Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Two-Faced Role of TANs in Tumor Progression
3. The Prognosis Value of TANs in CRC
4. Antitumor Effect of TANs in CRC
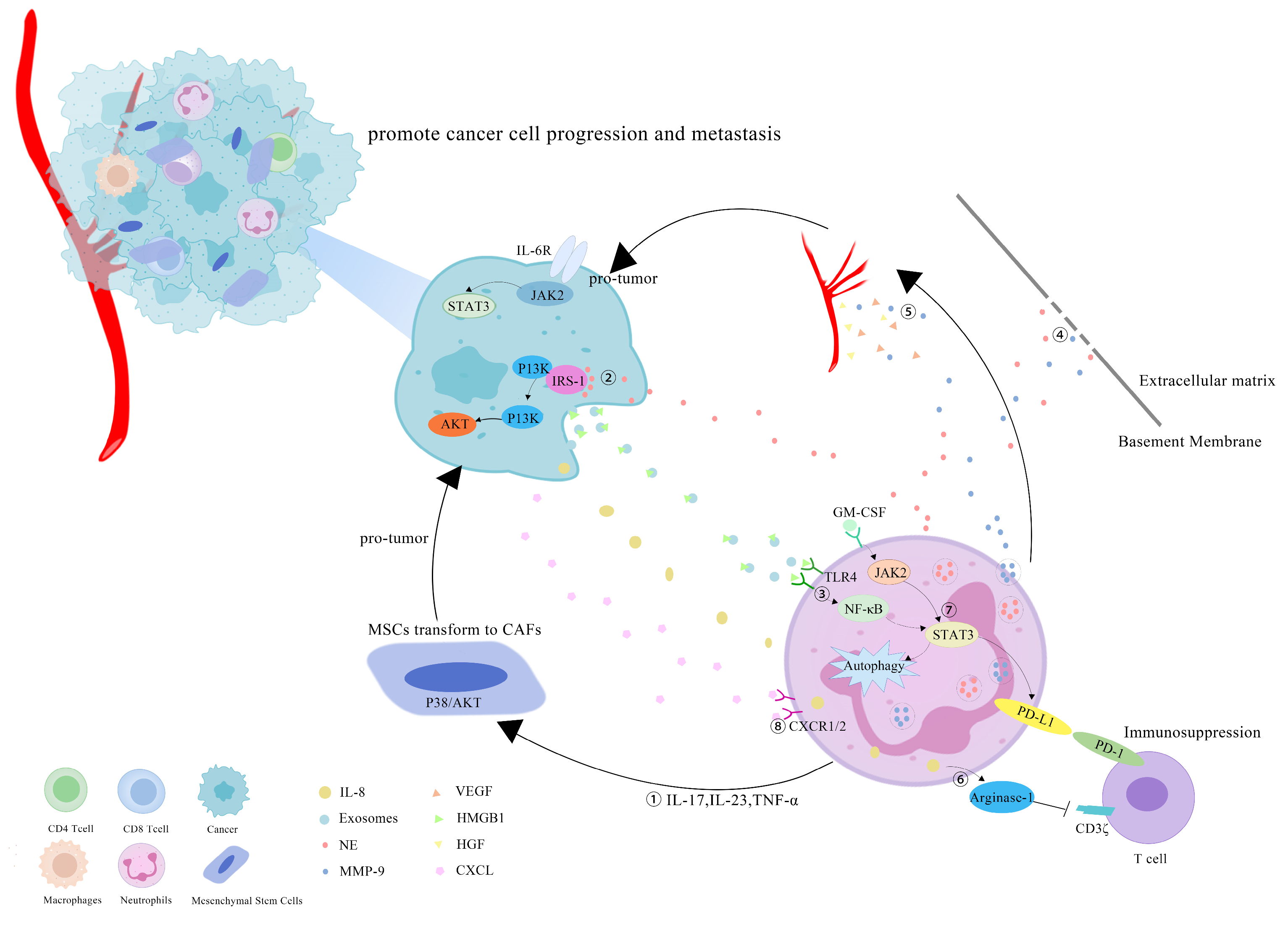
5. Tumor-Promoting Effect of TANs in CRC
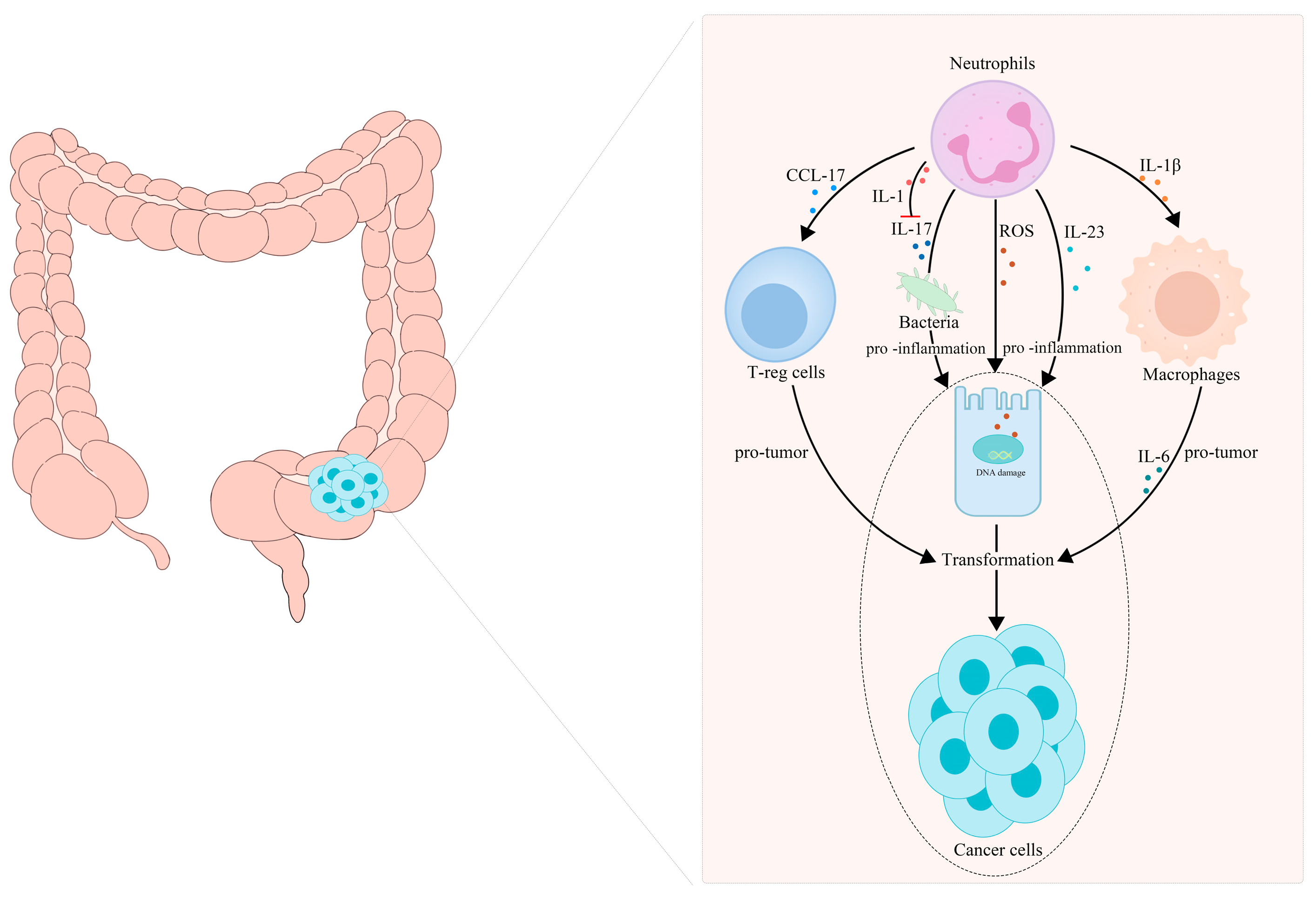
5.1. TANs Are Associated with the Transformation of Inflammation into CRC
5.2. TANs Boost Proliferation, Migration and Chemoradiotherapy Resistance of CRC
5.3. TANs Accelerate Liver Metastases of CRC
5.4. TANs Promote Angiogenesis in CRC
6. TANs May Become a Potential Adjuvant Target for CRC Immunotherapy
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Cao, S.; Xu, R. Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021, 41, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Lau, D.K.; Chau, I. HER2 targeted therapy in colorectal cancer: New horizons. Cancer Treat. Rev. 2022, 105, 102363. [Google Scholar] [CrossRef]
- Ganesh, K. Optimizing immunotherapy for colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 93–94. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Singhal, S.; Albelda, S.M. Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends Cancer 2017, 3, 149–160. [Google Scholar] [CrossRef]
- Ballesteros, I.; Rubio-Ponce, A.; Genua, M.; Lusito, E.; Kwok, I.; Fernandez-Calvo, G.; Khoyratty, T.E.; van Grinsven, E.; Gonzalez-Hernandez, S.; Nicolas-Avila, J.A.; et al. Co-Option of Neutrophil Fates by Tissue Environments. Cell 2020, 183, 1282–1297.e18. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Taucher, E.; Taucher, V.; Fink-Neuboeck, N.; Lindenmann, J.; Smolle-Juettner, F.M. Role of Tumor-Associated Neutrophils in the Molecular Carcinogenesis of the Lung. Cancers 2021, 13, 5972. [Google Scholar] [CrossRef]
- Singhal, S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Garfall, A.L.; Rao, A.S.; Quatromoni, J.G.; Stephen, T.L.; Litzky, L.; Deshpande, C.; et al. Origin and Role of a Subset of Tumor-Associated Neutrophils with Antigen-Presenting Cell Features in Early-Stage Human Lung Cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Gershkovitz, M.; Caspi, Y.; Fainsod-Levi, T.; Katz, B.; Michaeli, J.; Khawaled, S.; Lev, S.; Polyansky, L.; Shaul, M.E.; Sionov, R.V.; et al. TRPM2 Mediates Neutrophil Killing of Disseminated Tumor Cells. Cancer Res. 2018, 78, 2680–2690. [Google Scholar] [CrossRef]
- Finisguerra, V.; Di Conza, G.; Di Matteo, M.; Serneels, J.; Costa, S.; Thompson, A.A.; Wauters, E.; Walmsley, S.; Prenen, H.; Granot, Z.; et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 2015, 522, 349–353. [Google Scholar] [CrossRef]
- Tanaka, H.; Ito, T.; Kyo, T.; Kimura, A. Treatment with IFNα in vivo up-regulates serum-soluble TNF-related apoptosis inducing ligand (sTRAIL) levels and TRAIL mRNA expressions in neutrophils in chronic myelogenous leukemia patients. Eur. J. Haematol. 2007, 78, 389–398. [Google Scholar] [CrossRef]
- Tecchio, C.; Huber, V.; Scapini, P.; Calzetti, F.; Margotto, D.; Todeschini, G.; Pilla, L.; Martinelli, G.; Pizzolo, G.; Rivoltini, L.; et al. IFNα-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood 2004, 103, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Riise, R.E.; Bernson, E.; Aurelius, J.; Martner, A.; Pesce, S.; Della Chiesa, M.; Marcenaro, E.; Bylund, J.; Hellstrand, K.; Moretta, L.; et al. TLR-Stimulated Neutrophils Instruct NK Cells To Trigger Dendritic Cell Maturation and Promote Adaptive T Cell Responses. J. Immunol. 2015, 195, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulou, S.; Valadez-Cosmes, P.; Mihalic, Z.N.; Schicho, R.; Kargl, J. Tumor-Mediated Neutrophil Polarization and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 3218. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Luo, J.; Li, D.; Shu, Y.; Luo, C.; Wang, S.S.; Qin, J.; Zhang, G.M.; Feng, Z.H. Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal NK cells. Oncotarget 2014, 5, 12621–12634. [Google Scholar] [CrossRef]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef]
- Loffredo, S.; Borriello, F.; Iannone, R.; Ferrara, A.L.; Galdiero, M.R.; Gigantino, V.; Esposito, P.; Varricchi, G.; Lambeau, G.; Cassatella, M.A.; et al. Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front. Immunol. 2017, 8, 443. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, C.; Li, W.; Mao, Z.; Shi, Y.; Shi, H.; Ji, R.; Qian, H.; Xu, W.; Zhang, X. Tumor-Educated Neutrophils Activate Mesenchymal Stem Cells to Promote Gastric Cancer Growth and Metastasis. Front. Cell Dev. Biol. 2020, 8, 788. [Google Scholar] [CrossRef]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 6. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer 2018, 17, 146. [Google Scholar] [CrossRef]
- Rotondo, R.; Barisione, G.; Mastracci, L.; Grossi, F.; Orengo, A.M.; Costa, R.; Truini, M.; Fabbi, M.; Ferrini, S.; Barbieri, O. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int. J. Cancer 2009, 125, 887–893. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Swierczak, A.; Mouchemore, K.A.; Hamilton, J.A.; Anderson, R.L. Neutrophils: Important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015, 34, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Martin, T.A.; Parr, C.; Davies, G.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit. Rev. Oncol. Hematol. 2005, 53, 35–69. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 128, 3446–3458. [Google Scholar] [CrossRef]
- Quail, D.F.; Olson, O.C.; Bhardwaj, P.; Walsh, L.A.; Akkari, L.; Quick, M.L.; Chen, I.C.; Wendel, N.; Ben-Chetrit, N.; Walker, J.; et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat. Cell Biol. 2017, 19, 974–987. [Google Scholar] [CrossRef]
- Xiao, Y.; Cong, M.; Li, J.; He, D.; Wu, Q.; Tian, P.; Wang, Y.; Yang, S.; Liang, C.; Liang, Y.; et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 2021, 39, 423–437.e7. [Google Scholar] [CrossRef]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhao, Y.L.; Peng, L.S.; Chen, N.; Chen, W.; Lv, Y.P.; Mao, F.Y.; Zhang, J.Y.; Cheng, P.; Teng, Y.S.; et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017, 66, 1900–1911. [Google Scholar] [CrossRef]
- Sparmann, A.; Bar-Sagi, D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 2004, 6, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Faget, J.; Peters, S.; Quantin, X.; Meylan, E.; Bonnefoy, N. Neutrophils in the era of immune checkpoint blockade. J. Immunother. Cancer 2021, 9, e002242. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wei, G.; Li, N.; Niu, M.; Gong, S.; Wu, G.; Wang, T.; Jiang, Y.; Chen, P. CCR2 and CCR5 promote diclofenac-induced hepatotoxicity in mice. Naunyn Schmiedeb. Arch. Pharmacol. 2019, 392, 287–297. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, J.; Wang, H.; Wang, G.; Wang, C.Y.; Zhang, J. CCR2 dependent neutrophil activation and mobilization rely on TLR4-p38 axis during liver ischemia-reperfusion injury. Am. J. Transl. Res. 2017, 9, 2878–2890. [Google Scholar] [PubMed]
- Berry, R.S.; Xiong, M.J.; Greenbaum, A.; Mortaji, P.; Nofchissey, R.A.; Schultz, F.; Martinez, C.; Luo, L.; Morris, K.T.; Hanson, J.A. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS ONE 2017, 12, e0188799. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Bianchi, P.; Grizzi, F.; Di Caro, G.; Basso, G.; Ponzetta, A.; Bonavita, E.; Barbagallo, M.; Tartari, S.; Polentarutti, N.; et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int. J. Cancer 2016, 139, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Chen, J.W.; Li, M.; Xiao, Y.B.; Fu, J.; Zeng, Y.X.; Cai, M.Y.; Xie, D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE 2012, 7, e30806. [Google Scholar] [CrossRef]
- Jakubowska, K.; Koda, M.; Grudzinska, M.; Kisielewski, W.; Lomperta, K.; Famulski, W. Neutrophil infiltration combined with necrosis in the primary tumor is a useful prognostic indicator for three-year disease-free survival time in patients with colorectal cancer. Oncol. Lett. 2022, 23, 199. [Google Scholar] [CrossRef]
- Su, H.; Cai, T.; Zhang, S.; Yan, X.; Zhou, L.; He, Z.; Xue, P.; Li, J.; Zheng, M.; Yang, X.; et al. Identification of hub genes associated with neutrophils infiltration in colorectal cancer. J. Cell. Mol. Med. 2021, 25, 3371–3380. [Google Scholar] [CrossRef]
- Rottmann, B.G.; Patel, N.; Ahmed, M.; Deng, Y.; Ciarleglio, M.; Vyas, M.; Jain, D.; Zhang, X. Clinicopathological significance of neutrophil-rich colorectal carcinoma. J. Clin. Pathol. 2021. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.Q.; Ma, X.J.; Zhang, L.; Cai, S.J.; Peng, J.J. A Risk Signature with Inflammatory and T Immune Cells Infiltration in Colorectal Cancer Predicting Distant Metastases and Efficiency of Chemotherapy. Front. Oncol. 2019, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Jian, F.; Yanhong, J.; Limeng, W.; Guoping, N.; Yiqing, T.; Hao, L.; Zhaoji, P. TIMP2 is associated with prognosis and immune infiltrates of gastric and colon cancer. Int. Immunopharmacol. 2022, 110, 109008. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lai, H.; Liao, J.; Cai, J.; Li, B.; Meng, L.; Wang, W.; Mo, X.; Qin, H. Upregulation of ADAM12 Is Associated with a Poor Survival and Immune Cell Infiltration in Colon Adenocarcinoma. Front. Oncol. 2021, 11, 729230. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Okugawa, Y.; Yamamoto, A.; Kitajima, T.; Shimura, T.; Kawamura, M.; Tsujiura, M.; Okita, Y.; Ohi, M.; Toiyama, Y. Prognostic significance of CD8+ tumor-infiltrating lymphocytes and CD66b+ tumor-associated neutrophils in the invasive margins of stages I-III colorectal cancer. Oncol. Lett. 2022, 24, 212. [Google Scholar] [CrossRef]
- Xu, X.; Ma, J.; Yu, G.; Qiu, Q.; Zhang, W.; Cao, F. Effective Predictor of Colorectal Cancer Survival Based on Exclusive Expression Pattern among Different Immune Cell Infiltration. J. Histochem. Cytochem. 2021, 69, 271–286. [Google Scholar] [CrossRef]
- Vayrynen, J.P.; Lau, M.C.; Haruki, K.; Vayrynen, S.A.; Dias Costa, A.; Borowsky, J.; Zhao, M.; Fujiyoshi, K.; Arima, K.; Twombly, T.S.; et al. Prognostic Significance of Immune Cell Populations Identified by Machine Learning in Colorectal Cancer Using Routine Hematoxylin and Eosin-Stained Sections. Clin. Cancer Res. 2020, 26, 4326–4338. [Google Scholar] [CrossRef]
- Edin, S.; Kaprio, T.; Hagstrom, J.; Larsson, P.; Mustonen, H.; Bockelman, C.; Strigard, K.; Gunnarsson, U.; Haglund, C.; Palmqvist, R. The Prognostic Importance of CD20+ B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets. Sci. Rep. 2019, 9, 19997. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, T.; Kang, Z.; Guo, G.; Sun, Y.; Lin, K.; Huang, Q.; Shi, X.; Ni, Z.; Ding, N.; et al. Tumor-Infiltrating Immune Cells Act as a Marker for Prognosis in Colorectal Cancer. Front. Immunol. 2019, 10, 2368. [Google Scholar] [CrossRef]
- Governa, V.; Trella, E.; Mele, V.; Tornillo, L.; Amicarella, F.; Cremonesi, E.; Muraro, M.G.; Xu, H.; Droeser, R.; Daster, S.R.; et al. The Interplay between Neutrophils and CD8+ T Cells Improves Survival in Human Colorectal Cancer. Clin. Cancer Res. 2017, 23, 3847–3858. [Google Scholar] [CrossRef]
- Wikberg, M.L.; Ling, A.; Li, X.; Oberg, A.; Edin, S.; Palmqvist, R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum. Pathol. 2017, 68, 193–202. [Google Scholar] [CrossRef]
- Droeser, R.A.; Hirt, C.; Eppenberger-Castori, S.; Zlobec, I.; Viehl, C.T.; Frey, D.M.; Nebiker, C.A.; Rosso, R.; Zuber, M.; Amicarella, F.; et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS ONE 2013, 8, e64814. [Google Scholar] [CrossRef] [PubMed]
- Hirt, C.; Eppenberger-Castori, S.; Sconocchia, G.; Iezzi, G.; Tornillo, L.; Terracciano, L.; Spagnoli, G.C.; Droeser, R.A. Colorectal carcinoma infiltration by myeloperoxidase-expressing neutrophil granulocytes is associated with favorable prognosis. Oncoimmunology 2013, 2, e25990. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.J.; Hansen, U.; Christensen, I.J.; Reimert, C.M.; Brunner, N.; Moesgaard, F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J. Pathol. 1999, 189, 487–495. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Dai, Q.; Shang, B.; Xiao, T.; Di, X.; Zhang, K.; Feng, L.; Shou, J.; Wang, Y. A signature for pan-cancer prognosis based on neutrophil extracellular traps. J. Immunother. Cancer 2022, 10, e004210. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, K.; Zhou, H.; Peng, L.; You, W.; Fu, Z. Profiles of immune infiltration in colorectal cancer and their clinical significant: A gene expression-based study. Cancer Med. 2018, 7, 4496–4508. [Google Scholar] [CrossRef]
- Zhu, B.; Luo, J.; Jiang, Y.; Yu, L.; Liu, M.; Fu, J. Prognostic significance of nomograms integrating IL-37 expression, neutrophil level, and MMR status in patients with colorectal cancer. Cancer Med. 2018, 7, 3682–3694. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Feng, Q.; Zheng, P.; Yang, L.; Liu, T.; Xu, Y.; Zhu, D.; Chang, W.; Ji, M.; Ren, L.; et al. Low tumor purity is associated with poor prognosis, heavy mutation burden, and intense immune phenotype in colon cancer. Cancer Manag. Res. 2018, 10, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Su, H.; Zhong, W.; Yuan, Y.; Yu, Z.; Fang, Y.; Zhou, H.; Li, C.; Huang, K. Interleukin-17 is a favorable prognostic marker for colorectal cancer. Clin. Transl. Oncol. 2015, 17, 50–56. [Google Scholar] [CrossRef]
- Richards, C.H.; Flegg, K.M.; Roxburgh, C.S.; Going, J.J.; Mohammed, Z.; Horgan, P.G.; McMillan, D.C. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br. J. Cancer 2012, 106, 2010–2015. [Google Scholar] [CrossRef]
- Khanh, D.T.; Mekata, E.; Mukaisho, K.; Sugihara, H.; Shimizu, T.; Shiomi, H.; Murata, S.; Naka, S.; Yamamoto, H.; Endo, Y.; et al. Prognostic role of CD10+ myeloid cells in association with tumor budding at the invasion front of colorectal cancer. Cancer Sci. 2011, 102, 1724–1733. [Google Scholar] [CrossRef]
- Bergenfelz, C.; Leandersson, K. The Generation and Identity of Human Myeloid-Derived Suppressor Cells. Front. Oncol. 2020, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Terada, A.; Kita, H. CD66b regulates adhesion and activation of human eosinophils. J. Immunol. 2007, 179, 8454–8462. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Yue, Y.; Wu, D.; Zhou, C.; Guo, M.; Sun, C.; Liao, Q.; Sun, M.; Zhou, D.; Miao, C. Increased MPO in Colorectal Cancer Is Associated with High Peripheral Neutrophil Counts and a Poor Prognosis: A TCGA with Propensity Score-Matched Analysis. Front. Oncol. 2022, 12, 940706. [Google Scholar] [CrossRef] [PubMed]
- Quaas, A.; Pamuk, A.; Klein, S.; Quantius, J.; Rehkaemper, J.; Barutcu, A.G.; Rueschoff, J.; Zander, T.; Gebauer, F.; Hillmer, A.; et al. Sex-specific prognostic effect of CD66b-positive tumor-infiltrating neutrophils (TANs) in gastric and esophageal adenocarcinoma. Gastric Cancer 2021, 24, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Triner, D.; Devenport, S.N.; Ramakrishnan, S.K.; Ma, X.; Frieler, R.A.; Greenson, J.K.; Inohara, N.; Nunez, G.; Colacino, J.A.; Mortensen, R.M.; et al. Neutrophils Restrict Tumor-Associated Microbiota to Reduce Growth and Invasion of Colon Tumors in Mice. Gastroenterology 2019, 156, 1467–1482. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [PubMed]
- Wang, Y.; Wang, K.; Han, G.C.; Wang, R.X.; Xiao, H.; Hou, C.M.; Guo, R.F.; Dou, Y.; Shen, B.F.; Li, Y.; et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014, 7, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Kvedaraite, E.; Lourda, M.; Idestrom, M.; Chen, P.; Olsson-Akefeldt, S.; Forkel, M.; Gavhed, D.; Lindforss, U.; Mjosberg, J.; Henter, J.I.; et al. Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD. Gut 2016, 65, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, S.; Lartey, D.A.; D’Haens, G.R.; Grootjans, J. The Role of the Immune System in IBD-Associated Colorectal Cancer: From Pro to Anti-Tumorigenic Mechanisms. Int. J. Mol. Sci. 2021, 22, 12739. [Google Scholar] [CrossRef] [PubMed]
- Wera, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Schins, R.P.; Polat, D.; Becker, A.; Borm, P.J. Mechanisms of neutrophil-induced DNA damage in respiratory tract epithelial cells. Mol. Cell. Biochem. 2002, 234, 143–151. [Google Scholar] [CrossRef]
- Metzger, R.; Maruskova, M.; Krebs, S.; Janssen, K.P.; Krug, A.B. Increased Incidence of Colon Tumors in AOM-Treated Apc1638N/+ Mice Reveals Higher Frequency of Tumor Associated Neutrophils in Colon Than Small Intestine. Front. Oncol. 2019, 9, 1001. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Eruslanov, E.; Michaeli, J.; Levy, L.; Zolotarov, L.; Singhal, S.; Albelda, S.M.; Granot, Z.; Fridlender, Z.G. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—A new mechanism of impaired antitumor immunity. Int. J. Cancer 2014, 135, 1178–1186. [Google Scholar] [CrossRef]
- Wei, T.T.; Lin, Y.T.; Tseng, R.Y.; Shun, C.T.; Lin, Y.C.; Wu, M.S.; Fang, J.M.; Chen, C.C. Prevention of Colitis and Colitis-Associated Colorectal Cancer by a Novel Polypharmacological Histone Deacetylase Inhibitor. Clin. Cancer Res. 2016, 22, 4158–4169. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Dzutsev, A.; Posocco, D.F.; Hou, V.; Yuan, W.; Thovarai, V.; Mufazalov, I.A.; Gunzer, M.; Shilovskiy, I.P.; Khaitov, M.R.; et al. Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity 2019, 50, 166–180.e7. [Google Scholar] [CrossRef] [Green Version]
- Shang, K.; Bai, Y.P.; Wang, C.; Wang, Z.; Gu, H.Y.; Du, X.; Zhou, X.Y.; Zheng, C.L.; Chi, Y.Y.; Mukaida, N.; et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS ONE 2012, 7, e51848. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Chae, C. Expression of cyclooxygenase-2 and nitric oxide synthase 2 in swine ulcerative colitis caused by Salmonella typhimurium. Vet. Pathol. 2004, 41, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Singer, I.I.; Kawka, D.W.; Schloemann, S.; Tessner, T.; Riehl, T.; Stenson, W.F. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998, 115, 297–306. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Brown, J.; Daikoku, T.; Ning, W.; Shi, Q.; Richmond, A.; Strieter, R.; Dey, S.K.; DuBois, R.N. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J. Exp. Med. 2006, 203, 941–951. [Google Scholar] [CrossRef]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of Prostaglandin E2-EP2 Signals in the Colon Tumor Microenvironment That Amplify Inflammation and Tumor Growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar] [CrossRef]
- Schimek, V.; Strasser, K.; Beer, A.; Gober, S.; Walterskirchen, N.; Brostjan, C.; Muller, C.; Bachleitner-Hofmann, T.; Bergmann, M.; Dolznig, H.; et al. Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment. Cell Death Dis. 2022, 13, 113. [Google Scholar] [CrossRef]
- Shang, A.; Gu, C.; Zhou, C.; Yang, Y.; Chen, C.; Zeng, B.; Wu, J.; Lu, W.; Wang, W.; Sun, Z.; et al. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell Commun. Signal. 2020, 18, 52. [Google Scholar] [CrossRef]
- Luley, K.; Noack, F.; Lehnert, H.; Homann, N. Local calprotectin production in colorectal cancer and polyps—Active neutrophil recruitment in carcinogenesis. Int. J. Colorectal Dis. 2011, 26, 603–607. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, I.; Ning, Y.; Kim, N.Y.; Khatchadourian, V.; Yang, D.; Chung, H.K.; Choi, D.; LaBonte, M.J.; Ladner, R.D.; et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br. J. Cancer 2012, 106, 1833–1841. [Google Scholar] [CrossRef]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef] [Green Version]
- Rayes, R.F.; Vourtzoumis, P.; Bou Rjeily, M.; Seth, R.; Bourdeau, F.; Giannias, B.; Berube, J.; Huang, Y.H.; Rousseau, S.; Camilleri-Broet, S.; et al. Neutrophil Extracellular Trap-Associated CEACAM1 as a Putative Therapeutic Target to Prevent Metastatic Progression of Colon Carcinoma. J. Immunol. 2020, 204, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chu, Y.; Hu, J.; Ding, X.; Liu, Z.; Fu, D.; Yuan, Y.; Deng, Y.; Wang, G.; Wang, L.; et al. Tumour-associated neutrophils secrete AGR2 to promote colorectal cancer metastasis via its receptor CD98hc-xCT. Gut 2022. [Google Scholar] [CrossRef] [PubMed]
- Lepsenyi, M.; Algethami, N.; Al-Haidari, A.A.; Algaber, A.; Syk, I.; Rahman, M.; Thorlacius, H. CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells. Clin. Exp. Metastasis 2021, 38, 401–410. [Google Scholar] [CrossRef]
- He, Y.; Li, T.; Liu, J.; Ou, Q.; Zhou, J. Early onset neutropenia: A useful predictor of chemosensitivity and favorable prognosis in patients with serous ovarian cancer. BMC Cancer 2020, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Imaoka, H.; Watanabe, K.; Sasaki, M.; Takahashi, H.; Hashimoto, Y.; Ohno, I.; Mitsunaga, S.; Umemoto, K.; Kimura, G.; et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with pancreatic cancer treated with gemcitabine plus nab-paclitaxel: A retrospective cohort study. Cancer Chemother. Pharmacol. 2020, 86, 203–210. [Google Scholar] [CrossRef]
- Meisel, A.; von Felten, S.; Vogt, D.R.; Liewen, H.; de Wit, R.; de Bono, J.; Sartor, O.; Stenner-Liewen, F. Severe neutropenia during cabazitaxel treatment is associated with survival benefit in men with metastatic castration-resistant prostate cancer (mCRPC): A post-hoc analysis of the TROPIC phase III trial. Eur. J. Cancer 2016, 56, 93–100. [Google Scholar] [CrossRef]
- Yoshino, T.; Cleary, J.M.; Van Cutsem, E.; Mayer, R.J.; Ohtsu, A.; Shinozaki, E.; Falcone, A.; Yamazaki, K.; Nishina, T.; Garcia-Carbonero, R.; et al. Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann. Oncol. 2020, 31, 88–95. [Google Scholar] [CrossRef]
- Ramachandran, I.R.; Condamine, T.; Lin, C.; Herlihy, S.E.; Garfall, A.; Vogl, D.T.; Gabrilovich, D.I.; Nefedova, Y. Bone marrow PMN-MDSCs and neutrophils are functionally similar in protection of multiple myeloma from chemotherapy. Cancer Lett. 2016, 371, 117–124. [Google Scholar] [CrossRef]
- Shinde-Jadhav, S.; Mansure, J.J.; Rayes, R.F.; Marcq, G.; Ayoub, M.; Skowronski, R.; Kool, R.; Bourdeau, F.; Brimo, F.; Spicer, J.; et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat. Commun. 2021, 12, 2776. [Google Scholar] [CrossRef]
- Germann, M.; Zangger, N.; Sauvain, M.O.; Sempoux, C.; Bowler, A.D.; Wirapati, P.; Kandalaft, L.E.; Delorenzi, M.; Tejpar, S.; Coukos, G.; et al. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFβ. EMBO Mol. Med. 2020, 12, e10681. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Narayan, R.R.; Kemeny, N.E.; D’Angelica, M.I. Role of Hepatic Artery Infusion Chemotherapy in Treatment of Initially Unresectable Colorectal Liver Metastases: A Review. JAMA Surg. 2019, 154, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-Metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Andzinski, L.; Kasnitz, N.; Kroger, A.; Klawonn, F.; Lienenklaus, S.; Weiss, S.; Jablonska, J. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int. J. Cancer 2015, 137, 837–847. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Seubert, B.; Grunwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N.; et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef]
- Jackstadt, R.; van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e7. [Google Scholar] [CrossRef]
- Ruland, J. Colon Cancer: Epithelial Notch Signaling Recruits Neutrophils to Drive Metastasis. Cancer Cell 2019, 36, 213–214. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Fujishita, T.; Kakizaki, F.; Hirai, H.; Matsumoto, T.; Iwamoto, M.; Inamoto, S.; Hatano, E.; Hasegawa, S.; et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology 2013, 145, 1064–1075.e11. [Google Scholar] [CrossRef] [Green Version]
- Inamoto, S.; Itatani, Y.; Yamamoto, T.; Minamiguchi, S.; Hirai, H.; Iwamoto, M.; Hasegawa, S.; Taketo, M.M.; Sakai, Y.; Kawada, K. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through the CCL15-CCR1 Chemokine Axis. Clin. Cancer Res. 2016, 22, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Itatani, Y.; Inamoto, S.; Okamura, R.; Iwamoto, M.; Miyamoto, E.; Chen-Yoshikawa, T.F.; Hirai, H.; Hasegawa, S.; et al. Loss of SMAD4 Promotes Lung Metastasis of Colorectal Cancer by Accumulation of CCR1+ Tumor-Associated Neutrophils through CCL15-CCR1 Axis. Clin. Cancer Res. 2017, 23, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Yamamoto, T.; Hirai, H.; Hanada, K.; Kiyasu, Y.; Nishikawa, G.; Mizuno, R.; Inamoto, S.; Itatani, Y.; Sakai, Y.; et al. Loss of SMAD4 Promotes Colorectal Cancer Progression by Recruiting Tumor-Associated Neutrophils via the CXCL1/8-CXCR2 Axis. Clin. Cancer Res. 2019, 25, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, L.; Zhang, R.; Hong, J.; Wang, Y.; Wang, J.; Zuo, J.; Zhang, J.; Chen, J.; Hao, H. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J. Cancer 2020, 11, 4384–4396. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Weeks, A.N.; Lim, S.Y.; Yuzhalin, A.E.; Jones, K.; Markelc, B.; Kim, K.J.; Buzzelli, J.N.; Fokas, E.; Cao, Y.; Smart, S.; et al. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology 2017, 65, 1920–1935. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Lopez, A.; Harada, K.; Vasilakopoulou, M.; Shanbhag, N.; Ajani, J.A. Targeting Angiogenesis in Colorectal Carcinoma. Drugs 2019, 79, 63–74. [Google Scholar] [CrossRef]
- Palmieri, V.; Lazaris, A.; Mayer, T.Z.; Petrillo, S.K.; Alamri, H.; Rada, M.; Jarrouj, G.; Park, W.Y.; Gao, Z.H.; McDonald, P.P.; et al. Neutrophils expressing lysyl oxidase-like 4 protein are present in colorectal cancer liver metastases resistant to anti-angiogenic therapy. J. Pathol. 2020, 251, 213–223. [Google Scholar] [CrossRef]
- Itatani, Y.; Yamamoto, T.; Zhong, C.; Molinolo, A.A.; Ruppel, J.; Hegde, P.; Taketo, M.M.; Ferrara, N. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 21598–21608. [Google Scholar] [CrossRef]
- Wu, D.W.; Huang, H.Y.; Tang, Y.; Zhao, Y.; Yang, Z.M.; Wang, J.; Wang, S.H.; Yu, Y.; Fang, Y.; Fang, H.; et al. Clinical development of immuno-oncology in China. Lancet Oncol. 2020, 21, 1013–1016. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Nieto-Jimenez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 141. [Google Scholar] [CrossRef]
- Deng, H.; Kan, A.; Lyu, N.; He, M.; Huang, X.; Qiao, S.; Li, S.; Lu, W.; Xie, Q.; Chen, H.; et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, e002305. [Google Scholar] [CrossRef] [PubMed]
- Yajuk, O.; Baron, M.; Toker, S.; Zelter, T.; Fainsod-Levi, T.; Granot, Z. The PD-L1/PD-1 Axis Blocks Neutrophil Cytotoxicity in Cancer. Cells 2021, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Clavijo, P.E.; Robbins, Y.; Patel, P.; Friedman, J.; Greene, S.; Das, R.; Silvin, C.; Van Waes, C.; Horn, L.A.; et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 2019, 4, e126853. [Google Scholar] [CrossRef]
- Lin, H.; Wu, Y.; Chen, J.; Huang, S.; Wang, Y. (−)-4-O-(4-O-β-D-glucopyranosylcaffeoyl) Quinic Acid Inhibits the Function of Myeloid-Derived Suppressor Cells to Enhance the Efficacy of Anti-PD1 against Colon Cancer. Pharm. Res. 2018, 35, 183. [Google Scholar] [CrossRef]
- Tavazoie, M.F.; Pollack, I.; Tanqueco, R.; Ostendorf, B.N.; Reis, B.S.; Gonsalves, F.C.; Kurth, I.; Andreu-Agullo, C.; Derbyshire, M.L.; Posada, J.; et al. LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer. Cell 2018, 172, 825–840.e18. [Google Scholar] [CrossRef]
- Bergerot, P.; Lamb, P.; Wang, E.; Pal, S.K. Cabozantinib in Combination with Immunotherapy for Advanced Renal Cell Carcinoma and Urothelial Carcinoma: Rationale and Clinical Evidence. Mol. Cancer Ther. 2019, 18, 2185–2193. [Google Scholar] [CrossRef]
- Bierie, B.; Moses, H.L. Tumour microenvironment: TGFβ: The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 2006, 6, 506–520. [Google Scholar] [CrossRef]
- Zhong, Z.; Carroll, K.D.; Policarpio, D.; Osborn, C.; Gregory, M.; Bassi, R.; Jimenez, X.; Prewett, M.; Liebisch, G.; Persaud, K.; et al. Anti-transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin. Cancer Res. 2010, 16, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.L.; Singh, S.; Li, A.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011, 300, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol. 2019, 40, 228–242. [Google Scholar] [CrossRef] [PubMed]
| Prognosis | First Author | Year | Journal | Model | Keynote | Reference |
|---|---|---|---|---|---|---|
| Better | Chengzeng Yin | 2022 | Oncology Letters | Human | High density of CD66b+ TANs in the invasive margin significantly correlated with better prognoses for OS and DFS of patients with stages I–III CRC. | [54] |
| Xiaowen Xu | 2021 | The Journal of Histochemistry and Cytochemistry | Human | Higher numbers of tumor-infiltrating CD66b+ neutrophils were significantly associated with both longer DFS and OS for CRC patients. | [55] | |
| Juha P Väyrynen | 2020 | Clinical Cancer Research | Human | Intraepithelial TANs and stromal TANs were significantly associated with better CSS and OS, respectively. | [56] | |
| Sofia Edin | 2019 | Scientific Reports | Human | Those highly infiltrated by CD66b+ cells had a significantly improved DSS. | [57] | |
| Lele Ye | 2019 | Frontiers in Immunology | Human | High-CD66b+ TANs were significantly related with better OS and DFS in CRC patients based on GEO and TCGA databases. | [58] | |
| Valeria Governa | 2017 | Clinical Cancer Research | Human | CD66b+ cell infiltration in CRC is significantly associated with increased OS. | [59] | |
| Maria L Wikberg | 2017 | Human Pathology | Human | Infiltration of CD66b+ cells in the tumor front indicated statistically favorable prognoses in patients with stages I–II colon cancer. | [60] | |
| Ryan S Berry | 2017 | PloS ONE | Human | High levels of TANs were associated with improved OS in patients with stage II CRC. | [45] | |
| Maria Rosaria Galdiero | 2016 | International Journal of Cancer | Human | CD66b was found to be a reliable marker to identify TANs in CRC tissues, whereas MPO also identified a subset of CD68+ macrophages. Higher TAN density was associated with better prognosis. | [46] | |
| Raoul A Droeser | 2013 | PloS One | Human | High MPO+ cell infiltration was significantly associated with better prognosis. | [61] | |
| Christian Hirt | 2014 | Oncoimmunology | Human | A high density of MPO+ infiltrating cells was significantly associated with increased 5-year OS. | [62] | |
| Hans Jorgen Nielsen | 1999 | Journal of Pathology | Human | High counts of neutrophils infiltrated in the peritumoral were significant predictors of good OS. | [63] | |
| Worse | Yi Zhang | 2022 | Journal for Immunotherapy of Cancer | Human | The higher proportion of MPO+ cells in tumor-infiltrating stromal cells was significantly associated with worse prognosis. | [64] |
| Katarzyna Jakubowska | 2022 | Oncology Letters | Human | Patients in the low-stroma TAN level group exhibited significantly longer 3- and 5-year DFS rates compared with those in patients in the high-stroma TAN level group. | [48] | |
| Bruce G Rottmann | 2021 | Journal of Clinical Pathology | Human | Patients with neutrophil-rich CRCs showed significantly poorer 5-year RFS compared with patients with neutrophil-intermediate or neutrophil-poor CRCs. | [50] | |
| Hao Su | 2021 | Journal of Cellular and Molecular Medicine | Human | Increased neutrophil infiltration in CRC was associated with a poorer prognosis based on data from GEO and TCGA databases. | [49] | |
| Xiang Hu | 2019 | Frontiers in Oncology | Human | CEACAM8 was used to detect tumor-infiltrating neutrophils within CRC. High-CEACAM8+ tumor-infiltrating neutrophils were associated with worse DFS. | [51] | |
| Yongfu Xiong | 2018 | Cancer Medicine | Human | Tumor-infiltrating neutrophils were significantly associated with poorer prognosis based on data from GEO and TCGA databases. | [65] | |
| Bing Zhu | 2018 | Cancer Medicine | Human | Increased CD66b+ TANs showed statistically unfavorable DFS and OS. | [66] | |
| Yihao Mao | 2018 | Cancer Management and Research | Human | High relative proportion of tumor-infiltrating neutrophils in colon cancer indicated poor OS based on data from GEO and TCGA databases. | [67] | |
| Hui-Lan Rao | 2012 | PloS One | Human | Increased intratumoral CD66b+ neutrophils were correlated with adverse OS. | [47] | |
| No significance | Fang Jian | 2022 | International Immunopharmacology | Human | Tumor-infiltrating neutrophils had no significant effect on the OS of colon adenocarcinoma patients based on TIMER database (p = 0.406). | [52] |
| Zigao Huang | 2021 | Frontiers in Oncology | Human | No significant association was found between tumor-infiltrating neutrophils and survival rates of patients with colon adenocarcinoma based on TIMER database (p = 0.789). | [53] | |
| Y Lin | 2015 | Clinical and Translational Oncology | Human | The number of tumor-infiltrating neutrophils (CD15+ neutrophils) did not significantly affect the overall survival. | [68] | |
| C H Richards | 2012 | British Journal of Cancer | Human | Peritumoral neutrophil infiltration was not significantly associated with CSS (p = 0.27). | [69] | |
| Do Trong Khanh | 2011 | Cancer Science | Human | The infiltration of neutrophils was not significant in predicting either RFS or OS in stages I–III CRCs (p = 0.410/p = 0.080, respectively). | [70] |
| Ongoing Trials | Neutrophil Biology Targets | Agents | ICI Targets | ICIs | Cancer | First Posted | Phase | Status | Results |
|---|---|---|---|---|---|---|---|---|---|
| NCT02851004 | STAT3 | BBI-608 | PD-1 | Pembrolizumab | Metastatic CRC | 2016 | Phase Ib/II | Terminated | / |
| NCT03647839 | PD-1 | Nivolumab | MSS Metastatic CRC | 2018 | Phase II | Completed | Not available | ||
| NCT02983578 | Danvatirsen/AZD9150 | PD-L1 | Durvalumab | dMMR CRC | 2016 | Phase II | Active, not recruiting | / | |
| NCT03168139 | CXCL12/CXCR4/CXCR7 | NOX-A12/Olaptesed | PD-1 | Pembrolizumab | Metastatic CRC | 2017 | Phase I/II | Completed | A total of 70% were still alive at 24 weeks, and 50% at 36 weeks. A total of 27% CRC patients achieved SD based on data from the 2018 ESMO Immuno-Oncology Congress. |
| NCT03473925 | CXCR1/2 | Navarixin | PD-1 | Pembrolizumab | Advanced/Metastatic Solid Tumors (MSS CRC) | 2018 | Phase II | Completed | A total of 19 participants with MSS CRC were enrolled in a low-dose group (30 mg navarixin plus 200 mg pembrolizumab), and 21 participants in a high-dose group (100 mg navarixin plus 200 mg pembrolizumab). The median PFS was 1.8 months (95% CI, from 1.0 to 2.0) in the low-dose group, and 1.9 months (95% CI, from 1.6 to 2.0) in the high-dose group. The median OS was 6.5 months (95% CI, from 3.0 to 9.7) in the low-dose group, and 8.0 months (95% CI, from 5.7 to 14.4) in the high-dose group. |
| NCT04599140 | SX-682 | PD-1 | Nivolumab | RAS-Mutated, MSS Metastatic CRC | 2020 | Phase Ib/II | Recruiting | / | |
| NCT03184870 | CCR2/5 | BMS-813160 | PD-1 | Nivolumab | Advanced Solid Tumors (CRC) | 2017 | Phase Ib/II | Active, not recruiting | / |
| NCT03631407 | CCR5 | Vicriviroc | PD-1 | Pembrolizumab | MSS Metastatic CRC | 2018 | Phase II | Completed | A total of 41 participants with MSS CRC were randomized to receive vicriviroc (low-dose: 150 mg; high-dose: 250 mg) in combination with pembrolizumab (200 mg). The ORRs of the two groups were both 5.0% (95% CI, from 0.1 to 24.9%). The median PFS was 2.1 months (95% CI, from 1.8 to 3.0) in the low-dose group, and 2.1 months (95% CI, from 1.6 to 3.9) in the high-dose group. The median OS was 4.6 months (95% CI, from 2.7 to 12.6) in the low-dose group, and 5.3 months (95% CI, from 3.2 to 8.0) in the high-dose group. |
| NCT04721301 | Maraviroc | PD-1/CTLA-4 | Nivolumab + Ipilimumab | Advanced Metastatic CRC | 2021 | Phase I | Active, not recruiting | / | |
| NCT03274804 | PD-1 | Pembrolizumab | MSS Metastatic CRC | 2017 | Phase I | Completed | A total of 20 patients with MSS CRC received a pembrolizumab plus maraviroc treatment. After the core treatment period of 8 cycles, the DCR and ORR were both 5.3%. Median PFS was 9 weeks (95% CI, from 7.0 to 10.0), and median OS was 9 months (95% CI, from 6.0 to 20.0). | ||
| NCT03711058 | PI3K | Copanlisib | PD-1 | Nivolumab | MSS Metastatic CRC | 2018 | Phase I/II | Active, not recruiting | / |
| NCT02646748 | PI3K-delta | INCB050465 | PD-1 | Pembrolizumab | Advanced Solid Tumors (CRC) | 2016 | Phase I | Completed | Not available |
| NCT05205330 | PGE2 | CR6086 | PD-1 | AGEN2034 | pMMR-MSS Metastatic CRC | 2022 | Phase Ib/IIa | Recruiting | / |
| NCT03658772 | PGE2-receptor/EP4 | Grapiprant | PD-1 | Pembrolizumab | MSS CRC | 2018 | Phase I | Active, not recruiting | / |
| NCT04432857 | AN0025 | PD-1 | Pembrolizumab | Advanced Solid Tumors (MSS CRC) | 2020 | Phase Ib | Recruiting | / | |
| NCT05205330 | CR6086 | PD-1 | AGEN2034 /Balstilimab | pMMR-MSS Metastatic CRC | 2022 | Phase Ib/IIa | Recruiting | / | |
| NCT04344795 | EP2/EP4 | TPST-1495 | PD-1 | Pembrolizumab | Solid Tumors (CRC) | 2020 | Phase Ia/Ib | Recruiting | / |
| NCT03026140 | COX2 | Celecoxib | PD-1/CTLA-4 | Nivolumab/Ipilimumab | Early-Stage Colon Cancer | 2017 | Phase II | Recruiting | / |
| NCT03926338 | Celecoxib | PD-L1 | Toripalimab | CRC | 2019 | Phase I/II | Recruiting | / | |
| NCT03638297 | COX | aspirin | PD-1 | BAT1306/pembrolizumab | MSI-H/dMMR Colorectal Cancer | 2018 | Phase II | Unknown | / |
| NCT02903914 | Arginase | INCB001158/CB-1158 | PD-1 | Pembrolizumab | Advanced/Metastatic Solid Tumors (CRC) | 2016 | Phase I/II | Active, not recruiting | / |
| NCT03436563 | TGF-βRII | M7824 | PD-L1 | M7824 | CRC | 2018 | Phase Ib/II | Active, not recruiting | / |
| NCT02947165 | TGF-β | NIS793 | PD-1 | PDR001 | Advanced Malignancies (CRC) | 2016 | Phase I/Ib | Completed | Not available |
| NCT04429542 | EGFR + TGF-β | BCA101 | PD-1 | Pembrolizumab | EGFR-driven Advanced Solid Tumors (CRC) | 2020 | Phase I | Recruiting | / |
| NCT04166383 | TNF-α | VB-111 | PD-1 | Nivolumab | Metastatic CRC | 2019 | Phase II | Active, not recruiting | / |
| NCT04060342 | CD11b | GB1275 | PD-1 | Pembrolizumab | MSS CRC | 2019 | Phase I | Terminated | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Wu, J.; Peng, Y.; Sun, J.; Cheng, P.; Huang, Q. Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy. Cancers 2022, 14, 4755. https://doi.org/10.3390/cancers14194755
Zheng W, Wu J, Peng Y, Sun J, Cheng P, Huang Q. Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy. Cancers. 2022; 14(19):4755. https://doi.org/10.3390/cancers14194755
Chicago/Turabian StyleZheng, Wei, Jingjing Wu, Yao Peng, Jing Sun, Pu Cheng, and Qi Huang. 2022. "Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy" Cancers 14, no. 19: 4755. https://doi.org/10.3390/cancers14194755
APA StyleZheng, W., Wu, J., Peng, Y., Sun, J., Cheng, P., & Huang, Q. (2022). Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy. Cancers, 14(19), 4755. https://doi.org/10.3390/cancers14194755






