A Bloody Conspiracy— Blood Vessels and Immune Cells in the Tumor Microenvironment
Abstract
:Simple Summary
Abstract
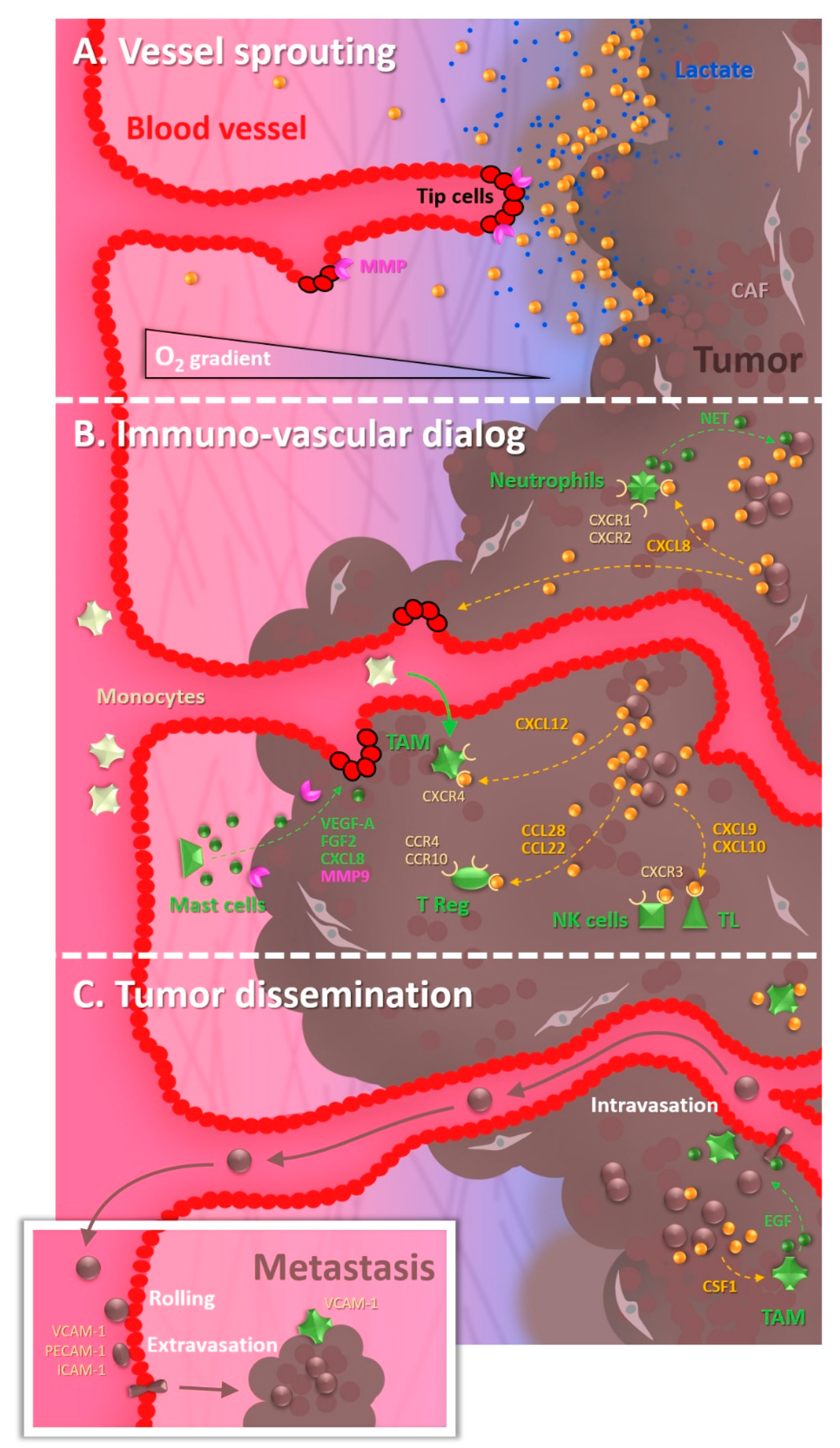
1. The Tumor Microenvironment: The Octopus Spreading Its Tentacles
1.1. The Tumor Vascularization, a Key Partner in the Malignant Progression
1.2. The Cocktail Driving Tumor Angiogenesis
1.3. The Recruitment of Undercover Agents
| Cytokines | Effects | References |
|---|---|---|
| IL-6/IL-8 | Angiogenesis promotion/inflammation | [18] |
| GM-CSF | Angiogenesis promotion | [18] |
| CCL2 | Monocyte recruitment | [23] |
| CXCL5 | Monocyte recruitment | [23] |
| CXCL12 (also called SDF1) | TAM recruitment/ | [23,24] |
| CXCL12 (also called SDF1) | Dendritic cell migration | [32] |
| CXCL9/CXCL10 | TL and NK recruitment | [23] |
| CCL22/CCL28 | T-reg recruitment | [25] |
| IL-1/TNFα | Inflammation/Haptotaxis | [33] |
| CCL11 | Eosinophil recruitment | [26] |
| CXCL1/CXCL2/CXCL8 | Neutrophil recruitment | [27] |
1.4. The Underground Environment: Of Hypoxia and Its Malignant Consequences
1.5. The Remote Expansion of Activities
2. Chips, Patrolling to Catch Specific Scenes of the Bloody Conspiracy
2.1. Tumor Angiogenesis under the Spotlight Thanks to VoC, Vessels-on-Chip
2.2. A Successful Business Requires a Steady Cash Flow
2.3. What about Particular Supply Routes?
2.4. The Choreography of Immune Cells as Undercover Agents among Gangsters
2.5. Crossing the Legal Line
3. Future Is Coming, Giving a New Dimension to the Trade
3.1. Spying on the Double Game of Endothelial Cells within Tumors
3.2. Unmasking the Interplay between Tumoral Thugs and Undercover Immune Agents
3.3. Fueling the System, a Vital Issue
4. Discussion/Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Marx, J. Cancer Biology. All in the Stroma: Cancer’s Cosa Nostra. Science 2008, 320, 38–41. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Bear, H.D.; Tang, G.; Rastogi, P.; Geyer, C.E.; Robidoux, A.; Atkins, J.N.; Baez-Diaz, L.; Brufsky, A.M.; Mehta, R.S.; Fehrenbacher, L.; et al. Bevacizumab Added to Neoadjuvant Chemotherapy for Breast Cancer. N. Engl. J. Med. 2012, 366, 310–320. [Google Scholar] [CrossRef]
- Aalders, K.C.; Tryfonidis, K.; Senkus, E.; Cardoso, F. Anti-Angiogenic Treatment in Breast Cancer: Facts, Successes, Failures and Future Perspectives. Cancer Treat. Rev. 2017, 53, 98–110. [Google Scholar] [CrossRef]
- Sivridis, E.; Giatromanolaki, A.; Koukourakis, M.I. The Vascular Network of Tumours--What Is It Not for? J. Pathol. 2003, 201, 173–180. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Germain, S.; Monnot, C.; Muller, L.; Eichmann, A. Hypoxia-Driven Angiogenesis: Role of Tip Cells and Extracellular Matrix Scaffolding. Curr. Opin. Hematol. 2010, 17, 245–251. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Lu, K.V.; Chang, J.P.; Parachoniak, C.A.; Pandika, M.M.; Aghi, M.K.; Meyronet, D.; Isachenko, N.; Fouse, S.D.; Phillips, J.J.; Cheresh, D.A.; et al. VEGF Inhibits Tumor Cell Invasion and Mesenchymal Transition through a MET/VEGFR2 Complex. Cancer Cell 2012, 22, 21–35. [Google Scholar] [CrossRef]
- Pàez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Viñals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef]
- Furlan, A.; Kherrouche, Z.; Montagne, R.; Copin, M.-C.; Tulasne, D. Thirty Years of Research on Met Receptor to Move a Biomarker from Bench to Bedside. Cancer Res. 2014, 74, 6737–6744. [Google Scholar] [CrossRef]
- Mira, E.; Lacalle, R.A.; Buesa, J.M.; de Buitrago, G.G.; Jiménez-Baranda, S.; Gómez-Moutón, C.; Martínez-A, C.; Mañes, S. Secreted MMP9 Promotes Angiogenesis More Efficiently than Constitutive Active MMP9 Bound to the Tumor Cell Surface. J. Cell Sci. 2004, 117, 1847–1857. [Google Scholar] [CrossRef]
- Giraudo, E.; Inoue, M.; Hanahan, D. An Amino-Bisphosphonate Targets MMP-9-Expressing Macrophages and Angiogenesis to Impair Cervical Carcinogenesis. J. Clin. Investig. 2004, 114, 623–633. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Bourcy, M.; Lesage, J.; Leroi, N.; Syne, L.; Blacher, S.; Hubert, P.; Erpicum, C.; Foidart, J.-M.; Delvenne, P.; et al. Soluble Factors Regulated by Epithelial-Mesenchymal Transition Mediate Tumour Angiogenesis and Myeloid Cell Recruitment. J. Pathol. 2015, 236, 491–504. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical Roles of the Immune System during Cancer Development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Akkari, L.; Bowman, R.L.; Tessier, J.; Klemm, F.; Handgraaf, S.M.; de Groot, M.; Quail, D.F.; Tillard, L.; Gadiot, J.; Huse, J.T.; et al. Dynamic Changes in Glioma Macrophage Populations after Radiotherapy Reveal CSF-1R Inhibition as a Strategy to Overcome Resistance. Sci. Transl. Med. 2020, 12, eaaw7843. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Cancer and Inflammation: An Old Intuition with Rapidly Evolving New Concepts. Annu. Rev. Immunol. 2012, 30, 677–706. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the Cancer Microenvironment and Their Relevance in Cancer Immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Wang, S.-C.; Hong, J.-H.; Hsueh, C.; Chiang, C.-S. Tumor-Secreted SDF-1 Promotes Glioma Invasiveness and TAM Tropism toward Hypoxia in a Murine Astrocytoma Model. Lab. Investig. 2012, 92, 151–162. [Google Scholar] [CrossRef]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.-P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour Hypoxia Promotes Tolerance and Angiogenesis via CCL28 and T(Reg) Cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Cormier, S.A.; Taranova, A.G.; Bedient, C.; Nguyen, T.; Protheroe, C.; Pero, R.; Dimina, D.; Ochkur, S.I.; O’Neill, K.; Colbert, D.; et al. Pivotal Advance: Eosinophil Infiltration of Solid Tumors Is an Early and Persistent Inflammatory Host Response. J. Leukoc. Biol. 2006, 79, 1131–1139. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory Mast Cells Up-Regulate Angiogenesis during Squamous Epithelial Carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef]
- Stockmann, C.; Doedens, A.; Weidemann, A.; Zhang, N.; Takeda, N.; Greenberg, J.I.; Cheresh, D.A.; Johnson, R.S. Deletion of Vascular Endothelial Growth Factor in Myeloid Cells Accelerates Tumorigenesis. Nature 2008, 456, 814–818. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix Metalloproteinase-9 Triggers the Angiogenic Switch during Carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Scott, E.N.; Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Regulatory T Cells: Barriers of Immune Infiltration Into the Tumor Microenvironment. Front. Immunol. 2021, 12, 702726. [Google Scholar] [CrossRef]
- Parlato, S.; De Ninno, A.; Molfetta, R.; Toschi, E.; Salerno, D.; Mencattini, A.; Romagnoli, G.; Fragale, A.; Roccazzello, L.; Buoncervello, M.; et al. 3D Microfluidic Model for Evaluating Immunotherapy Efficacy by Tracking Dendritic Cell Behaviour toward Tumor Cells. Sci. Rep. 2017, 7, 1093. [Google Scholar] [CrossRef]
- de Graaf, M.N.S.; Cochrane, A.; van den Hil, F.E.; Buijsman, W.; van der Meer, A.D.; van den Berg, A.; Mummery, C.L.; Orlova, V.V. Scalable Microphysiological System to Model Three-Dimensional Blood Vessels. APL Bioeng. 2019, 3, 026105. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Barrett, R.L.; Puré, E. Cancer-Associated Fibroblasts and Their Influence on Tumor Immunity and Immunotherapy. eLife 2020, 9, e57243. [Google Scholar] [CrossRef]
- Sewell-Loftin, M.K.; Bayer, S.V.H.; Crist, E.; Hughes, T.; Joison, S.M.; Longmore, G.D.; George, S.C. Cancer-Associated Fibroblasts Support Vascular Growth through Mechanical Force. Sci. Rep. 2017, 7, 12574. [Google Scholar] [CrossRef]
- Allen, E.; Miéville, P.; Warren, C.M.; Saghafinia, S.; Li, L.; Peng, M.-W.; Hanahan, D. Metabolic Symbiosis Enables Adaptive Resistance to Anti-Angiogenic Therapy That Is Dependent on MTOR Signaling. Cell Rep. 2016, 15, 1144–1160. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate Influx through the Endothelial Cell Monocarboxylate Transporter MCT1 Supports an NF-ΚB/IL-8 Pathway That Drives Tumor Angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Cantelmo, A.R.; Conradi, L.-C.; Brajic, A.; Goveia, J.; Kalucka, J.; Pircher, A.; Chaturvedi, P.; Hol, J.; Thienpont, B.; Teuwen, L.-A.; et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell 2016, 30, 968–985. [Google Scholar] [CrossRef]
- Maione, F.; Oliaro-Bosso, S.; Meda, C.; Di Nicolantonio, F.; Bussolino, F.; Balliano, G.; Viola, F.; Giraudo, E. The Cholesterol Biosynthesis Enzyme Oxidosqualene Cyclase Is a New Target to Impair Tumour Angiogenesis and Metastasis Dissemination. Sci. Rep. 2015, 5, 9054. [Google Scholar] [CrossRef] [Green Version]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; D’Ascenzo, S.; Giusti, I.; Marchetti, D.; Borsotti, P.; Millimaggi, D.; Giavazzi, R.; Pavan, A.; Dolo, V. Bioavailability of VEGF in Tumor-Shed Vesicles Depends on Vesicle Burst Induced by Acidic PH. Neoplasia 2006, 8, 96–103. [Google Scholar] [CrossRef]
- Caslin, H.L.; Abebayehu, D.; Pinette, J.A.; Ryan, J.J. Lactate Is a Metabolic Mediator That Shapes Immune Cell Fate and Function. Front. Physiol. 2021, 12, 688485. [Google Scholar] [CrossRef]
- Xia, H.; Green, D.R.; Zou, W. Autophagy in Tumour Immunity and Therapy. Nat. Rev. Cancer 2021, 21, 281–297. [Google Scholar] [CrossRef]
- Oshi, M.; Newman, S.; Tokumaru, Y.; Yan, L.; Matsuyama, R.; Endo, I.; Nagahashi, M.; Takabe, K. Intra-Tumoral Angiogenesis Is Associated with Inflammation, Immune Reaction and Metastatic Recurrence in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 6708. [Google Scholar] [CrossRef]
- Wyckoff, J.; Wang, W.; Lin, E.Y.; Wang, Y.; Pixley, F.; Stanley, E.R.; Graf, T.; Pollard, J.W.; Segall, J.; Condeelis, J. A Paracrine Loop between Tumor Cells and Macrophages Is Required for Tumor Cell Migration in Mammary Tumors. Cancer Res. 2004, 64, 7022–7029. [Google Scholar] [CrossRef]
- Wyckoff, J.B.; Wang, Y.; Lin, E.Y.; Li, J.; Goswami, S.; Stanley, E.R.; Segall, J.E.; Pollard, J.W.; Condeelis, J. Direct Visualization of Macrophage-Assisted Tumor Cell Intravasation in Mammary Tumors. Cancer Res. 2007, 67, 2649–2656. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.H.-F.; Massagué, J. Macrophage Binding to Receptor VCAM-1 Transmits Survival Signals in Breast Cancer Cells That Invade the Lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [Green Version]
- Kaczanowska, S.; Beury, D.W.; Gopalan, V.; Tycko, A.K.; Qin, H.; Clements, M.E.; Drake, J.; Nwanze, C.; Murgai, M.; Rae, Z.; et al. Genetically Engineered Myeloid Cells Rebalance the Core Immune Suppression Program in Metastasis. Cell 2021, 184, 2033–2052.e21. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune Surveillance Properties of Human NK Cell-Derived Exosomes. J. Immunol. Baltim. Md 1950 2012, 189, 2833–2842. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting Metastatic Cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Azimi, M.S.; Lacey, M.; Mondal, D.; Murfee, W.L. An Ex Vivo Tissue Culture Model for Anti-Angiogenic Drug Testing. Methods Mol. Biol. Clifton NJ 2016, 1464, 85–95. [Google Scholar] [CrossRef]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of Perfused, Functional Microvascular Tubes in Vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef]
- Bischel, L.L.; Lee, S.-H.; Beebe, D.J. A Practical Method for Patterning Lumens through ECM Hydrogels via Viscous Finger Patterning. J. Lab. Autom. 2012, 17, 96–103. [Google Scholar] [CrossRef]
- Delannoy, E.; Tellier, G.; Cholet, J.; Leroy, A.M.; Treizebré, A.; Soncin, F. Multi-Layered Human Blood Vessels-on-Chip Design Using Double Viscous Finger Patterning. Biomedicines 2022, 10, 797. [Google Scholar] [CrossRef]
- Pauty, J.; Usuba, R.; Cheng, I.G.; Hespel, L.; Takahashi, H.; Kato, K.; Kobayashi, M.; Nakajima, H.; Lee, E.; Yger, F.; et al. A Vascular Endothelial Growth Factor-Dependent Sprouting Angiogenesis Assay Based on an In Vitro Human Blood Vessel Model for the Study of Anti-Angiogenic Drugs. EBioMedicine 2018, 27, 225–236. [Google Scholar] [CrossRef]
- Usuba, R.; Pauty, J.; Soncin, F.; Matsunaga, Y.T. EGFL7 Regulates Sprouting Angiogenesis and Endothelial Integrity in a Human Blood Vessel Model. Biomaterials 2019, 197, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, M.; Hoffman, L.M.; Jensen, C.C.; Yost, H.J.; Beckerle, M.C. Mechanical Force Mobilizes Zyxin from Focal Adhesions to Actin Filaments and Regulates Cytoskeletal Reinforcement. J. Cell Biol. 2005, 171, 209–215. [Google Scholar] [CrossRef]
- Song, J.W.; Munn, L.L. Fluid Forces Control Endothelial Sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 15342–15347. [Google Scholar] [CrossRef]
- Akbari, E.; Spychalski, G.B.; Rangharajan, K.K.; Prakash, S.; Song, J.W. Competing Fluid Forces Control Endothelial Sprouting in a 3-D Microfluidic Vessel Bifurcation Model. Micromachines 2019, 10, 451. [Google Scholar] [CrossRef]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-Chip Platform to Investigate Progression and Drug Sensitivity in Cell Lines and Patient-Derived Organoids. Lab. Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.F.; Ingram, M.; et al. Quantitative Prediction of Human Pharmacokinetic Responses to Drugs via Fluidically Coupled Vascularized Organ Chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef]
- Vandenhaute, E.; Drolez, A.; Sevin, E.; Gosselet, F.; Mysiorek, C.; Dehouck, M.-P. Adapting Coculture in Vitro Models of the Blood-Brain Barrier for Use in Cancer Research: Maintaining an Appropriate Endothelial Monolayer for the Assessment of Transendothelial Migration. Lab. Investig. J. Tech. Methods Pathol. 2016, 96, 588–598. [Google Scholar] [CrossRef]
- Deligne, C.; Hachani, J.; Duban-Deweer, S.; Meignan, S.; Leblond, P.; Carcaboso, A.M.; Sano, Y.; Shimizu, F.; Kanda, T.; Gosselet, F.; et al. Development of a Human in Vitro Blood-Brain Tumor Barrier Model of Diffuse Intrinsic Pontine Glioma to Better Understand the Chemoresistance. Fluids Barriers CNS 2020, 17, 37. [Google Scholar] [CrossRef]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-Enhanced Blood-Brain Barrier Chip Recapitulates Human Barrier Function and Shuttling of Drugs and Antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.F.; Ingber, D.E. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H.; et al. A Bioprinted Human-Glioblastoma-on-a-Chip for the Identification of Patient-Specific Responses to Chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef]
- Arts, J.J.; Mahlandt, E.K.; Grönloh, M.L.; Schimmel, L.; Noordstra, I.; Gordon, E.; van Steen, A.C.; Tol, S.; Walzog, B.; van Rijssel, J.; et al. Endothelial Junctional Membrane Protrusions Serve as Hotspots for Neutrophil Transmigration. eLife 2021, 10, e66074. [Google Scholar] [CrossRef]
- Riddle, R.B.; Jennbacken, K.; Hansson, K.M.; Harper, M.T. Endothelial Inflammation and Neutrophil Transmigration Are Modulated by Extracellular Matrix Composition in an Inflammation-on-a-Chip Model. Sci. Rep. 2022, 12, 6855. [Google Scholar] [CrossRef]
- Um, E.; Oh, J.M.; Park, J.; Song, T.; Kim, T.-E.; Choi, Y.; Shin, C.; Kolygina, D.; Jeon, J.-H.; Grzybowski, B.A.; et al. Immature Dendritic Cells Navigate Microscopic Mazes to Find Tumor Cells. Lab. Chip 2019, 19, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Surendran, V.; Rutledge, D.; Colmon, R.; Chandrasekaran, A. A Novel Tumor-Immune Microenvironment (TIME)-on-Chip Mimics Three Dimensional Neutrophil-Tumor Dynamics and Neutrophil Extracellalar Traps (NETs)- Mediated Collective Tumor Invasion. Biofabrication 2021, 13, 035029. [Google Scholar] [CrossRef]
- Cui, X.; Ma, C.; Vasudevaraja, V.; Serrano, J.; Tong, J.; Peng, Y.; Delorenzo, M.; Shen, G.; Frenster, J.; Morales, R.-T.T.; et al. Dissecting the Immunosuppressive Tumor Microenvironments in Glioblastoma-on-a-Chip for Optimized PD-1 Immunotherapy. eLife 2020, 9, e52253. [Google Scholar] [CrossRef]
- Cui, X.; Morales, R.-T.T.; Qian, W.; Wang, H.; Gagner, J.-P.; Dolgalev, I.; Placantonakis, D.; Zagzag, D.; Cimmino, L.; Snuderl, M.; et al. Hacking Macrophage-Associated Immunosuppression for Regulating Glioblastoma Angiogenesis. Biomaterials 2018, 161, 164–178. [Google Scholar] [CrossRef]
- Ando, Y.; Siegler, E.L.; Ta, H.P.; Cinay, G.E.; Zhou, H.; Gorrell, K.A.; Au, H.; Jarvis, B.M.; Wang, P.; Shen, K. Evaluating CAR-T Cell Therapy in a Hypoxic 3D Tumor Model. Adv. Healthc. Mater. 2019, 8, 1900001. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Rehman, S.; Virumbrales-Munoz, M.; McMinn, P.H.; Geiger, P.; Fitzgerald, C.; Heaster, T.; Skala, M.C.; Beebe, D.J. Microfluidic Tumor-on-a-Chip Model to Evaluate the Role of Tumor Environmental Stress on NK Cell Exhaustion. Sci. Adv. 2021, 7, eabc2331. [Google Scholar] [CrossRef] [PubMed]
- Maulana, T.I.; Kromidas, E.; Wallstabe, L.; Cipriano, M.; Alb, M.; Zaupa, C.; Hudecek, M.; Fogal, B.; Loskill, P. Immunocompetent Cancer-on-Chip Models to Assess Immuno-Oncology Therapy. Adv. Drug Deliv. Rev. 2021, 173, 281–305. [Google Scholar] [CrossRef]
- Hajal, C.; Ibrahim, L.; Serrano, J.C.; Offeddu, G.S.; Kamm, R.D. The Effects of Luminal and Trans-Endothelial Fluid Flows on the Extravasation and Tissue Invasion of Tumor Cells in a 3D in Vitro Microvascular Platform. Biomaterials 2021, 265, 120470. [Google Scholar] [CrossRef]
- Song, J.; Miermont, A.; Lim, C.T.; Kamm, R.D. A 3D Microvascular Network Model to Study the Impact of Hypoxia on the Extravasation Potential of Breast Cell Lines. Sci. Rep. 2018, 8, 17949. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Whisler, J.A.; Fröse, J.; Yu, C.; Shin, Y.; Kamm, R.D. On-Chip Human Microvasculature Assay for Visualization and Quantitation of Tumor Cell Extravasation Dynamics. Nat. Protoc. 2017, 12, 865–880. [Google Scholar] [CrossRef]
- Chen, M.B.; Hajal, C.; Benjamin, D.C.; Yu, C.; Azizgolshani, H.; Hynes, R.O.; Kamm, R.D. Inflamed Neutrophils Sequestered at Entrapped Tumor Cells via Chemotactic Confinement Promote Tumor Cell Extravasation. Proc. Natl. Acad. Sci. USA 2018, 115, 7022–7027. [Google Scholar] [CrossRef]
- Mi, S.; Liu, Z.; Du, Z.; Yi, X.; Sun, W. Three-Dimensional Microfluidic Tumor-Macrophage System for Breast Cancer Cell Invasion. Biotechnol. Bioeng. 2019, 116, 1731–1741. [Google Scholar] [CrossRef]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-Dimensional Microfluidic Model for Tumor Cell Intravasation and Endothelial Barrier Function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef]
- Zhang, X.; Karim, M.; Hasan, M.M.; Hooper, J.; Wahab, R.; Roy, S.; Al-Hilal, T.A. Cancer-on-a-Chip: Models for Studying Metastasis. Cancers 2022, 14, 648. [Google Scholar] [CrossRef]
- Method of the Year 2017: Organoids. Nat. Methods 2018, 15, 1. [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Burdett, E.; Kasper, F.K.; Mikos, A.G.; Ludwig, J.A. Engineering Tumors: A Tissue Engineering Perspective in Cancer Biology. Tissue Eng. Part B Rev. 2010, 16, 351–359. [Google Scholar] [CrossRef]
- Young, J.L.; Holle, A.W.; Spatz, J.P. Nanoscale and Mechanical Properties of the Physiological Cell-ECM Microenvironment. Exp. Cell Res. 2016, 343, 3–6. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Anderson, S.M.; Siegman, S.N.; Segura, T. The Effect of Vascular Endothelial Growth Factor (VEGF) Presentation within Fibrin Matrices on Endothelial Cell Branching. Biomaterials 2011, 32, 7432–7443. [Google Scholar] [CrossRef]
- Wörsdörfer, P.; Dalda, N.; Kern, A.; Krüger, S.; Wagner, N.; Kwok, C.K.; Henke, E.; Ergün, S. Generation of Complex Human Organoid Models Including Vascular Networks by Incorporation of Mesodermal Progenitor Cells. Sci. Rep. 2019, 9, 15663. [Google Scholar] [CrossRef]
- Barrett, J.M.; Mangold, K.A.; Jilling, T.; Kaul, K.L. Bi-Directional Interactions of Prostate Cancer Cells and Bone Marrow Endothelial Cells in Three-Dimensional Culture. Prostate 2005, 64, 75–82. [Google Scholar] [CrossRef]
- Furlan, A.; Vercamer, C.; Heliot, L.; Wernert, N.; Desbiens, X.; Pourtier, A. Ets-1 Drives Breast Cancer Cell Angiogenic Potential and Interactions between Breast Cancer and Endothelial Cells. Int. J. Oncol. 2018, 54, 29–40. [Google Scholar] [CrossRef]
- Bahary, N.; Zon, L.I. Development. Endothelium--Chicken Soup for the Endoderm. Science 2001, 294, 530–531. [Google Scholar] [CrossRef]
- Lammert, E.; Cleaver, O.; Melton, D. Induction of Pancreatic Differentiation by Signals from Blood Vessels. Science 2001, 294, 564–567. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, W.; Zhao, Y.; Yang, Y.; Luo, H.; Ji, G.; Dong, E.; Deng, H.; Lin, S.; Wei, Y.; et al. Endothelial Cords Promote Tumor Initial Growth Prior to Vascular Function through a Paracrine Mechanism. Sci. Rep. 2016, 6, 19404. [Google Scholar] [CrossRef] [PubMed]
- Augustine, T.N. Analysis of Immune-Tumor Cell Interactions Using a 3D Co-Culture Model. Methods Mol. Biol. Clifton NJ 2020, 2184, 103–110. [Google Scholar] [CrossRef]
- DelNero, P.; Lane, M.; Verbridge, S.S.; Kwee, B.; Kermani, P.; Hempstead, B.; Stroock, A.; Fischbach, C. 3D Culture Broadly Regulates Tumor Cell Hypoxia Response and Angiogenesis via Pro-Inflammatory Pathways. Biomaterials 2015, 55, 110–118. [Google Scholar] [CrossRef]
- Lim, J.T.C.; Kwang, L.G.; Ho, N.C.W.; Toh, C.C.M.; Too, N.S.H.; Hooi, L.; Benoukraf, T.; Chow, P.K.-H.; Dan, Y.Y.; Chow, E.K.-H.; et al. Hepatocellular Carcinoma Organoid Co-Cultures Mimic Angiocrine Crosstalk to Generate Inflammatory Tumor Microenvironment. Biomaterials 2022, 284, 121527. [Google Scholar] [CrossRef]
- Chirivì, M.; Maiullari, F.; Milan, M.; Presutti, D.; Cordiglieri, C.; Crosti, M.; Sarnicola, M.L.; Soluri, A.; Volpi, M.; Święszkowski, W.; et al. Tumor Extracellular Matrix Stiffness Promptly Modulates the Phenotype and Gene Expression of Infiltrating T Lymphocytes. Int. J. Mol. Sci. 2021, 22, 5862. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Tian, Q.; Zhou, Y.; Zhu, L.; Lu, Y.; Ma, Y.; Feng, J.; Jiang, Y.; Wang, B. 3D Collagen Fiber Concentration Regulates Treg Cell Infiltration in Triple Negative Breast Cancer. Front. Immunol. 2022, 13, 904418. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.S.; Knútsdóttir, H.; Ramakrishnan, G.; Padmanaban, V.; Warrier, M.; Ramirez, J.C.; Dunworth, M.; Zhang, H.; Jaffee, E.M.; Bader, J.S.; et al. Cancer Cells Educate Natural Killer Cells to a Metastasis-Promoting Cell State. J. Cell Biol. 2020, 219, e202001134. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Berthier, E.; Craig, A.; de Groot, T.E.; Sparks, S.; Ingram, P.N.; Jarrard, D.F.; Huang, W.; Beebe, D.J.; Theberge, A.B. Reconfigurable Open Microfluidics for Studying the Spatiotemporal Dynamics of Paracrine Signalling. Nat. Biomed. Eng. 2019, 3, 830–841. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-Culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef]
- Zhou, G.; Lieshout, R.; van Tienderen, G.S.; de Ruiter, V.; van Royen, M.E.; Boor, P.P.C.; Magré, L.; Desai, J.; Köten, K.; Kan, Y.Y.; et al. Modelling Immune Cytotoxicity for Cholangiocarcinoma with Tumour-Derived Organoids and Effector T Cells. Br. J. Cancer 2022, 127, 649–660. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.P.; Pinto, C.; Martins, T.R.; Harrer, N.; Estrada, M.F.; Loza-Alvarez, P.; Cabeçadas, J.; Alves, P.M.; Gualda, E.J.; Sommergruber, W.; et al. 3D-3-Culture: A Tool to Unveil Macrophage Plasticity in the Tumour Microenvironment. Biomaterials 2018, 163, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Wu, Z.; Cai, H.; Hu, L.; Li, X.; Kaurich, C.; Chang, J.; Gu, M.; Liang, C.; Lu, X.; et al. Rapid Profiling of Tumor-Immune Interaction Using Acoustically Assembled Patient-Derived Cell Clusters. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, e2201478. [Google Scholar] [CrossRef] [PubMed]
- Pourtier-Manzanedo, A.; Vercamer, C.; Van Belle, E.; Mattot, V.; Mouquet, F.; Vandenbunder, B. Expression of an Ets-1 Dominant-Negative Mutant Perturbs Normal and Tumor Angiogenesis in a Mouse Ear Model. Oncogene 2003, 22, 1795–1806. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of Blood Vessel Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef]
- Shin, N.; Kim, Y.; Ko, J.; Choi, S.W.; Hyung, S.; Lee, S.-E.; Park, S.; Song, J.; Jeon, N.L.; Kang, K.-S. Vascularization of INSC Spheroid in a 3D Spheroid-on-a-Chip Platform Enhances Neural Maturation. Biotechnol. Bioeng. 2021, 119, 566–574. [Google Scholar] [CrossRef]
- Ahn, Y.; An, J.-H.; Yang, H.-J.; Lee, D.G.; Kim, J.; Koh, H.; Park, Y.-H.; Song, B.-S.; Sim, B.-W.; Lee, H.J.; et al. Human Blood Vessel Organoids Penetrate Human Cerebral Organoids and Form a Vessel-Like System. Cells 2021, 10, 2036. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid Casting of Patterned Vascular Networks for Perfusable Engineered Three-Dimensional Tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Lai, B.F.L.; Lu, R.X.Z.; Davenport Huyer, L.; Kakinoki, S.; Yazbeck, J.; Wang, E.Y.; Wu, Q.; Zhang, B.; Radisic, M. A Well Plate-Based Multiplexed Platform for Incorporation of Organoids into an Organ-on-a-Chip System with a Perfusable Vasculature. Nat. Protoc. 2021, 16, 2158–2189. [Google Scholar] [CrossRef]
- Andrique, L.; Recher, G.; Alessandri, K.; Pujol, N.; Feyeux, M.; Bon, P.; Cognet, L.; Nassoy, P.; Bikfalvi, A. A Model of Guided Cell Self-Organization for Rapid and Spontaneous Formation of Functional Vessels. Sci. Adv. 2019, 5, eaau6562. [Google Scholar] [CrossRef] [Green Version]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sekine, K.; Kin, T.; Takebe, T.; Taniguchi, H. Self-Condensation Culture Enables Vascularization of Tissue Fragments for Efficient Therapeutic Transplantation. Cell Rep. 2018, 23, 1620–1629. [Google Scholar] [CrossRef]
- Palikuqi, B.; Nguyen, D.-H.T.; Li, G.; Schreiner, R.; Pellegata, A.F.; Liu, Y.; Redmond, D.; Geng, F.; Lin, Y.; Gómez-Salinero, J.M.; et al. Adaptable Haemodynamic Endothelial Cells for Organogenesis and Tumorigenesis. Nature 2020, 585, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M. Tumors’ Do-It-Yourself Blood Vessels. Science 2016, 352, 1381–1383. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, V.L.; Henriet, E.; Linville, R.M.; Wong, A.D.; Searson, P.C.; Ewald, A.J. A Tissue-Engineered 3D Microvessel Model Reveals the Dynamics of Mosaic Vessel Formation in Breast Cancer. Cancer Res. 2020, 80, 4288–4301. [Google Scholar] [CrossRef]
- Landau, S.; Newman, A.; Edri, S.; Michael, I.; Ben-Shaul, S.; Shandalov, Y.; Ben-Arye, T.; Kaur, P.; Zheng, M.H.; Levenberg, S. Investigating Lymphangiogenesis in Vitro and in Vivo Using Engineered Human Lymphatic Vessel Networks. Proc. Natl. Acad. Sci. USA 2021, 118, e2101931118. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial Activation and Dysfunction in COVID-19: From Basic Mechanisms to Potential Therapeutic Approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial Dysfunction and Immunothrombosis as Key Pathogenic Mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Leiva, O.; Newcomb, R.; Connors, J.M.; Al-Samkari, H. Cancer and Thrombosis: New Insights to an Old Problem. J. Med. Vasc. 2020, 45, 6S8–6S16. [Google Scholar] [CrossRef]
- Gaertner, F.; Massberg, S. Patrolling the Vascular Borders: Platelets in Immunity to Infection and Cancer. Nat. Rev. Immunol. 2019, 19, 747–760. [Google Scholar] [CrossRef]
- Pandita, A.; Ekstrand, M.; Bjursten, S.; Zhao, Z.; Fogelstrand, P.; Le Gal, K.; Ny, L.; Bergo, M.O.; Karlsson, J.; Nilsson, J.A.; et al. Intussusceptive Angiogenesis in Human Metastatic Malignant Melanoma. Am. J. Pathol. 2021, 191, 2023–2038. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; De Rooij, L.P.M.H.; Cuypers, A.; Rohlenova, K.; Dumas, S.J.; García-Caballero, M.; Meta, E.; Amersfoort, J.; Taverna, F.; Becker, L.M.; et al. Tumor Vessel Co-Option Probed by Single-Cell Analysis. Cell Rep. 2021, 35, 109253. [Google Scholar] [CrossRef]
- Brown, J.M. Vasculogenesis: A Crucial Player in the Resistance of Solid Tumours to Radiotherapy. Br. J. Radiol. 2014, 87, 20130686. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Troiani, T.; Criscuolo, G.; Marone, G.; Ciardiello, F.; Tocchetti, C.G.; Varricchi, G. Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events. Front. Immunol. 2022, 13, 804597. [Google Scholar] [CrossRef]
- Wagner, D.L.; Fritsche, E.; Pulsipher, M.A.; Ahmed, N.; Hamieh, M.; Hegde, M.; Ruella, M.; Savoldo, B.; Shah, N.N.; Turtle, C.J.; et al. Immunogenicity of CAR T Cells in Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 379–393. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P.; et al. Combined Antiangiogenic and Anti-PD-L1 Therapy Stimulates Tumor Immunity through HEV Formation. Sci. Transl. Med. 2017, 9, eaak9679. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrassoux, L.; Claux, H.; Bacari, S.; Meignan, S.; Furlan, A. A Bloody Conspiracy— Blood Vessels and Immune Cells in the Tumor Microenvironment. Cancers 2022, 14, 4581. https://doi.org/10.3390/cancers14194581
Terrassoux L, Claux H, Bacari S, Meignan S, Furlan A. A Bloody Conspiracy— Blood Vessels and Immune Cells in the Tumor Microenvironment. Cancers. 2022; 14(19):4581. https://doi.org/10.3390/cancers14194581
Chicago/Turabian StyleTerrassoux, Lisa, Hugo Claux, Salimata Bacari, Samuel Meignan, and Alessandro Furlan. 2022. "A Bloody Conspiracy— Blood Vessels and Immune Cells in the Tumor Microenvironment" Cancers 14, no. 19: 4581. https://doi.org/10.3390/cancers14194581





