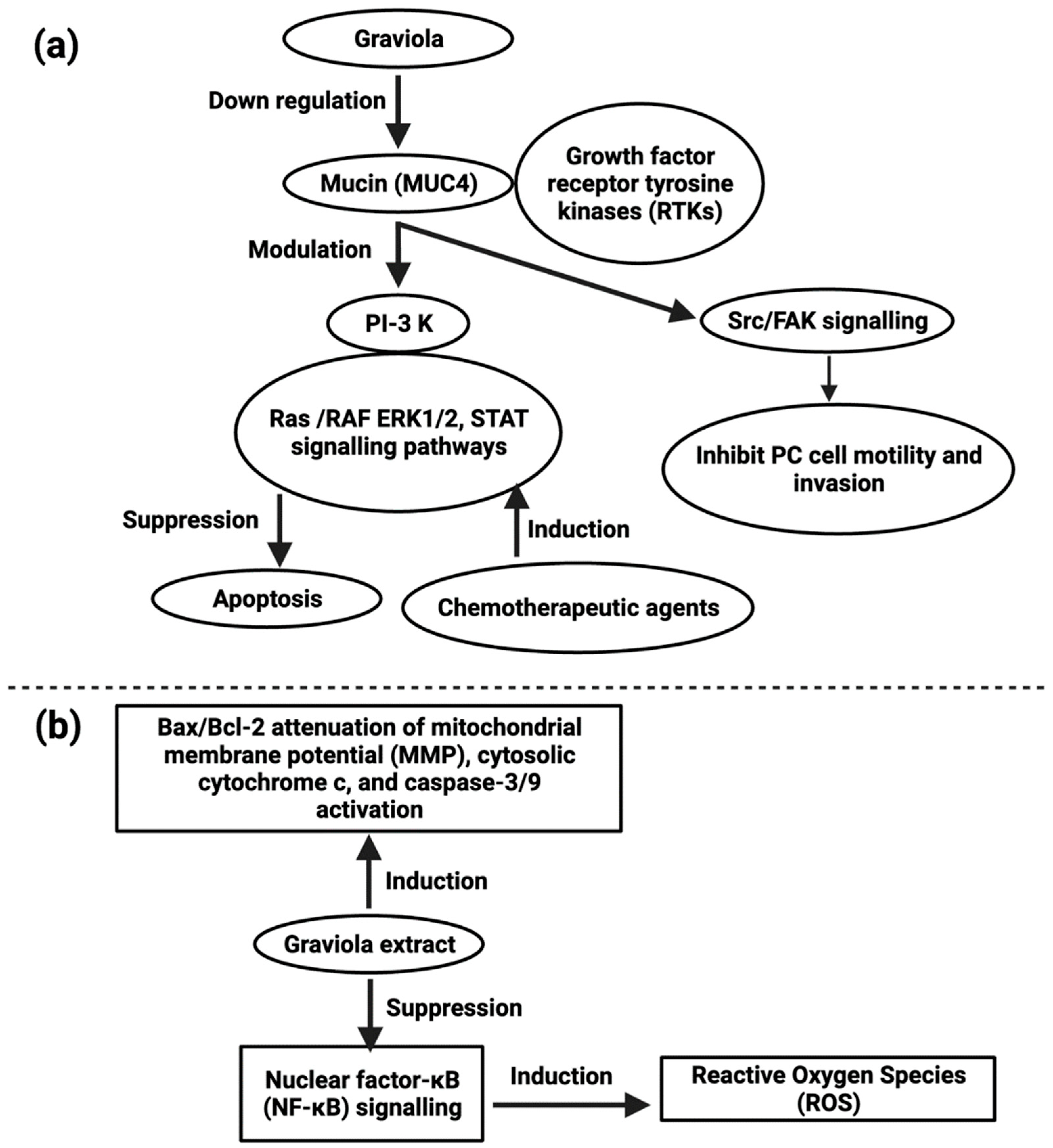
- Torres, M.P.; Rachagani, S.; Purohit, V.; Pandey, P.; Joshi, S.; Moore, E.D.; Johansson, S.L.; Singh, P.K.; Ganti, A.K.; Batra, S.K. Graviola: A Novel Promising Natural-Derived Drug That Inhibits Tumorigenicity and Metastasis of Pancreatic Cancer Cells In Vitro and In Vivo Through Altering Cell Metabolism. Cancer Lett. 2012, 323, 29–40. [Google Scholar] [CrossRef]
- Mohamad Rosdi, M.N.; Nik Mat Daud, N.N.N.; Zulkifli, R.M.; Ya’akob, H. Cytotoxic Effect of Annona muricata Linn Leaves Extract on Capan-1 Cells. J. Appl. Pharm. Sci. 2015, 5, 045–048. [Google Scholar] [CrossRef]
- Macha, M.A.; Krishn, S.R.; Jahan, R.; Banerjee, K.; Batra, S.K.; Jain, M. Emerging Potential of Natural Products for Targeting Mucins for Therapy Against Inflammation and Cancer. Cancer Treat. Rev. 2015, 41, 277. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Ghelli, A.; Ratta, M.; Cortes, D.; Estornell, E. Natural Substances (Acetogenins) from the Family Annonaceae Are Powerful Inhibitors of Mitochondrial NADH Dehydrogenase (Complex I). Biochem. J. 1994, 301 Pt 1, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656. [Google Scholar] [CrossRef]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sánchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene Ablation-Resistant Pancreatic Cancer Cells Depend on Mitochondrial Function. Nature 2014, 514, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Annona Muricata (Soursop). Available online: https://www.cabi.org/isc/datasheet/5812 (accessed on 2 August 2022).
- Meenakshisundaram, S.; Krishnamoorthy, V.; Jagadeesan, Y.; Vilwanathan, R.; Balaiah, A. Annona muricata Assisted Biogenic Synthesis of Silver Nanoparticles Regulates Cell Cycle Arrest in NSCLC Cell Lines. Bioorg. Chem. 2019, 95, 103451. [Google Scholar] [CrossRef] [PubMed]
- Shaniba, V.S.; Aziz, A.A.; Joseph, J.; Jayasree, P.R.; Manish Kumar, P.R. Synthesis, Characterization and Evaluation of Antioxidant and Cytotoxic Potential of Annona muricata Root Extract-Derived Biogenic Silver Nanoparticles. J. Clust. Sci. 2022, 33, 467–483. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Paydar, M.; Rouhollahi, E.; Karimian, H. Annona muricata Leaves Induced Apoptosis in A549 Cells through Mitochondrial-Mediated Pathway and Involvement of NF-ΚB. BMC Complement. Altern. Med. 2014, 14, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.X.; Rieser, M.J.; Hui, Y.H.; Miesbauer, L.R.; Smith, D.L.; McLaughlin, J.L. Biologically Active Acetogenins from Stem Bark of Asimina Triloba. Phytochemistry 1993, 33, 1065–1073. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive Natural Products for Chemoprevention and Treatment of Castration-Resistant Prostate Cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Zhou, N.; Zhu, W.; Dou, Q.P.; Zhou, K. Isolation of Three New Annonaceous Acetogenins from Graviola Fruit (Annona muricata) and Their Antiproliferation on Human Prostate Cancer Cell PC-3. Bioorg. Med. Chem. Lett. 2016, 26, 4282–4385. [Google Scholar] [CrossRef]
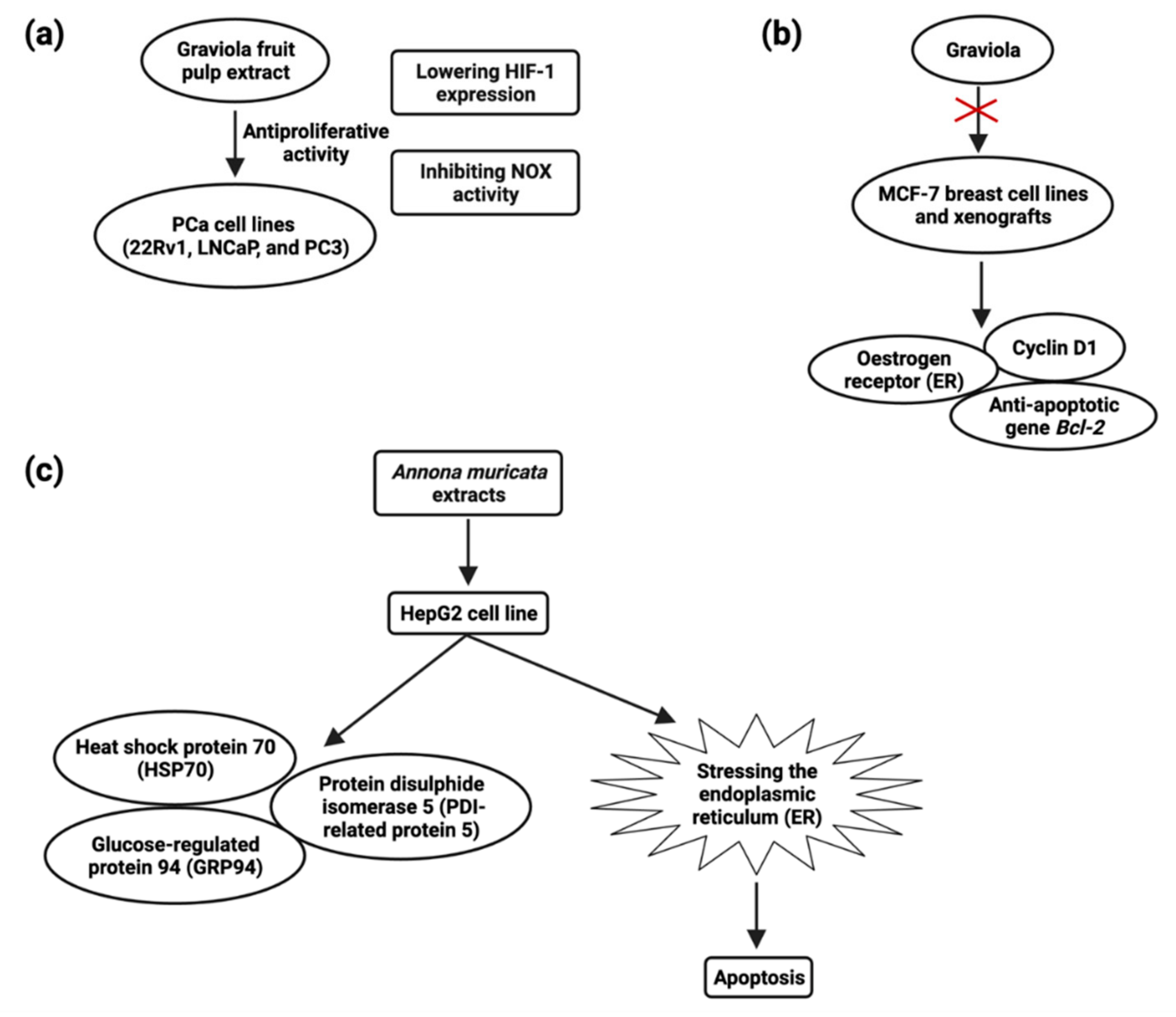
- Deep, G.; Kumar, R.; Jain, A.K.; Dhar, D.; Panigrahi, G.K.; Hussain, A.; Agarwal, C.; El-Elimat, T.; Sica, V.P.; Oberlies, N.H.; et al. Graviola Inhibits Hypoxia-Induced NADPH Oxidase Activity in Prostate Cancer Cells Reducing Their Proliferation and Clonogenicity. Sci. Rep. 2016, 6, 23135. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Kadouh, H.; Sun, X.; Zhou, K. Three New Anti-Proliferative Annonaceous Acetogenins with Monotetrahydrofuran Ring from Graviola Fruit (Annona Muricata). Bioorg. Med. Chem. Lett. 2014, 24, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic Interactions among Flavonoids and Acetogenins in Graviola (Annona muricata) Leaves Confer Protection against Prostate Cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Gavamukulya, Y.; Maina, E.N.; El-Shemy, H.A.; Meroka, A.M.; Kangogo, G.K.; Magoma, G.; Wamunyokoli, F. Annona muricata Silver Nanoparticles Exhibit Strong Anticancer Activities against Cervical and Prostate Adenocarcinomas through Regulation of CASP9 and the CXCL1/CXCR2 Genes Axis. Tumor Biol. 2021, 43, 37–55. [Google Scholar] [CrossRef]
- Minari, J.B.; Chikezie, C.C. Analysis of Annexin 7 Gene of Malignant Prostatic Hyperplasia-Induced Male Wistar Rats in the Presence of Annona muricata. J. Taibah Univ. Sci. 2019, 13, 460–467. [Google Scholar] [CrossRef]
- Foster, K.; Oyenihi, O.; Rademan, S.; Erhabor, J.; Matsabisa, M.; Barker, J.; Langat, M.K.; Kendal-Smith, A.; Asemota, H.; Delgoda, R. Selective Cytotoxic and Anti-Metastatic Activity in DU-145 Prostate Cancer Cells Induced by Annona muricata L. Bark Extract and Phytochemical, Annonacin. BMC Complementary Med. Ther. 2020, 20, 375. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Afriyie, D.; Ngala, R.A.; Abutiate, H.; Doku, D.; Mahmood, S.A.; Rahman, H. Antiproliferative Activity of Aqueous Leaf Extract of Annona muricata L. on the Prostate, BPH-1 Cells, and Some Target Genes. Integr. Cancer Ther. 2015, 14, 65–74. [Google Scholar] [CrossRef]
- de Melo, J.G.; de Sousa Araújo, T.A.; de Almeida Castro, V.T.N.; de Vasconcelos Cabral, D.L.; do Desterro Rodrigues, M.; do Nascimento, S.C.; de Amorim, E.L.C.; de Albuquerque, U.P. Antiproliferative Activity, Antioxidant Capacity and Tannin Content in Plants of Semi-Arid Northeastern Brazil. Molecules 2010, 15, 8534–8542. [Google Scholar] [CrossRef]
- Magadi, V.P.; Ravi, V.; Arpitha, A.; Litha, K.K.; Manjunath, K. Evaluation of Cytotoxicity of Aqueous Extract of Graviola Leaves on Squamous Cell Carcinoma Cell-25 Cell Lines by 3-(4,5-Dimethylthiazol-2-Yl) -2,5-Diphenyltetrazolium Bromide Assay and Determination of Percentage of Cell Inhibition at G2M Phase of Cell c. Contemp. Clin. Dent. 2015, 6, 529–533. [Google Scholar]
- Ko, E.-Y.; Moon, A. Natural Products for Chemoprevention of Breast Cancer. J. Cancer Prev. 2015, 20, 223. [Google Scholar] [CrossRef]
- Ko, Y.M.; Wu, T.Y.; Wu, Y.C.; Chang, F.R.; Guh, J.Y.; Chuang, L.Y. Annonacin Induces Cell Cycle-Dependent Growth Arrest and Apoptosis in Estrogen Receptor-α-Related Pathways in MCF-7 Cells. J. Ethnopharmacol. 2011, 137, 1283–1290. [Google Scholar] [CrossRef]
- Dai, Y.; Hogan, S.; Schmelz, E.M.; Ju, Y.H.; Canning, C.; Zhou, K. Selective Growth Inhibition of Human Breast Cancer Cells by Graviola Fruit Extract in Vitro and in Vivo Involving Downregulation of EGFR Expression. Nutr. Cancer 2011, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, N.H.; Croy, V.L.; Harrison, M.L.; McLaughlin, J.L. The Annonaceous Acetogenin Bullatacin Is Cytotoxic against Multidrug-Resistant Human Mammary Adenocarcinoma Cells. Cancer Lett. 1997, 115, 73–79. [Google Scholar] [CrossRef]
- Yuan, F.; Bai, G.; Miao, Y.; Chen, Y.; Li, X.; Chen, J. Annosquacin B Induces Mitochondrial Apoptosis in Multidrug Resistant Human Breast Cancer Cell Line MCF-7/ADR through Selectively Modulating MAPKs Pathways. Pharm. Biol. 2016, 54, 3040–3045. [Google Scholar] [CrossRef] [PubMed]
- González-Pedroza, M.G.; Argueta-Figueroa, L.; García-Contreras, R.; Jiménez-Martínez, Y.; Martínez-Martínez, E.; Navarro-Marchal, S.A.; Marchal, J.A.; Morales-Luckie, R.A.; Boulaiz, H. Silver Nanoparticles from Annona muricata Peel and Leaf Extracts as a Potential Potent, Biocompatible and Low Cost Antitumor Tool. Nanomaterials 2021, 11, 1273. [Google Scholar] [CrossRef]
- Sabapati, M.; Palei, N.N.; Ashok, A.K.; Molakpogu, R.B. Solid Lipid Nanoparticles of Annona muricata Fruit Extract: Formulation, Optimization and in Vitro Cytotoxicity Studies. Drug Dev. Ind. Pharm. 2019, 45, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green Synthesis of Silver Nanoparticles Using Annona muricata Extract as an Inducer of Apoptosis in Cancer Cells and Inhibitor for NLRP3 Inflammasome via Enhanced Autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef]
- Daddiouaissa, D.; Amid, A.; Kabbashi, N.A.; Fuad, F.A.A.; Elnour, A.M.; Epandy, M.A.K.M.S. Antiproliferative Activity of Ionic Liquid-Graviola Fruit Extract against Human Breast Cancer (MCF-7) Cell Lines Using Flow Cytometry Techniques. J. Ethnopharmacol. 2019, 236, 466–473. [Google Scholar] [CrossRef]
- Naik, A.V.; Sellappan, K. In Vitro Evaluation of Annona muricata L. (Soursop) Leaf Methanol Extracts on Inhibition of Tumorigenicity and Metastasis of Breast Cancer Cells. Biomarkers 2020, 25, 701–710. [Google Scholar] [CrossRef]
- Kim, Y.S. Annona muricata Leaf Extract Triggered Intrinsic Apoptotic Pathway to Attenuate Cancerous Features of Triple Negative Breast Cancer MDA-MB-231 Cells. Evid.-Based Complementary Altern. Med. 2018, 2018, 7972916. [Google Scholar] [CrossRef]
- Qazi, A.K.; Hussain, A.; Aga, M.A.; Ali, S.; Taneja, S.C.; Sharma, P.R.; Saxena, A.K.; Mondhe, D.M.; Hamid, A. Cell Specific Apoptosis by RLX Is Mediated by NFκB in Human Colon Carcinoma HCT-116 Cells. BMC Cell Biol. 2014, 15, 36. [Google Scholar] [CrossRef]
- Abdullah, M.; Syam, A.F.; Meilany, S.; Laksono, B.; Prabu, O.G.; Bekti, H.S.; Indrawati, L.; Makmun, D. The Value of Caspase-3 after the Application of Annona muricata Leaf Extract in COLO-205 Colorectal Cancer Cell Line. Gastroenterol. Res. Pr. 2017, 2017, 4357165. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Zeng, L.; Alali, F.; Rogers, L.L.; Wu, F.E.; McLaughlin, J.L.; Sastrodihardjo, S. Two New Mono-Tetrahydrofuran Ring Acetogenins, Annomuricin E and Muricapentocin, from the Leaves of Annona muricata. J. Nat. Prod. 1998, 61, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.E.; Gu, Z.M.; Zeng, L.; Zhao, G.X.; Zhang, Y.; McLaughlin, J.L.; Sastrodihardjo, S. Two New Cytotoxic Monotetrahydrofuran Annonaceous Acetogenins, Annomuricins A and B, from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.E.; Zeng, L.; Gu, Z.M.; Zhao, G.X.; Zhang, Y.; Schwedler, J.T.; McLaughlin, J.L.; Sastrodihardjo, S. New Bioactive Monotetrahydrofuran Annonaceous Acetogenins, Annomuricin C and Muricatocin C, from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Y.; Zhou, G.X.; Chen, T.; Gao, S.; Choi, M.Y.; Wong, Y.S. Desacetyluvaricin Induces S Phase Arrest in SW480 Colorectal Cancer Cells through Superoxide Overproduction. J. Cell. Biochem. 2014, 115, 464–475. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Qazi, A.K.; Siddiqui, J.A.; Jahan, R.; Chaudhary, S.; Walker, L.A.; Sayed, Z.; Jones, D.T.; Batra, S.K.; Macha, M.A. Emerging Therapeutic Potential of Graviola and Its Constituents in Cancers. Carcinogenesis 2018, 39, 522–533. [Google Scholar] [CrossRef] [Green Version]
- Csuka, O.; RemenÁr, É.; Koronczay, K.; Doleschall, Z.; NÉmeth, G. Predictive Value of P53, Bcl2 and Bax in the Radiotherapy of Head and Neck Cancer. Pathol. Oncol. Res. 1997, 3, 204–210. [Google Scholar] [CrossRef]
- Wahyudiono, A.; Istyawati, E.; Rahaju, P. Annona muricata (Soursop) Leaves Extract Increased Caspase 3 Expression in Patient-Derived WHO III Nasopharyngeal Cancer Cell. In Proceedings of the International Conference on Drug Discovery and Translational Medicine 2018 (ICDDTM ’18) “Seizing Opportunities and Addressing Challenges of Precision Medicine”, Putrajaya, Malaysia, 3 December–5 February 2019. [Google Scholar] [CrossRef]
- Lucas, D.M.; Still, P.C.; Pérez, L.B.; Grever, M.R.; Kinghorn, A.D. Potential of Plant-Derived Natural Products in the Treatment of Leukemia and Lymphoma. Curr. Drug Targets 2010, 11, 812. [Google Scholar] [CrossRef]
- Pieme, A.A.; Kumar, G.G.; Dongmo, S.S.; Moukette, M.M.; Boyoum, F.F.; Ngogang, Y.Y.; Saxena, K.K. Antiproliferative Activity and Induction of Apoptosis by Annona muricata (Annonaceae) Extract on Human Cancer Cells. BMC Complement. Altern. Med. 2014, 14, 516. [Google Scholar] [CrossRef]
- Ezirim, A.U.; Okochi, V.I.; James, A.B.; Adebeshi, O.A.; Ogunnowo, S.; Odeghe, O.B. Odeghe Induction of Apoptosis in Myelogenous Leukemic K562 Cells by Ethanolic Leaf Extract of Annona muricata L. Glob. J. Res. Med. Plants Indig. Med. 2013, 2, 142–151. [Google Scholar]
- de Castro Nascimento, J.; do Vale Bosso, R.M.; Anholeti, M.C.; da Silva Castro, E.; Junior, M.A.B.; do Nascimento, T.A.; de Paiva, S.R.; Fonte de Amorim, L.M. da Comparison of Anticancer Properties of Annona muricata L. Acetonic and Methanolic Leaf Extracts. Nat. Prod. J. 2018, 9, 312–320. [Google Scholar] [CrossRef]
- Yang, H.; Liu, N.; Lee, S. Ethanol Extract of Annona muricata L Induces Liver Cancer Cell Apoptosis through ROS Pathway. Biomed. Pharmacol. J. 2016, 9, 919–925. [Google Scholar] [CrossRef]
- Liu, N.; Yang, H.L.; Wang, P.; Lu, Y.C.; Yang, Y.J.; Wang, L.; Lee, S.C. Functional Proteomic Analysis Revels That the Ethanol Extract of Annona muricata L. Induces Liver Cancer Cell Apoptosis through Endoplasmic Reticulum Stress Pathway. J. Ethnopharmacol. 2016, 189, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.V.; Paul, J.; Shrungeswara, A.H.; Biswas, S.; Shah, A.A.; Subraya, C.K.; Nayak, Y. Annona muricata Fruit Extract Protects against Diethylnitrosamine-Induced Hepatocellular Cancer in Rats. Asian Pac. J. Trop. Med. 2019, 12, 272–282. [Google Scholar]
- Capes-Davis, A.; Theodosopoulos, G.; Atkin, I.; Drexler, H.G.; Kohara, A.; MacLeod, R.A.F.; Masters, J.R.; Nakamura, Y.; Reid, Y.A.; Reddel, R.R.; et al. Check Your Cultures! A List of Cross-Contaminated or Misidentified Cell Lines. Int. J. Cancer 2010, 127, 1–8. [Google Scholar] [CrossRef]
- Qorina, F.; Arsianti, A.; Fithrotunnisa, Q.; Tejaputri, N.; Azizah, N.N.; Putrianingsih, R. Cytotoxicity of Soursop Leaves (Annona muricata) against Cervical HeLa Cancer Cells. Pharmacogn. J. 2020, 12, 20–24. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef]
- Guo, W.; Wang, N.; Feng, Y. Recent Progress on the Molecular Mechanisms of Anti-Invasive and Metastatic Chinese Medicines for Cancer Therapy. In Unique Aspects of Anti-cancer Drug Development; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- De Craene, B.; Berx, G. Regulatory Networks Defining EMT during Cancer Initiation and Progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Chiarugi, P.; Giannoni, E. Anoikis: A Necessary Death Program for Anchorage-Dependent Cells. Biochem. Pharm. 2008, 76, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Wang, P.; Toh, A.; Thompson, E.W. New Insights into the Role of Phenotypic Plasticity and EMT in Driving Cancer Progression. Front. Mol. Biosci. 2020, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Redfern, A.D.; Spalding, L.J.; Thompson, E.W. The Kraken Wakes: Induced EMT as a Driver of Tumour Aggression and Poor Outcome. Clin. Exp. Metastasis 2018, 35, 285–308. [Google Scholar] [CrossRef]
- Brabletz, T. To Differentiate or Not—Routes towards Metastasis. Nat. Rev. Cancer 2012, 12, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.L.; Shepard, C.R.; Wells, A. Breast Carcinoma Cells Re-Express E-Cadherin during Mesenchymal to Epithelial Reverting Transition. Mol. Cancer 2010, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156. [Google Scholar] [CrossRef]
- Al-Medhtiy, M.H.; Jabbar, A.A.; Shareef, S.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Abdulla, M.A. Histopathological Evaluation of Annona muricata in TAA-Induced Liver Injury in Rats. Processes 2022, 10, 1613. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling Mechanisms of the Epithelial-Mesenchymal Transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.S.; Yung, K.K.L. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef]
- Escobar-Khondiker, M.; Höllerhage, M.; Muriel, M.P.; Champy, P.; Bach, A.; Depienne, C.; Respondek, G.; Yamada, E.S.; Lannuzel, A.; Yagi, T.; et al. Annonacin, a Natural Mitochondrial Complex I Inhibitor, Causes Tau Pathology in Cultured Neurons. J. Neurosci. 2007, 27, 7827. [Google Scholar] [CrossRef]
- Abd El-Kaream, S.A. Biochemical and Biophysical Study of Chemopreventive and Chemotherapeutic Anti-Tumor Potential of Some Egyptian Plant Extracts. Biochem. Biophys. Rep. 2019, 18, 100637. [Google Scholar] [CrossRef] [PubMed]
- Hamizah, S.; Roslida, A.H.; Fezah, O.; Tan, K.L.; Tor, Y.S.; Tan, C.I. Chemopreventive Potential of Annona muricata L. Leaves on Chemically-Induced Skin Papillomagenesis in Mice. Asian Pac. J. Cancer Prev. 2012, 13, 2533–2539. [Google Scholar] [CrossRef]
- Rajesh, V.; Baby Kala, M. Antiproliferative and Chemopreventive Effect of Annona muricata Linn. on Ehrlich Ascites Carcinoma and Benzo[a]Pyrene Induced Lung Carcinoma. Orient. Pharm. Exp. Med. 2015, 15, 239–256. [Google Scholar] [CrossRef]
- Eggadi, V.; Gundamedi, S.; Sheshagiri, S.B.B.; Revoori, S.K.; Jupally, V.R.; Kulandaivelu, U. Evaluation of Anticancer Activity of Annona muricata in 1, 2-Dimethyl Hydrazine Induced Colon Cancer. World Appl. Sci. J. 2014, 32, 444–450. [Google Scholar]
- Indrawati, L.; Purwantyastuti, P.; Abdullah, M.; Surono, I.S.; Basir, I. Safety of Annona muricata Extract Supplementation for Colorectal Cancer Patients. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2017, 17, 170–175. [Google Scholar] [CrossRef]
- Indrawati, L.; Ascobat, P.; Bela, B.; Abdullah, M.; Surono, I.S. The Effect of an Annona muricata Leaf Extract on Nutritional Status and Cytotoxicity in Colorectal Cancer: A Randomized Controlled Trial. Asia Pac. J. Clin. Nutr. 2017, 26, 606–612. [Google Scholar] [CrossRef]
- Surono, I.S.; Lienggonegoro, L.A.; Indrawati, L.; Wibowo, H. Inflammatory Response of Annona muricata Linn Leaves Extract in Colorectal Cancer Patients. J. Global. Pharma. Technol. 2017, 9 (Suppl. 7), 150–157. [Google Scholar]
- Knüpfer, H.; Preiss, R. Serum Interleukin-6 Levels in Colorectal Cancer Patients-a Summary of Published Results. Int. J. Colorectal. Dis. 2010, 25, 135–140. [Google Scholar] [CrossRef]
- Yap, S. Colon Cancer Reversed by Phyto-Nutritional Therapy: A Case Study. Int. J. Biotechnol. Wellness Ind. 2013, 2, 132–139. [Google Scholar] [CrossRef]
- Hansra, D.M.; Silva, O.; Mehta, A.; Ahn, E. Patient with Metastatic Breast Cancer Achieves Stable Disease for 5 Years on Graviola and Xeloda after Progressing on Multiple Lines of Therapy. Adv. Breast Cancer Res. 2014, 2014, 84–87. [Google Scholar] [CrossRef]
- Shahid, F.; Farooqui, Z.; Khan, F. Cisplatin-Induced Gastrointestinal Toxicity: An Update on Possible Mechanisms and on Available Gastroprotective Strategies. Eur. J. Pharm. 2018, 827, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Holanda, C.M.D.C.X.; Barbosa, D.A.; Demeda, V.F.; Bandeira, F.T.M.; de Medeiros, H.C.S.; Pereira, K.R.S.G.; de Barbosa, V.S.A.; Medeiros, A.C. Influence of Annona muricata (Soursop) on Biodistribution of Radiopharmaceuticals in Rats. Acta. Cir. Bras. 2014, 29, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Höllerhage, M.; Rösler, T.W.; Berjas, M.; Luo, R.; Tran, K.; Richards, K.M.; Sabaa-Srur, A.U.; Maia, J.G.S.; de Moraes, M.R.; Godoy, H.T.; et al. Neurotoxicity of Dietary Supplements from Annonaceae Species. Int. J. Toxicol. 2015, 34, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Ethnomedicinal Uses | Plant Parts Used | Graviola Extract/Chemical Compound | References |
|---|---|---|---|
| Insecticide | Seed, leaves, barks, stems, roots and flowers | Acetogenins | [79,80] |
| Parasiticide | Leaf | Ethanolic extract and its fractions, methanol extracts, and acetogenins, ethyl acetate extract | [81,82,83,84] |
| Hypotensive | Leaf, fruit | Aqueous extract, the alkaloids, isoquinoline, coreximine, and anomurine | [85] |
| Fever | Leaf | Flavonoids | [70,86] |
| Respiratory illness | Leaf | Essential Oil | [47,70] |
| Sedative | Leaf | Hydroalcoholic extract | [87] |
| Malaria | Seed, leaf | Ethanolic extract | [50,88] |
| Gastrointestinal disorders | Leaf | Ethyl acetate extract | [70,89] |
| Liver, heart, and renal disorders | Fruit, Leaf | Ethyl acetate and ethanol extracts | [50,89,90,91,92,93] |
| Hypoglycemic | Leaf, branch | Ethanolic extract | [91,94,95] |
| Cancer | Leaf, fruit, stem, bark and branch | Annonaceous acetogenins, alkaloids, flavonoids, sterols, and others | Discussed in detail in the following sections |
| Cancer Type | Models Used | Concentration | A. muricata Extract | Outcome | References |
|---|---|---|---|---|---|
| Lung cancer | In vitro (H1299) cell line | 146 μg/mL (IC50 Value) | A. muricata leaf extract | Cytotoxic activity | [118] |
| In vitro (A549) cell line | 194 μg/mL (IC50 Value) | A. muricata leaf extract | Cytotoxic activity | [118] | |
| In vitro (A549) cell line | 6 µg/mL | Green synthesis of silver nanoparticles using A. muricata leaves extract | Cell cycle arrest, elevated levels of apoptotic proteins, down regulation in Bcl-2 and cell cycle regulators, upregulation in apoptotic genes | [119] | |
| In vitro (A549) cell line | >100 ± 1 µg/mL (IC50 at 24 h treatment) | A. muricata Root Extract-derived Biogenic Silver Nanoparticles | Anti-proliferative activity | [120] | |
| >80 ± 1 µg/mL (IC50 at 48 h treatment) | |||||
| >70 ± 3 µg/mL (IC50 at 72 h treatment) |
| Cancer Type | Models Used | Concentration | Annona muricata Extract | Outcome | References |
|---|---|---|---|---|---|
| Prostate cancer | In vitro PC-3 cell line | 80 μg/mL (IC50 Value) | A. muricata leaf extract | Cytotoxic activity | [118] |
| In vitro PNT1-A cell line | 375.68 μg/mL (IC50 Value) | Green synthesized A. muricata fruit extract silver nanoparticles | Cytotoxic activity, upregulation of CASP9 at 1.37-fold, upregulation of CXCL1 (7.17-fold), CXCR2 down regulated (0.66-fold) | [128] | |
| 112.29 μg/mL (IC50 Value) | Green synthesized A. muricata leaf extract silver nanoparticles | Cytotoxic activity, upregulation of CASP9 at 16.78-fold, upregulation of CXCL1 (85.96-fold), CXCR2 up regulated | |||
| In vitro PC-3 cell line | 48.17 μg/mL (IC50 Value) | Green synthesized A. muricata fruit extract silver nanoparticles | Cytotoxic activity | ||
| 47.58 μg/mL (IC50 Value) | Green synthesized A. muricata leaf extract silver nanoparticles | Cytotoxic activity | |||
| In vivo | 90:22.5:100 mg/kg−1 | Combination of Arginine (L-ARG), Monosodium Glutamate (MSG), Ethanolic leaves extract | Induced annexin 7 gene mutation in malignant prostatic hyperplasia-induced male Wistar rats | [129] | |
| In vivo | 50 μM | Combination of hexane fraction (A. muricata seeds) and Finasteride | Prostate weight reduction in BPH induced rats | [103] | |
| In vitro DU-145 cell line | 0.1 ± 0.07 μM (IC50 Value) | Annonacin | Cytotoxic effect | [130] | |
| 0.5 μM | Angiogenesis | ||||
| 55.501 ± 0.55 μg/mL (IC50 Value) | Ethyl acetate bark extract | Cytotoxic effect | |||
| 50 μg/mL | Cell migration | ||||
| 100 μg/mL | Angiogenesis | ||||
| 0.0002 μg/mL | Docetaxel in combination with 100 μg/mL Ethyl acetate bark extract | Elevated intracellular ROS, mitochondrial membrane depolarization, 3/7 caspase activation. |
| Cancer Type | Models used | Concentration | Annona muricata Extract | Outcome | References |
|---|---|---|---|---|---|
| Breast cancer | In vitro (MCF-7) cell line | 2.996 µg/mL (IC50 Value) | Green synthesis of silver nanoparticles using A. muricata leaves extract | Antiproliferative activity | [139] |
| 3.109 µg/mL (IC50 Value) | Green synthesis of silver nanoparticles using A. muricata peel extract | ||||
| 1278 µg/mL (IC50 Value) | A. muricata peel extract | ||||
| 2280 µg/mL (IC50 Value) | A. muricata leaf extract | ||||
| In vitro (MDA-MB-468) cell line | 1.685 µg/mL (IC50 Value) | Green synthesis of silver nanoparticles using A. muricata leaves extract | Antiproliferative activity | [139] | |
| 1.910 µg/mL (IC50 Value) | Green synthesis of silver nanoparticles using A. muricata peel extract | ||||
| 264.9 µg/mL (IC50 Value) | A. muricata peel extract | ||||
| 776.4 µg/mL (IC50 Value) | A. muricata leaf extract | ||||
| In vitro (MCF-7) cell line | 12 µg/mL | Solid lipid nanoparticles of A. muricata fruit extract | Antiproliferative activity, Cell cycle arrest | [140] | |
| In vitro AMJ13 Cell line | 17.34 µg/mL | Green synthesized A. muricata silver nanoparticles | Anti-proliferation, apoptosis, Mitochondrial membrane potential, upregulated P53, down regulated caspase-1, IL-1β, ASC protein, NLRP3 degradation, autophagy | [141] | |
| In vitro (MCF-7) cell line | 4.75 μg/mL (IC50 Value) | Ionic liquid- A. muricata fruit extract | Cytotoxic activity, cell cycle arrest (G0/G1-phase), apoptosis | [142] | |
| 85.55 µg/mL | Methanolic leaf extracts of A. muricata | Cytotoxic activity | [143] | ||
| 100 µg/mL | Cell cycle arrest (G1-phase), apoptosis, elevated intracellular ROS, upregulation of caspase-3 | ||||
| 10 µg/mL | Annonacin | Genotoxic activity | [144] | ||
| 0, 50, 100 and 200 µg/mL | A. muricata leaf extract | ER-dependent mechanism of apoptosis | |||
| In vitro TNBC MDA-MB-231 cell line | 0, 50, 100 and 200 µg/mL | A. muricata leaf extract | Clonogenicity, Sub-G1 cell cycle arrest, impaired cell motility and invasiveness, intrinsic apoptotic pathway | ||
| In vitro MCF-7 cell line | 220 µg/mL (IC50 Value) | A. muricata leaf extract | Cytotoxic activity | [118] |
| Cancer Type | Models Used | Concentration | Annona muricata Extract | Outcome | References |
|---|---|---|---|---|---|
| Colon cancer | In vitro HCT-116 cell line | 1.285 µg/mL (IC50 Value) | Green synthesis of silver nanoparticles using A. muricata leaves extract | Antiproliferative activity | [139] |
| 2.004 µg/mL (IC50 Value) | Green synthesis of silver nanoparticles using A. muricata peel extract | ||||
| 309.3 µg/mL (IC50 Value) | A. muricata peel extract | ||||
| 404.8 µg/mL (IC50 Value) | A. muricata leaf extract | ||||
| In vitro HCT-116 cell line | 69 ± 2.0 µg/mL (IC50 at 24 h treatment) | A. muricata Root Extract-derived Biogenic Silver Nanoparticles | Anti-proliferative activity, cell cycle arrest, apoptosis | [120] | |
| 44 ± 1.5 µg/mL (IC50 at 48 h treatment) | |||||
| 8.5 ± 3 µg/mL (IC50 at 72 h treatment) |
| Cancer Type | Models Used | Concentration | Annona muricata Extract | Outcome | References |
|---|---|---|---|---|---|
| Hematological malignancies | In vitro k562 Cell line | 28.82 µg/mL (IC50 Value) | A. muricata acetone leaf extract | Cytotoxic effect, G0/G1 cell cycle arrest | [158] |
| 32.49 µg/mL (IC50 Value) | A. muricata methanol leaf extract | ||||
| In vitro THP-1 Cell line | 17.34 µg/mL | Green synthesized A. muricata silver nanoparticles | Anti-proliferation, apoptosis, Mitochondrial membrane potential, upregulated P53, down regulated caspase-1, IL-1β, ASC protein, NLRP3 degradation, autophagy | [141] | |
| In vitro Raji (Human Burkitt’s Lymphoma B-lymphoblastoid) cell line | 19.1 ± 1.4 µg/mL (IC50 Value) | Hexane leaf extract | Cytotoxic effect | [53] | |
| 4.7 ± 1.4 µg/mL (IC50 Value) | Dichloromethane leaves extract | ||||
| 80.4 ± 1.2 µg/mL (IC50 Value) | Methanol leaves extract | ||||
| 193.8 ± 1.9 µg/mL (IC50 Value) | Ethanol leaves extract | ||||
| 73.1 ± 1.4 µg/mL (IC50 Value) | Aqueous leaves extract | ||||
| 385.2 ± 1.7 µg/mL (IC50 Value) | Aqueous fruit extract | ||||
| 2.89 ± 1.3 µg/mL (IC50 Value) | Annonacin |
| Cancer Type | Models Used | Concentration | Annona muricata Extract | Outcome | References |
|---|---|---|---|---|---|
| Liver cancer | In vitro HepG-2 cell line | 62.699 µg/mL (IC50 Value) | Aqueous fruit extract | Anti-proliferative activity | [74] |
| 63.710 µg/mL (IC50 Value) | Chloroform fruit extract | ||||
| 20.617 µg/mL (IC50 Value) | Ethyl acetate fruit extract | ||||
| 44.553 µg/mL (IC50 Value) | Hexane fruit extract | ||||
| 13.104 µg/mL (IC50 Value) | Methanol fruit extract | ||||
| 13.104 µǥ/mL and 1000 µǥ/mL (IC50 and maximum concentration) | Methanol fruit extract | Nuclear condensation | |||
| (13.104 µǥ/mL and 1000 µǥ/mL) (IC50 and maximum concentration) | Methanol fruit extract | Apoptosis | |||
| In vitro Hep2 cell line | 17.98 µg/mL (IC50 Value) | Green synthesized A. muricata fruit pulp mediated gold nanoparticles | Antiproliferative activity | [76] | |
| 13.08 µg/mL (IC50 Value) | Green synthesized A. muricata fruit peel mediated gold nanoparticles | ||||
| In vivo Mono sodium glutamate-induced hepatic injury in rats | 200 mg/kg (Body Weight) | A. muricata extract | Upregulated SIRT1 expression, down regulated FAS, ROS, IL-6, P53, Caspase-3, Bax levels when compared to Mono Sodium Glutamate induced rats | [92] | |
| In vitro HepG2 cell line | 274.9 ± 8.3 µg/mL (IC50 Value) | A. muricata fruit extract (AMF) | Cytotoxic activity | [161] | |
| 53.7 ± 4.3 µg/mL (IC50 Value) | Chloroform fraction of AMF | Cytotoxic activity, Significant inhibition of cell migration, apoptosis, G0/G1 phase cell cycle arrest | |||
| 55 µg/mL | |||||
| 341.4 ± 6.7 µg/mL (IC50 Value) | Ethyl acetate fraction of AMF | Cytotoxic activity | |||
| 928.8 ± 10.5 µg/mL (IC50 Value) | Petroleum ether fraction of AMF | Cytotoxic activity |
| Cancer Type | Models Used | Concentration | Annona muricata Extract | Outcome | References |
|---|---|---|---|---|---|
| Cervical cancer | In vitro HeLa cell line | 100 μg/mL (IC50 Value) | A. muricata leaf extract | Cytotoxic activity | [118] |
| In vitro HeLa cell line | >70 ± 1.0 µg/mL (IC50 at 24 h treatment) | A. muricata Root Extract-derived Biogenic Silver Nanoparticles | Anti-proliferative activity | [120] | |
| >70 ± 2 µg/mL (IC50 at 48 h treatment) | |||||
| 60 ± 2.0 µg/mL (IC50 at 72 h treatment) | |||||
| In vitro HeLa cell line | 38.58 μg/mL (IC50 Value) | Green synthesized A. muricata fruit extract silver nanoparticles | Cytotoxic activity, CXCL1 down regulated (1.53-fold), CXCR2 down regulated (0.64-fold) | [128] | |
| 57.63 μg/mL (IC50 Value) | Green synthesized A. muricata leaf extract silver nanoparticles | Cytotoxic activity, CXCL1 was down regulated (1.52-fold), CXCR2 down regulated (1.12-fold) | |||
| In vitro HeLa cell line | 5.91 μg/mL (IC50 Value) | Ethanolic leaf extract | Cytotoxic activity | [163] | |
| 7.56 μg/mL (IC50 Value) | Ethyl acetate leaf extract | ||||
| 8.39 μg/mL (IC50 Value) | Hexane leaf extract |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilango, S.; Sahoo, D.K.; Paital, B.; Kathirvel, K.; Gabriel, J.I.; Subramaniam, K.; Jayachandran, P.; Dash, R.K.; Hati, A.K.; Behera, T.R.; et al. A Review on Annona muricata and Its Anticancer Activity. Cancers 2022, 14, 4539. https://doi.org/10.3390/cancers14184539
Ilango S, Sahoo DK, Paital B, Kathirvel K, Gabriel JI, Subramaniam K, Jayachandran P, Dash RK, Hati AK, Behera TR, et al. A Review on Annona muricata and Its Anticancer Activity. Cancers. 2022; 14(18):4539. https://doi.org/10.3390/cancers14184539
Chicago/Turabian StyleIlango, Suganya, Dipak Kumar Sahoo, Biswaranjan Paital, Kavibharathi Kathirvel, Jerrina Issac Gabriel, Kalyani Subramaniam, Priyanka Jayachandran, Rajendra Kumar Dash, Akshaya Kumar Hati, Tapas Ranjan Behera, and et al. 2022. "A Review on Annona muricata and Its Anticancer Activity" Cancers 14, no. 18: 4539. https://doi.org/10.3390/cancers14184539
APA StyleIlango, S., Sahoo, D. K., Paital, B., Kathirvel, K., Gabriel, J. I., Subramaniam, K., Jayachandran, P., Dash, R. K., Hati, A. K., Behera, T. R., Mishra, P., & Nirmaladevi, R. (2022). A Review on Annona muricata and Its Anticancer Activity. Cancers, 14(18), 4539. https://doi.org/10.3390/cancers14184539








