Altered Plasma, Urine, and Tissue Profiles of Sulfatides and Sphingomyelins in Patients with Renal Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
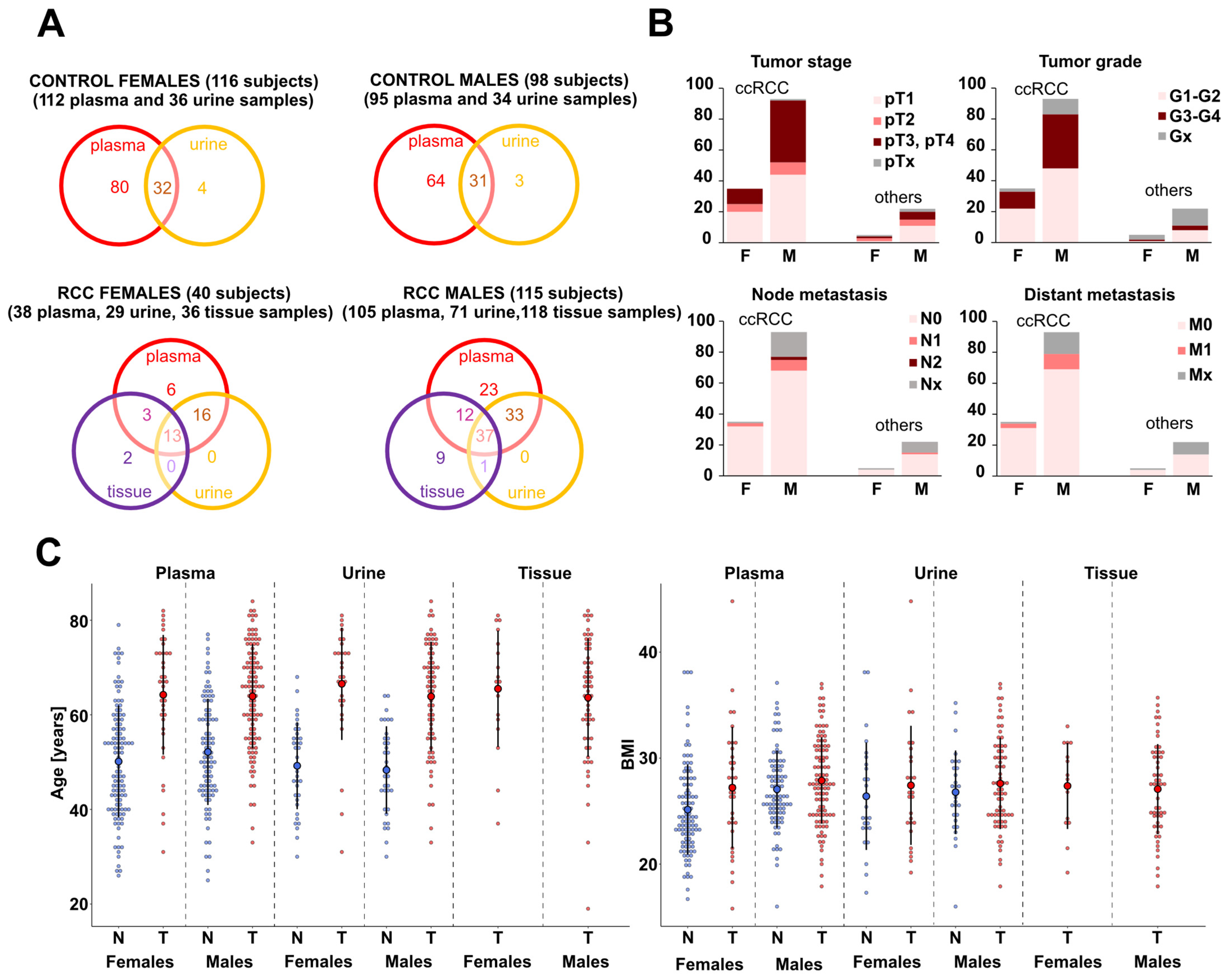
2.1. Patients and Sample Collection
2.2. Sample Processing
2.3. Mass Spectrometry Analysis
2.4. Data Processing
2.5. Statistical Data Analysis
3. Results
3.1. Study Design and Analytical Validation
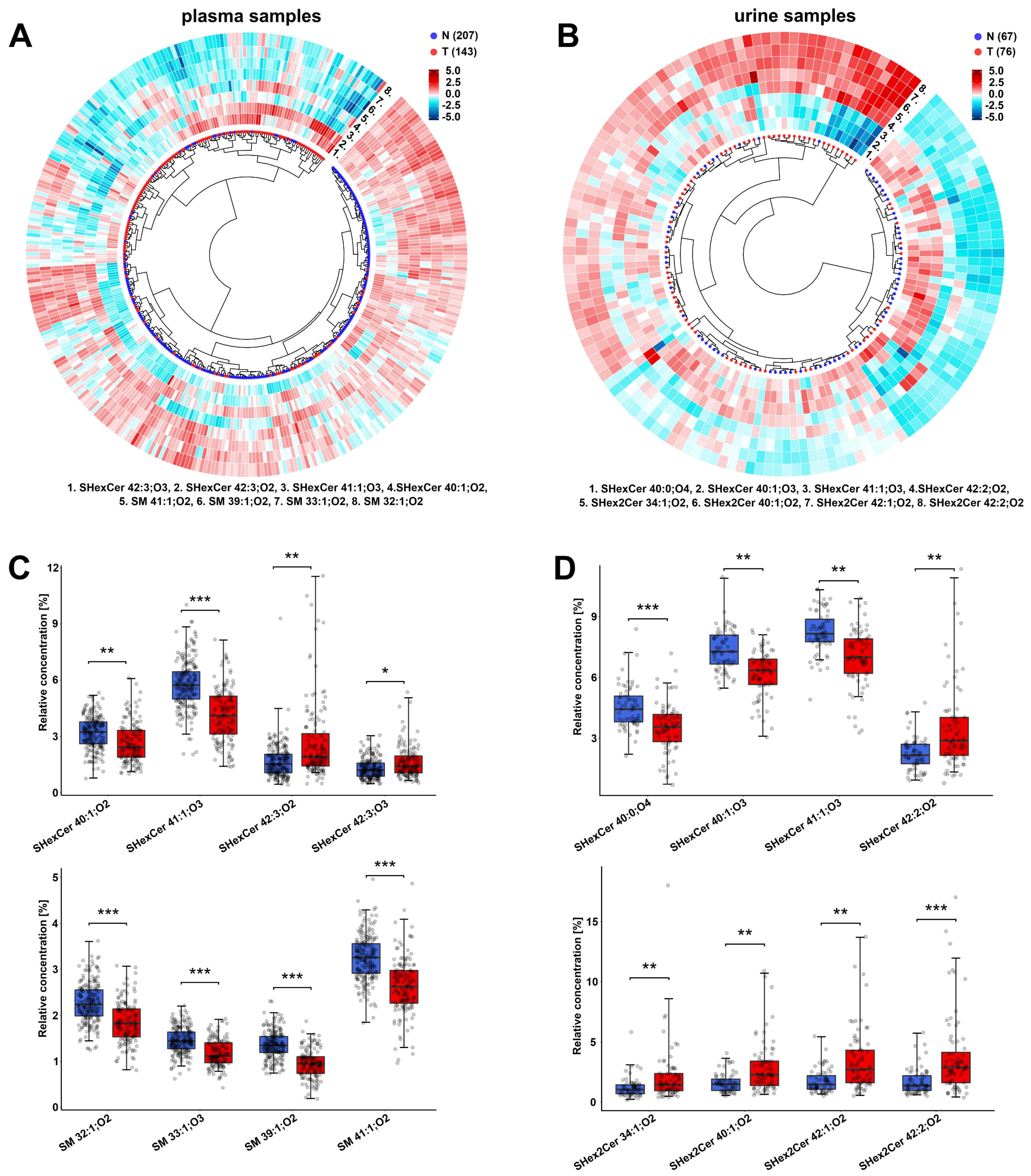
3.2. Alterations in the Plasma Lipid Profiles of Cancer Patients
3.3. Alterations in the Urine Lipid Profiles of Cancer Patients
3.4. Different Sulfatide and Sphingomyelin Levels in Cancer Patients by Advanced Tumor Stage and Grade
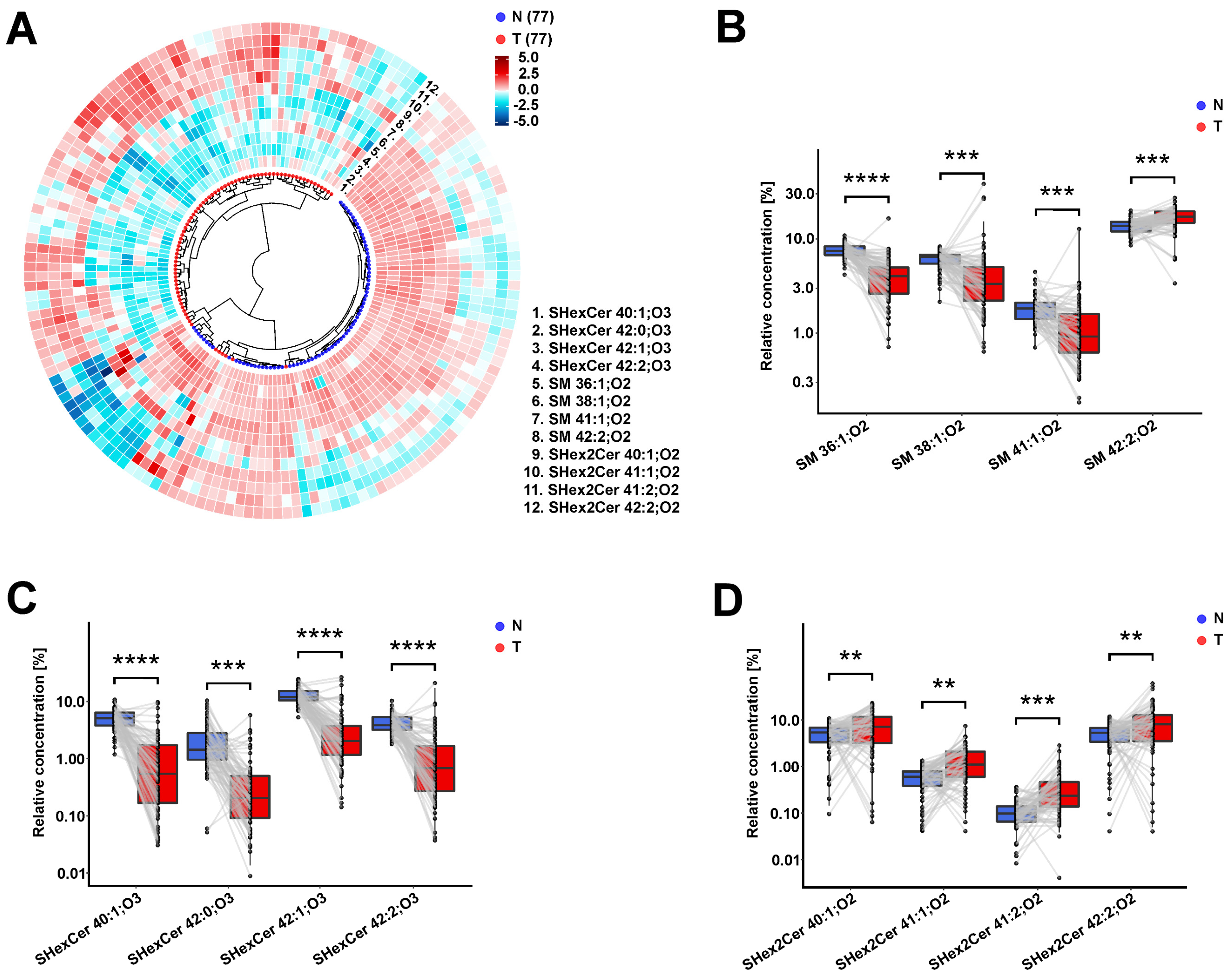
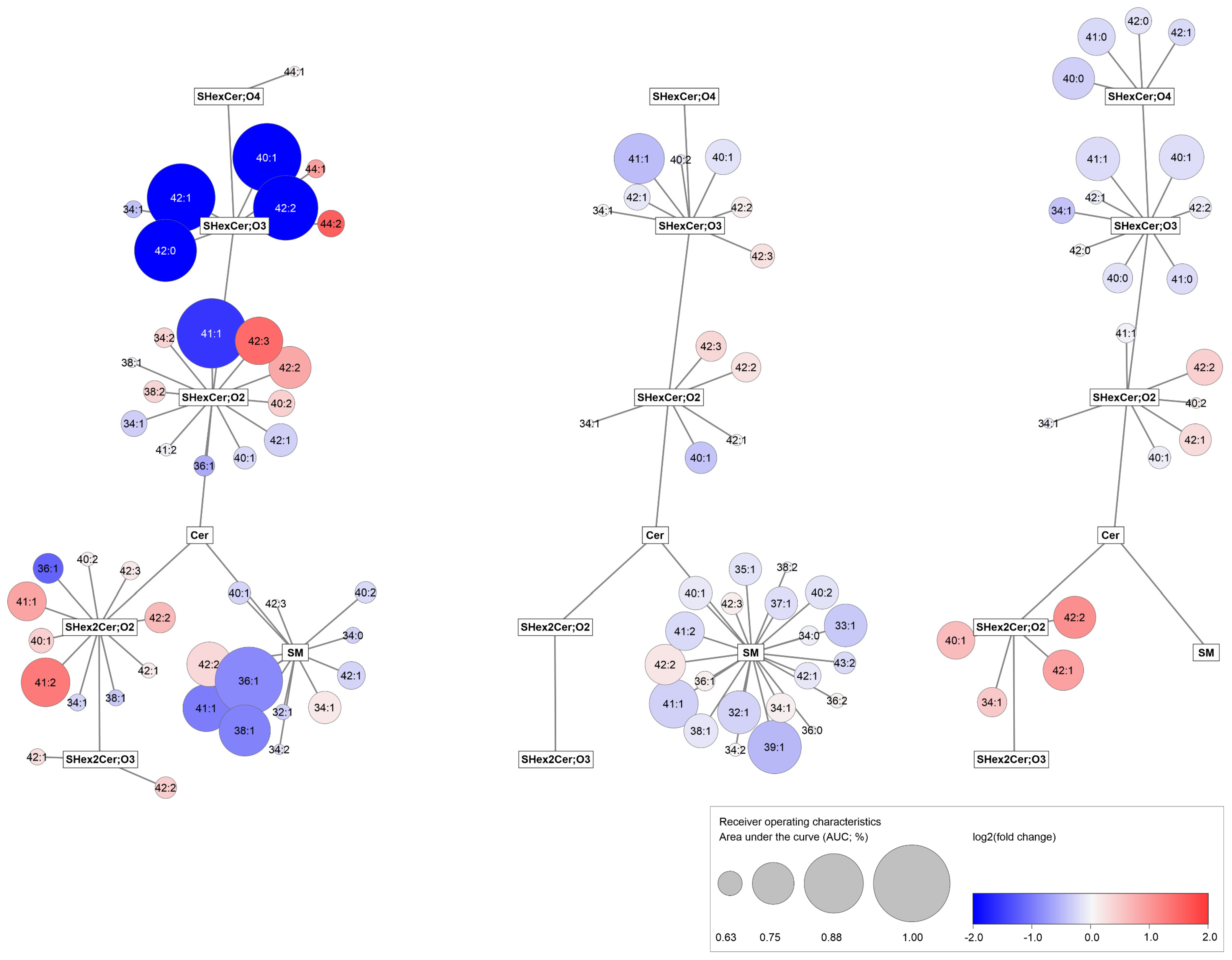
3.5. Observed Sulfatide and Sphingomyelin Dysregulations in RCC Patients Are Similar in Body Fluids and Tumor Tissues
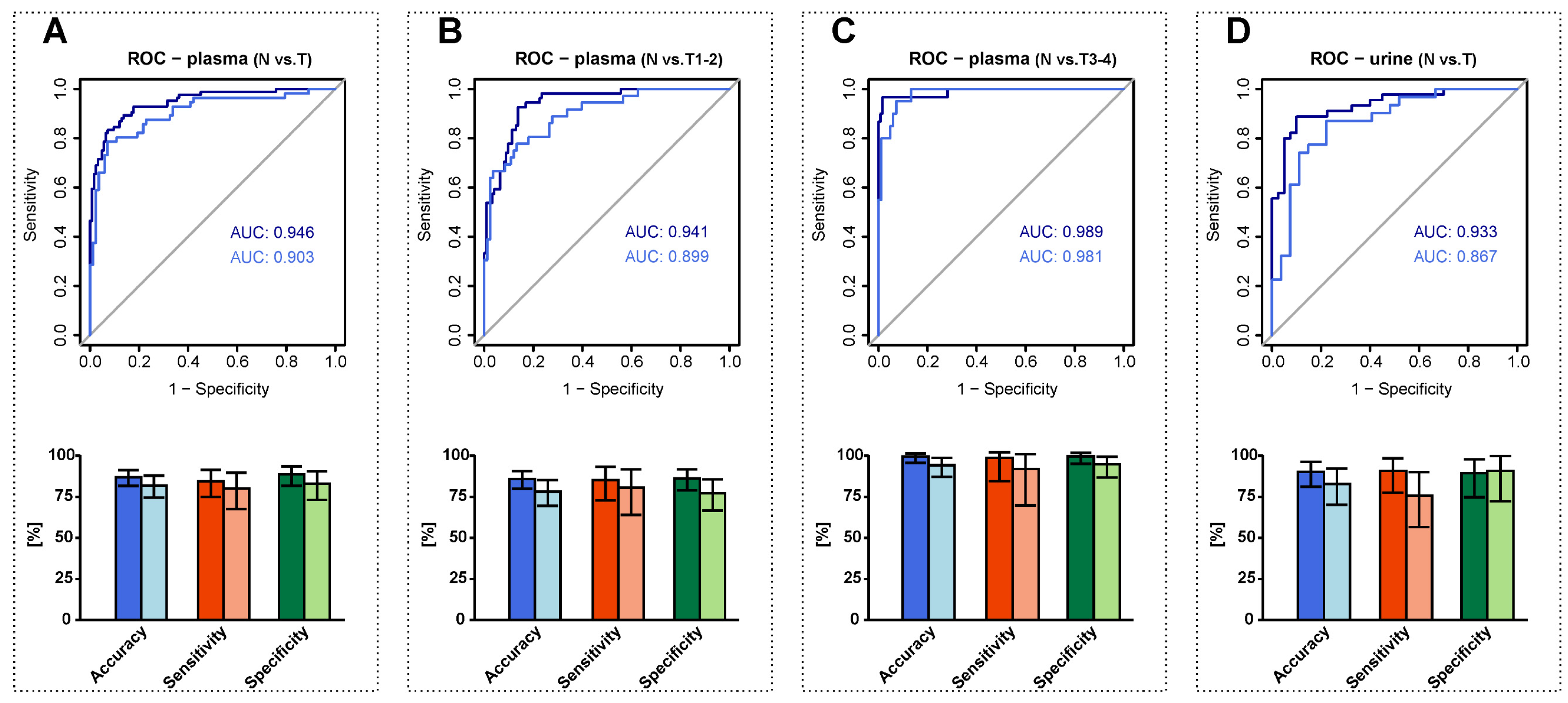
3.6. RCC Detection Based on Alterations in Plasma and Urine Sphingolipid Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Znaor, A.; Holcatova, I.; Fabianova, E.; Mates, D.; Wozniak, M.B.; Ferlay, J.; Scelo, G. Regional Geographic Variations in Kidney Cancer Incidence Rates in European Countries. Eur. Urol. 2015, 67, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Cairns, P. Renal cell carcinoma. Cancer Biomark. 2010, 9, 461–473. [Google Scholar] [CrossRef]
- National Cancer Institute. Tumor Markers. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-list (accessed on 29 July 2022).
- Wolrab, D.; Jirásko, R.; Chocholoušková, M.; Peterka, O.; Holčapek, M. Oncolipidomics: Mass spectrometric quantitation of lipids in cancer research. Trac-Trends Anal. Chem. 2019, 120, 115480. [Google Scholar] [CrossRef]
- Schaeffeler, E.; Büttner, F.; Reustle, A.; Klumpp, V.; Winter, S.; Rausch, S.; Fisel, P.; Hennenlotter, J.; Kruck, S.; Stenzl, A.; et al. Metabolic and Lipidomic Reprogramming in Renal Cell Carcinoma Subtypes Reflects Regions of Tumor Origin. Eur. Urol. Focus 2019, 5, 608–618. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Wolrab, D.; Jirásko, R.; Cífková, E.; Höring, M.; Mei, D.; Chocholoušková, M.; Peterka, O.; Idkowiak, J.; Hrnčiarová, T.; Kuchař, L.; et al. Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat. Commun. 2022, 13, 124. [Google Scholar] [CrossRef]
- Wolrab, D.; Jirásko, R.; Peterka, O.; Idkowiak, J.; Chocholoušková, M.; Vaňková, Z.; Hořejší, K.; Brabcová, I.; Vrána, D.; Študentová, H.; et al. Plasma lipidomic profiles of kidney, breast and prostate cancer patients differ from healthy controls. Sci. Rep. 2021, 11, 20322. [Google Scholar] [CrossRef]
- Porubsky, S.; Nientiedt, M.; Kriegmair, M.C.; Siemoneit, J.H.H.; Sandhoff, R.; Jennemann, R.; Borgmann, H.; Gaiser, T.; Weis, C.A.; Erben, P.; et al. The prognostic value of galactosylceramide-sulfotransferase (Gal3ST1) in human renal cell carcinoma. Sci. Rep. 2021, 11, 10926. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Suzuki, T. Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.C.; Bang, G.; Lee, J.H.; Kim, K.P.; Kim, Y.H.; Kim, H.K.; Chung, J. Low C24-OH and C22-OH sulfatides in human renal cell carcinoma. J. Mass Spectrom. 2014, 49, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.M.; Fathalla, M.M.; Zakhary, M.A.; Makarem, M.H. Sulfatides in ovarian tumors: Clinicopathological correlates. Int. J. Gynecol. Cancer 2004, 14, 89–93. [Google Scholar] [CrossRef]
- Morichika, H.; Hamanaka, Y.; Tai, T.; Ishizuka, I. Sulfatides as a predictive factor of lymph node metastasis in patients with colorectal adenocarcinoma. Cancer 1996, 78, 43–47. [Google Scholar] [CrossRef]
- Sakakibara, N.; Gasa, S.; Kamio, K.; Makita, A.; Koyanagi, T. Association of Elevated Sulfatides and Sulfotransferase Activities with Human Renal-Cell Carcinoma. Cancer Res. 1989, 49, 335–339. [Google Scholar]
- Tanaka, K.; Mikami, M.; Aoki, D.; Kiguchi, K.; Ishiwata, I.; Iwamori, M. Expression of sulfatide and sulfated lactosylceramide among histological types of human ovarian carcinomas. Hum. Cell 2015, 28, 37–43. [Google Scholar] [CrossRef]
- Jirásko, R.; Holčapek, M.; Khalikova, M.; Vrána, D.; Študent, V.; Prouzová, Z.; Melichar, B. MALDI Orbitrap Mass Spectrometry Profiling of Dysregulated Sulfoglycosphingolipids in Renal Cell Carcinoma Tissues. J. Am. Soc. Mass Spectrom. 2017, 28, 1562–1574. [Google Scholar] [CrossRef]
- Honke, K.; Tsuda, M.; Hirahara, Y.; Miyao, N.; Tsukamoto, T.; Satoh, M.; Wada, Y. Cancer-associated expression of glycolipid sulfotransferase gene in human renal cell carcinoma cells. Cancer Res. 1998, 58, 3800–3805. [Google Scholar]
- Kobayashi, T.; Honke, K.; Kamio, K.; Sakakibara, N.; Gasa, S.; Miyao, N.; Tsukamoto, T.; Ishizuka, I.; Miyazaki, T.; Makita, A. Sulfolipids and Glycolipid Sulfotransferase Activities in Human Renal-Cell Carcinoma-Cells. Br. J. Cancer 1993, 67, 76–80. [Google Scholar] [CrossRef]
- Wu, X.Z.; Honke, K.; Zhang, Y.L.; Zha, X.L.; Taniguchi, N. Lactosylsulfatide expression in hepatocellular carcinoma cells enhances cell adhesion to vitronectin and intrahepatic metastasis in nude mice. Int. J. Cancer 2004, 110, 504–510. [Google Scholar] [CrossRef]
- Hubert, M.; Vandervieren, E. An adjusted boxplot for skewed distributions. Comput. Stat. Data Anal. 2008, 52, 5186–5201. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Staging. Available online: https://www.cancer.org/treatment/understanding-your-diagnosis/staging.html (accessed on 29 July 2022).
- Lipid Maps. Lipid Classsification System. Available online: https://www.lipidmaps.org/data/classification/LM_classification_exp.php (accessed on 29 July 2022).
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Kofeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Vizcaino, J.A.; Kofeler, H.; Trotzmuller, M.; Griffiths, W.J.; Schmitz, G.; Spener, F.; Wakelam, M.J.O. Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 2013, 54, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.; Ogretmen, B. The Role of Ceramide Metabolism and Signaling in the Regulation of Mitophagy and Cancer Therapy. Cancers 2021, 13, 2475. [Google Scholar] [CrossRef]
- Pruett, S.T.; Bushnev, A.; Hagedorn, K.; Adiga, M.; Haynes, C.A.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H. Thematic review series: Sphingolipids—Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 2008, 49, 1621–1639. [Google Scholar] [CrossRef]
- Alderson, N.L.; Rembiesa, B.M.; Walla, M.D.; Bielawska, A.; Bielawski, J.; Hama, H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 2004, 279, 48562–48568. [Google Scholar] [CrossRef]
- Yao, Y.Z.; Yang, X.Q.; Sun, L.; Sun, S.S.; Huang, X.H.; Zhou, D.Y.; Li, T.T.; Zhang, W.; Abumrad, N.A.; Zhu, X.G.; et al. Fatty acid 2-hydroxylation inhibits tumor growth and increases sensitivity to cisplatin in gastric cancer. Ebiomedicine 2019, 41, 256–267. [Google Scholar] [CrossRef]
- Lemay, A.M.; Courtemanche, O.; Couttas, T.A.; Jamsari, G.; Gagne, A.; Bosse, Y.; Joubert, P.; Don, A.S.; Marsolais, D. High FA2H and UGT8 transcript levels predict hydroxylated hexosylceramide accumulation in lung adenocarcinoma. J. Lipid Res. 2019, 60, 1776–1786. [Google Scholar] [CrossRef]
- Qi, T.; Wu, D.D.; Duan, Z.P.; Chen, C.; Qiu, J.J.; Kang, J. Overexpression of Fatty Acid 2-Hydroxylase is Associated with an Increased Sensitivity to Cisplatin by Ovarian Cancer and Better Prognoses. Genet. Test. Mol. Biomark. 2020, 24, 632–640. [Google Scholar] [CrossRef]
- Sun, L.; Yang, X.Q.; Huang, X.H.; Yao, Y.Z.; Wei, X.Y.; Yang, S.G.; Zhou, D.Y.; Zhang, W.; Long, Z.M.; Xu, X.Y.; et al. 2-Hydroxylation of Fatty Acids Represses Colorectal Tumorigenesis and Metastasis via the YAP Transcriptional Axis. Cancer Res. 2021, 81, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.F.; Zhang, S.; Cheng, H.Y.; Cai, D.Y.; Chen, X.; Huang, Z.H. FA2H Exhibits Tumor Suppressive Roles on Breast Cancers via Cancer Stemness Control. Front. Oncol. 2019, 9, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.F.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef]
- Pandey, N.; Lanke, V.; Vinod, P.K. Network-based metabolic characterization of renal cell carcinoma. Sci. Rep. 2020, 10, 5955. [Google Scholar] [CrossRef]
- Shroff, E.H.; Eberlin, L.S.; Dang, V.M.; Gouw, A.M.; Gabay, M.; Adam, S.J.; Bellovin, D.I.; Tran, P.T.; Philbrick, W.M.; Garcia-Ocana, A.; et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 6539–6544. [Google Scholar] [CrossRef]
- Stettner, P.; Bourgeois, S.; Marsching, C.; Traykova-Brauch, M.; Porubsky, S.; Nordstrom, V.; Hopf, C.; Kosters, R.; Sandhoff, R.; Wiegandt, H.; et al. Sulfatides are required for renal adaptation to chronic metabolic acidosis. Proc. Natl. Acad. Sci. USA 2013, 110, 9998–10003. [Google Scholar] [CrossRef]
- Marsching, C.; Rabionet, M.; Mathow, D.; Jennemann, R.; Kremser, C.; Porubsky, S.; Bolenz, C.; Willecke, K.; Grone, H.J.; Hopf, C.; et al. Renal sulfatides: Sphingoid base-dependent localization and region-specific compensation of CerS2-dysfunction. J. Lipid Res. 2014, 55, 2354–2369. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.J.; Lee, Y.J.; Yang, Y.R.; Park, S.; Suh, P.G.; Follo, M.Y.; Cocco, L.; Ryu, S.H. Molecular Mechanisms Underlying Psychological Stress and Cancer. Curr. Pharm. Des. 2016, 22, 2389–2402. [Google Scholar] [CrossRef]
- Trah, J.; Arand, J.; Oh, J.; Pagerols-Raluy, L.; Trochimiuk, M.; Appl, B.; Heidelbach, H.; Vincent, D.; Saleem, M.A.; Reinshagen, K.; et al. Lithocholic bile acid induces apoptosis in human nephroblastoma cells: A non-selective treatment option. Sci. Rep. 2020, 10, 20349. [Google Scholar] [CrossRef]
- Saito, K.; Arai, E.; Maekawa, K.; Ishikawa, M.; Fujimoto, H.; Taguchi, R.; Matsumoto, K.; Kanai, Y.; Saito, Y. Lipidomic Signatures and Associated Transcriptomic Profiles of Clear Cell Renal Cell Carcinoma. Sci. Rep. 2016, 6, 28932. [Google Scholar] [CrossRef]
- Ding, T.; Li, Z.Q.; Hailemariam, T.; Mukherjee, S.; Maxfield, F.R.; Wu, M.P.; Jiang, X.C. SMS overexpression and knockdown: Impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. J. Lipid Res. 2008, 49, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zarisfi, M.; Nguyen, T.; Nedrow, J.R.; Le, A. The Heterogeneity Metabolism of Renal Cell Carcinomas. Adv. Exp. Med. Biol. 2021, 1311, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Israel, G.M.; Bosniak, M.A. How I Do It: Evaluating Renal Masses. Radiology 2005, 236, 441–450. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Bioanalytical Method Validation, Committee for Medicinal Products for Human Use, 192217/2009, Rev. 1 Corr.2, 21 July 2011. Available online: https://www.ema.europa.eu/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 29 July 2022).
- Food and Drug Administration. Guidance for Industry on Bioloanalytical Method Validation, Federal Register, 23 May 2001. Available online: https://www.federalregister.gov/documents/2001/05/23/01-12908/guidance-for-industry-on-bioanalytical-method-validation-availability (accessed on 29 July 2022).
- Bowden, J.A.; Heckert, A.; Ulmer, C.Z.; Jones, C.M.; Koelmel, J.P.; Abdullah, L.; Ahonen, L.; Alnouti, Y.; Armando, A.M.; Asara, J.M.; et al. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950–Metabolites in Frozen Human Plasma. J. Lipid Res. 2017, 58, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Barlow, C.K.; Jayawardana, K.S.; Weir, J.M.; Mellett, N.A.; Cinel, M.; Magliano, D.J.; Shaw, J.E.; Drew, B.G.; Meikle, P.J. High-Throughput Plasma Lipidomics: Detailed Mapping of the Associations with Cardiometabolic Risk Factors. Cell Chem. Biol. 2019, 26, 71–84. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirásko, R.; Idkowiak, J.; Wolrab, D.; Kvasnička, A.; Friedecký, D.; Polański, K.; Študentová, H.; Študent, V.; Melichar, B.; Holčapek, M. Altered Plasma, Urine, and Tissue Profiles of Sulfatides and Sphingomyelins in Patients with Renal Cell Carcinoma. Cancers 2022, 14, 4622. https://doi.org/10.3390/cancers14194622
Jirásko R, Idkowiak J, Wolrab D, Kvasnička A, Friedecký D, Polański K, Študentová H, Študent V, Melichar B, Holčapek M. Altered Plasma, Urine, and Tissue Profiles of Sulfatides and Sphingomyelins in Patients with Renal Cell Carcinoma. Cancers. 2022; 14(19):4622. https://doi.org/10.3390/cancers14194622
Chicago/Turabian StyleJirásko, Robert, Jakub Idkowiak, Denise Wolrab, Aleš Kvasnička, David Friedecký, Krzysztof Polański, Hana Študentová, Vladimír Študent, Bohuslav Melichar, and Michal Holčapek. 2022. "Altered Plasma, Urine, and Tissue Profiles of Sulfatides and Sphingomyelins in Patients with Renal Cell Carcinoma" Cancers 14, no. 19: 4622. https://doi.org/10.3390/cancers14194622
APA StyleJirásko, R., Idkowiak, J., Wolrab, D., Kvasnička, A., Friedecký, D., Polański, K., Študentová, H., Študent, V., Melichar, B., & Holčapek, M. (2022). Altered Plasma, Urine, and Tissue Profiles of Sulfatides and Sphingomyelins in Patients with Renal Cell Carcinoma. Cancers, 14(19), 4622. https://doi.org/10.3390/cancers14194622





