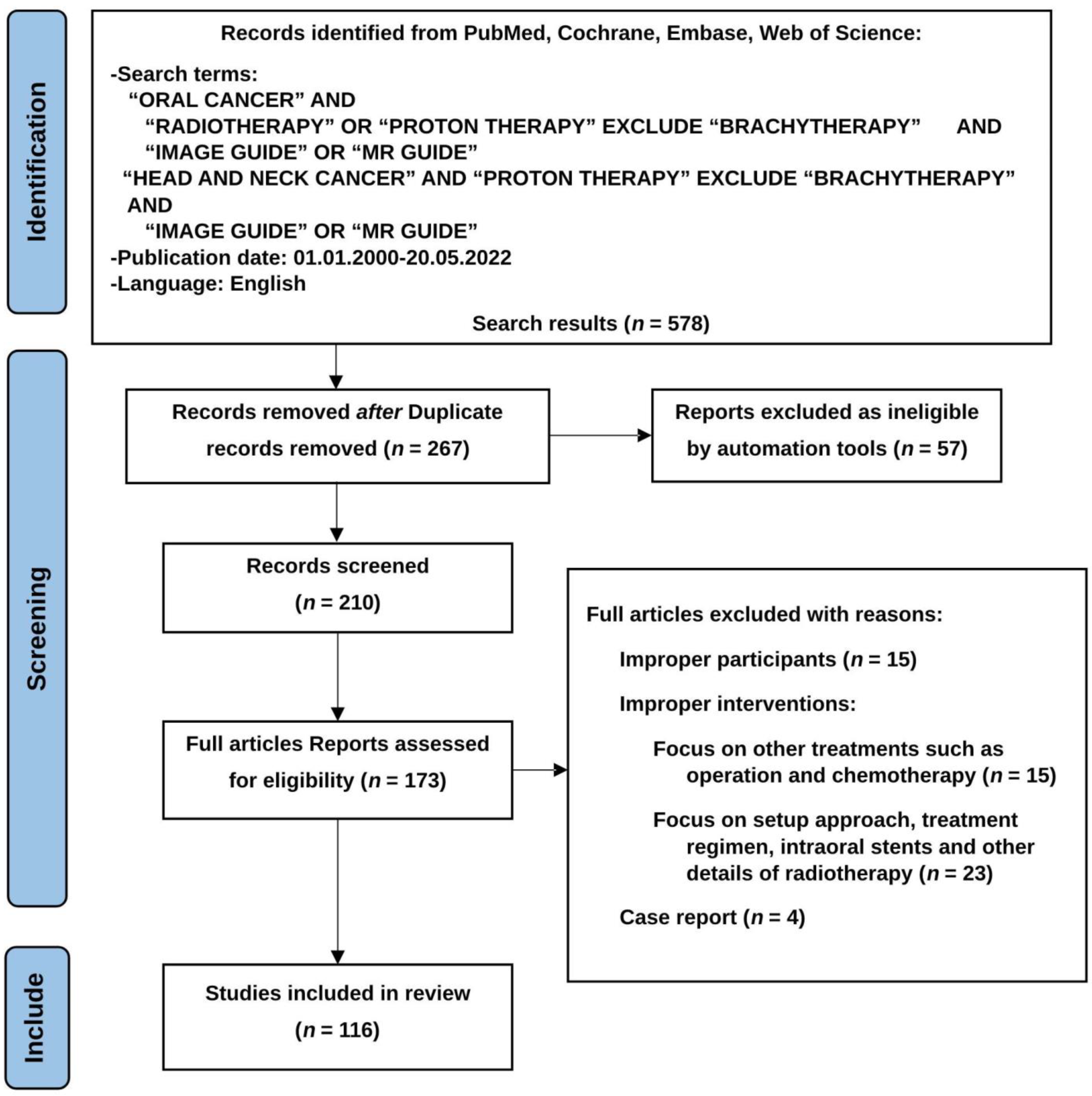
Advances in Image-Guided Radiotherapy in the Treatment of Oral Cavity Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Populations and Inclusion/Exclusion Criteria
2.2. Intervension and Comparison
2.3. Outcome
2.4. Timing and Setting
2.5. Searching Strategy
2.6. Results
3. Image-Guided Radiotherapy Techniques
3.1. Two-Dimensional (2D) X-ray Plain-Film Image Guidance
3.2. Three-Dimensional Computed (3D) Tomography Image Guidance
3.3. Magnetic Resonance Imaging Guidance
3.4. Other Imaging Guidance Techniques
4. Benefits of IGRT for OCC Patients
4.1. Benefits in Overall Survival and Locoregional Control
| Selected Published Series | Number of Postoperative Patients | Modality | Follow-Up Period | OS | DFS | LR PF | DMF |
|---|---|---|---|---|---|---|---|
| Chen AM et al. [58] | 78 (OCC: 30) | 2DRT | 3 years | 69% | - | 70% | 66% |
| 52 (OCC: 25) | IMRT | 3 years | 72% | - | 73% | 70% | |
| Wang ZH et al. [64] | 44 (OCC: 38) | 2DRT | 4 years | 56.8% | 52.3% | - | - |
| 44 (OCC: 39) | IMRT | 4 years | 70.5% | 68.2% | - | - | |
| Chen PY et al. [60] | 42 | 2DRT | 3 years | 51.2% | 47.8% | 53.5% | - |
| 72 | IMRT | 3 years | 69.4% | 70.0% | 76.3% | - | |
| Yao et al. [65] | 55 | IMRT | 2 years | 68% | 74% | 82% | 89% |
| Gomez et al. [52] | 35 | IMRT | 3 years | 74% | 64% | 77% | 85% |
| Chen WC et al. [57] | 27 | 2DRT | 3 years | 77% | 66% | - | - |
| 22 | IMRT | 3 years | 67% | 64% | - | - | |
| Lin CS et al. [61] | 91 | 2DRT | 5 years | 30.0% | - | 30.0% | - |
| 83 | IMRT | 5 years | 53.5% | - | 40.5% | - | |
| Hoffmann M et al. [66] | 18 | IMRT | 5 years | 77% | 72% | 78% | 80% |
| EORTC 22931 [11] | 167 (OCC: 41) | CCRT | 5 years | 53% | 47% | ||
| RTOG 9501 [12] | 206 (OCC: 50) | CCRT | 2 years | 82% | |||
| RTOG 9501 [15] | 50 (206) | CCRT | 5 years | 46% | 30% | ||
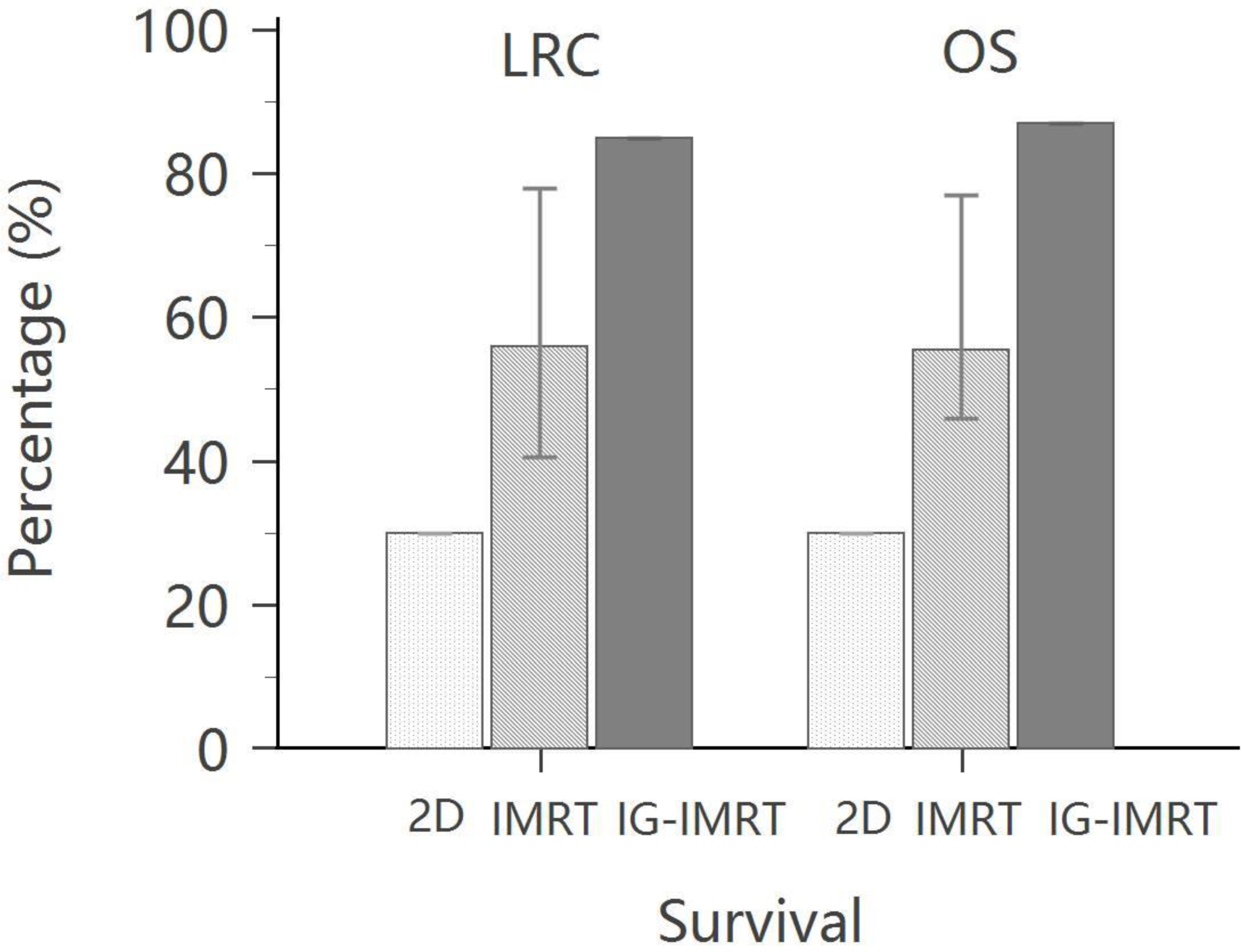
| Hsieh et al. [63] | 79 | IMRT | 5 years | 48% | 39% | 58% | 83% |
| 73 | IG-IMRT (HT) | 87% | 74% | 85% | 80% |
4.2. Benefits for High-Risk Patients
4.3. Benefits in Treatment Compliance
5. Contributing Factors
5.1. Increased Marginal Failure Control
5.2. Reduced Treatment Side Effects
5.3. Xerostomia
5.4. Posttreatment Esophageal Stricture and Gastrostomy Tube Dependence
5.5. Performance Status and Quality of Life
6. Limitations of IGRT
6.1. Extra Time for Imaging Guidance
6.2. Intrafraction Motion
6.3. Comparison between CT-Guided and MR-Guided Radiotherapy
6.4. Application of IGRT in Proton Therapy
7. Future Prospects
7.1. Cooperation between IGRT and PET-CT
7.2. IGRT with Dose Painting for Biologic Mapping
7.3. IGRT for Reirradiation
7.4. SBRT in Oral Cavity Cancer
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.; Ferlay, J. Cancer Incidence and Mortality in Europe, 2004. Ann. Oncol. 2005, 16, 481–488. [Google Scholar] [CrossRef]
- Cancer Registry Annual Report, 2019 Taiwan; Health Promotion Administration Ministry of Health and Welfare: Taipei, Taiwan, December 2021.
- Moon, S.H.; Jung, Y.-S.; Ryu, J.S.; Choi, S.W.; Park, J.Y.; Yun, T.; Lee, S.H.; Cho, K.H. Outcomes of Postoperative Simultaneous Modulated Accelerated Radiotherapy for Head-and-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Cruz, O.S.; Tsoutsou, P.; Castella, C.; Khanfir, K.; Anchisi, S.; Bouayed, S.; Matzinger, O.; Ozsahin, M. Locoregional Control and Toxicity in Head and Neck Carcinoma Patients Following Helical Tomotherapy-Delivered Intensity-Modulated Radiation Therapy Compared with 3D-CRT Data. Oncology 2018, 95, 61–68. [Google Scholar] [CrossRef]
- Lin, C.-S.; Jen, Y.-M.; Cheng, M.-F.; Lin, Y.-S.; Su, W.-F.; Hwang, J.-M.; Chang, L.-P.; Chao, H.-L.; Liu, D.-W.; Lin, H.-Y.; et al. Squamous Cell Carcinoma of the Buccal Mucosa: An Aggressive Cancer Requiring Multimodality Treatment. Head Neck 2006, 28, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.M., Jr.; Holsinger, F.C.; Zuniga, E.R.; Roberts, D.B.; Sorensen, D.M. Squamous Cell Carcinoma of the Buccal Mucosa: One Institution’s Experience with 119 Previously Untreated Patients. Head Neck 2003, 25, 267–273. [Google Scholar] [CrossRef]
- Eisbruch, A.; Marsh, L.H.; Dawson, L.A.; Bradford, C.R.; Teknos, T.N.; Chepeha, D.B.; Worden, F.P.; Urba, S.; Lin, A.; Schipper, M.J.; et al. Recurrences near Base of Skull after IMRT for Head-and-Neck Cancer: Implications for Target Delineation in High Neck and for Parotid Gland Sparing. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.J.; Parsons, J.T.; Mendenhall, W.M.; Million, R.R.; Stringer, S.P.; Cassisi, N.J. Postoperative Irradiation for Squamous Cell Carcinoma of the Head and Neck: An Analysis of Treatment Results and Complications. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 25–36. [Google Scholar] [CrossRef]
- Bernier, J.; Ozsahin, M.; Lefèbvre, J.-L.; Maingon, P.; Cognetti, F.; van Glabbeke, M. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Wendt, C.D.; Peters, L.J.; Delclos, L.; Ang, K.K.; Morrison, W.H.; Maor, M.H.; Robbins, K.T.; Byers, R.M.; Carlson, L.S.; Oswald, M.J. Primary Radiotherapy in the Treatment of Stage I and II Oral Tongue Cancers: Importance of the Proportion of Therapy Delivered with Interstitial Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 1287–1292. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.-K.; et al. Defining Risk Levels in Locally Advanced Head and Neck Cancers: A Comparative Analysis of Concurrent Postoperative Radiation plus Chemotherapy Trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Cooper, J.S.; Zhang, Q.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Long-Term Follow-up of the RTOG 9501/Intergroup Phase III Trial: Postoperative Concurrent Radiation Therapy and Chemotherapy in High-Risk Squamous Cell Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1198–1205. [Google Scholar] [CrossRef]
- Eich, H.T.; Löschcke, M.; Scheer, M.; Kocher, M.; Bongartz, R.; Wacker, S.; Zöller, J.E.; Müller, R.-P. Neoadjuvant Radiochemotherapy and Radical Resection for Advanced Squamous Cell Carcinoma of the Oral Cavity: Outcome of 134 Patients. Strahlenther. Onkol. 2008, 184, 23–29. [Google Scholar] [CrossRef]
- von der Grün, J.; Winkelmann, R.; Burck, I.; Martin, D.; Rödel, F.; Wild, P.J.; Bankov, K.; Weigert, A.; Kur, I.-M.; Brandts, C.; et al. Neoadjuvant Chemoradiotherapy for Oral Cavity Cancer: Predictive Factors for Response and Interim Analysis of the Prospective INVERT-Trial. Front. Oncol. 2022, 12, 817692. [Google Scholar] [CrossRef]
- Dreizen, S. Oral Complications of Cancer Therapies. Description and Incidence of Oral Complications. NCI Monogr. 1990, 9, 11–15. [Google Scholar]
- Dreizen, S.; Daly, T.E.; Drane, J.B.; Brown, L.R. Oral Complications of Cancer Radiotherapy. Postgrad. Med. 1977, 61, 85–92. [Google Scholar] [CrossRef]
- Oba, M.K.; Innocentini, L.M.A.R.; Viani, G.; Ricz, H.M.A.; de Carvalho Reis, T.; Ferrari, T.C.; de Macedo, L.D. Evaluation of the Correlation between Side Effects to Oral Mucosa, Salivary Glands, and General Health Status with Quality of Life during Intensity-Modulated Radiotherapy for Head and Neck Cancer. Support. Care Cancer 2021, 29, 127–134. [Google Scholar] [CrossRef]
- Jabbari, S.; Kim, H.M.; Feng, M.; Lin, A.; Tsien, C.; Elshaikh, M.; Terrel, J.E.; Murdoch-Kinch, C.; Eisbruch, A. Matched Case–Control Study of Quality of Life and Xerostomia after Intensity-Modulated Radiotherapy or Standard Radiotherapy for Head-and-Neck Cancer: Initial Report. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 725–731. [Google Scholar] [CrossRef]
- Murdoch-Kinch, C.-A.; Kim, H.M.; Vineberg, K.A.; Ship, J.A.; Eisbruch, A. Dose-Effect Relationships for the Submandibular Salivary Glands and Implications for Their Sparing by Intensity Modulated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 373–382. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Yan, C.; Zhang, Z.-Y.; Zhang, C.-P.; Hu, H.-S.; Tu, W.-Y.; Kirwan, J.; Mendenhall, W.M. Impact of Salivary Gland Dosimetry on Post-IMRT Recovery of Saliva Output and Xerostomia Grade for Head-and-Neck Cancer Patients Treated With or Without Contralateral Submandibular Gland Sparing: A Longitudinal Study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1479–1487. [Google Scholar] [CrossRef]
- Hawkins, P.G.; Lee, J.Y.; Mao, Y.; Li, P.; Green, M.; Worden, F.P.; Swiecicki, P.L.; Mierzwa, M.L.; Spector, M.E.; Schipper, M.J.; et al. Sparing All Salivary Glands with IMRT for Head and Neck Cancer: Longitudinal Study of Patient-Reported Xerostomia and Head-and-Neck Quality of Life. Radiother. Oncol. 2018, 126, 68–74. [Google Scholar] [CrossRef]
- Ma, C.-M.C.; Paskalev, K. In-Room CT Techniques for Image-Guided Radiation Therapy. Med. Dosim. 2006, 31, 30–39. [Google Scholar] [CrossRef]
- Bhide, S.A.; Davies, M.; Burke, K.; McNair, H.A.; Hansen, V.; Barbachano, Y.; El-Hariry, I.A.; Newbold, K.; Harrington, K.J.; Nutting, C.M. Weekly Volume and Dosimetric Changes During Chemoradiotherapy With Intensity-Modulated Radiation Therapy for Head and Neck Cancer: A Prospective Observational Study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1360–1368. [Google Scholar] [CrossRef]
- Beltran, M.; Ramos, M.; Rovira, J.J.; Perez-Hoyos, S.; Sancho, M.; Puertas, E.; Benavente, S.; Ginjaume, M.; Giralt, J. Dose Variations in Tumor Volumes and Organs at Risk during IMRT for Head-and-neck Cancer. J. Appl. Clin. Med. Phys. 2012, 13, 101–111. [Google Scholar] [CrossRef]
- Wang, W.; Yang, H.; Hu, W.; Shan, G.; Ding, W.; Yu, C.; Wang, B.; Wang, X.; Xu, Q. Clinical Study of the Necessity of Replanning Before the 25th Fraction During the Course of Intensity-Modulated Radiotherapy for Patients With Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 617–621. [Google Scholar] [CrossRef]
- Loo, H.; Fairfoul, J.; Chakrabarti, A.; Dean, J.C.; Benson, R.J.; Jefferies, S.J.; Burnet, N.G. Tumour Shrinkage and Contour Change during Radiotherapy Increase the Dose to Organs at Risk but Not the Target Volumes for Head and Neck Cancer Patients Treated on the TomoTherapy HiArtTM System. Clin. Oncol. 2011, 23, 40–47. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Yan, C.; Zhang, Z.-Y.; Zhang, C.-P.; Hu, H.-S.; Kirwan, J.; Mendenhall, W.M. Radiation-Induced Volume Changes in Parotid and Submandibular Glands in Patients with Head and Neck Cancer Receiving Postoperative Radiotherapy: A Longitudinal Study. Laryngoscope 2009, 119, 1966–1974. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, J.; Zhou, D.; Krauss, D.J.; Chen, P.Y.; Yan, D. Dosimetric Evaluation of Incorporating Patient Geometric Variations Into Adaptive Plan Optimization Through Probabilistic Treatment Planning in Head and Neck Cancers. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 985–997. [Google Scholar] [CrossRef]
- Mahmoud, O.; Reis, I.M.; Samuels, M.M.; Elsayyad, N.; Bossart, E.; Both, J.; ELGhoneimy, E.; Moustafa, M.; AbdAllah, M.; Takita, C. Prospective Pilot Study Comparing the Need for Adaptive Radiotherapy in Unresected Bulky Disease and in Postoperative Patients with Head and Neck Cancer. Technol. Cancer Res. Treat. 2017, 16, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Castelli, J.; Simon, A.; Louvel, G.; Henry, O.; Chajon, E.; Nassef, M.; Haigron, P.; Cazoulat, G.; Ospina, J.D.; Jegoux, F.; et al. Impact of Head and Neck Cancer Adaptive Radiotherapy to Spare the Parotid Glands and Decrease the Risk of Xerostomia. Radiat. Oncol. 2015, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Castelli, J.; Simon, A.; Rigaud, B.; Lafond, C.; Chajon, E.; Ospina, J.D.; Haigron, P.; Laguerre, B.; Loubière, A.R.; Benezery, K.; et al. A Nomogram to Predict Parotid Gland Overdose in Head and Neck IMRT. Radiat. Oncol. 2016, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.L.; Garden, A.S.; Thomas, J.; Chen, Y.; Zhang, Y.; Lewin, J.; Chambers, M.S.; Dong, L. Adaptive Radiotherapy for Head-and-Neck Cancer: Initial Clinical Outcomes From a Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 986–993. [Google Scholar] [CrossRef]
- Menten, M.J.; Wetscherek, A.; Fast, M.F. MRI-Guided Lung SBRT: Present and Future Developments. Phys. Med. Eur. J. Med. Phys. 2017, 44, 139–149. [Google Scholar] [CrossRef]
- Fallone, B.G. The Rotating Biplanar Linac–Magnetic Resonance Imaging System. Semin. Radiat. Oncol. 2014, 24, 200–202. [Google Scholar] [CrossRef]
- Keall, P.J.; Barton, M.; Crozier, S. The Australian Magnetic Resonance Imaging–Linac Program. Semin. Radiat. Oncol. 2014, 24, 203–206. [Google Scholar] [CrossRef]
- Jaffray, D.A.; Carlone, M.C.; Milosevic, M.F.; Breen, S.L.; Stanescu, T.; Rink, A.; Alasti, H.; Simeonov, A.; Sweitzer, M.C.; Winter, J.D. A Facility for Magnetic Resonance–Guided Radiation Therapy. Semin. Radiat. Oncol. 2014, 24, 193–195. [Google Scholar] [CrossRef]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef]
- Lagendijk, J.J.W.; Raaymakers, B.W.; van Vulpen, M. The Magnetic Resonance Imaging–Linac System. Semin. Radiat. Oncol. 2014, 24, 207–209. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Jürgenliemk-Schulz, I.M.; Bol, G.H.; Glitzner, M.; Kotte, A.N.T.J.; van Asselen, B.; de Boer, J.C.J.; Bluemink, J.J.; Hackett, S.L.; Moerland, M.A.; et al. First Patients Treated with a 1.5 T MRI-Linac: Clinical Proof of Concept of a High-Precision, High-Field MRI Guided Radiotherapy Treatment. Phys. Med. Biol. 2017, 62, L41–L50. [Google Scholar] [CrossRef] [PubMed]
- Buatti, J.M.; Bova, F.J.; Friedman, W.A.; Meeks, S.L.; Marcus, R.B.; Mickle, J.P.; Ellis, T.L.; Mendenhall, W.M. Preliminary Experience with Frameless Stereotactic Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 591–599. [Google Scholar] [CrossRef]
- Meeks, S.L.; Bova, F.J.; Wagner, T.H.; Buatti, J.M.; Friedman, W.A.; Foote, K.D. Image Localization for Frameless Stereotactic Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1291–1299. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Grantham, K.; Zhou, Y.; Cui, T.; Zhang, Y.; Liu, B.; Wang, X.; Vergalasova, I.; Reyhan, M.; et al. Performance Assessment of Two Motion Management Systems for Frameless Stereotactic Radiosurgery. Strahlenther. Onkol. 2021, 197, 150–157. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Tung, S.; Dimmitt, A.W.; Wong, P.F.; Edson, M.A.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Gunn, G.B.; et al. Improved Setup and Positioning Accuracy Using a Three-point Customized Cushion/Mask/Bite-block Immobilization System for Stereotactic Reirradiation of Head and Neck Cancer. J. Appl. Clin. Med. Phys. 2016, 17, 180–189. [Google Scholar] [CrossRef]
- Zhao, L.R.; Qian, J.D.; Duan, X.J.; Yang, D.Q.; Zhou, Y.B.; Sun, J.G. The Clinical Feasibility and Effect of Online ExacTrac 6 Degree-of-Freedom System for Head-and-Neck Cancer. Digit Med 2019, 5, 119–125. [Google Scholar] [CrossRef]
- Covington, E.L.; Fiveash, J.B.; Wu, X.; Brezovich, I.; Willey, C.D.; Riley, K.; Popple, R.A. Optical Surface Guidance for Submillimeter Monitoring of Patient Position during Frameless Stereotactic Radiotherapy. J. Appl. Clin. Med. Phys. 2019, 20, 91–98. [Google Scholar] [CrossRef]
- Hoisak, J.D.P.; Pawlicki, T. The Role of Optical Surface Imaging Systems in Radiation Therapy. Semin. Radiat. Oncol. 2018, 28, 185–193. [Google Scholar] [CrossRef]
- Freislederer, P.; Kügele, M.; Öllers, M.; Swinnen, A.; Sauer, T.-O.; Bert, C.; Giantsoudi, D.; Corradini, S.; Batista, V. Recent Advances in Surface Guided Radiation Therapy. Radiat. Oncol. 2020, 15, 187. [Google Scholar] [CrossRef]
- Chow, V.U.Y.; Cheung, M.L.M.; Kan, M.W.K.; Chan, A.T.C. Shift Detection Discrepancy between ExacTrac Dynamic System and Cone-Beam Computed Tomography. J. Appl. Clin. Med. Phys. 2022, 23, e13567. [Google Scholar] [CrossRef]
- Gomez, D.R.; Zhung, J.E.; Gomez, J.; Chan, K.; Wu, A.J.; Wolden, S.L.; Pfister, D.G.; Shaha, A.; Shah, J.P.; Kraus, D.H.; et al. Intensity-Modulated Radiotherapy in Postoperative Treatment of Oral Cavity Cancers. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, G.O.; Amdur, R.J.; Morris, C.G.; Li, J.G.; Hinerman, R.W.; Mendenhall, W.M. Patterns of Failure and Toxicity after Intensity-Modulated Radiotherapy for Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.S.C.; Ozyigit, G.; Tran, B.N.; Cengiz, M.; Dempsey, J.F.; Low, D.A. Patterns of Failure in Patients Receiving Definitive and Postoperative IMRT for Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 312–321. [Google Scholar] [CrossRef]
- Claus, F.; Duthoy, W.; Boterberg, T.; De Gersem, W.; Huys, J.; Vermeersch, H.; De Neve, W. Intensity Modulated Radiation Therapy for Oropharyngeal and Oral Cavity Tumors: Clinical Use and Experience. Oral Oncol. 2002, 38, 597–604. [Google Scholar] [CrossRef]
- Verdonck, H.W.D.; de Jong, J.M.A.; Granzier, M.E.P.G.; Nieman, F.H.; de Baat, C.; Stoelinga, P.J.W. Intensity-Modulated Radiation Therapy for Oropharyngeal Cancer: Radiation Dosage Constraint at the Anterior Mandible. Oral Oncol. 2009, 45, 511–514. [Google Scholar] [CrossRef]
- Chen, W.-C.; Hwang, T.-Z.; Wang, W.-H.; Lu, C.-H.; Chen, C.-C.; Chen, C.-M.; Weng, H.-H.; Lai, C.-H.; Chen, M.-F. Comparison between Conventional and Intensity-Modulated Post-Operative Radiotherapy for Stage III and IV Oral Cavity Cancer in Terms of Treatment Results and Toxicity. Oral Oncol. 2009, 45, 505–510. [Google Scholar] [CrossRef]
- Chen, A.M.; Farwell, D.G.; Luu, Q.; Chen, L.M.; Vijayakumar, S.; Purdy, J.A. Misses and Near-Misses after Postoperative Radiation Therapy for Head and Neck Cancer: Comparison of IMRT and Non-IMRT Techniques in the CT-Simulation Era. Head Neck 2010, 32, 1452–1459. [Google Scholar] [CrossRef]
- Clavel, S.; Nguyen, D.H.A.; Fortin, B.; Després, P.; Khaouam, N.; Donath, D.; Soulières, D.; Guertin, L.; Nguyen-Tan, P.F. Simultaneous Integrated Boost Using Intensity-Modulated Radiotherapy Compared With Conventional Radiotherapy in Patients Treated With Concurrent Carboplatin and 5-Fluorouracil for Locally Advanced Oropharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 582–589. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Chen, H.H.W.; Hsiao, J.-R.; Yang, M.-W.; Hsueh, W.-T.; Tasi, S.-T.; Lin, F.-C.; Wu, Y.-H. Intensity-Modulated Radiotherapy Improves Outcomes in Postoperative Patients with Squamous Cell Carcinoma of the Oral Cavity. Oral Oncol. 2012, 48, 747–752. [Google Scholar] [CrossRef]
- Lin, C.; Jen, Y.; Kao, W.; Ho, C.; Dai, M.; Shih, C.; Cheng, J.; Chang, P.; Huang, W.; Su, Y. Improved Outcomes in Buccal Squamous Cell Carcinoma. Head Neck 2013, 35, 65–71. [Google Scholar] [CrossRef]
- Shueng, P.-W.; Wu, L.-J.; Chen, S.-Y.; Hsiao, C.-H.; Tien, H.-J.; Cheng, P.-W.; Kuo, Y.-S.; Chen, Y.-J.; Chen, C.-A.; Hsieh, P.-Y.; et al. Concurrent Chemoradiotherapy With Helical Tomotherapy for Oropharyngeal Cancer: A Preliminary Result. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Shueng, P.-W.; Wang, L.-Y.; Huang, Y.-C.; Liao, L.-J.; Lo, W.-C.; Lin, Y.-C.; Wu, L.-J.; Tien, H.-J. Impact of Postoperative Daily Image-Guided Intensity-Modulated Radiotherapy on Overall and Local Progression-Free Survival in Patients with Oral Cavity Cancer. BMC Cancer 2016, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Yan, C.; Zhang, Z.-Y.; Zhang, C.-P.; Hu, H.-S.; Tu, W.-Y.; Kirwan, J.; Mendenhall, W.M. Outcomes and Xerostomia after Postoperative Radiotherapy for Oral and Oropharyngeal Carcinoma. Head Neck 2014, 36, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Chang, K.; Funk, G.F.; Lu, H.; Tan, H.; Wacha, J.; Dornfeld, K.J.; Buatti, J.M. The Failure Patterns of Oral Cavity Squamous Cell Carcinoma After Intensity-Modulated Radiotherapy—The University of Iowa Experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Saleh-Ebrahimi, L.; Zwicker, F.; Haering, P.; Schwahofer, A.; Debus, J.; Huber, P.E.; Roeder, F. Long Term Results of Postoperative Intensity-Modulated Radiation Therapy (IMRT) in the Treatment of Squamous Cell Carcinoma (SCC) Located in the Oropharynx or Oral Cavity. Radiat. Oncol. 2015, 10, 251. [Google Scholar] [CrossRef]
- Ang, K.K.; Trotti, A.; Brown, B.W.; Garden, A.S.; Foote, R.L.; Morrison, W.H.; Geara, F.B.; Klotch, D.W.; Goepfert, H.; Peters, L.J. Randomized Trial Addressing Risk Features and Time Factors of Surgery plus Radiotherapy in Advanced Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 571–578. [Google Scholar] [CrossRef]
- Rosenthal, D.I.; Liu, L.; Lee, J.H.; Vapiwala, N.; Chalian, A.A.; Weinstein, G.S.; Chilian, I.; Weber, R.S.; Machtay, M. Importance of the Treatment Package Time in Surgery and Postoperative Radiation Therapy for Squamous Carcinoma of the Head and Neck. Head Neck 2002, 24, 115–126. [Google Scholar] [CrossRef]
- Hinerman, R.W.; Mendenhall, W.M.; Morris, C.G.; Amdur, R.J.; Werning, J.W.; Villaret, D.B. Postoperative Irradiation for Squamous Cell Carcinoma of the Oral Cavity: 35-Year Experience. Head Neck 2004, 26, 984–994. [Google Scholar] [CrossRef]
- Ghanem, A.I.; Schymick, M.; Bachiri, S.; Mannari, A.; Sheqwara, J.; Burmeister, C.; Chang, S.; Ghanem, T.; Siddiqui, F. The Effect of Treatment Package Time in Head and Neck Cancer Patients Treated with Adjuvant Radiotherapy and Concurrent Systemic Therapy. World J. Otorhinolaryngol. Head Neck Surg. 2019, 5, 160–167. [Google Scholar] [CrossRef]
- Muriel, V.P.; Tejada, M.R.G.; de Dios Luna del Castillo, J. Time–Dose–Response Relationships in Postoperatively Irradiated Patients with Head and Neck Squamous Cell Carcinomas. Radiother. Oncol. 2001, 60, 137–145. [Google Scholar] [CrossRef]
- Marcus, R.B.; Million, R.R.; Cassissi, N.J. Postoperative Irradiation for Squamous Cell Carcinomas of the Head and Neck: Analysis of Time-Dose Factors Related to Control above the Clavicles. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 1943–1949. [Google Scholar] [CrossRef]
- Suwinski, R.; Sowa, A.; Rutkowski, T.; Wydmanski, J.; Tarnawski, R.; Maciejewski, B. Time Factor in Postoperative Radiotherapy: A Multivariate Locoregional Control Analysis in 868 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 399–412. [Google Scholar] [CrossRef]
- Langendijk, J.A.; de Jong, M.A.; Leemans, C.R.; de Bree, R.; Smeele, L.E.; Doornaert, P.; Slotman, B.J. Postoperative Radiotherapy in Squamous Cell Carcinoma of the Oral Cavity: The Importance of the Overall Treatment Time. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 693–700. [Google Scholar] [CrossRef]
- Fujiwara, R.J.T.; Judson, B.L.; Yarbrough, W.G.; Husain, Z.; Mehra, S. Treatment Delays in Oral Cavity Squamous Cell Carcinoma and Association with Survival. Head Neck 2017, 39, 639–646. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Kuo, Y.-S.; Liao, L.-J.; Hu, K.-Y.; Lin, S.-C.; Wu, L.-J.; Lin, Y.-C.; Chen, Y.-J.; Wang, L.-Y.; Hsieh, Y.-P.; et al. Image-Guided Intensity Modulated Radiotherapy with Helical Tomotherapy for Postoperative Treatment of High-Risk Oral Cavity Cancer. BMC Cancer 2011, 11, 37. [Google Scholar] [CrossRef]
- Daly, M.E.; Lieskovsky, Y.; Pawlicki, T.; Yau, J.; Pinto, H.; Kaplan, M.; Fee, W.E.; Koong, A.; Goffinet, D.R.; Xing, L.; et al. Evaluation of Patterns of Failure and Subjective Salivary Function in Patients Treated with Intensity Modulated Radiotherapy for Head and Neck Squamous Cell Carcinoma. Head Neck 2007, 29, 211–220. [Google Scholar] [CrossRef]
- Daly, M.E.; Le, Q.-T.; Kozak, M.M.; Maxim, P.G.; Murphy, J.D.; Hsu, A.; Loo, B.W.; Kaplan, M.J.; Fischbein, N.J.; Chang, D.T. Intensity-Modulated Radiotherapy for Oral Cavity Squamous Cell Carcinoma: Patterns of Failure and Predictors of Local Control. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1412–1422. [Google Scholar] [CrossRef]
- Bachar, G.; Goldstein, D.P.; Barker, E.; Lea, J.; O’Sullivan, B.; Brown, D.H.; Gullane, P.J.; Gilbert, R.W.; Xu, W.; Su, J.; et al. Squamous Cell Carcinoma of the Buccal Mucosa: Outcomes of Treatment in the Modern Era. Laryngoscope 2012, 122, 1552–1557. [Google Scholar] [CrossRef]
- Chen, A.M.; Farwell, D.G.; Luu, Q.; Chen, L.M.; Vijayakumar, S.; Purdy, J.A. Marginal Misses After Postoperative Intensity-Modulated Radiotherapy for Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1423–1429. [Google Scholar] [CrossRef]
- Geretschläger, A.; Bojaxhiu, B.; Crowe, S.; Arnold, A.; Manser, P.; Hallermann, W.; Aebersold, D.M.; Ghadjar, P. Outcome and Patterns of Failure after Postoperative Intensity Modulated Radiotherapy for Locally Advanced or High-Risk Oral Cavity Squamous Cell Carcinoma. Radiat. Oncol. 2012, 7, 175. [Google Scholar] [CrossRef]
- Chan, A.K.; Huang, S.H.; Le, L.W.; Yu, E.; Dawson, L.A.; Kim, J.J.; John Cho, B.C.; Bayley, A.J.; Ringash, J.; Goldstein, D.; et al. Postoperative Intensity-Modulated Radiotherapy Following Surgery for Oral Cavity Squamous Cell Carcinoma: Patterns of Failure. Oral Oncol. 2013, 49, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ooishi, M.; Motegi, A.; Kawashima, M.; Arahira, S.; Zenda, S.; Nakamura, N.; Ariji, T.; Tokumaru, S.; Sakuraba, M.; Tahara, M.; et al. Patterns of Failure after Postoperative Intensity-Modulated Radiotherapy for Locally Advanced and Recurrent Head and Neck Cancer. Jpn. J. Clin. Oncol. 2016, 46, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Mallick, I.; Das, P.; Shrimali, R.K.; Achari, R.; Chatterjee, S. Evaluating the Need for Daily Image Guidance in Head and Neck Cancers Treated with Helical Tomotherapy: A Retrospective Analysis of a Large Number of Daily Imaging-Based Corrections. Clin. Oncol. 2016, 28, 178–184. [Google Scholar] [CrossRef]
- Zeidan, O.A.; Langen, K.M.; Meeks, S.L.; Manon, R.R.; Wagner, T.H.; Willoughby, T.R.; Jenkins, D.W.; Kupelian, P.A. Evaluation of Image-Guidance Protocols in the Treatment of Head and Neck Cancers. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Farwell, D.G.; Luu, Q.; Donald, P.J.; Perks, J.; Purdy, J.A. Evaluation of the Planning Target Volume in the Treatment of Head and Neck Cancer With Intensity-Modulated Radiotherapy: What Is the Appropriate Expansion Margin in the Setting of Daily Image Guidance? Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Yu, Y.; Daly, M.E.; Farwell, D.G.; Benedict, H.S.; Purdy, J.A. Long-Term Experience with Reduced Planning Target Volume Margins and Intensity-Modulated Radiotherapy with Daily Image-Guidance for Head and Neck Cancer. Head Neck 2014, 36, 1766–1772. [Google Scholar] [CrossRef]
- Farrag, A.; Voordeckers, M.; Tournel, K.; De Coninck, P.; Storme, G. Pattern of Failure after Helical Tomotherapy in Head and Neck Cancer. Strahlenther. Onkol. 2010, 186, 511–516. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Shueng, P.-W.; Wang, L.-Y.; Liao, L.-J.; Lin, Y.-C.; Kuo, Y.-S.; Lo, W.-C.; Tseng, C.-F.; Tien, H.-J.; Chou, H.-L.; et al. Clinical Effectiveness, Toxicity, and Failure Patterns of Helical Tomotherapy for Postoperative Oral Cavity Cancer Patients. OncoTargets Ther. 2014, 7, 405–414. [Google Scholar] [CrossRef][Green Version]
- Seung, S.; Bae, J.; Solhjem, M.; Bader, S.; Gannett, D.; Hansen, E.K.; Louie, J.; Underhill, K.; Cha, C. Intensity-Modulated Radiotherapy for Head-and-Neck Cancer in the Community Setting. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1075–1081. [Google Scholar] [CrossRef]
- Capuano, G.; Grosso, A.; Gentile, P.C.; Battista, M.; Bianciardi, F.; Di Palma, A.; Pavese, I.; Satta, F.; Tosti, M.; Palladino, A.; et al. Influence of Weight Loss on Outcomes in Patients with Head and Neck Cancer Undergoing Concomitant Chemoradiotherapy. Head Neck 2008, 30, 503–508. [Google Scholar] [CrossRef]
- Pacholke, H.D.; Amdur, R.J.; Morris, C.G.; Li, J.G.; Dempsey, J.F.; Hinerman, R.W.; Mendenhall, W.M. Late Xerostomia After Intensity-Modulated Radiation Therapy Versus Conventional Radiotherapy. Am. J. Clin. Oncol. 2005, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Saarilahti, K.; Kouri, M.; Collan, J.; Hämäläinen, T.; Atula, T.; Joensuu, H.; Tenhunen, M. Intensity Modulated Radiotherapy for Head and Neck Cancer: Evidence for Preserved Salivary Gland Function. Radiother. Oncol. 2005, 74, 251–258. [Google Scholar] [CrossRef]
- Blanco, A.I.; Chao, K.S.C.; El Naqa, I.; Franklin, G.E.; Zakarian, K.; Vicic, M.; Deasy, J.O. Dose–Volume Modeling of Salivary Function in Patients with Head-and-Neck Cancer Receiving Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.S.C.; Deasy, J.O.; Markman, J.; Haynie, J.; Perez, C.A.; Purdy, J.A.; Low, D.A. A Prospective Study of Salivary Function Sparing in Patients with Head-and-Neck Cancers Receiving Intensity-Modulated or Three-Dimensional Radiation Therapy: Initial Results. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 907–916. [Google Scholar] [CrossRef]
- Lin, A.; Kim, H.M.; Terrell, J.E.; Dawson, L.A.; Ship, J.A.; Eisbruch, A. Quality of Life after Parotid-Sparing IMRT for Head-and-Neck Cancer: A Prospective Longitudinal Study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 61–70. [Google Scholar] [CrossRef]
- Amosson, C.M.; Teh, B.S.; Van, T.J.; Uy, N.; Huang, E.; Mai, W.-Y.; Frolov, A.; Woo, S.Y.; Chiu, J.K.; Carpenter, L.S.; et al. Dosimetric Predictors of Xerostomia for Head-and-Neck Cancer Patients Treated with the Smart (Simultaneous Modulated Accelerated Radiation Therapy) Boost Technique. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 136–144. [Google Scholar] [CrossRef]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A Systematic Review of Salivary Gland Hypofunction and Xerostomia Induced by Cancer Therapies: Management Strategies and Economic Impact. Support. Care Cancer 2010, 18, 1061–1079. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef]
- Nguyen, N.P.; Vos, P.; Vinh-Hung, V.; Ceizyk, M.; Smith-Raymond, L.; Stevie, M.; Slane, B.; Chi, A.; Desai, A.; Krafft, S.P.; et al. Feasibility of Image-Guided Radiotherapy Based on Helical Tomotherapy to Reduce Contralateral Parotid Dose in Head and Neck Cancer. BMC Cancer 2012, 12, 175. [Google Scholar] [CrossRef]
- Beck, A.-J.C.C.; Kieffer, J.M.; Retèl, V.P.; van Overveld, L.F.J.; Takes, R.P.; van den Brekel, M.W.M.; van Harten, W.H.; Stuiver, M.M. Mapping the EORTC QLQ-C30 and QLQ-H&N35 to the EQ-5D for Head and Neck Cancer: Can Disease-Specific Utilities Be Obtained? PLoS ONE 2019, 14, e0226077. [Google Scholar] [CrossRef]
- Bjordal, K.; de Grae, A.; Fayers, P.M.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.J.; Meyza, J.W.; Brédart, A.; et al. A 12 Country field Study of the EORTC QLQ-C30 (Version 3.0) and the Head and Neck Cancer Specific Module (EORTC QLQ-H&N35) in Head and Neck Patients. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar]
- Wan Leung, S.; Lee, T.-F.; Chien, C.-Y.; Chao, P.-J.; Tsai, W.-L.; Fang, F.-M. Health-Related Quality of Life in 640 Head and Neck Cancer Survivors after Radiotherapy Using EORTC QLQ-C30 and QLQ-H&N35 Questionnaires. BMC Cancer 2011, 11, 128. [Google Scholar] [CrossRef]
- Voordeckers, M.; Farrag, A.; Everaert, H.; Tournel, K.; Storme, G.; Verellen, D.; De Ridder, M. Parotid Gland Sparing With Helical Tomotherapy in Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 443–448. [Google Scholar] [CrossRef]
- Engelsman, M.; Rosenthal, S.J.; Michaud, S.L.; Adams, J.A.; Schneider, R.J.; Bradley, S.G.; Flanz, J.B.; Kooy, H.M. Intra- and Interfractional Patient Motion for a Variety of Immobilization Devices. Med. Phys. 2005, 32, 3468–3474. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, T.; Stemkens, B.; Terhaard, C.H.J.; Lagendijk, J.J.W.; Raaijmakers, C.P.J.; Tijssen, R.H.N. Intrafraction Motion Quantification and Planning Target Volume Margin Determination of Head-and-Neck Tumors Using Cine Magnetic Resonance Imaging. Radiother. Oncol. 2019, 130, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Gurney-Champion, O.J.; McQuaid, D.; Dunlop, A.; Wong, K.H.; Welsh, L.C.; Riddell, A.M.; Koh, D.-M.; Oelfke, U.; Leach, M.O.; Nutting, C.M.; et al. MRI-Based Assessment of 3D Intrafractional Motion of Head and Neck Cancer for Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.; Licht, N.; Rübe, C.; Dzierma, Y. Image Guidance and Positioning Accuracy in Clinical Practice: Influence of Positioning Errors and Imaging Dose on the Real Dose Distribution for Head and Neck Cancer Treatment. Radiat. Oncol. 2018, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.X.; Malcolm, A.W. An Optically Stimulated Luminescence Dosimeter for Measuring Patient Exposure from Imaging Guidance Procedures. Phys. Med. Biol. 2013, 58, 5885–5897. [Google Scholar] [CrossRef]
- Spezi, E.; Downes, P.; Jarvis, R.; Radu, E.; Staffurth, J. Patient-Specific Three-Dimensional Concomitant Dose From Cone Beam Computed Tomography Exposure in Image-Guided Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 419–426. [Google Scholar] [CrossRef]
- Gupta, T.; Narayan, C.A. Image-Guided Radiation Therapy: Physician’s Perspectives. J. Med. Phys. 2012, 37, 174–182. [Google Scholar] [CrossRef]
- Deshpande, S.; Dhote, D.S.; Kumar, R.; Naidu, S.; Sutar, A.; Kannan, V. Use of Image Guided Radiation Therapy Techniques and Imaging Dose Measurement at Indian Hospitals: A Survey. J. Med. Phys. 2015, 40, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Alaei, P.; Spezi, E. Imaging Dose from Cone Beam Computed Tomography in Radiation Therapy. Phys. Med. 2015, 31, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Otazo, R.; Lambin, P.; Pignol, J.-P.; Ladd, M.E.; Schlemmer, H.-P.; Baumann, M.; Hricak, H. MRI-Guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology. Radiology 2021, 298, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Boeke, S.; Mönnich, D.; van Timmeren, J.E.; Balermpas, P. MR-Guided Radiotherapy for Head and Neck Cancer: Current Developments, Perspectives, and Challenges. Front. Oncol. 2021, 11, 616156. [Google Scholar] [CrossRef]
- Gupta, A.; Dunlop, A.; Mitchell, A.; McQuaid, D.; Nill, S.; Barnes, H.; Newbold, K.; Nutting, C.; Bhide, S.; Oelfke, U.; et al. Online Adaptive Radiotherapy for Head and Neck Cancers on the MR Linear Accelerator: Introducing a Novel Modified Adapt-to-Shape Approach. Clin. Transl. Radiat. Oncol. 2022, 32, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Chuter, R.W.; Pollitt, A.; Whitehurst, P.; MacKay, R.I.; van Herk, M.; McWilliam, A. Assessing MR-Linac Radiotherapy Robustness for Anatomical Changes in Head and Neck Cancer. Phys. Med. Biol. 2018, 63, 125020. [Google Scholar] [CrossRef]
- Raaijmakers, A.J.E.; Raaymakers, B.W.; Lagendijk, J.J.W. Integrating a MRI Scanner with a 6 MV Radiotherapy Accelerator: Dose Increase at Tissue-Air Interfaces in a Lateral Magnetic Field Due to Returning Electrons. Phys. Med. Biol. 2005, 50, 1363–1376. [Google Scholar] [CrossRef]
- Raaijmakers, A.J.E.; Raaymakers, B.W.; van der Meer, S.; Lagendijk, J.J.W. Integrating a MRI Scanner with a 6 MV Radiotherapy Accelerator: Impact of the Surface Orientation on the Entrance and Exit Dose Due to the Transverse Magnetic Field. Phys. Med. Biol. 2007, 52, 929–939. [Google Scholar] [CrossRef]
- Keyvanloo, A.; Burke, B.; Warkentin, B.; Tadic, T.; Rathee, S.; Kirkby, C.; Santos, D.M.; Fallone, B.G. Skin Dose in Longitudinal and Transverse Linac-MRIs Using Monte Carlo and Realistic 3D MRI Field Models. Med. Phys. 2012, 39, 6509–6521. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Raaijmakers, A.J.E.; Kotte, A.N.T.J.; Jette, D.; Lagendijk, J.J.W. Integrating a MRI Scanner with a 6 MV Radiotherapy Accelerator: Dose Deposition in a Transverse Magnetic Field. Phys. Med. Biol. 2004, 49, 4109–4118. [Google Scholar] [CrossRef]
- Kirkby, C.; Stanescu, T.; Fallone, B.G. Magnetic Field Effects on the Energy Deposition Spectra of MV Photon Radiation. Phys. Med. Biol. 2009, 54, 243–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oborn, B.M.; Metcalfe, P.E.; Butson, M.J.; Rosenfeld, A.B. High Resolution Entry and Exit Monte Carlo Dose Calculations from a Linear Accelerator 6 MV Beam under the Influence of Transverse Magnetic Fields. Med. Phys. 2009, 36, 3549–3559. [Google Scholar] [CrossRef] [PubMed]
- Oborn, B.M.; Metcalfe, P.E.; Butson, M.J.; Rosenfeld, A.B. Monte Carlo Characterization of Skin Doses in 6 MV Transverse Field MRI-Linac Systems: Effect of Field Size, Surface Orientation, Magnetic Field Strength, and Exit Bolus. Med. Phys. 2010, 37, 5208–5217. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, T.; Stemkens, B.; Lagendijk, J.J.W.; van den Berg, C.A.T.; Tijssen, R.H.N. Multiresolution Radial MRI to Reduce IDLE Time in Pre-Beam Imaging on an MR-Linac (MR-RIDDLE). Phys. Med. Biol. 2019, 64, 055011. [Google Scholar] [CrossRef] [PubMed]
- Ng-Cheng-Hin, B.; Nutting, C.; Newbold, K.; Bhide, S.; McQuaid, D.; Dunlop, A.; Harrington, K.; Wong, K.H. The Impact of Restricted Length of Treatment Field and Anthropometric Factors on Selection of Head and Neck Cancer Patients for Treatment on the MR-Linac. Br. J. Radiol. 2020, 93, 20200023. [Google Scholar] [CrossRef]
- Sayan, M.; Serbez, I.; Teymur, B.; Gur, G.; Zoto Mustafayev, T.; Gungor, G.; Atalar, B.; Ozyar, E. Patient-Reported Tolerance of Magnetic Resonance-Guided Radiation Therapy. Front. Oncol. 2020, 10, 1782. [Google Scholar] [CrossRef]
- Romesser, P.B.; Cahlon, O.; Scher, E.D.; Hug, E.B.; Sine, K.; DeSelm, C.; Fox, J.L.; Mah, D.; Garg, M.K.; Han-Chih Chang, J.; et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-Institutional Report on Feasibility and Early Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 386–395. [Google Scholar] [CrossRef]
- Phan, J.; Sio, T.T.; Nguyen, T.P.; Takiar, V.; Gunn, G.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Beadle, B.; et al. Reirradiation of Head and Neck Cancers with Proton Therapy: Outcomes and Analyses. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 30–41. [Google Scholar] [CrossRef]
- Lin, A.; Swisher-McClure, S.; Millar, L.B.; Kirk, M.; Yeager, C.; Kassaee, A.; Teo, B.-K.K.; Hahn, S.M. Proton Therapy for Head and Neck Cancer: Current Applications and Future Directions. Transl. Cancer Res. 2013, 1. [Google Scholar] [CrossRef]
- Kandula, S.; Zhu, X.; Garden, A.S.; Gillin, M.; Rosenthal, D.I.; Ang, K.-K.; Mohan, R.; Amin, M.V.; Garcia, J.A.; Wu, R.; et al. Spot-Scanning Beam Proton Therapy vs Intensity-Modulated Radiation Therapy for Ipsilateral Head and Neck Malignancies: A Treatment Planning Comparison. Med. Dosim. 2013, 38, 390–394. [Google Scholar] [CrossRef]
- Steneker, M.; Lomax, A.; Schneider, U. Intensity Modulated Photon and Proton Therapy for the Treatment of Head and Neck Tumors. Radiother. Oncol. 2006, 80, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.J.; Cox, J.D.; Gillin, M.; Mohan, R.; Garden, A.S.; Rosenthal, D.I.; Gunn, G.B.; Weber, R.S.; Kies, M.S.; Lewin, J.S.; et al. Multi-Field Optimization Intensity-Modulated Proton Therapy for Head and Neck Tumors—A Translation to Practice. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Nakamura, T.; Takada, A.; Makita, C.; Suzuki, M.; Azami, Y.; Kato, T.; Hayashi, Y.; Ono, T.; Toyomasu, Y.; et al. Treatment Results of Alternating Chemoradiotherapy Followed by Proton Beam Therapy Boost Combined with Intra-Arterial Infusion Chemotherapy for Stage III–IVB Tongue Cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.C.; Mitra, N.; Harton, J.G.; Xiao, Y.; Wojcieszynski, A.P.; Gabriel, P.E.; Zhong, H.; Geng, H.; Doucette, A.; Wei, J.; et al. Comparative Effectiveness of Proton vs Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer. JAMA Oncol. 2020, 6, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.C.; Frank, S.J.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Gunn, G.B.; Reddy, J.P.; Morrison, W.H.; Williamson, T.D.; Holliday, E.B.; et al. Intensity Modulated Proton Therapy (IMPT)—The Future of IMRT for Head and Neck Cancer. Oral Oncol. 2019, 88, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Stuschke, M.; Kaiser, A.; Jawad, J.A.; Pöttgen, C.; Levegrün, S.; Farr, J. Multi-Scenario Based Robust Intensity-Modulated Proton Therapy (IMPT) Plans Can Account for Set-up Errors More Effectively in Terms of Normal Tissue Sparing than Planning Target Volume (PTV) Based Intensity-Modulated Photon Plans in the Head and Neck Region. Radiat. Oncol. 2013, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.S.; Duma, M.N.; Kampfer, S.; Nill, S.; Oelfke, U.; Geinitz, H.; Wilkens, J.J. Impact of Interfractional Changes in Head and Neck Cancer Patients on the Delivered Dose in Intensity Modulated Radiotherapy with Protons and Photons. Phys. Med. 2015, 31, 266–272. [Google Scholar] [CrossRef][Green Version]
- Stützer, K.; Jakobi, A.; Bandurska-Luque, A.; Barczyk, S.; Arnsmeyer, C.; Löck, S.; Richter, C. Potential Proton and Photon Dose Degradation in Advanced Head and Neck Cancer Patients by Intratherapy Changes. J. Appl. Clin. Med. Phys. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Placidi, L.; Bolsi, A.; Lomax, A.J.; Schneider, R.A.; Malyapa, R.; Weber, D.C.; Albertini, F. Effect of Anatomic Changes on Pencil Beam Scanned Proton Dose Distributions for Cranial and Extracranial Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 616–623. [Google Scholar] [CrossRef]
- Beddok, A.; Vela, A.; Calugaru, V.; Tessonnier, T.; Kubes, J.; Dutheil, P.; Gerard, A.; Vidal, M.; Goudjil, F.; Florescu, C.; et al. Proton Therapy for Head and Neck Squamous Cell Carcinomas: A Review of the Physical and Clinical Challenges. Radiother. Oncol. 2020, 147, 30–39. [Google Scholar] [CrossRef]
- MacKay, R.I. Image Guidance for Proton Therapy. Clin. Oncol. 2018, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Oborn, B.; Moteabbed, M.; Yan, S.; Bortfeld, T.; Knopf, A.; Fuchs, H.; Georg, D.; Seco, J.; Spadea, M.F.; et al. MR-Guided Proton Therapy: A Review and a Preview. Radiat. Oncol. 2020, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.S.C.; McHugh, L.; Aznar, M.; Lindsay, J.; Eccles, C. Streamlining the Image-Guided Radiotherapy Process for Proton Beam Therapy. Br. J. Radiol. 2021, 94, 20210764. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.; Nijhuis, R.; Dedes, G.; Handrack, J.; Thieke, C.; Janssens, G.; Orban de Xivry, J.; Reiner, M.; Kamp, F.; Wilkens, J.J.; et al. Investigating CT to CBCT Image Registration for Head and Neck Proton Therapy as a Tool for Daily Dose Recalculation. Med. Phys. 2015, 42, 1354–1366. [Google Scholar] [CrossRef]
- Harms, J.; Lei, Y.; Wang, T.; McDonald, M.; Ghavidel, B.; Stokes, W.; Curran, W.J.; Zhou, J.; Liu, T.; Yang, X. Cone-Beam CT-Derived Relative Stopping Power Map Generation via Deep Learning for Proton Radiotherapy. Med. Phys. 2020, 47, 4416–4427. [Google Scholar] [CrossRef]
- Arai, K.; Kadoya, N.; Kato, T.; Endo, H.; Komori, S.; Abe, Y.; Nakamura, T.; Wada, H.; Kikuchi, Y.; Takai, Y.; et al. Feasibility of CBCT-Based Proton Dose Calculation Using a Histogram-Matching Algorithm in Proton Beam Therapy. Phys. Med. 2017, 33, 68–76. [Google Scholar] [CrossRef]
- Burigo, L.N.; Oborn, B.M. MRI-Guided Proton Therapy Planning: Accounting for an Inline MRI Fringe Field. Phys. Med. Biol. 2019, 64, 215015. [Google Scholar] [CrossRef]
- Santos, D.M.; Wachowicz, K.; Burke, B.; Fallone, B.G. Proton Beam Behavior in a Parallel Configured MRI-Proton Therapy Hybrid: Effects of Time-Varying Gradient Magnetic Fields. Med. Phys. 2019, 46, 822–838. [Google Scholar] [CrossRef]
- Pham, T.T.; Whelan, B.; Oborn, B.M.; Delaney, G.P.; Vinod, S.; Brighi, C.; Barton, M.; Keall, P. Magnetic Resonance Imaging (MRI) Guided Proton Therapy: A Review of the Clinical Challenges, Potential Benefits and Pathway to Implementation. Radiother. Oncol. 2022, 170, 37–47. [Google Scholar] [CrossRef]
- Servagi-Vernat, S.; Differding, S.; Sterpin, E.; Hanin, F.-X.; Labar, D.; Bol, A.; Lee, J.A.; Grégoire, V. Hypoxia-Guided Adaptive Radiation Dose Escalation in Head and Neck Carcinoma: A Planning Study. Acta Oncol. 2015, 54, 1008–1016. [Google Scholar] [CrossRef]
- Hodolič, M.; Fettich, J.; Kairemo, K. Hypoxia PET Tracers in EBRT Dose Planning in Head and Neck Cancer. Curr. Radiopharm. 2015, 8, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Johansen, J.; Kallehauge, J.; Primdahl, H.; Busk, M.; Lassen, P.; Alsner, J.; Sørensen, B.S.; Toustrup, K.; Jakobsen, S.; et al. FAZA PET/CT Hypoxia Imaging in Patients with Squamous Cell Carcinoma of the Head and Neck Treated with Radiotherapy: Results from the DAHANCA 24 Trial. Radiother. Oncol. 2012, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.G.; Schwartz, D.L.; O’Sullivan, J.; Peterson, L.M.; Ng, P.; Scharnhorst, J.; Grierson, J.R.; Krohn, K.A. Tumor Hypoxia Imaging with [F-18] Fluoromisonidazole Positron Emission Tomography in Head and Neck Cancer. Clin. Cancer Res. 2006, 12, 5435–5441. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting Tumour Hypoxia in Cancer Treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic Value of Tumor Oxygenation in 397 Head and Neck Tumors after Primary Radiation Therapy. An International Multi-Center Study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J. Hypoxic Modification of Radiotherapy in Squamous Cell Carcinoma of the Head and Neck—A Systematic Review and Meta-Analysis. Radiother. Oncol. 2011, 100, 22–32. [Google Scholar] [CrossRef]
- Spanier, G.; Weidt, D.; Hellwig, D.; Meier, J.K.H.; Reichert, T.E.; Grosse, J. Total Lesion Glycolysis in Oral Squamous Cell Carcinoma as a Biomarker Derived from Pre-Operative FDG PET/CT Outperforms Established Prognostic Factors in a Newly Developed Multivariate Prediction Model. Oncotarget 2021, 12, 37–48. [Google Scholar] [CrossRef]
- Berwouts, D.; Olteanu, L.A.M.; Duprez, F.; Vercauteren, T.; De Gersem, W.; De Neve, W.; Van de Wiele, C.; Madani, I. Three-Phase Adaptive Dose-Painting-by-Numbers for Head-and-Neck Cancer: Initial Results of the Phase I Clinical Trial. Radiother. Oncol. 2013, 107, 310–316. [Google Scholar] [CrossRef]
- Duprez, F.; De Neve, W.; De Gersem, W.; Coghe, M.; Madani, I. Adaptive Dose Painting by Numbers for Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1045–1055. [Google Scholar] [CrossRef]
- Lambrecht, M.; Van Calster, B.; Vandecaveye, V.; De Keyzer, F.; Roebben, I.; Hermans, R.; Nuyts, S. Integrating Pretreatment Diffusion Weighted MRI into a Multivariable Prognostic Model for Head and Neck Squamous Cell Carcinoma. Radiother. Oncol. 2014, 110, 429–434. [Google Scholar] [CrossRef]
- Hatakenaka, M.; Nakamura, K.; Yabuuchi, H.; Shioyama, Y.; Matsuo, Y.; Ohnishi, K.; Sunami, S.; Kamitani, T.; Setoguchi, T.; Yoshiura, T.; et al. Pretreatment Apparent Diffusion Coefficient of the Primary Lesion Correlates with Local Failure in Head-and-Neck Cancer Treated With Chemoradiotherapy or Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Hatakenaka, M.; Shioyama, Y.; Nakamura, K.; Yabuuchi, H.; Matsuo, Y.; Sunami, S.; Kamitani, T.; Yoshiura, T.; Nakashima, T.; Nishikawa, K.; et al. Apparent Diffusion Coefficient Calculated with Relatively High B-Values Correlates with Local Failure of Head and Neck Squamous Cell Carcinoma Treated with Radiotherapy. AJNR Am. J. Neuroradiol. 2011, 32, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Loevner, L.; Quon, H.; Sherman, E.; Weinstein, G.; Kilger, A.; Poptani, H. Diffusion Weighted MRI for Predicting and Detecting Early Response to Chemoradiation Therapy of Squamous Cell Carcinomas of the Head and Neck. Clin. Cancer Res. 2009, 15, 986–994. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Chow, K.-K.; Yu, K.-H.; Mo, F.K.F.; Yeung, D.K.W.; Yuan, J.; Bhatia, K.S.; Vlantis, A.C.; Ahuja, A.T. Head and Neck Squamous Cell Carcinoma: Diagnostic Performance of Diffusion-Weighted MR Imaging for the Prediction of Treatment Response. Radiology 2013, 266, 531–538. [Google Scholar] [CrossRef]
- Morgan, H.E.; Sher, D.J. Adaptive Radiotherapy for Head and Neck Cancer. Cancers Head Neck 2020, 5, 1. [Google Scholar] [CrossRef]
- Keall, P.J.; Brighi, C.; Glide-Hurst, C.; Liney, G.; Liu, P.Z.Y.; Lydiard, S.; Paganelli, C.; Pham, T.; Shan, S.; Tree, A.C.; et al. Integrated MRI-Guided Radiotherapy—Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2022, 19, 458–470. [Google Scholar] [CrossRef]
- Ohnleiter, T.; Antoni, D.; Lefebvre, F.; Truntzer, P.; Schultz, P.; Burgy, M.; Borel, C.; Noël, G. Factors Improving the Outcome of Patients Re-Irradiated with Intensity-Modulated Radiotherapy (IMRT) for Relapse or New Head and Neck Cancer Developed in Irradiated Areas. Chin. Clin. Oncol. 2018, 7, 60. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Fan, K.-H.; Lin, C.-Y.; Kang, C.-J.; Huang, S.-F.; Wang, H.-M.; Cheng, A.-J.; Chang, J.T.-C. Outcomes of Re-Irradiation for Oral Cavity Squamous Cell Carcinoma. Biomed. J. 2021, S2319417021001773. [Google Scholar] [CrossRef]
- Strojan, P.; Corry, J.; Eisbruch, A.; Vermorken, J.B.; Mendenhall, W.M.; Lee, A.W.M.; Haigentz, M., Jr.; Beitler, J.J.; de Bree, R.; Takes, R.P.; et al. Recurrent and Second Primary Squamous Cell Carcinoma of the Head and Neck: When and How to Reirradiate. Head Neck 2015, 37, 134–150. [Google Scholar] [CrossRef]
- Tortochaux, J.; Tao, Y.; Tournay, E.; Lapeyre, M.; Lesaunier, F.; Bardet, E.; Janot, F.; Lusinchi, A.; Benhamou, E.; Bontemps, P.; et al. Randomized Phase III Trial (GORTEC 98-03) Comparing Re-Irradiation plus Chemotherapy versus Methotrexate in Patients with Recurrent or a Second Primary Head and Neck Squamous Cell Carcinoma, Treated with a Palliative Intent. Radiother. Oncol. 2011, 100, 70–75. [Google Scholar] [CrossRef]
- Rühle, A.; Sprave, T.; Kalckreuth, T.; Stoian, R.; Haehl, E.; Zamboglou, C.; Laszig, R.; Knopf, A.; Grosu, A.-L.; Nicolay, N.H. The Value of Moderate Dose Escalation for Re-Irradiation of Recurrent or Second Primary Head-and-Neck Cancer. Radiat. Oncol. 2020, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Chan, K.; Bekelman, J.E.; Zhung, J.; Mechalakos, J.; Narayana, A.; Wolden, S.; Venkatraman, E.S.; Pfister, D.; Kraus, D.; et al. Salvage Re-Irradiation for Recurrent Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Duprez, F.; Madani, I.; Bonte, K.; Boterberg, T.; Vakaet, L.; Derie, C.; De Gersem, W.; De Neve, W. Intensity-Modulated Radiotherapy for Recurrent and Second Primary Head and Neck Cancer in Previously Irradiated Territory. Radiother. Oncol. 2009, 93, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Vargo, J.A.; Ferris, R.L.; Ohr, J.; Clump, D.A.; Davis, K.S.; Duvvuri, U.; Kim, S.; Johnson, J.T.; Bauman, J.E.; Gibson, M.K.; et al. A Prospective Phase 2 Trial of Reirradiation With Stereotactic Body Radiation Therapy Plus Cetuximab in Patients With Previously Irradiated Recurrent Squamous Cell Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 480–488. [Google Scholar] [CrossRef]
- Stanisce, L.; Koshkareva, Y.; Xu, Q.; Patel, A.; Squillante, C.; Ahmad, N.; Rajagopalan, K.; Kubicek, G.J. Stereotactic Body Radiotherapy Treatment for Recurrent, Previously Irradiated Head and Neck Cancer. Technol. Cancer Res. Treat. 2018, 17, 1533033818780086. [Google Scholar] [CrossRef]
- Rwigema, J.-C.M.; Heron, D.E.; Ferris, R.L.; Andrade, R.S.; Gibson, M.K.; Yang, Y.; Ozhasoglu, C.; Argiris, A.E.; Grandis, J.R.; Burton, S.A. The Impact of Tumor Volume and Radiotherapy Dose on Outcome in Previously Irradiated Recurrent Squamous Cell Carcinoma of the Head and Neck Treated With Stereotactic Body Radiation Therapy. Am. J. Clin. Oncol. 2011, 34, 372–379. [Google Scholar] [CrossRef]
- Ansinelli, H.; Singh, R.; Sharma, D.L.; Jenkins, J.; Davis, J.; Vargo, J.A.; Sharma, S. Salvage Stereotactic Body Radiation Therapy for Locally Recurrent Previously Irradiated Head and Neck Squamous Cell Carcinoma: An Analysis from the RSSearch® Registry. Cureus 2018, 10, e3237. [Google Scholar] [CrossRef]
- Salama, J.K.; Vokes, E.E.; Chmura, S.J.; Milano, M.T.; Kao, J.; Stenson, K.M.; Witt, M.E.; Haraf, D.J. Long-Term Outcome of Concurrent Chemotherapy and Reirradiation for Recurrent and Second Primary Head-and-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 382–391. [Google Scholar] [CrossRef]
- Krstevska, V.; Tolevska, C.; Zafirova-Ivanova, B.; Stojkovski, I.; Crvenkova, S. Prognostic Factors in Patients with Recurrent Head and Neck Cancer Treated with Reirradiation. J. BUON 2008, 13, 369–376. [Google Scholar]
- Kasperts, N.; Slotman, B.; Leemans, C.R.; Langendijk, J.A. A Review on Re-Irradiation for Recurrent and Second Primary Head and Neck Cancer. Oral Oncol. 2005, 41, 225–243. [Google Scholar] [CrossRef]
- Wang, K.; Heron, D.E.; Clump, D.A.; Flickinger, J.C.; Kubicek, G.J.; Rwigema, J.-C.M.; Ferris, R.L.; Ohr, J.P.; Quinn, A.E.; Ozhasoglu, C.; et al. Target Delineation in Stereotactic Body Radiation Therapy for Recurrent Head and Neck Cancer: A Retrospective Analysis of the Impact of Margins and Automated PET-CT Segmentation. Radiother. Oncol. 2013, 106, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Embring, A.; Onjukka, E.; Mercke, C.; Lax, I.; Berglund, A.; Bornedal, S.; Wennberg, B.; Friesland, S. Overlapping Volumes in Re-Irradiation for Head and Neck Cancer—An Important Factor for Patient Selection. Radiat. Oncol. 2020, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Keeling, V.; Hossain, S.; Jin, H.; Algan, O.; Ahmad, S.; Ali, I. Quantitative Evaluation of Patient Setup Uncertainty of Stereotactic Radiotherapy with the Frameless 6D ExacTrac System Using Statistical Modeling. J. Appl. Clin. Med. Phys. 2016, 17, 111–127. [Google Scholar] [CrossRef]
- Mesko, S.; Wang, H.; Tung, S.; Wang, C.; Pasalic, D.; Chapman, B.V.; Moreno, A.C.; Reddy, J.P.; Garden, A.S.; Rosenthal, D.I.; et al. Estimating PTV Margins in Head and Neck Stereotactic Ablative Radiation Therapy (SABR) through Target Site Analysis of Positioning and Intrafractional Accuracy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 185–193. [Google Scholar] [CrossRef]
- Heron, D.E.; Ferris, R.L.; Karamouzis, M.; Andrade, R.S.; Deeb, E.L.; Burton, S.; Gooding, W.E.; Branstetter, B.F.; Mountz, J.M.; Johnson, J.T.; et al. Stereotactic Body Radiotherapy for Recurrent Squamous Cell Carcinoma of the Head and Neck: Results of a Phase I Dose-Escalation Trial. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Lartigau, E.F.; Tresch, E.; Thariat, J.; Graff, P.; Coche-Dequeant, B.; Benezery, K.; Schiappacasse, L.; Degardin, M.; Bondiau, P.-Y.; Peiffert, D.; et al. Multi Institutional Phase II Study of Concomitant Stereotactic Reirradiation and Cetuximab for Recurrent Head and Neck Cancer. Radiother. Oncol. 2013, 109, 281–285. [Google Scholar] [CrossRef]
- Eckstein, J.; Sidiqi, B.U.; Gogineni, E.; Lisser, N.; Teckie, S.; Marrero, M.; Malsevic, V.; Antone, J.; Seetharamu, N.; Frank, D.; et al. Stereotactic Body Radiation Therapy (SBRT) in Oropharynx and Oral Cavity Cancer: Toxicity and Local Control. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e391. [Google Scholar] [CrossRef]
- Biau, J.; Thivat, E.; Millardet, C.; Saroul, N.; Pham-Dang, N.; Molnar, I.; Pereira, B.; Durando, X.; Bourhis, J.; Lapeyre, M. A Multicenter Prospective Phase II Study of Postoperative Hypofractionated Stereotactic Body Radiotherapy (SBRT) in the Treatment of Early-Stage Oropharyngeal and Oral Cavity Cancers with High Risk Margins: The STEREO POSTOP GORTEC 2017-03 Trial. BMC Cancer 2020, 20, 730. [Google Scholar] [CrossRef]

| Landmarks | Dimensionality | Advantages | Disadvantages | |
|---|---|---|---|---|
| Two-dimensional X-ray plain-film imaging guidance | Bone structure | 2D |
|
|
| Three-dimensional computed tomography imaging guidance | Soft tissue and bone structure | 3D |
|
|
| Magnetic resonance imaging guidance | Water and fat distribution | 3D |
|
|
| Infrared markers for image guidance | Infrared marker position | others |
|
|
| Body surface for image guidance | Surface shape | others |
|
|
| Body temperature for image guidance | Body temperature mapping | others |
|
|
| Modality | Number of Enrolled Patients | Percentage of Oral Cavity Cancer | Margin of PTV | No. of Marginal Failures/No. of Locoregional Failures | Percentage of Marginal Failure | |
|---|---|---|---|---|---|---|
| Bern University Hospital, Switzerland [81] | IMRT | 53 | 100% | 3 mm | 10/12 | 83% |
| University of Iowa Health Care, USA [65] | IMRT | 49 | 100% | 5 mm | 2/8 | 25% |
| Princess Margaret Hospital, Canada [82] | IMRT | 180 | 100% | - | 12/38 | 32% |
| University of California Davis School of Medicine, Canada [80] | IMRT | 90 | 48% | 3–5 mm | 6/17 | 35% |
| University of California Davis School of Medicine, Canada [58] | IMRT | 52 | 48% | 3–5 mm | 4/13 | 31% |
| Stanford University Medical Center, Canada [78] | IMRT | 30 | 100% | 3–5 mm | 2/11 | 18% |
| National Cancer Center Hospital East, Japan [83] | IMRT | 122 | 48% | 5 mm | 5/32 | 16% |
| Far Eastern Memorial Hospital, Taiwan [63] | IMRT | 79 | 100% | 5 mm | 10/19 | 53% |
| Far Eastern Memorial Hospital, Taiwan [63] | IG-IMRT | 73 | 100% | 3 mm | 0/5 | 0% |
| University of California Davis Comprehensive Cancer Center, USA [86] | IG-IMRT | 103 | 31% | 5 mm | 5/76 | 7% |
| University of California Davis Comprehensive Cancer Center, USA [86] | IG-IMRT | 264 | 21% | 3 mm | 4/76 | 5% |
| University of California Davis Comprehensive Cancer Center, USA [86] | IG-IMRT | 367 (103–5 mm, 264–3 mm) | 24% | 3–5 mm | 9/76 (5–5 mm, 4–3 mm) | 12% |
| Side Effect | Modality | Number of Enrolled Patients | Percentage of Oral Cavity Cancer | Gr.1 | Gr.2 | Gr.3 | Gr.4 | Significance |
|---|---|---|---|---|---|---|---|---|
| Body weight loss | ||||||||
| Hsieh et al. [63] | IMRT | 79 | 100% | 51 | 27 | 1 | 0 | |
| IG-IMRT | 73 | 100% | 62 | 11 | 0 | 0 | p = 0.004 | |
| Xerostomia | ||||||||
| Chen PY et al. [60] | CRT | 42 | 100% | - | 10 (34.5%) | 0 | - | |
| Chen WC et al. [57] | CRT | 27 | 100% | - | −82% | - | ||
| IMRT | 22 | 100% | - | −36% | - | |||
| Chen PY et al. [60] | IMRT | 72 | 100% | - | 8 (14.0%) | 0 | ||
| Moon et al. [5] | IMRT | 51 | 45.1% | - | - | 10 (19.6%) | - | |
| Wang et al. [23] | IMRT | 26 | 92.3% | - | - | 3 (11.5%) | - | |
| Seung et al. [90] | IMRT | 69 | 26% | 0 | 29 | 40 (58%) | 0 | |
| Hsieh et al. [76] | IG-IMRT | 19 | 100% | 10 | 9 | 0 | 0 | |
| Hsieh et al. [89] | IG-IMRT | 53 | 100% | (66.7%) | (33.3%) | 0 | 0 | |
| Leucopenia | ||||||||
| Hsieh et al. [63] | IMRT | 79 | 100% | 49 | 9 | 5 | 2 | |
| IG-IMRT | 73 | 100% | 25 | 17 | 6 | 1 | p = 0.007 | |
| Thrombocytopenia | ||||||||
| Hsieh et al. [63] | IMRT | 79 | 100% | 59 | 3 | 2 | 0 | |
| IG-IMRT | 73 | 100% | 41 | 1 | 0 | 0 | p = 0.003 | |
| Modality | Pros | Cons |
|---|---|---|
| Image-guided radiotherapy | Improve set-up accuracy Safe smaller margin -Clinical benefits including avoid marginal failure, reduce treatment side effects, good treatment tolerance, and overcome poor prognostic factors Understand tumor condition during treatment period Online adaptive plan Opportunities of biological mapping for dose painting. | Increase immobility duration -Not suitable for patients with claustrophobia, unstable conditions, or severe painIncrease ionizing radiation exposure (2D, 3D CT image) Disturbing noise during image scanning (MRI) The registration between the original CT contouring and the IGRT images and the decisions to interrupt the treatment due to bulk motion highly relies on the physician’s experience. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nien, H.-H.; Wang, L.-Y.; Liao, L.-J.; Lin, P.-Y.; Wu, C.-Y.; Shueng, P.-W.; Chung, C.-S.; Lo, W.-C.; Lin, S.-C.; Hsieh, C.-H. Advances in Image-Guided Radiotherapy in the Treatment of Oral Cavity Cancer. Cancers 2022, 14, 4630. https://doi.org/10.3390/cancers14194630
Nien H-H, Wang L-Y, Liao L-J, Lin P-Y, Wu C-Y, Shueng P-W, Chung C-S, Lo W-C, Lin S-C, Hsieh C-H. Advances in Image-Guided Radiotherapy in the Treatment of Oral Cavity Cancer. Cancers. 2022; 14(19):4630. https://doi.org/10.3390/cancers14194630
Chicago/Turabian StyleNien, Hsin-Hua, Li-Ying Wang, Li-Jen Liao, Ping-Yi Lin, Chia-Yun Wu, Pei-Wei Shueng, Chen-Shuan Chung, Wu-Chia Lo, Shih-Chiang Lin, and Chen-Hsi Hsieh. 2022. "Advances in Image-Guided Radiotherapy in the Treatment of Oral Cavity Cancer" Cancers 14, no. 19: 4630. https://doi.org/10.3390/cancers14194630
APA StyleNien, H.-H., Wang, L.-Y., Liao, L.-J., Lin, P.-Y., Wu, C.-Y., Shueng, P.-W., Chung, C.-S., Lo, W.-C., Lin, S.-C., & Hsieh, C.-H. (2022). Advances in Image-Guided Radiotherapy in the Treatment of Oral Cavity Cancer. Cancers, 14(19), 4630. https://doi.org/10.3390/cancers14194630






