Clinical and Prognostic Significance of the Eighth Edition Oral Cancer Staging System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
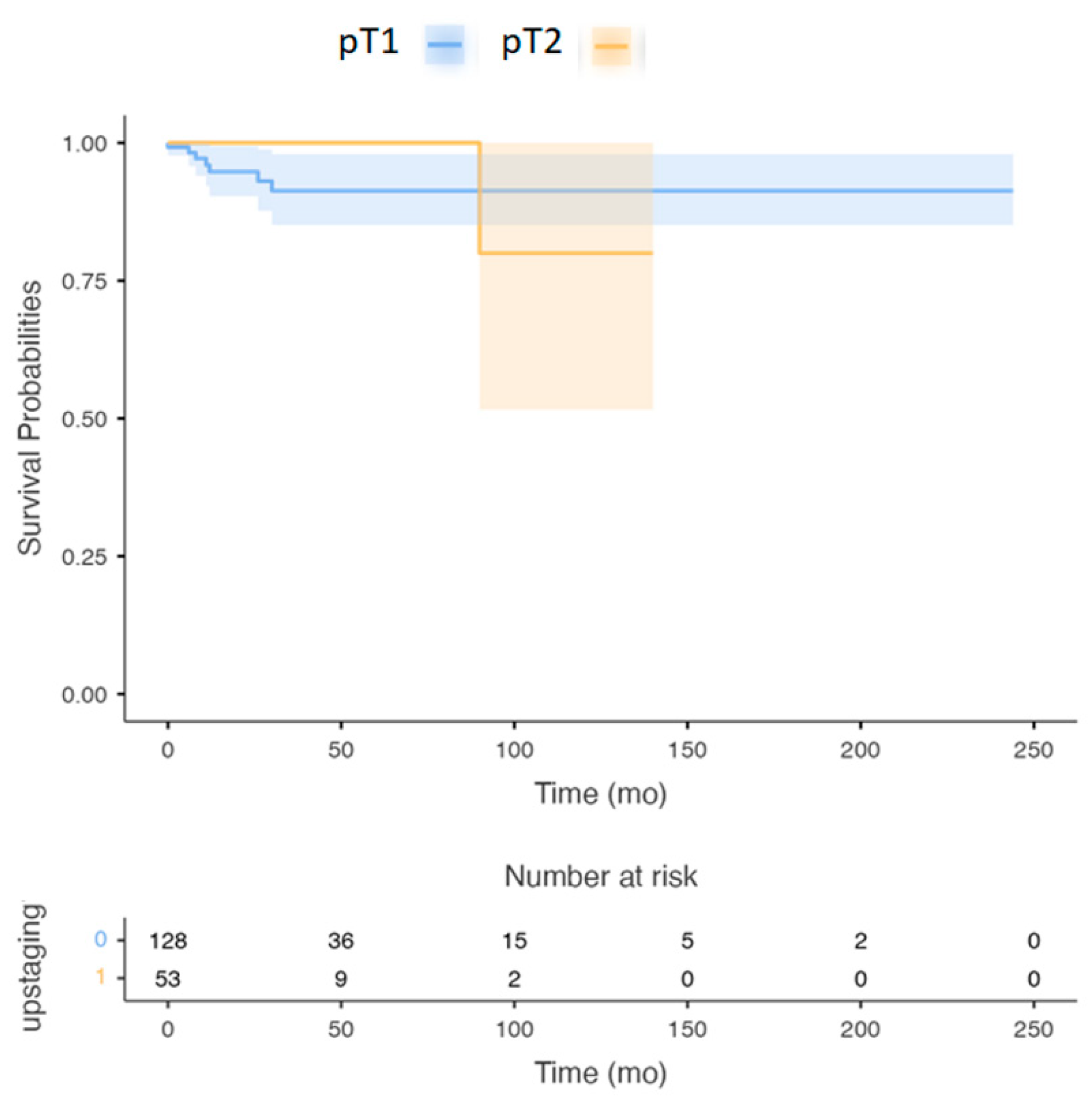
3. Results
3.1. Clinical and Pathological Results
3.1.1. Patients Clinicopathologic Data
3.1.2. Upstaged Patients According to the 8th AJCC Edition of the Study Group
3.1.3. The OS and Five Years DSS Were Analyzed between the Sub-Groups
3.1.4. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patterson, R.H.; Fischman, V.G.; Wasserman, I.; Siu, J.; Shrime, M.G.; Fagan, J.J.; Koch, W.; Alkire, B.C. Global burden of head and neck cancer: Economic consequences, health, and the role of surgery. Otolaryngol.-Head Neck Surg. 2020, 162, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Wahab, A.; Onkamo, O.; Pirinen, M.; Almangush, A.; Salo, T. The budding and depth of invasion model in oral cancer: A systematic review and meta-analysis. Oral Dis. 2020, 28, 275–283. [Google Scholar] [CrossRef]
- Berdugo, J.; Thompson, L.D.R.; Purgina, B.; Sturgis, C.D.; Tuluc, M.; Seethala, R.; Chiosea, S.I. Measuring Depth of Invasion in Early Squamous Cell Carcinoma of the Oral Tongue: Positive Deep Margin, Extratumoral Perineural Invasion, and Other Challenges. Head Neck Pathol. 2019, 13, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zenga, J.; Divi, V.; Stadler, M.; Massey, B.; Campbell, B.; Shukla, M.; Awan, M.; Schultz, C.J.; Shreenivas, A.; Wong, S.; et al. Lymph node yield, depth of invasion, and survival in node-negative oral cavity cancer. Oral Oncol. 2019, 98, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dünne, A.A.; Müller, H.H.; Eisele, D.W.; Keßel, K.; Moll, R.; Werner, J.A. Meta-analysis of the prognostic significance of perinodal spread in head and neck squamous cell carcinomas (HNSCC) patients. Eur. J. Cancer 2006, 42, 1863–1868. [Google Scholar] [CrossRef]
- Mermod, M.; Tolstonog, G.; Simon, C.; Monnier, Y. Extracapsular spread in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2016, 62, 60–71. [Google Scholar] [CrossRef]
- Moore, C.; Kuhns, J.G.; Greenberg, R.A. Thickness as Prognostic Aid in Upper Aerodigestive Tract Cancer. Arch. Surg. 1986, 121, 1410–1414. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Frederick, L.; Greene, A.T. (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Bray, F.; Ren, J.S.; Masuyer, E.; Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 2013, 132, 1133–1145. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Lauer, C.S.; Fredricks, S.; Clifford, A.R.; McNeil, E.B.; Bagia, J.S.; Koulmandas, C. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer—But what thickness? Head Neck 2003, 25, 937–945. [Google Scholar] [CrossRef]
- Ganly, I.; Goldstein, D.P.; Carlson, D.L.; Patel, S.G.; O’Sullivan, B.; Lee, N.; Gullane, P.J.; Shah, J. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: The importance of tumor thickness. Cancer 2013, 119, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, N.; Khafaga, Y.; El-Husseiny, J.; Saleem, M.; Mourad, W.; Al-Otieschan, A.; Al-Amro, A. Early stage carcinoma of oral tongue: Prognostic factors for local control and survival. Oral Oncol. 2000, 36, 508–514. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Esteban, F.; Rodriguez-Archilla, A.; Ruiz-Avila, I.; Gonzalez-Moles, S. Importance of tumour thickness measurement in prognosis of tongue cancer. Oral Oncol. 2002, 38, 394–397. [Google Scholar] [CrossRef]
- Piazza, C.; Bresciani, L.; Giannini, L. Depth of invasion for prognostic stratification in oral cavity cancer: Do we need further validation? Ann. Transl. Med. 2019, 7, S84. [Google Scholar] [CrossRef]
- de Matos, L.L.; Manfro, G.; dos Santos, R.V.; Stabenow, E.; de Mello, E.S.; Alves, V.A.F.; Pinto, F.R.; Kulcsar, M.A.V.; Brandão, L.G.; Cernea, C.R. Tumor thickness as a predictive factor of lymph node metastasis and disease recurrence in T1N0 and T2N0 squamous cell carcinoma of the oral tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.L.; Dedivitis, R.A.; Kulcsar, M.A.V.; de Mello, E.S.; Alves, V.A.F.; Cernea, C.R. External validation of the AJCC Cancer Staging Manual, 8th edition, in an independent cohort of oral cancer patients. Oral Oncol. 2017, 71, 47–53. [Google Scholar] [CrossRef]
- Faisal, M.; Abu Bakar, M.; Sarwar, A.; Adeel, M.; Batool, F.; Malik, K.I.; Jamshed, A.; Hussain, R. Depth of invasion (DOI) as a predictor of cervical nodal metastasis and local recurrence in early stage squamous cell carcinoma of oral tongue (ESSCOT). PLoS ONE 2018, 13, e0202632. [Google Scholar] [CrossRef]
- Kano, S.; Sakashita, T.; Tsushima, N.; Mizumachi, T.; Nakazono, A.; Suzuki, T.; Yasukawa, S.; Homma, A. Validation of the 8th edition of the AJCC/UICC TNM staging system for tongue squamous cell carcinoma. Int. J. Clin. Oncol. 2018, 23, 844–850. [Google Scholar] [CrossRef]
- Shinn, J.R.; Wood, C.B.; Colazo, J.M.; Harrell, F.E.; Rohde, S.L.; Mannion, K. Cumulative incidence of neck recurrence with increasing depth of invasion. Oral Oncol. 2018, 87, 36–42. [Google Scholar] [CrossRef]
- Huang, S.H.; Hwang, D.; Lockwood, G.; Goldstein, D.P.; O’Sullivan, B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: A Meta-analysis of reported studies. Cancer 2009, 115, 1489–1497. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. Cancer/Radiother. 2005, 9, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Wreesmann, V.B.; Katabi, N.; Palmer, F.L.; Montero, P.H.; Migliacci, J.C.; Goenen, M.; Carlson, D.; Ganly, I.; Shah, J.P.; Ghossein, R.; et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck 2014, 36, 1391. [Google Scholar] [CrossRef]
- Jeffrey, N.; Myers, M.D.; Greenberg, J.S.; Vivian Mo, B.A.; Roberts, D. Extracapsular Spread A Significant Predictor of Treatment Failure in Patients with Squamous Cell Carcinoma of the Tongue. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2001, 92, 3030–3036. [Google Scholar] [CrossRef]
- Künzel, J.; Psychogios, G.; Mantsopoulos, K.; Grundtner, P.; Waldfahrer, F.; Iro, H. Lymph node ratio as a predictor of outcome in patients with oropharyngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 1171–1180. [Google Scholar] [CrossRef] [PubMed]


| Variable | Num. (%) | p Value (t-Test) |
|---|---|---|
| Sex: | 0.08 | |
| female | 94 | |
| male | 171 | |
| Mean age at diagnosis | 63 (range 22–90) | 0.3 |
| Tobacco consumption | 127 (48%) | 0.06 |
| Alcohol consumption | 77 (29%) | 0.02 |
| pT staging 7th edition: | ||
| T1 | 165 | |
| T2 | 97 | |
| T3 | 3 | |
| T4 | - | |
| pT staging 8th edition: | ||
| T1 | 107 | |
| T2 | 133 | |
| T3 | 20 | |
| T4 | - | |
| Pathological N staging 7th edition: | ||
| N0 | 204 | |
| N1 | 60 | |
| N2a | - | |
| N2b | - | |
| N3 | - | |
| Pathological N staging 8th edition: | ||
| N0 | 205 | |
| N1 | 42 | |
| N2a | 18 | |
| N2b | - | |
| N3 | - | |
| Tumor sub-site: | ||
| Oral tongue | 164 (62%) | |
| Floor of mouth | 60 (23%) | |
| Buccal mucosa | 21 (8%) | |
| Lower alveolus | 9 (3%) | |
| Upper alveolus | 7 (3%) | |
| Retro-molar trigone | 4 (1%) | |
| Neck Dissection | 159 (60%) | 0.37 |
| Stage I | 64 | |
| Stage II | 41 | |
| Stage III | 53 | |
| Level I-III | 39 | |
| Level IV | 120 | |
| Extra-capsular extension | 18 | |
| TNM staging 7th edition: | ||
| Stage I | 140 | |
| Stage II | 62 | |
| Stage III | 63 | |
| Stage IVa | - | |
| Stage IVb | - | |
| TNM staging 8th edition: | ||
| Stage I | 87 | |
| Stage II | 98 | |
| Stage III | 62 | |
| Stage IVa | 18 | |
| Stage IVb | - | |
| Variable | Disease-Specific Survival p Value | HR CI 95% | Overall Survival p Value | HR CI 95% |
|---|---|---|---|---|
| pT staging 7th edition: (Reference T1) | ||||
| T1 | - | - | - | - |
| T2 | 0.043 | 2.64 (1.19–5.83) | 0.036 | 2.02 (1.09–3.37) |
| T3 | 0.67 | 0.5 (0.03–501) | 0.6 | 0.85 (0.03–19.8) |
| T4 | - | - | - | - |
| pT staging 8th edition: (Reference T1) | ||||
| T1 | - | - | - | - |
| T2 | 0.023 | 2.1 (1.3–4.1) | 0.03 | 2.5 (1.3–5.2) |
| T3 | 0.43 | 0.49 (0.65–501) | 0.56 | 0.84 (0.02–24.6) |
| T4 | - | - | - | - |
| Pathological N staging 7th edition: (Reference T1) | ||||
| N0 | 0.37 | 0.89 (0.92–1.04) | 0.38 | 0.85 (0.26–2.57) |
| N1 | <0.001 | 4.08 (1.96–8.48) | <0.001 | 3.83 (2.12–6.94) |
| N2a | - | - | - | - |
| N2b | - | - | - | - |
| N3 | - | - | - | - |
| Pathological N staging 8th edition: (Reference T1) | ||||
| N0 | 0.49 | 0.26 (0.04–1.65) | 0.12 | 0.42 (0.14–1.26) |
| N2a | <0.0001 | 5.97 (2.84–9.54) | <0.0001 | 5.1 (2.91–8.95) |
| N2b | 0.04 | 3.6 (1.9–8.95) | 0.032 | 4.55 (3.1–9.32) |
| N3 | - | - | - | - |
| Tumor subsite: (Reference T1) | ||||
| Oral tongue | 0.056 | 1.76 (0.49–2.3) | 0.042 | 1.8 (1.1–3.21) |
| Floor of mouth | 0.32 | 1.3 (0.01–4.5) | 0.41 | 2.1 (0.3–6.67) |
| Buccal mucosa | 0.07 | 2.3 (0.1–3.5) | 0.034 | 1.98 (0.3–3.7) |
| Lower alveolus | 0.9 | 0.95 (0.2–4.6) | 0.85 | 1.3 (0.5–3.8) |
| Upper alveolus | 0.57 | 3.1 (0.6–7.1) | 0.8 | 3.6 (0.7–4.1) |
| Retro-molar trigone | 0.87 | 2.01 (0.5–8.9) | 0.69 | 1.9 (0.3–8.1) |
| Neck Dissection (Reference T1) | ||||
| Stage I | 0.89 | 0.91 (0.17–3.62) | 0.77 | 0.85 (0.26–2.57) |
| Stage II | 0.8 | 1.3 (0.7–2.1) | 0.85 | 1.6 (0.4–3.1) |
| Stage III | 0.12 | 1.1 (0.1–3.7) | 0.3 | 1.8 (0.09–3.5) |
| Extra-capsular extension | <0.001 | 4.39 (1.76–8.32) | <0.001 | 5.97 (2.84–9.91) |
| TNM staging 7th edition: | ||||
| Stage I | - | - | - | - |
| Stage II | 0.65 | 2.1 (0.3–4.1) | 0.54 | 2.3 (0.9–3.6) |
| Stage III | 0.047 | 1.203 (1.044–2.094) | 0.042 | 1.12 (1.001–3.01) |
| Stage IVa | 0.03 | 1.2 (1.1–3.4) | 0.035 | 1.5 (1.1–4.2) |
| Stage IVb | - | - | - | - |
| TNM staging 8th edition: | ||||
| Stage I | 0.45 | 1.7 (0.6–4.2) | 0.44 | 0.82 (0.02–24.6) |
| Stage II | 0.2 | 1.17 (0.65–4.66) | 0.12 | 0.98 (0.94–1.1) |
| Stage III | 0.056 | 0.18 (0.0–1886) | 0.084 | 0.3 (0.02–24.6) |
| Stage IVa | - | - | - | - |
| Stage IVb | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghantous, Y.; Nashef, A.; Sidransky, D.; Abdelraziq, M.; Alkeesh, K.; Araidy, S.; Koch, W.; Brait, M.; Abu El-Naaj, I. Clinical and Prognostic Significance of the Eighth Edition Oral Cancer Staging System. Cancers 2022, 14, 4632. https://doi.org/10.3390/cancers14194632
Ghantous Y, Nashef A, Sidransky D, Abdelraziq M, Alkeesh K, Araidy S, Koch W, Brait M, Abu El-Naaj I. Clinical and Prognostic Significance of the Eighth Edition Oral Cancer Staging System. Cancers. 2022; 14(19):4632. https://doi.org/10.3390/cancers14194632
Chicago/Turabian StyleGhantous, Yasmin, Aysar Nashef, David Sidransky, Murad Abdelraziq, Kutaiba Alkeesh, Shareef Araidy, Wayne Koch, Mariana Brait, and Imad Abu El-Naaj. 2022. "Clinical and Prognostic Significance of the Eighth Edition Oral Cancer Staging System" Cancers 14, no. 19: 4632. https://doi.org/10.3390/cancers14194632
APA StyleGhantous, Y., Nashef, A., Sidransky, D., Abdelraziq, M., Alkeesh, K., Araidy, S., Koch, W., Brait, M., & Abu El-Naaj, I. (2022). Clinical and Prognostic Significance of the Eighth Edition Oral Cancer Staging System. Cancers, 14(19), 4632. https://doi.org/10.3390/cancers14194632








