Functional Downregulation of PD-L1 and PD-L2 by CpG and non-CpG Oligonucleotides in Melanoma Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Fluorescence-Activated Cell Sorting Analysis
2.4. Western Blot Analysis
2.5. DNA Synthesis
2.6. Membrane Integrity
2.7. Histone-Associated DNA Fragments
2.8. Real-Time RT-PCR Analysis
2.9. PD-L1/2 Promoter Transient Reporter Assay
2.10. Chromatin Immunoprecipitation (ChIP) Assays
2.11. Cellular Uptake, Quadruplex (G4) Formation and Binding to the IFNGR1
2.12. Statistical Analysis
3. Results
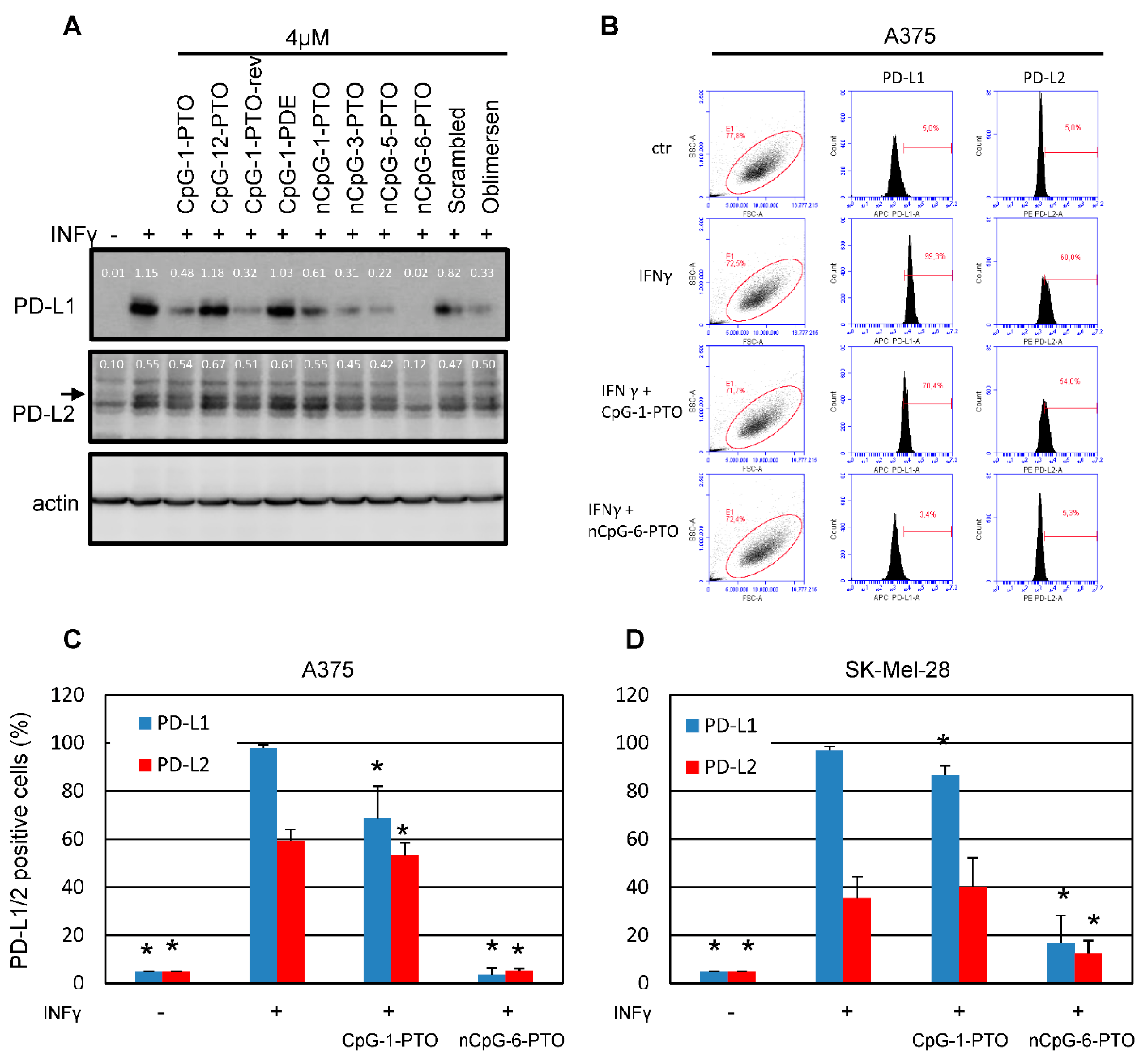
3.1. Oligonucleotides Suppress the Protein Expression of PD-L1 and PD-L2 in Melanoma Cells
3.2. PD-L1/2 Protein Suppression by CpG-1-PTO and nCpG-6-PTO
3.3. PD-L1/2 Protein Suppression by CpG-1-PTO and nCpG-6-PTO: Dependence on Concentration and Molecule Length
3.4. CpG-1-PTO and nCpG-6-PTO Suppress PD-L1/2 mRNA and Promoter Activation
3.5. CpG-1-PTO and nCpG-6-PTO Inhibit Signaling Molecules of the IFN Type I and II Pathway
3.6. nCpG-6-PTO Enters the Cell, Forms Quadruplexes (G4) and Binds to the IFNGR2
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dreno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandala, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Colombino, M.; Capone, M.; Lissia, A.; Cossu, A.; Rubino, C.; De Giorgi, V.; Massi, D.; Fonsatti, E.; Staibano, S.; Nappi, O.; et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J. Clin. Oncol. 2012, 30, 2522–2529. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a therapeutic target in metastatic melanoma. JAMA J. Am. Med. Assoc. 2011, 305, 2327–2334. [Google Scholar] [CrossRef]
- Merelli, B.; Massi, D.; Cattaneo, L.; Mandala, M. Targeting the PD1/PD-L1 axis in melanoma: Biological rationale, clinical challenges and opportunities. Crit. Rev. Oncol. Hematol. 2014, 89, 140–165. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Prasad, V.; Kaestner, V. Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin. Oncol. 2017, 44, 132–135. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Shimada, S.; Yano, O.; Inoue, H.; Kuramoto, E.; Fukuda, T.; Yamamoto, H.; Kataoka, T.; Tokunaga, T. Antitumor activity of the DNA fraction from Mycobacterium bovis BCG. II. Effects on various syngeneic mouse tumors. J. Natl. Cancer Inst. 1985, 74, 681–688. [Google Scholar] [PubMed]
- Ballas, Z.K.; Rasmussen, W.L.; Krieg, A.M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 1996, 157, 1840–1845. [Google Scholar]
- Kumagai, Y.; Takeuchi, O.; Akira, S. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 2008, 60, 795–804. [Google Scholar] [CrossRef]
- Melisi, D.; Frizziero, M.; Tamburrino, A.; Zanotto, M.; Carbone, C.; Piro, G.; Tortora, G. Toll-Like Receptor 9 Agonists for Cancer Therapy. Biomedicines 2014, 2, 211–228. [Google Scholar] [CrossRef]
- Ribas, A.; Medina, T.; Kummar, S.; Amin, A.; Kalbasi, A.; Drabick, J.J.; Barve, M.; Daniels, G.A.; Wong, D.J.; Schmidt, E.V.; et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov. 2018, 8, 1250–1257. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Flaherty, K.; Goff, S. Emerging Strategies in Systemic Therapy for the Treatment of Melanoma. In American Society of Clinical Oncology Educational Book; Annual Meeting; American Society of Clinical Oncology: Orlando, FL, USA, 2018; Volume 38, pp. 751–758. [Google Scholar] [CrossRef]
- Diab, A.; Haymaker, C.; Uemura, M.; Murthy, R.; James, M.; Geib, J.; Cornfeld, M.; Swann, S.; Yee, C.; Wargo, J.; et al. 1187PA Phase 1/2 trial of intratumoral (i.t.) IMO-2125 (IMO) in combination with checkpoint inhibitors (CPI) in PD-(L)1-refractory melanoma. Ann. Oncol. 2017, 28, v421. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, G.; Wojdyla, L.; Frakes, M.; Schrank, Z.; Leviskas, B.; Ivancich, M.; Vinay, P.; Ganapathy, R.; Ramirez, B.E.; Puri, N. Mechanism of Action of G-Quadruplex-Forming Oligonucleotide Homologous to the Telomere Overhang in Melanoma. J. Investig. Dermatol. 2018, 138, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Moruno-Manchon, J.F.; Koellhoffer, E.C.; Gopakumar, J.; Hambarde, S.; Kim, N.; McCullough, L.D.; Tsvetkov, A.S. The G-quadruplex DNA stabilizing drug pyridostatin promotes DNA damage and downregulates transcription of Brca1 in neurons. Aging 2017, 9, 1957–1970. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Kirkwood, J.M. Oblimersen in the treatment of metastatic melanoma. Future Oncol. 2007, 3, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Dampmann, M.; Görgens, A.; Möllmann, M.; Murke, F.; Dührsen, U.; Giebel, B.; Dürig, J. CpG stimulation of chronic lymphocytic leukemia cells induces a polarized cell shape and promotes migration in vitro and in vivo. PLoS ONE 2020, 15, e0228674. [Google Scholar] [CrossRef]
- Erika, A.B.; Aguet, M.; Schreiber, R.D. THE IFNγ RECEPTOR:A Paradigm for Cytokine Receptor Signaling. Annu. Rev. Immunol. 1997, 15, 563–591. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Obeid, J.M.; Erdag, G.; Smolkin, M.E.; Deacon, D.H.; Patterson, J.W.; Chen, L.; Bullock, T.N.; Slingluff, C.L. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology 2016, 5, e1235107. [Google Scholar] [CrossRef]
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef]
- Karunarathne, D.S.; Horne-Debets, J.M.; Huang, J.X.; Faleiro, R.; Leow, C.Y.; Amante, F.; Watkins, T.S.; Miles, J.J.; Dwyer, P.J.; Stacey, K.J.; et al. Programmed Death-1 Ligand 2-Mediated Regulation of the PD-L1 to PD-1 Axis Is Essential for Establishing CD4+ T Cell Immunity. Immunity 2016, 45, 333–345. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [Green Version]
- Avery, O.T.; Macleod, C.M.; McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 1944, 79, 137–158. [Google Scholar] [CrossRef]
- Travers, A.; Muskhelishvili, G. DNA structure and function. FEBS J. 2015, 282, 2279–2295. [Google Scholar] [CrossRef]
- Lin, J.; Kaur, P.; Countryman, P.; Opresko, P.L.; Wang, H. Unraveling secrets of telomeres: One molecule at a time. DNA Repair 2014, 20, 142–153. [Google Scholar] [CrossRef]
- Palumbo, S.L.; Ebbinghaus, S.W.; Hurley, L.H. Formation of a unique end-to-end stacked pair of G-quadruplexes in the hTERT core promoter with implications for inhibition of telomerase by G-quadruplex-interactive ligands. J. Am. Chem. Soc. 2009, 131, 10878–10891. [Google Scholar] [CrossRef]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Vollmer, J.; Weeratna, R.D.; Jurk, M.; Samulowitz, U.; McCluskie, M.J.; Payette, P.; Davis, H.L.; Schetter, C.; Krieg, A.M. Oligodeoxynucleotides lacking CpG dinucleotides mediate Toll-like receptor 9 dependent T helper type 2 biased immune stimulation. Immunology 2004, 113, 212–223. [Google Scholar] [CrossRef]
- Eiro, N.; Ovies, C.; Fernandez-Garcia, B.; Alvarez-Cuesta, C.C.; Gonzalez, L.; Gonzalez, L.O.; Vizoso, F.J. Expression of TLR3, 4, 7 and 9 in cutaneous malignant melanoma: Relationship with clinicopathological characteristics and prognosis. Arch. Dermatol. Res. 2013, 305, 59–67. [Google Scholar] [CrossRef]
- Srivastava, R.; Geng, D.; Liu, Y.; Zheng, L.; Li, Z.; Joseph, M.A.; McKenna, C.; Bansal, N.; Ochoa, A.; Davila, E. Augmentation of therapeutic responses in melanoma by inhibition of IRAK-1,-4. Cancer Res. 2012, 72, 6209–6216. [Google Scholar] [CrossRef]
- Wagner, H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004, 25, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Y.; Zhang, W. G-quadruplex structures and their interaction diversity with ligands. Chem. Med. Chem. 2014, 9, 899–911. [Google Scholar] [CrossRef]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Moye, A.L.; Porter, K.C.; Cohen, S.B.; Phan, T.; Zyner, K.G.; Sasaki, N.; Lovrecz, G.O.; Beck, J.L.; Bryan, T.M. Telomeric G-quadruplexes are a substrate and site of localization for human telomerase. Nat. Commun. 2015, 6, 7643. [Google Scholar] [CrossRef]
- Platella, C.; Riccardi, C.; Montesarchio, D.; Roviello, G.N.; Musumeci, D. G-quadruplex-based aptamers against protein targets in therapy and diagnostics. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 1429–1447. [Google Scholar] [CrossRef]
- Bates, P.J.; Reyes-Reyes, E.M.; Malik, M.T.; Murphy, E.M.; O’Toole, M.G.; Trent, J.O. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: Uses and mechanisms. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 1414–1428. [Google Scholar] [CrossRef]
- Stuart, R.K.; Stockerl-Goldstein, K.; Cooper, M.; Devetten, M.; Herzig, R.; Medeiros, B.; Schiller, G.; Wei, A.; Acton, G.; Rizzieri, D. Randomized phase II trial of the nucleolin targeting aptamer AS1411 combined with high-dose cytarabine in relapsed/refractory acute myeloid leukemia (AML). J. Clin. Oncol. 2009, 27, 7019. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.M.; Drabkin, H.A.; Lara, P.N., Jr.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A.; et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Inv. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef]
- Smith, C.I.E.; Zain, R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630. [Google Scholar] [CrossRef]





| CpG-1-PTO | 5′-TCC ATG ACG TTC CTG ACG TT-3′ |
| CpG-14-PTO | 5′-TCC ATG ACG TTC CTG A-3′ |
| CpG-12-PTO | 5′-CAT GAC GTT CCT-3′ |
| CpG-9-PTO | 5′-GAC GTT-3′ |
| CpG-1-PDE | 5′-tcc atg acg ttc ctg acg tt-3′ |
| CpG-1-PTO-rev | 5′-AAC GTC AGG AAC GTC ATG GA-3′ |
| nCpG-1-PTO | 5′-TCC ATG AGC TTC CTG AGT CT-3′ |
| nCpG-3-PTO | 5′-TTT TTT TTT TTT TTT TTT TT-3′ |
| nCpG-5-PTO | 5′-CCC CCC CCC CCC CCC CCC CC-3′ |
| Scrambled | 5′-CTC TAG GAC TCT CTG GAC TT-3′ |
| Oblimersen (G3139) | 5′-TCT CCC AGC GTG CGC CAT-3′ |
| nCpG-6-PTO | 5′-GGG GGG GGG GGG GGG GGG GG-3′ |
| nCpG-6B-PTO | 5′-GGG GGG GGG GGG GGG G-3′ |
| nCpG-6D-PTO | 5′-GGG GGG GGG GGG-3′ |
| nCpG-6G-PTO | 5′-GGG GGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleemann, J.; Steinhorst, K.; König, V.; Zöller, N.; Cinatl, J., Jr.; Özistanbullu, D.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Functional Downregulation of PD-L1 and PD-L2 by CpG and non-CpG Oligonucleotides in Melanoma Cells. Cancers 2022, 14, 4698. https://doi.org/10.3390/cancers14194698
Kleemann J, Steinhorst K, König V, Zöller N, Cinatl J Jr., Özistanbullu D, Kaufmann R, Meissner M, Kippenberger S. Functional Downregulation of PD-L1 and PD-L2 by CpG and non-CpG Oligonucleotides in Melanoma Cells. Cancers. 2022; 14(19):4698. https://doi.org/10.3390/cancers14194698
Chicago/Turabian StyleKleemann, Johannes, Katja Steinhorst, Veronika König, Nadja Zöller, Jindrich Cinatl, Jr., Deniz Özistanbullu, Roland Kaufmann, Markus Meissner, and Stefan Kippenberger. 2022. "Functional Downregulation of PD-L1 and PD-L2 by CpG and non-CpG Oligonucleotides in Melanoma Cells" Cancers 14, no. 19: 4698. https://doi.org/10.3390/cancers14194698
APA StyleKleemann, J., Steinhorst, K., König, V., Zöller, N., Cinatl, J., Jr., Özistanbullu, D., Kaufmann, R., Meissner, M., & Kippenberger, S. (2022). Functional Downregulation of PD-L1 and PD-L2 by CpG and non-CpG Oligonucleotides in Melanoma Cells. Cancers, 14(19), 4698. https://doi.org/10.3390/cancers14194698







