Pharmacogenomics for Prediction of Cardiovascular Toxicity: Landscape of Emerging Data in Breast Cancer Therapies
Abstract
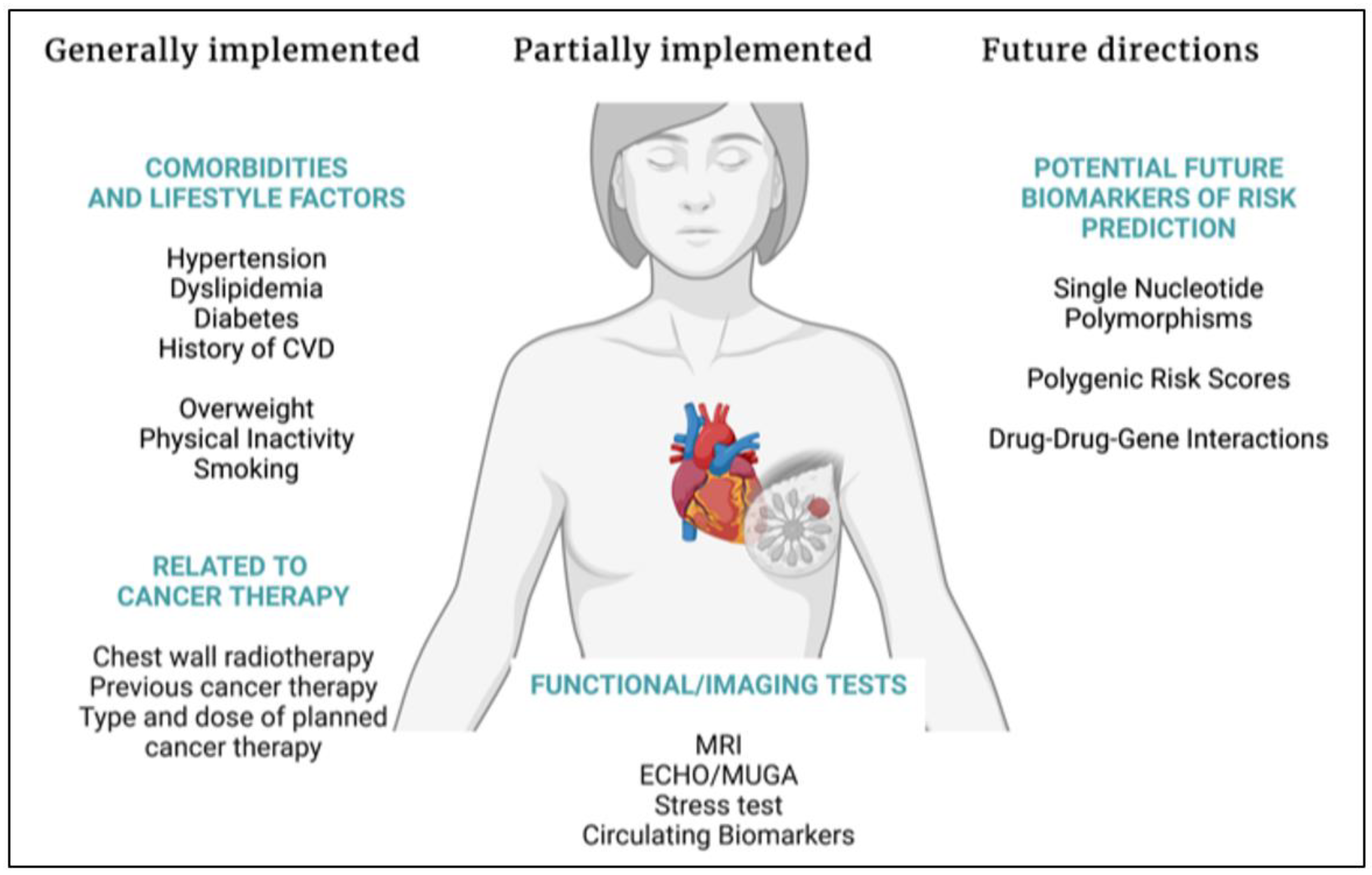
Simple Summary
Abstract
1. Introduction
2. Chemotherapy
3. Targeted Therapies
3.1. Anti-HER2
Trastuzumab
3.2. CDK-4/6 Inhibitors
3.3. Phosphatidylinositol 4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha Isoform (PIK3CA) Inhibitors
3.4. Mammalian Target of Rapamycin (mTOR) Inhibitors
3.5. Immune Checkpoint Inhibitors
4. Endocrine Therapy
5. Radiotherapy
6. Polygenic Risk Scores
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-an Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Muñoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, T.; Xiong, X.; Liu, N.; Pang, B.; Ruan, Y.; Gao, Y.; Shang, H.; Xing, Y. Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104577. [Google Scholar] [CrossRef]
- Sim, S.; Lövrot, J.; Lindh, J.D.; Bergh, J.; Xie, H. Effect of CYP2C19 and CYP2D6 genotype on tamoxifen treatment outcome indicates endogenous and exogenous interplay. Pharmacogenomics 2018, 19, 1027–1037. [Google Scholar] [CrossRef]
- Margolin, S.; Lindh, J.D.; Thorén, L.; Xie, H.; Koukel, L.; Dahl, M.-L.; Eliasson, E. CYP2D6 and adjuvant tamoxifen: Possible differences of outcome in pre- and post-menopausal patients. Pharmacogenomics 2013, 14, 613–622. [Google Scholar] [CrossRef]
- Sim, S.; Bergh, J.; Hellström, M.; Hatschek, T.; Xie, H. Pharmacogenetic impact of docetaxel on neoadjuvant treatment of breast cancer patients. Pharmacogenomics 2018, 19, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kimler, B.F.; O’Dea, A.P.; Nye, L.; Wang, Y.Y.; Yoder, R.; Staley, J.M.; Prochaska, L.; Wagner, J.; Amin, A.L.; et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin. Cancer Res. 2021, 27, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Tasaka, H.; Yu, K.-P.; Murphy, M.L.; Karnofsky, D.A. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 1967, 20, 333–353. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Huang, H.; Shafaattalab, S.; Maillet, A.; Lin, E.; Huang, K.; Laksman, Z.; Davis, M.K.; Tibbits, G.F.; Brunham, L.R. Variation in RARG increases susceptibility to doxorubicin-induced cardiotoxicity in patient specific induced pluripotent stem cell-derived cardiomyocytes. Sci. Rep. 2020, 10, 10363. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.K.; Karthikeyan, B.; Quiñones-Lombraña, A.; Blair, R.H.; Early, A.P.; Levine, E.G.; Sharma, U.C.; Blanco, J.G.; O’Connor, T. CBR3 V244M is associated with LVEF reduction in breast cancer patients treated with doxorubicin. Cardiooncology 2021, 7, 17. [Google Scholar] [CrossRef]
- Hertz, D.L.; Caram, M.V.; Kidwell, K.M.; Thibert, J.N.; Gersch, C.; Seewald, N.J.; Smerage, J.; Rubenfire, M.; Henry, N.L.; A Cooney, K.; et al. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics 2016, 17, 231–240. [Google Scholar] [CrossRef]
- Vulsteke, C.; Pfeil, A.M.; Maggen, C.; Schwenkglenks, M.; Pettengell, R.; Szucs, T.D.; Lambrechts, D.; Dieudonné, A.-S.; Hatse, S.; Neven, P.; et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res. Treat. 2015, 152, 67–76. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Guo, Z.; Jiang, X.; Su, X.; Zhang, X. Correlation of UGT2B7 Polymorphism with Cardiotoxicity in Breast Cancer Patients Undergoing Epirubicin/Cyclophosphamide-Docetaxel Adjuvant Chemotherapy. Yonsei Med. J. 2019, 60, 30–37. [Google Scholar] [CrossRef]
- Leong, S.L.; Chaiyakunapruk, N.; Lee, S.W.H. Candidate Gene Association Studies of Anthracycline-induced Cardiotoxicity: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, G.; Guan, M.; Bapat, A.; Dai, Q.; Zhong, C.; Yang, T.; Luo, C.; An, N.; Liu, W.; et al. Potential Gene Association Studies of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 651269. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Trastuzumab Cardiotoxicity After Anthracycline Exposure Constitutes a Complex and Clinically Important Entity. JACC Heart Fail. 2019, 7, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; De La Haba-Rodríguez, J.R.; Im, S.-A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Krop, I.E.; Suter, T.M.; Dang, C.T.; Dirix, L.; Romieu, G.; Zamagni, C.; Citron, M.L.; Campone, M.; Xu, N.; Smitt, M.; et al. Feasibility and Cardiac Safety of Trastuzumab Emtansine After Anthracycline-Based Chemotherapy As (neo) Adjuvant Therapy for Human Epidermal Growth Factor Receptor 2-Positive Early-Stage Breast Cancer. J. Clin. Oncol. 2015, 33, 1136–1142. [Google Scholar] [CrossRef]
- Pondé, N.; Ameye, L.; Lambertini, M.; Paesmans, M.; Piccart, M.; de Azambuja, E. Trastuzumab emtansine (T-DM1)-associated cardiotoxicity: Pooled analysis in advanced HER2-positive breast cancer. Eur. J. Cancer 2020, 126, 65–73. [Google Scholar] [CrossRef]
- Leong, S.L.; Chaiyakunapruk, N.; Tassaneeyakul, W.; Arunmanakul, P.; Nathisuwan, S.; Lee, S.W.H. Roles of pharmacogenomics in non-anthracycline antineoplastic-induced cardiovascular toxicities: A systematic review and meta-analysis of genotypes effect. Int. J. Cardiol. 2019, 280, 190–197. [Google Scholar] [CrossRef]
- Tan, L.; Su, X.; Li, X.; Li, H.; Hu, B. Correlation of HER2 codon 655 polymorphism with cardiotoxicity risk in Chinese HER2-positive breast cancer patients undergoing epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab adjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2020, 13, 286–294. [Google Scholar]
- Stanton, S.E.; Ward, M.M.; Christos, P.; Sanford, R.; Lam, C.; Cobham, M.V.; Donovan, D.; Scheff, R.J.; Cigler, T.; Moore, A.; et al. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer 2015, 15, 267. [Google Scholar] [CrossRef]
- Vazdar, L.; Gabrić, I.D.; Kruljac, I.; Pintarić, H.; Šeparović, R.; Biloš, L.S.K.; Pavlović, M.; Vuger, A.T.; Štefanović, M. Influence of Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in early-stage HER2 positive breast cancer. Sci. Rep. 2021, 11, 14395. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.G.; Dávila-Fajardo, C.L.; Martínez-González, L.J.; Carmona-Saez, P.; Pino, M.J.S.; Ramos, J.S.; Escobar, E.M.; Blancas, I.; Fernández, J.J.; Fernández, D.; et al. Influence of the HER2 Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients: A meta-analysis. Pharm. Genom. 2015, 25, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Beauclair, S.; Formento, P.; Fischel, J.; Lescaut, W.; Largillier, R.; Chamorey, E.; Hofman, P.; Ferrero, J.; Pagès, G.; Milano, G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann. Oncol. 2007, 18, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.; Diorio, C.; Côté, M.-A.; Provencher, L.; Barabé, F.; Jacob, S.; St-Pierre, C.; Demers, E.; Tremblay-Lemay, R.; Nadeau-Larochelle, C.; et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013, 33, 2569–2576. [Google Scholar]
- Roca, L.; Diéras, V.; Roché, H.; Lappartient, E.; Kerbrat, P.; Cany, L.; Chieze, S.; Canon, J.-L.; Spielmann, M.; Penault-Llorca, F.; et al. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER-PACS 04 trial. Breast Cancer Res. Treat. 2013, 139, 789–800. [Google Scholar] [CrossRef]
- Watrowski, R.; Castillo-Tong, D.C.; Wolf, A.; Schuster, E.; Fischer, M.B.; Speiser, P.; Zeillinger, R. HER2 Codon 655 (Ile/Val) Polymorphism and Breast Cancer in Austrian Women. Anticancer Res. 2015, 35, 5901–5904. [Google Scholar]
- Escórcio-Dourado, C.S.; Alves-Ribeiro, F.A.; Lima-Dourado, J.C.; dos Santos, A.R.; Pereira, R.D.O.; Tavares, C.B.; Silva, V.C.; Costa, P.V.L.; Soares-Júnior, J.M.; da Silva, B.B. Human Epidermal Growth Factor Receptor-2 gene polymorphism and breast cancer risk in women from the Northeastern region of Brazil. Clinics 2020, 75, e2360. [Google Scholar] [CrossRef]
- Dahabreh, I.J.; Murray, S. Lack of replication for the association between HER2 I655V polymorphism and breast cancer risk: A systematic review and meta-analysis. Cancer Epidemiol. 2011, 35, 503–509. [Google Scholar] [CrossRef]
- Peddi, P.F.; Fasching, P.A.; Liu, D.; Quinaux, E.; Robert, N.J.; Valero, V.; Crown, J.; Falkson, C.; Brufsky, A.; Cunningham, J.M.; et al. Genetic Polymorphisms and Correlation with Treatment-Induced Cardiotoxicity and Prognosis in Patients with Breast Cancer. Clin. Cancer Res. 2022, 28, 1854–1862. [Google Scholar] [CrossRef]
- Marinko, T.; Konda, J.T.S.; Dolžan, V.; Goričar, K. Genetic Variability of Antioxidative Mechanisms and Cardiotoxicity after Adjuvant Radiotherapy in HER2-Positive Breast Cancer Patients. Dis. Markers 2020, 2020, 6645588. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial—PEARL☆. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Martel, S.; Maurer, C.; Lambertini, M.; Pondé, N.; De Azambuja, E. Breast cancer treatment-induced cardiotoxicity. Expert Opin. Drug Saf. 2017, 16, 1021–1038. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Åberg, K.; Adkins, D.E.; Liu, Y.; McClay, J.L.; Bukszár, J.; Jia, P.; Zhao, Z.; Perkins, D.; Stroup, T.S.; Lieberman, J.A.; et al. Genome-wide association study of antipsychotic-induced QTc interval prolongation. Pharm. J. 2012, 12, 165–172. [Google Scholar] [CrossRef]
- Seyerle, A.A.; Sitlani, C.M.; Noordam, R.; Gogarten, S.M.; Li, J.; Li, X.; Evans, D.S.; Sun, F.; A Laaksonen, M.; Isaacs, A.; et al. Pharmacogenomics study of thiazide diuretics and QT interval in multi-ethnic populations: The cohorts for heart and aging research in genomic epidemiology. Pharm. J. 2018, 18, 215–226. [Google Scholar] [CrossRef]
- Niemeijer, M.N.; Berg, M.E.V.D.; Eijgelsheim, M.; Rijnbeek, P.R.; Stricker, B.H. Pharmacogenetics of Drug-Induced QT Interval Prolongation: An Update. Drug Saf. 2015, 38, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.E.; E R, N.; Danesi, R.; Girmenia, C.; Invernizzi, P.; Elvevi, A.; Uguccioni, M. Management of toxicities associated with targeted therapies for HR-positive metastatic breast cancer: A multidisciplinary approach is the key to success. Breast Cancer Res. Treat. 2019, 176, 483–494. [Google Scholar] [CrossRef]
- De Mattia, E.; Cecchin, E.; Guardascione, M.; Foltran, L.; Di Raimo, T.; Angelini, F.; D’Andrea, M.; Toffoli, G. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 3870–3896. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves First PI3K Inhibitor for Breast Cancer. 2019. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-pi3k-inhibitor-breast-cancer (accessed on 12 April 2022).
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.-S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Meoli, D.F.; Moslehi, J.; Roden, D.M. Inhibition of the α-Subunit of Phosphoinositide 3-Kinase in Heart Increases Late Sodium Current and Is Arrhythmogenic. J. Pharmacol. Exp. Ther. 2018, 365, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Ezeani, M.; Elom, S. Necessity to evaluate PI3K/Akt signalling pathway in proarrhythmia. Open Heart 2017, 4, e000596. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., III; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2011, 366, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Karvelas, G.; Roumpi, A.; Komporozos, C.; Syrigos, K. Everolimus as cancer therapy: Cardiotoxic or an unexpected antiatherogenic agent? A narrative review. Hell. J. Cardiol. 2018, 59, 196–200. [Google Scholar] [CrossRef]
- Pascual, T.; Apellaniz-Ruiz, M.; Pernaut, C.; Cueto-Felgueroso, C.; Villalba, P.; Álvarez, C.; Manso, L.; Inglada-Pérez, L.; Robledo, M.; Rodriguez-Antona, C.; et al. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS ONE 2017, 12, e0180192. [Google Scholar] [CrossRef]
- Castrillon, J.A.; Eng, C.; Cheng, F. Pharmacogenomics for immunotherapy and immune-related cardiotoxicity. Hum. Mol. Genet. 2020, 29, R186–R196. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.-E.; A Sosman, J.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Breunis, W.B.; Tarazona, E.; Chen, R.; Kiley, M.; Rosenberg, S.A.; Chanock, S.J. Influence of cytotoxic T lymphocyte-associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J. Immunother. 2008, 31, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Shek, D.; Read, S.A.; Ahlenstiel, G.; Piatkov, I. Pharmacogenetics of anticancer monoclonal antibodies. Cancer Drug Resist. 2019, 2, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Hoefsmit, E.P.; Rozeman, E.A.; Haanen, J.B.; Blank, C.U. Susceptible loci associated with autoimmune disease as potential biomarkers for checkpoint inhibitor-induced immune-related adverse events. ESMO Open 2019, 4, e000472. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Filion, K.; Al-Qurashi, S.; Torabi, N.; Bouganim, N.; Suissa, S.; Azoulay, L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 2017, 28, 487–496. [Google Scholar] [CrossRef]
- Johansson, H.; The TEXT principal investigators; Gray, K.P.; Pagani, O.; Regan, M.M.; Viale, G.; Aristarco, V.; Macis, D.; Puccio, A.; Roux, S.; et al. Impact of CYP19A1 and ESR1 variants on early-onset side effects during combined endocrine therapy in the TEXT trial. Breast Cancer Res. 2016, 18, 110. [Google Scholar] [CrossRef]
- Borrie, A.; Rose, F.A.; Choi, Y.-H.; Perera, F.E.; Read, N.; Sexton, T.; Lock, M.; Vandenberg, T.A.; Hahn, K.; Younus, J.; et al. Genetic and clinical predictors of arthralgia during letrozole or anastrozole therapy in breast cancer patients. Breast Cancer Res. Treat. 2020, 183, 365–372. [Google Scholar] [CrossRef]
- Baatjes, K.; Peeters, A.; McCaul, M.; Conradie, M.M.; Apffelstaedt, J.; Conradie, M.; Kotze, M.J. CYP19A1 rs10046 Pharmacogenetics in Postmenopausal Breast Cancer Patients Treated with Aromatase Inhibitors: One-year Follow-up. Curr. Pharm. Des. 2020, 26, 6007–6012. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Mccarty, C.A.; Wilke, R.A.; Glurich, I.; Engel, J.M.; Flockhart, D.A.; Nguyen, A.; Li, L.; Mi, D.; Skaar, T.C.; et al. Estrogen receptor genotype is associated with risk of venous thromboembolism during tamoxifen therapy. Breast Cancer Res. Treat. 2009, 115, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Holmberg, L.; Garmo, H.; Duvernoy, O.; Sjögren, I.; Lagerqvist, B.; Blomqvist, C. Distribution of coronary artery stenosis after radiation for breast cancer. J. Clin. Oncol. 2012, 30, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Perentesis, J.P. Polymorphisms of drug metabolizing enzymes and markers of genotoxicity to identify patients with Hodgkin’s lymphoma at risk of treatment-related complications. Ann. Oncol. 2002, 13 (Suppl. 1), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.G. The common variants/multiple disease hypothesis of common complex genetic disorders. Med. Hypotheses 2004, 62, 309–317. [Google Scholar] [CrossRef]
- Sugrue, L.P.; Desikan, R.S. What Are Polygenic Scores and Why Are They Important? JAMA 2019, 321, 1820–1821. [Google Scholar] [CrossRef] [PubMed]
- Liou, L.; Kaptoge, S.; Dennis, J.; Shah, M.; Tyrer, J.; Inouye, M.; Easton, D.F.; Pharoah, P.D.P. Genomic risk prediction of coronary artery disease in women with breast cancer: A prospective cohort study. Breast Cancer Res. 2021, 23, 94. [Google Scholar] [CrossRef]
- Elliott, J.; Bodinier, B.; Bond, T.A.; Chadeau-Hyam, M.; Evangelou, E.; Moons, K.G.M.; Dehghan, A.; Muller, D.; Elliott, P.; Tzoulaki, I. Predictive Accuracy of a Polygenic Risk Score-Enhanced Prediction Model vs. a Clinical Risk Score for Coronary Artery Disease. JAMA 2020, 323, 636–645. [Google Scholar] [CrossRef]
- Bruckmueller, H.; Cascorbi, I. Drug-Drug-Gene Interactions: A Call for Clinical Consideration. Clin. Pharmacol. Ther. 2021, 110, 549–551. [Google Scholar] [CrossRef]
- Meulendijks, D.; Henricks, L.; Sonke, G.; Deenen, M.J.; Froehlich, T.K.; Amstutz, U.; Largiader, C.; Jennings, B.; Marinaki, A.M.; Sanderson, J.D.; et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 1639–1650. [Google Scholar] [CrossRef]
- Han, X.; Diao, L.; Xu, Y.; Xue, W.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; et al. Association between the HER2 Ile655Val polymorphism and response to trastuzumab in women with operable primary breast cancer. Ann. Oncol. 2014, 25, 1158–1164. [Google Scholar] [CrossRef]
- Wasielewski, M.; van Spaendonck-Zwarts, K.Y.; Westerink, N.-D.L.; Jongbloed, J.D.H.; Postma, A.; A Gietema, J.; van Tintelen, J.P.; Berg, M.P.V.D. Potential genetic predisposition for anthracycline-associated cardiomyopathy in families with dilated cardiomyopathy. Open Heart 2014, 1, e000116. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Number of Patients | Gene | SNPs | Correlation to Cardiotoxicity Endpoint | Odds Ratio (OR) (95% CI) | Definition of Cardiotoxicity Endpoints |
|---|---|---|---|---|---|---|
| Drug: anthracyclines | ||||||
| Yang, 2021 | 41 studies | CYBA RAC2 CYP3A5 ABCC1 ABCC2 HER2 codon 655 | rs4673 rs13058338 rs776746 rs45511401 rs8187710 rs1136201 | ⇑ ⇑ ⇑ ⇑ ⇑ ⇑ | 1.93 (1.13–3.30) 2.05 (1.11–3.7) 2.15 (1.00–4.62) 1.46 (1.05–2.01) 2.19 (1.38–3.48) 2.48 (1.53–4.02) | Depending on the study |
| Leong, 2017 | 28 studies | ABCC2 CYBA RAC2 | rs8187710 rs4673 rs13058338 | ⇑ ⇑ ⇑ | 2.2 (1.36–3.54) 1.55 (1.05–2.30) 1.79 (1.27–2.52) | Depending on the study |
| Vulsteke, 2015 | 877 | ABCC1 | rs246221 | ⇑ | 1.6 (1.1–2.3) | Asymptomatic decrease in LVEF >10% or cardiac failure grade ≥3 |
| Li, 2019 | 427 | UGT2B7 codon 161 | rs7668258 | ⇑ | NR | LVEF decline ≥10% to <53%, heart failure, acute coronary artery syndrome, or fatal arrhythmia |
| Hertz, 2016 | 166 | CBR3 ABCB1 | rs1056892 rs1045642 | ⇑ ⇓ | NR | LVEF reduction to <55% |
| Lang, 2021 | 92 | CBR3 | rs1056892 | LVEF reduction from baseline but no correlation to cardiotoxicity | 2.55 (0.26–25.17) for GG vs. AA; 1.43 (0.15–13.26) for GA vs. AA | Asymptomatic LVEF reduction of ≥10% to levels < 55% |
| Drug: trastuzumab | ||||||
| Leong, 2019 | 35 studies | HER2 codon 655 | rs1136201 | ⇔ | 2.43 (1.17–5.06) | LVEF decline ≥10% from baseline and <53% or heart failure, acute coronary artery syndrome, or fatal arrhythmia |
| Gómez Peña, 2015 | 4 studies | HER2 codon 655 | rs1136201 | ⇑ | 5.35 (2.55–11.73) | Depending on the study |
| Peddi, 2022 | 666 | No polymorphisms correlated with cardiotoxicity were identified | LVEF decline ≥10% or symptomatic heart failure | |||
| Vazdar, 2021 | 177 | HER2 codon 655 | rs1136201 | ⇔ | NR | LVEF and diastolic dysfunction; cut-offs not clarified |
| Stanton, 2015 | 140 | HER2 HER2 codon 655 | Pro1170Ala rs1136201 | ⇑ ⇔ | 2.60 (1.02–6.62) NR | Symptomatic heart failure or LVEF decline ≥15% or LVEF decline ≥10% and <55% and discontinuation of trastuzumab (temporary or permanent) |
| Marinko, 2022 | 101 | PON1 PON1 GSTP1 | rs854560 rs662 rs1695 | ⇓ ⇑ ⇓ | 0.35 (0.15–0.83) 5.41 (2.12–13.78) 0.36 (0.15–0.88) | NT-proBNP increase to ≥125 ng/L |
| Tan, 2020 | 91 | HER2 codon 655 | rs1136201 | ⇑ | 7.99 (95% CI NA; p = 0.007 | LVEF decline ≥ 10% from baseline and <53%, or heart failure, acute coronary artery syndrome, or fatal arrhythmia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altena, R.; Bajalica-Lagercrantz, S.; Papakonstantinou, A. Pharmacogenomics for Prediction of Cardiovascular Toxicity: Landscape of Emerging Data in Breast Cancer Therapies. Cancers 2022, 14, 4665. https://doi.org/10.3390/cancers14194665
Altena R, Bajalica-Lagercrantz S, Papakonstantinou A. Pharmacogenomics for Prediction of Cardiovascular Toxicity: Landscape of Emerging Data in Breast Cancer Therapies. Cancers. 2022; 14(19):4665. https://doi.org/10.3390/cancers14194665
Chicago/Turabian StyleAltena, Renske, Svetlana Bajalica-Lagercrantz, and Andri Papakonstantinou. 2022. "Pharmacogenomics for Prediction of Cardiovascular Toxicity: Landscape of Emerging Data in Breast Cancer Therapies" Cancers 14, no. 19: 4665. https://doi.org/10.3390/cancers14194665
APA StyleAltena, R., Bajalica-Lagercrantz, S., & Papakonstantinou, A. (2022). Pharmacogenomics for Prediction of Cardiovascular Toxicity: Landscape of Emerging Data in Breast Cancer Therapies. Cancers, 14(19), 4665. https://doi.org/10.3390/cancers14194665







