Avoiding Pitfalls in Thermal Dose Effect Relationship Studies: A Review and Guide Forward
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
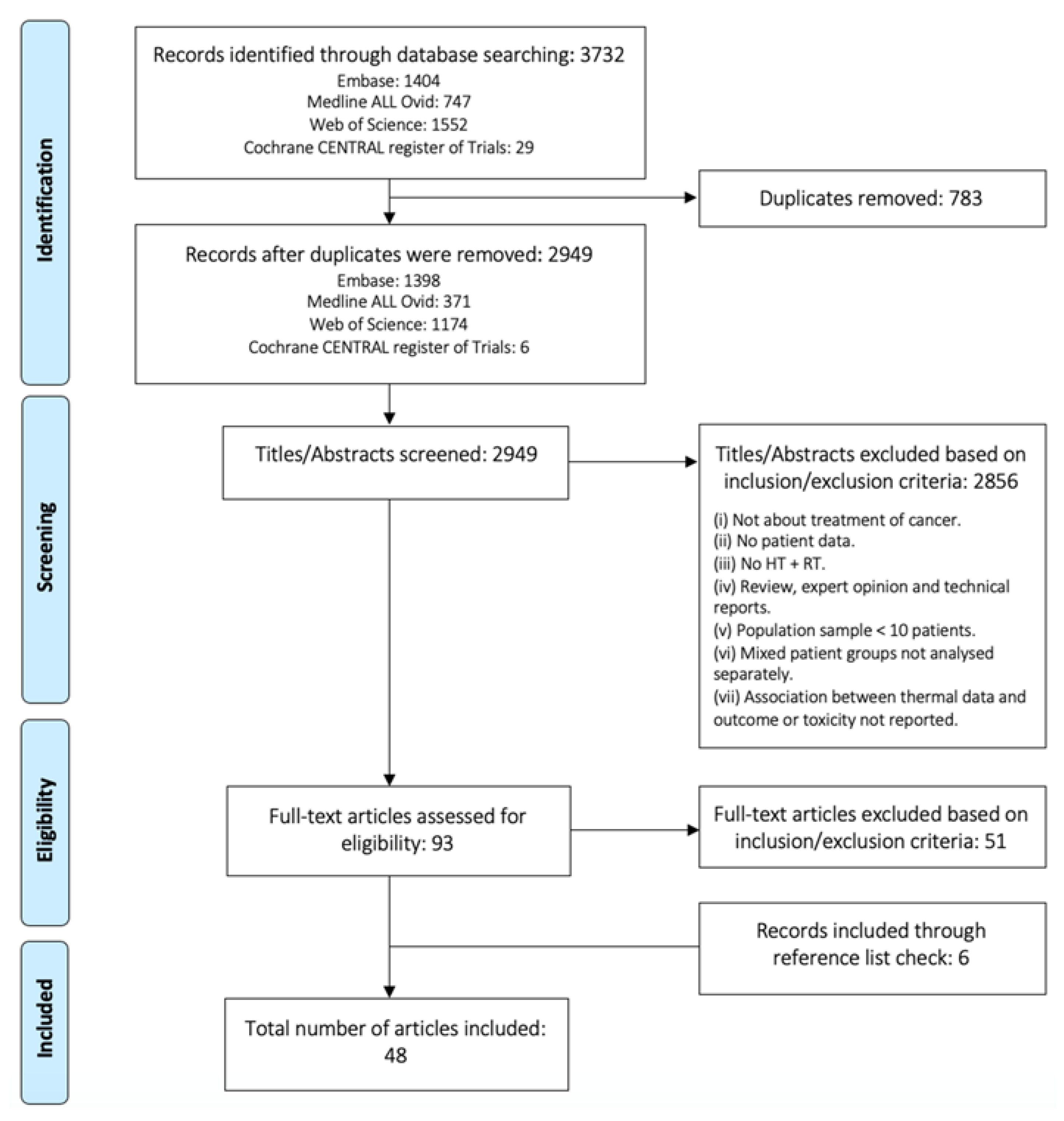
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Study Selection
2.4. Data Extraction and Evaluation
2.5. Critical Appraisal and Risk of Bias
3. Results
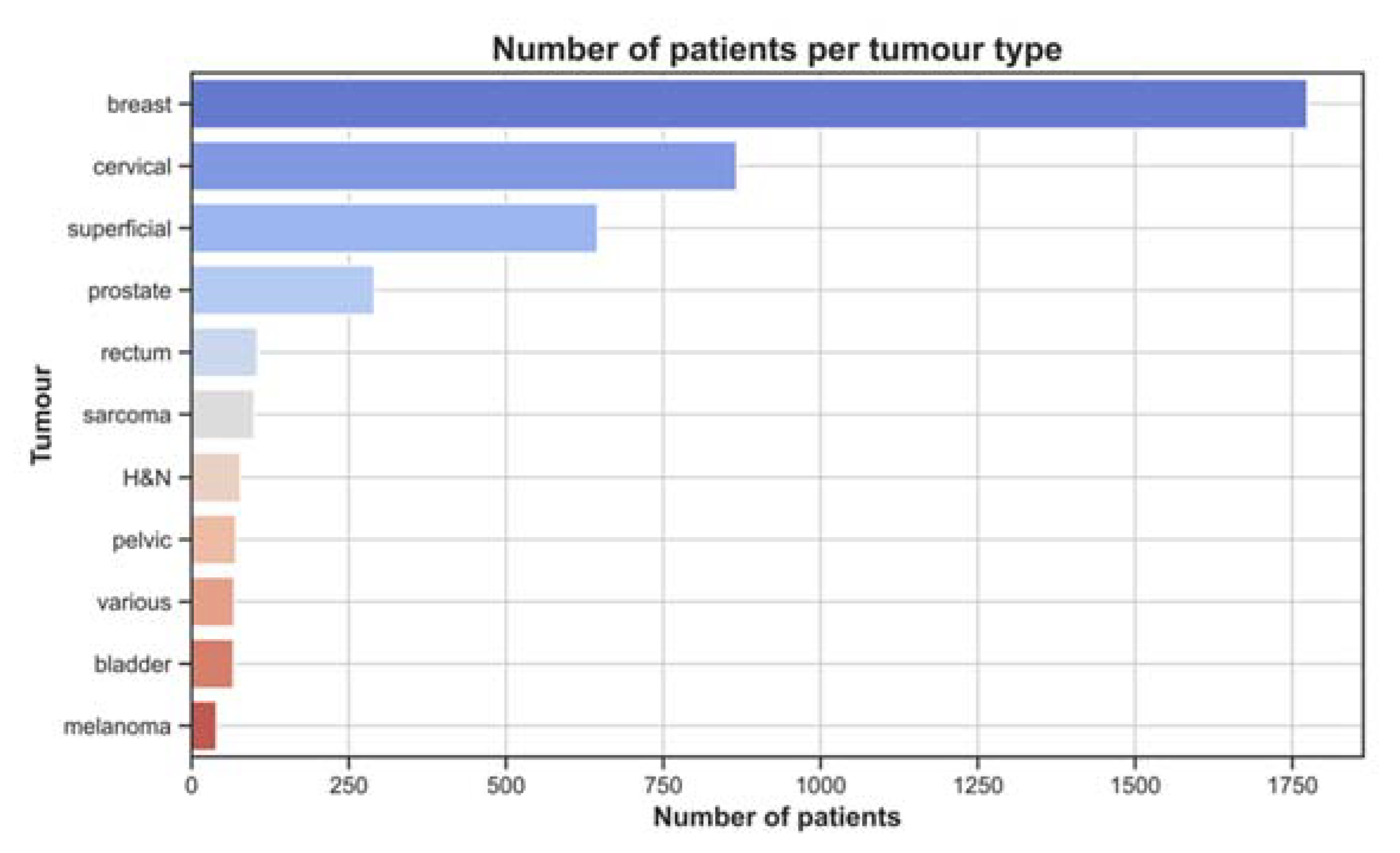
3.1. Treatment Characteristics
3.2. HT System and Thermometry
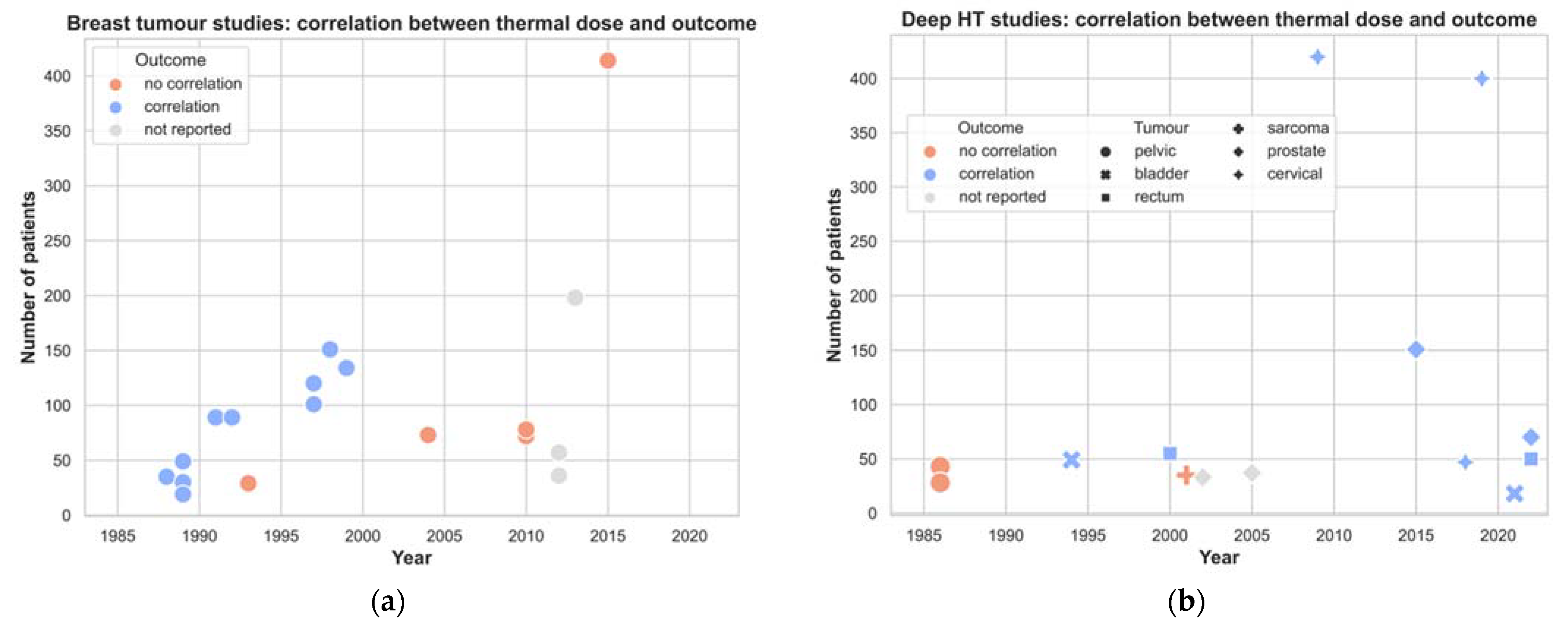
3.3. Temperature and Thermal Dose Parameters
4. Discussion
4.1. Impact of Time
4.2. Treatment Characteristics
4.3. Impact of HT Systems
4.4. Quality of Thermometry
4.5. Other Factors
4.6. How to Proceed
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewey, W.C.; Hopwood, L.E.; Sapareto, S.A.; Gerweck, L.E. Cellular Responses to Combinations of Hyperthermia and Radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Field, S.B.; Bleehen, N.M. Hyperthermia in the Treatment of Cancer. Cancer Treat. Rev. 1979, 6, 63–94. [Google Scholar] [CrossRef]
- Kampigna, H.H.; Dikomey, E. Hyperthermic Radiosensitization: Mode of Action and Clinical Relevance. Int. J. Radiat. Biol. 2001, 77, 399–408. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of Hyperthermia on DNA Repair Pathways: One Treatment to Inhibit Them All. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef]
- Van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving Efficacy of Hyperthermia in Oncology by Exploiting Biological Mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef]
- Perez, C.A.; Kuske, R.R.; Emami, B.; Fineberg, B. Irradiation Alone or Combined with Hyperthermia in the Treatment of Recurrent Carcinoma of the Breast in the Chest Wall: A Nonrandomized Comparison. Int. J. Hyperth. 1986, 2, 179–187. [Google Scholar] [CrossRef]
- Sneed, P.K.; Stauffer, P.R.; Gutin, P.H.; Phillips, T.L.; Suen, S.; Weaver, K.A.; Lamb, S.A.; Ham, B.; Prados, M.D.; Larson, D.A.; et al. Interstitial Irradiation and Hyperthermia for the Treatment of Recurrent Malignant Brain Tumors. Neurosurgery 1991, 28, 206–215. [Google Scholar] [CrossRef]
- Overgaard, J.; Bentzen, S.M.; Overgaard, J.; Gonzalez Gonzalez, D.; Hulshof, M.C.C.M.; Arcangeli, G.; Dahl, O.; Mella, O. Randomised Trial of Hyperthermia as Adjuvant to Radiotherapy for Recurrent or Metastatic Malignant Melanoma. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal Dose Determination in Cancer Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Vujaskovic, Z.; Jones, E.; Thrall, D. Re-Setting the Biologic Rationale for Thermal Therapy. Int. J. Hyperth. 2005, 21, 779–790. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Oleson, J.R.; Kirkpatrick, J.; Secomb, T.W. Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy. Cancers 2022, 14, 1701. [Google Scholar] [CrossRef] [PubMed]
- Van Rhoon, G.C. Is CEM43 Still a Relevant Thermal Dose Parameter for Hyperthermia Treatment Monitoring? Int. J. Hyperth. 2016, 32, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Franckena, M.; Fatehi, D.; de Bruijne, M.; Canters, R.A.M.; van Norden, Y.; Mens, J.W.; va Rhoon, G.C.; van der Zee, J. Hyperthermia Dose-Effect Relationship in 420 Patients with Cervical Cancer Treated with Combined Radiotherapy and Hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Marder, D.; Datta, S.; Meister, A.; Puric, E.; Stutz, E.; Rogers, S.; Eberle, B.; Timm, O.; Staruch, M.; et al. Quantification of Thermal Dose in Moderate Clinical Hyperthermia with Radiotherapy: A Relook Using Temperature–Time Area under the Curve (AUC). Int. J. Hyperth. 2021, 38, 296–307. [Google Scholar] [CrossRef] [PubMed]
- DeWhirst, M.W.; Phillips, T.L.; Samulski, T.V.; Stauffer, P.; Shrivastava, P.; Paliwal, B.; Pajak, T.; Gillim, M.; Sapozink, M.; Myerson, R.; et al. RTOG Quality Assurance Guidelines for Clinical Trials Using Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 1249–1259. [Google Scholar] [CrossRef]
- Wust, P.; Gellermann, J.; Harder, C.; Tilly, W.; Rau, B.; Dinges, S.; Schlag, P.; Budach, V.; Felix, R. Rationale for Using Invasive Thermometry for Regional Hyperthermia of Pelvic Tumors. Int. J. Radiat. Oncol. 1998, 41, 1129–1137. [Google Scholar] [CrossRef]
- Sapozink, M.D.; Gibbs, F.A.; Egger, M.J.; Stewart, J.R. Abdominal Regional Hyperthermia with an Annular Phased Array. J. Clin. Oncol. 1986, 4, 775–783. [Google Scholar] [CrossRef]
- Perez, C.A.; Gillespie, B.; Pajak, T.; Hornback, N.B.; Emami, D.; Rubin, P. Quality Assurance Problems in Clinical Hyperthermia and Their Impact on Therapeutic Outcome: A Report by the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 551–558. [Google Scholar] [CrossRef]
- Van Der Zee, J.; Vujaskovic, Z.; Kondo, M.; Sugahara, T. The Kadota Fund International Forum 2004-Clinical Group Consensus*. Int. J. Hyperth. 2008, 24, 111–122. [Google Scholar] [CrossRef]
- Lagendijk, J.J.W.; Van Rhoon, G.C.; Hornsleth, S.N.; Wust, P.; De Leeuw, A.C.C.; Schneider, C.J.; Van Dijk, J.D.P.; Van Der Zee, J.; Van Heek-Romanowski, R.; Rahman, S.A.; et al. ESHO Quality Assurance Guidelines for Regional Hyperthermia. Int. J. Hyperth. 1998, 14, 125–133. [Google Scholar] [CrossRef]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Quality Assurance for Clinical Studies in Regional Deep Hyperthermia. Strahlenther. Onkol. 2011, 187, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Bruggmoser, G. Some Aspects of Quality Management in Deep Regional Hyperthermia. Int. J. Hyperth. 2012, 28, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Trefná, H.D.; Crezee, H.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Hartmann, J.; Nadobny, J.; Gellermann, J.; van Holthe, N.; et al. Quality Assurance Guidelines for Superficial Hyperthermia Clinical Trials: I. Clinical Requirements. Int. J. Hyperth. 2017, 33, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Dobšíček Trefná, H.; Crezee, J.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Nadobny, J.; Hartmann, J.; Lomax, N.; Abdel-Rahman, S.; et al. Quality Assurance Guidelines for Superficial Hyperthermia Clinical Trials: II. Technical Requirements for Heating Devices. Strahlenther. Onkol. 2017, 193, 351–366. [Google Scholar] [CrossRef]
- Dobšíček Trefná, H.; Schmidt, M.; van Rhoon, G.C.; Kok, H.P.; Gordeyev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality Assurance Guidelines for Interstitial Hyperthermia. Int. J. Hyperth. 2019, 36, 277–294. [Google Scholar] [CrossRef]
- Van Der Zee, J.; Peer-Valstar, J.N.; Rietveld, P.J.M.; De Graaf-Strukowska, L.; Van Rhoon, G.C. Practical Limitations of Interstitial Thermometry during Deep Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1205–1212. [Google Scholar] [CrossRef]
- De Bruijne, M.; Holt, B.; Van Rhoon, G.C.; Van Der Zee, J. Evaluation of CEM43°CT90 Thermal Dose in Superficial Hyperthermia: A Retrospective Analysis. Strahlenther. Onkol. 2010, 186, 436–443. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. Evaluation of a New Method for Librarian-Mediated Literature Searches for Systematic Reviews. In Proceedings of the Research Synthesis Methods; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 9, pp. 510–520. [Google Scholar]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013, 158, 280. [Google Scholar] [CrossRef]
- Luk, K.H.; Purser, P.R.; Castro, J.R.; Meyler, T.S.; Phillips, T.L. Clinical Experiences with Local Microwave Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 615–619. [Google Scholar] [CrossRef]
- Arcangeli, G.; Arcangeli, G.; Guerra, A.; Lovisolo, G.; Cividalli, A.; Marino, C.; Mauro, F. Tumour Response to Heat and Radiation: Prognostic Variables in the Treatment of Neck Node Metastases from Head and Neck Cancer. Int. J. Hyperth. 1985, 1, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Van Der Zee, J.; Van Rhoon, G.C.; Wike-Hooley, J.L. Clinically Derived Dose Effect Relationship for Hyperthermia given in Combination with Low Dose Radiotherapy. Br. J. Radiol. 1985, 58, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sapozink, M.D.; Gibbs, F.A.; Egger, M.J.; Stewart, J.R. Regional Hyperthermia for Clinically Advanced Deep-Seated Pelvic Malignancy. Am. J. Clin. Oncol. Cancer Clin. Trials 1986, 9, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, G.; Benassi, M.; Cividalli, A.; Lovisolo, G.A.; Mauro, F. Radiotherapy and Hyperthermia. Analysis of Clinical Results and Identification of Prognostic Variables. Cancer 1987, 60, 950–956. [Google Scholar] [CrossRef]
- Gonzalez, D.G.; van Dijk, J.D.P.; Blank, L.E.C.M. Chestwall Recurrences of Breast Cancer: Results of Combined Treatment with Radiation and Hyperthermia. Radiother. Oncol. 1988, 12, 95–103. [Google Scholar] [CrossRef]
- Dragovic, J.; Seydel, H.G.; Sandhu, T.; Kolosvary, A.; Blough, J. Local Superficial Hyperthermia in Combination with Low-Dose Radiation Therapy for Palliation of Locally Recurrent Breast Carcinoma. J. Clin. Oncol. 1989, 7, 30–35. [Google Scholar] [CrossRef]
- Leopold, K.A.; Harrelson, J.; Prosnitz, L.; Samulski, T.V.; Dewhirst, M.W.; Oleson, J.R. Preoperative Hyperthermia and Radiation for Soft Tissue Sarcomas: Advantage of Two vs One Hyperthermia Treatments per Week. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 107–115. [Google Scholar] [CrossRef]
- Sannazzari, G.L.; Gabriele, P.; Orecchia, R.; Tseroni, V. Results of Hyperthermia, Alone or Combined with Irradiation, in Chest Wall Recurrences of Breast Cancer. Tumori 1989, 75, 284–288. [Google Scholar] [CrossRef]
- Seegenschmiedt, H.M.; Karlsson, U.L.; Sauer, R.; Brady, L.W.; Herbst, M.; Amendola, B.E.; Markoe, A.M.; Fisher, S.A.; Micaily, B. Superficial Chest Wall Recurrences of Breast Cancer: Prognostic Treatment Factors for Combined Radiation Therapy and Hyperthermia. Radiology 1989, 173, 551–558. [Google Scholar] [CrossRef]
- Myerson, R.J.; Perez, C.A.; Emami, B.; Straube, W.; Kuske, R.R.; Leybovich, L.; Von Gerichten, D. Tumor Control in Long-Term Survivors Following Superficial Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 1123–1129. [Google Scholar] [CrossRef]
- Kapp, D.S.; Petersen, I.A.; Cox, R.S.; Hahn, G.M.; Fessenden, P.; Prionas, S.D.; Lee, E.R.; Meyer, J.L.; Samulski, T.V.; Bagshaw, M.A. Two or Six Hyperthermia Treatments as an Adjunct to Radiation Therapy Yield Similar Tumor Responses: Results of a Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1481–1495. [Google Scholar] [CrossRef]
- Kapp, D.S.; Barnett, T.A.; Cox, R.S.; Lee, E.R.; Lohrbach, A.; Fessenden, P. Hyperthermia and Radiation Therapy of Local-Regional Recurrent Breast Cancer: Prognostic Factors for Response and Local Control of Diffuse or Nodular Tumors. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 1147–1164. [Google Scholar] [CrossRef]
- Kapp, D.S.; Cox, R.S.; Barnett, T.A.; Ben-Yosef, R. Thermoradiotherapy for Residual Microscopic Cancer: Elective or Post-Excisional Hyperthermia and Radiation Therapy in the Management of Local-Regional Recurrent Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 261–277. [Google Scholar] [CrossRef]
- Leopold, K.A.; Dewhirst, M.; Samulski, T.; Harrelson, J.; Alan Tucker, J.; George, S.L.; Dodge, R.K.; Grant, W.; Clegg, S.; Prosnitz, L.R.; et al. Relationships among Tumor Temperature, Treatment Time, and Histopathological Outcome Using Preoperative Hyperthermia with Radiation in Soft Tissue Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 989–998. [Google Scholar] [CrossRef]
- Leopold, K.A.; Dewhirst, M.W.; Samulski, T.V.; Dodge, R.K.; George, S.L.; Blivin, J.L.; Prosnitz, L.R.; Oleson, J.R. Cumulative Minutes with T90 Greater than Tempindex Is Predictive of Response of Superficial Malignancies to Hyperthermia and Radiation. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 841–847. [Google Scholar] [CrossRef]
- Bornstein, B.A.; Zouranjian, P.S.; Hansen, J.L.; Fraser, S.M.; Gelwan, L.A.; Teicher, B.A.; Svensson, G.K. Local Hyperthermia, Radiation Therapy, and Chemotherapy in Patients with Local-Regional Recurrence of Breast Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 79–85. [Google Scholar] [CrossRef]
- Engin, K.; Leeper, D.B.; Tupchong, L.; Waterman, F.M.; Mansfield, C.M. Thermoradiation Therapy for Superficial Malignant Tumors. Cancers 1993, 1, 287–296. [Google Scholar] [CrossRef]
- Engin, K.; Tupchong, L.; Moylan, D.J.; Alexander, G.A.; Waterman, F.M.; Komarnicky, L.; Nerlinger, R.E.; Leeper, D.B. Randomized Trial of One versus Two Adjuvant Hyperthermia Treatments per Week in Patients with Superficial Tumours. Int. J. Hyperth. 1993, 9, 327–340. [Google Scholar] [CrossRef]
- Engin, K.; Tupchong, L.; Phil, D.; Waterman, F.M.; Moylan, D.T.; Nerlinger, R.E.; Leeper, D.B. Hyperthermia and Radiation in Advanced Malignant Melanoma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 87–94. [Google Scholar] [CrossRef]
- Masunaga, S.I.; Hiraoka, M.; Akuta, K.; Nishimura, Y.; Nagata, Y.; Jo, S.; Takahashi, M.; Abe, M.; Terachi, T.; Oishi, K.; et al. Phase i/II Trial of Preoperative Thermoradiotherapy in the Treatment of Urinary Bladder Cancer. Int. J. Hyperth. 1994, 10, 31–40. [Google Scholar] [CrossRef]
- Hand, J.W.; Machin, D.; Vernon, C.C.; Whaley, J.B. Analysis of Thermal Parameters Obtained during Phase III Trials of Hyperthermia as an Adjunct to Radiotherapy in the Treatment of Breast Carcinoma. Int. J. Hyperth. 1997, 13, 343–364. [Google Scholar] [CrossRef] [PubMed]
- Sherar, M.; Liu, F.F.; Pintilie, M.; Levin, W.; Hunt, J.; Hill, R.; Hand, J.; Vernon, C.; Van Rhoon, G.; Van Der Zee, J.; et al. Relationship between Thermal Dose and Outcome in Thermoradiotherapy Treatments for Superficial Recurrences of Breast Cancer: Data from a Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 371–380. [Google Scholar] [CrossRef]
- Lee, H.K.; Antell, A.G.; Perez, C.A.; Straube, W.L.; Ramachandran, G.; Myerson, R.J.; Emami, B.; Molmenti, E.P.; Buckner, A.; Lockett, M.A. Superficial Hyperthermia and Irradiation for Recurrent Breast Carcinoma of the Chest Wall: Prognostic Factors in 196 Tumors. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 365–375. [Google Scholar] [CrossRef]
- Sneed, P.K.; Stauffer, P.R.; McDermott, M.W.; Diederich, C.J.; Lamborn, K.R.; Prados, M.D.; Chang, S.; Weaver, K.A.; Spry, L.; Malec, M.K.; et al. Survival Benefit of Hyperthermia in a Prospective Randomized Trial of Brachytherapy Boost ± Hyperthermia for Glioblastoma Multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 287–295. [Google Scholar] [CrossRef]
- Myerson, R.J.; Straube, W.L.; Moros, E.G.; Emami, B.N.; Lee, H.K.; Perez, C.A.; Taylor, M.E. Simultaneous Superficial Hyperthermia and External Radiotherapy: Report of Thermal Dosimetry and Tolerance to Treatment. Int. J. Hyperth. 1999, 15, 251–266. [Google Scholar] [CrossRef]
- Van Der Zee, J.; Van Der Holt, B.; Rietveld, P.J.M.; Helle, P.A.; Wijnmaalen, A.J.; Van Putten, W.L.J.; Van Rhoon, G.C. Reirradiation Combined with Hyperthermia in Recurrent Breast Cancer Results in a Worthwhile Local Palliation. Br. J. Cancer 1999, 79, 483–490. [Google Scholar] [CrossRef]
- Rau, B.; Wust, P.; Tilly, W.; Gellermann, J.; Harder, C.; Riess, H.; Budach, V.; Felix, R.; Schlag, P.M. Preoperative Radiochemotherapy in Locally Advanced or Recurrent Rectal Cancer: Regional Radiofrequency Hyperthermia Correlates with Clinical Parameters. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 381–391. [Google Scholar] [CrossRef]
- Maguire, P.D.; Samulski, T.V.; Prosnitz, L.R.; Jones, E.L.; Rosner, G.L.; Powers, B.; Layfields, L.W.; Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; et al. A Phase II Trial Testing the Thermal Dose Parameter CEM43°T90 as a Predictor of Response in Soft Tissue Sarcomas Treated with Pre-Operative Thermoradiotherapy. Int. J. Hyperth. 2001, 17, 283–290. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Kaplan, I.D.; Hansen, J.L.; Prokopios-Davos, S.; Topulos, G.P.; Wishnow, K.; Manola, J.; Bornstein, B.A.; Hynynen, K. Association of Rectal Toxicity with Thermal Dose Parameters in Treatment of Locally Advanced Prostate Cancer with Radiation and Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 913–918. [Google Scholar] [CrossRef]
- Li, G.; Mitsumori, M.; Ogura, M.; Horii, N.; Kawamura, S.; Masunaga, S.I.; Nagata, Y.; Hiraoka, M. Local Hyperthermia Combined with External Irradiation for Regional Recurrent Breast Carcinoma. Int. J. Clin. Oncol. 2004, 9, 179–183. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Kaplan, I.D.; Hansen, J.L.; Prokopios-Davos, S.; Topulos, G.P.; Wishnow, K.; Manola, J.; Bornstein, B.A.; Hynynen, K. Hyperthermia Combined with Radiation in Treatment of Locally Advanced Prostate Cancer Is Associated with a Favourable Toxicity Profile. Int. J. Hyperth. 2005, 21, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized Trial of Hyperthermia and Radiation for Superficial Tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, P.; Ferrara, T.; Baiotto, B.; Garibaldi, E.; Marini, P.G.; Penduzzu, G.; Giovannini, V.; Bardati, F.; Guiot, C. Radio Hyperthermia for Re-Treatment of Superficial Tumours. Int. J. Hyperth. 2009, 25, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, S.; Van Os, R.M.; Van Rij, C.M.; Crezee, J.; Van De Kamer, J.B.; Rutgers, E.J.T.; Geijsen, E.D.; Zum Vörde Sive Vörding, P.J.; Koning, C.C.E.; Van Tienhoven, G. Elective Re-Irradiation and Hyperthermia Following Resection of Persistent Locoregional Recurrent Breast Cancer: A Retrospective Study. Int. J. Hyperth. 2010, 26, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Linthorst, M.; Van Rhoon, G.C.; Van Geel, A.N.; Baaijens, M.; Ghidey, W.; Broekmeyer-Reurink, M.P.; Van Der Zee, J. The Tolerance of Reirradiation and Hyperthermia in Breast Cancer Patients with Reconstructions. Int. J. Hyperth. 2012, 28, 267–277. [Google Scholar] [CrossRef]
- Varma, S.; Myerson, R.; Moros, E.; Taylor, M.; Straube, W.; Zoberi, I. Simultaneous Radiotherapy and Superficial Hyperthermia for High-Risk Breast Carcinoma: A Randomised Comparison of Treatment Sequelae in Heated versus Non-Heated Sectors of the Chest Wall Hyperthermia. Int. J. Hyperth. 2012, 28, 583–590. [Google Scholar] [CrossRef]
- Linthorst, M.; Van Geel, A.N.; Baaijens, M.; Ameziane, A.; Ghidey, W.; Van Rhoon, G.C.; Van Der Zee, J. Re-Irradiation and Hyperthermia after Surgery for Recurrent Breast Cancer. Radiother. Oncol. 2013, 109, 188–193. [Google Scholar] [CrossRef]
- Oldenborg, S.; Griesdoorn, V.; Van Os, R.; Kusumanto, Y.H.; Oei, B.S.; Venselaar, J.L.; Zum Vörde Sive Vörding, P.J.; Heymans, M.W.; Kolff, M.W.; Rasch, C.R.N.; et al. Reirradiation and Hyperthermia for Irresectable Locoregional Recurrent Breast Cancer in Previously Irradiated Area: Size Matters. Radiother. Oncol. 2015, 117, 223–228. [Google Scholar] [CrossRef]
- Yahara, K.; Ohguri, T.; Yamaguchi, S.; Imada, H.; Narisada, H.; Ota, S.; Tomura, K.; Sakagami, M.; Fujimoto, N.; Korogi, Y. Definitive Radiotherapy plus Regional Hyperthermia for High-Risk and Very High-Risk Prostate Carcinoma: Thermal Parameters Correlated with Biochemical Relapse-Free Survival. Int. J. Hyperth. 2015, 31, 600–608. [Google Scholar] [CrossRef]
- Ohguri, T.; Harima, Y.; Imada, H.; Sakurai, H.; Ohno, T.; Hiraki, Y.; Tuji, K.; Tanaka, M.; Terashima, H. Relationships between Thermal Dose Parameters and the Efficacy of Definitive Chemoradiotherapy plus Regional Hyperthermia in the Treatment of Locally Advanced Cervical Cancer: Data from a Multicentre Randomised Clinical Trial. Int. J. Hyperth. 2018, 34, 461–468. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Oomen-de Hoop, E.; Vernhout, R.M.; Lutgens, L.C.; et al. The Effect of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in 400 Locally Advanced Cervical Carcinoma Patients. Front. Oncol. 2019, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Ohguri, T.; Kakinouchi, S.; Itamura, H.; Morisaki, T.; Tani, S.; Yahara, K.; Fujimoto, N. Intensity-Modulated Radiotherapy with Regional Hyperthermia for High-Risk Localized Prostate Carcinoma. Cancers 2022, 14, 400. [Google Scholar] [CrossRef]
- Schem, B.C.; Pfeffer, F.; Ott, M.A.; Wiig, J.N.; Sletteskog, N.; Frøystein, T.; Myklebust, M.P.; Leh, S.; Dahl, O.; Mella, O. Long-Term Outcome in a Phase II Study of Regional Hyperthermia Added to Preoperative Radiochemotherapy in Locally Advanced and Recurrent Rectal Adenocarcinomas. Cancers 2022, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.R. A Probe for Measuring Temperature in Radio-Frequency-Heated Material. IEEE Trans. Microw. Theory Tech. 1976, 24, 43–45. [Google Scholar] [CrossRef]
- Ademaj, A.; Veltsista, D.P.; Ghadjar, P.; Marder, D.; Oberacker, E.; Ott, O.J.; Wust, P.; Puric, E.; Hälg, R.A.; Rogers, S.; et al. Clinical Evidence for Thermometric Parameters to Guide Hyperthermia Treatment. Cancers 2022, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, D.; Whaley, J.B.; Van Der Zee, J.; Van Putten, W.L.J.; Van Rhoon, G.C.; Van Dijk, J.D.P.; González González, D.; et al. Radiotherapy with or without Hyperthermia in the Treatment of Superficial Localized Breast Cancer: Results from Five Randomized Controlled Trials. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar] [CrossRef]
- Paulides, M.M.; Verduijn, G.M.; Van Holthe, N. Status Quo and Directions in Deep Head and Neck Hyperthermia. Radiat. Oncol. 2016, 11, 21. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; ter Haar, G.; Wust, P.; Crezee, J. Heating Technology for Malignant Tumors: A Review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Van Der Zee, J.; González, D.G.; Van Rhoon, G.C.; Van Dijk, J.D.P.; Putten, W.L.J. Van Comparison of Radiotherapy Alone with Radiotherapy plus Hyperthermia in Locally Advanced Pelvic Tumors. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.M.; Jamshidi-Parsian, A.; Song, C.W. Mild Temperature Hyperthermia and Radiation Therapy: Role of Tumour Vascular Thermotolerance and Relevant Physiological Factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P. Hyperthermia in Anticancer Treatment. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Rietveld, P.J.M.; Van Putten, W.L.J.; Van Der Zee, J.; Van Rhoon, G.C. Comparison of the Clinical Effectiveness of the 433 MHz Lucite Cone Applicator with That of a Conventional Waveguide Applicator in Applications of Superficial Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 681–687. [Google Scholar] [CrossRef]
- Kroeze, H.; Van Vulpen, M.; De Leeuw, A.A.C.; Van De Kamer, J.B.; Lagendijk, J.J.W. Improvement of Absorbing Structures Used in Regional Hyperthermia. Int. J. Hyperth. 2003, 19, 598–616. [Google Scholar] [CrossRef]
- Johnson, J.E.; Neuman, D.G.; MacCarini, P.F.; Juang, T.; Stauffer, P.R.; Turner, P. Evaluation of a Dual-Arm Archimedean Spiral Array for Microwave Hyperthermia. Int. J. Hyperth. 2006, 22, 475–490. [Google Scholar] [CrossRef]
- Kok, H.P.; Navarro, F.; Strigari, L.; Cavagnaro, M.; Crezee, J. Locoregional Hyperthermia of Deep-Seated Tumours Applied with Capacitive and Radiative Systems: A Simulation Study. Int. J. Hyperth. 2018, 34, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Van Der Gaag, M.L.; De Bruijne, M.; Samaras, T.; Van Der Zee, J.; Van Rhoon, G.C. Development of a Guideline for the Water Bolus Temperature in Superficial Hyperthermia. Int. J. Hyperth. 2006, 22, 637–656. [Google Scholar] [CrossRef]
- Kokuryo, D.; Kumamoto, E.; Kuroda, K. Recent Technological Advancements in Thermometry. Adv. Drug Deliv. Rev. 2020, 163–164, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Feddersen, T.V.; Hernandez-Tamames, J.A.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia. Cancers 2020, 13, 31. [Google Scholar] [CrossRef]
- Kok, H.P.; Van Den Berg, C.A.T.; Van Haaren, P.M.A.; Crezee, J. Artefacts in Intracavitary Temperature Measurements during Regional Hyperthermia. Phys. Med. Biol. 2007, 52, 5157–5171. [Google Scholar] [CrossRef]
- Van Haaren, P.M.A.; Kok, H.P.; van den Berg, C.A.T.; Zum Vörde Sive Vörding, P.J.; Oldenborg, S.; Stalpers, L.J.A.; Schilthuis, M.S.; de Leeuw, A.A.C.; Crezee, J. On Verification of Hyperthermia Treatment Planning for Cervical Carcinoma Patients. Int. J. Hyperth. 2007, 23, 303–314. [Google Scholar] [CrossRef]
- De Leeuw, A.A.C.; Crezee, J.; Lagendijk, J.J.W. Temperature and SAR Measurements in Deep-Body Hyperthermia with Thermocouple Thermometry. Int. J. Hyperth. 1993, 9, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Chou, C.K.; Mcdougall, J.A.; Luk, K.H. Perturbations Due to the Use of Catheters with Non-Perturbing Thermometry Probes. Int. J. Hyperth. 1988, 4, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Maccarini, P.; Juang, T.; Gaeta, C.; Stauffer, P.R. Performance Evaluation of a Conformal Thermal Monitoring Sheet Sensor Array for Measurement of Surface Temperature Distributions during Superficial Hyperthermia Treatments. Int. J. Hyperth. 2008, 24, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.; Holman, R.; Rodrigues, D.B.; Dobšíček Trefná, H.; Stauffer, P.R.; van Tienhoven, G.; Rasch, C.R.N.; Crezee, H. Analysis of Clinical Data to Determine the Minimum Number of Sensors Required for Adequate Skin Temperature Monitoring of Superficial Hyperthermia Treatments. Int. J. Hyperth. 2018, 34, 910–917. [Google Scholar] [CrossRef]
- Juang, T.; Stauffer, P.R.; Craciunescu, O.A.; Maccarini, P.F.; Yuan, Y.; Das, S.K.; Dewhirst, M.W.; Inman, B.A.; Vujaskovic, Z. Thermal Dosimetry Characteristics of Deep Regional Heating of Non-Muscle Invasive Bladder Cancer. Int. J. Hyperth. 2014, 30, 176–183. [Google Scholar] [CrossRef]
- Dewhirst, M.; Gross, J.F.; Sim, D.; Arnold, P.; Boyer, D. The Effect of Rate of Heating or Cooling Prior to Heating on Tumor and Normal Tissue Microcirculatory Blood Flow. Biorheology 1984, 21, 539–558. [Google Scholar] [CrossRef]
- Siemann, D.W.; Horsman, M.R. Modulation of the Tumor Vasculature and Oxygenation to Improve Therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef]
- Bakker, A.; van der Zee, J.; van Tienhoven, G.; Kok, H.P.; Rasch, C.R.N.; Crezee, H. Temperature and Thermal Dose during Radiotherapy and Hyperthermia for Recurrent Breast Cancer Are Related to Clinical Outcome and Thermal Toxicity: A Systematic Review. Int. J. Hyperth. 2019, 36, 1024–1039. [Google Scholar]
- Chou, C.K. Use of Heating Rate and Specific Absorption Rate in the Hyperthermia Clinic. Int. J. Hyperth. 1990, 6, 367–370. [Google Scholar] [CrossRef]
- Wust, P.; Stahl, H.; Löffel, J.; Seebass, M.; Riess, H.; Felix, R. Clinical, Physiological and Anatomical Determinants for Radiofrequency Hyperthermia. Int. J. Hyperth. 1995, 11, 151–167. [Google Scholar] [CrossRef]
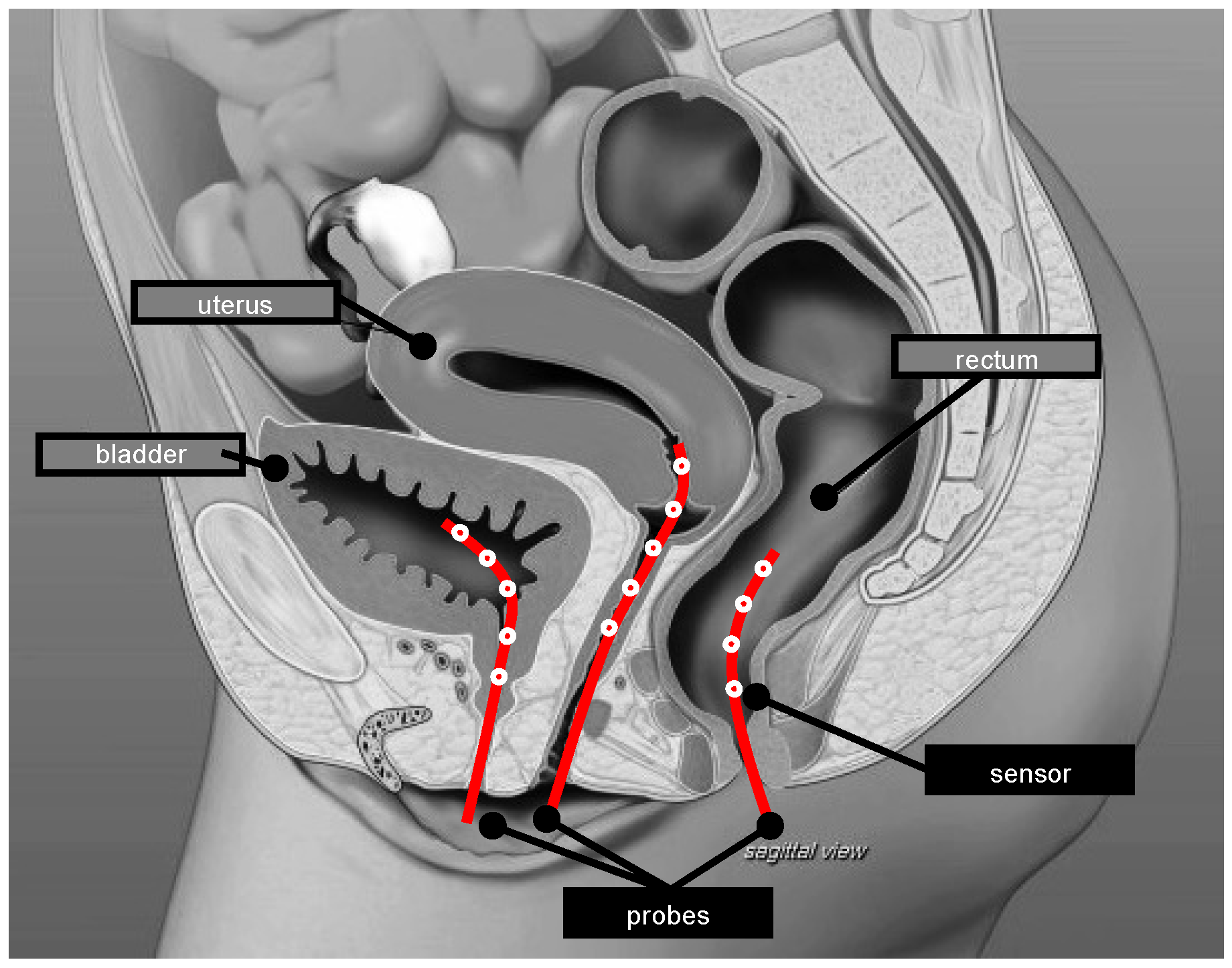
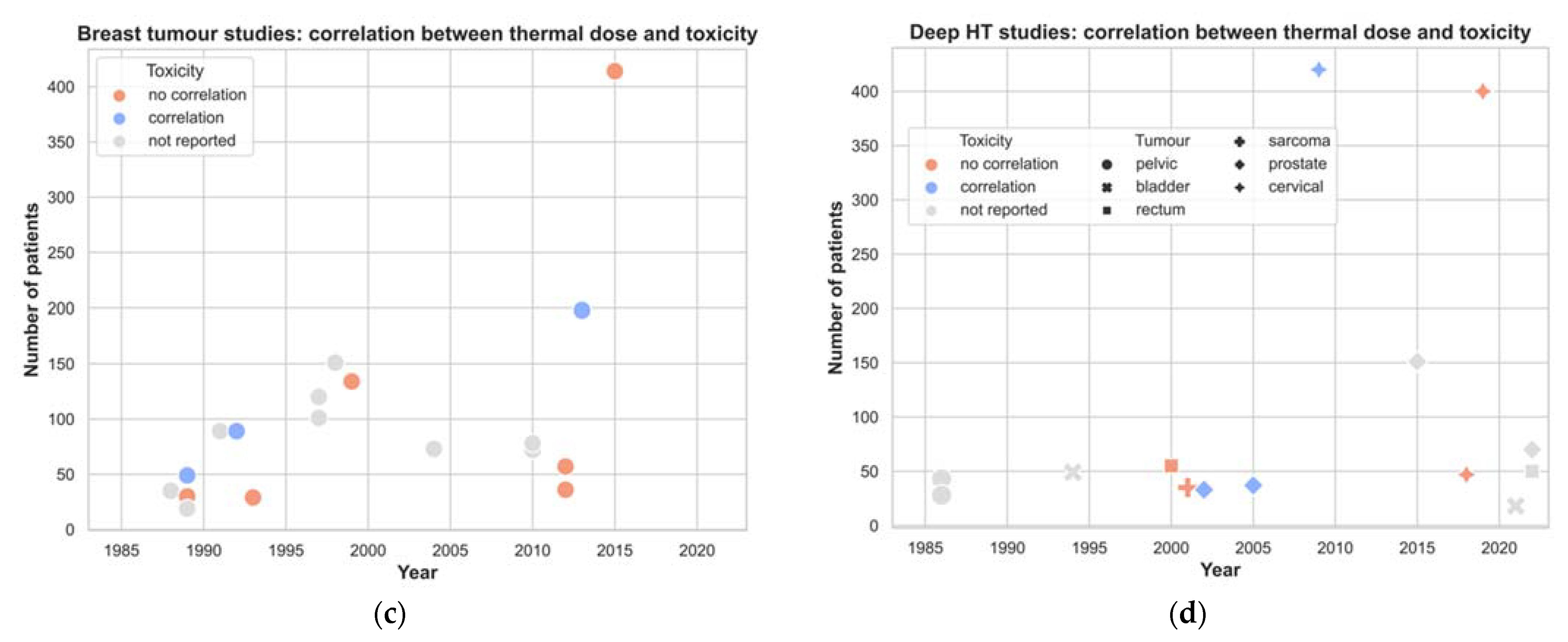
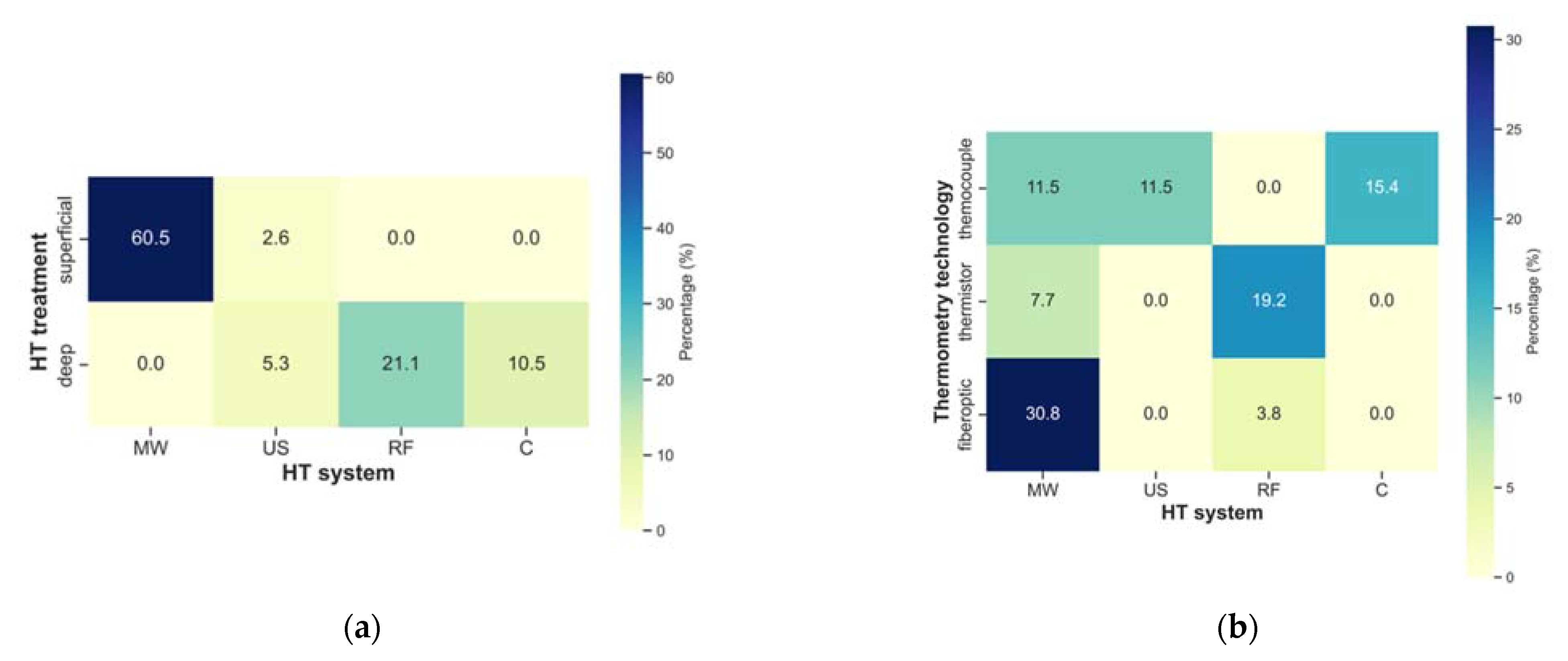
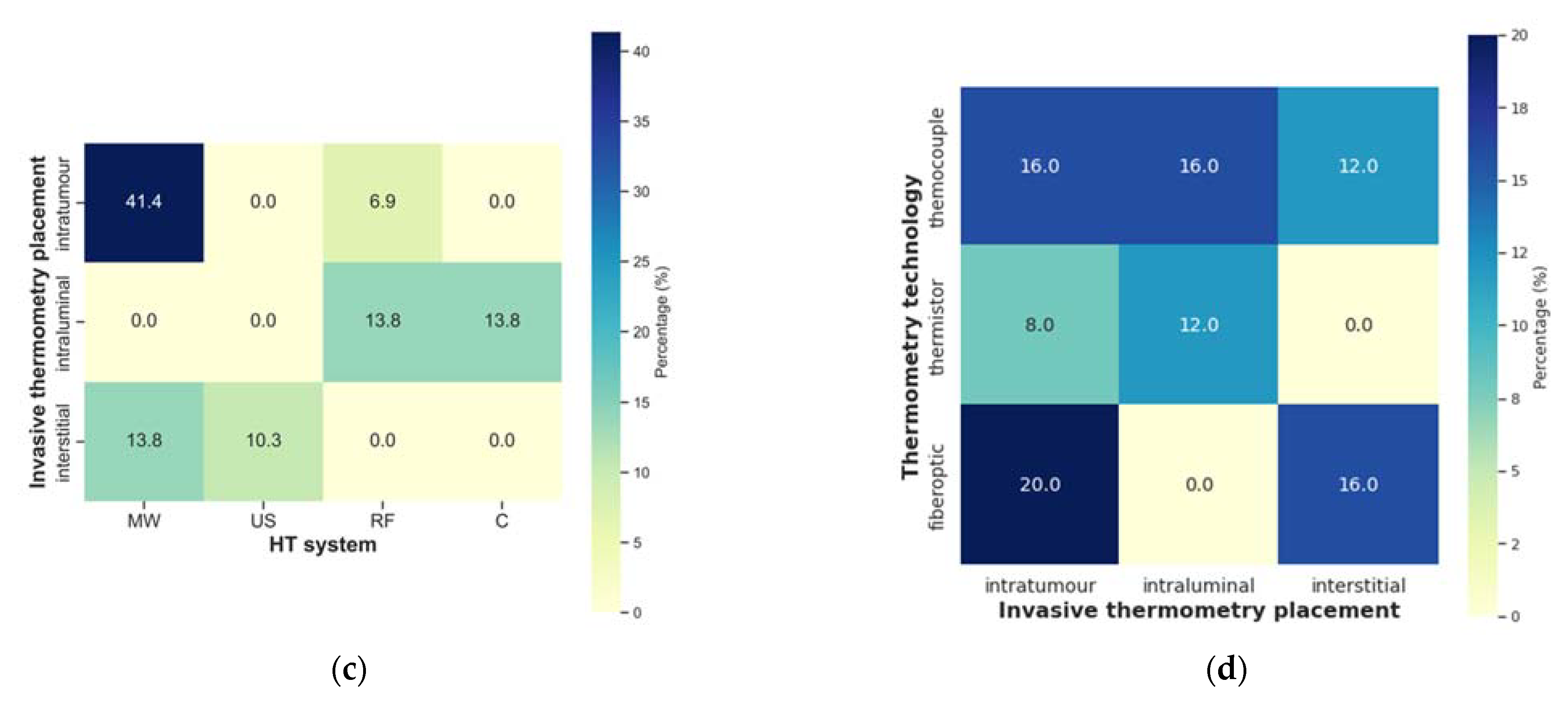
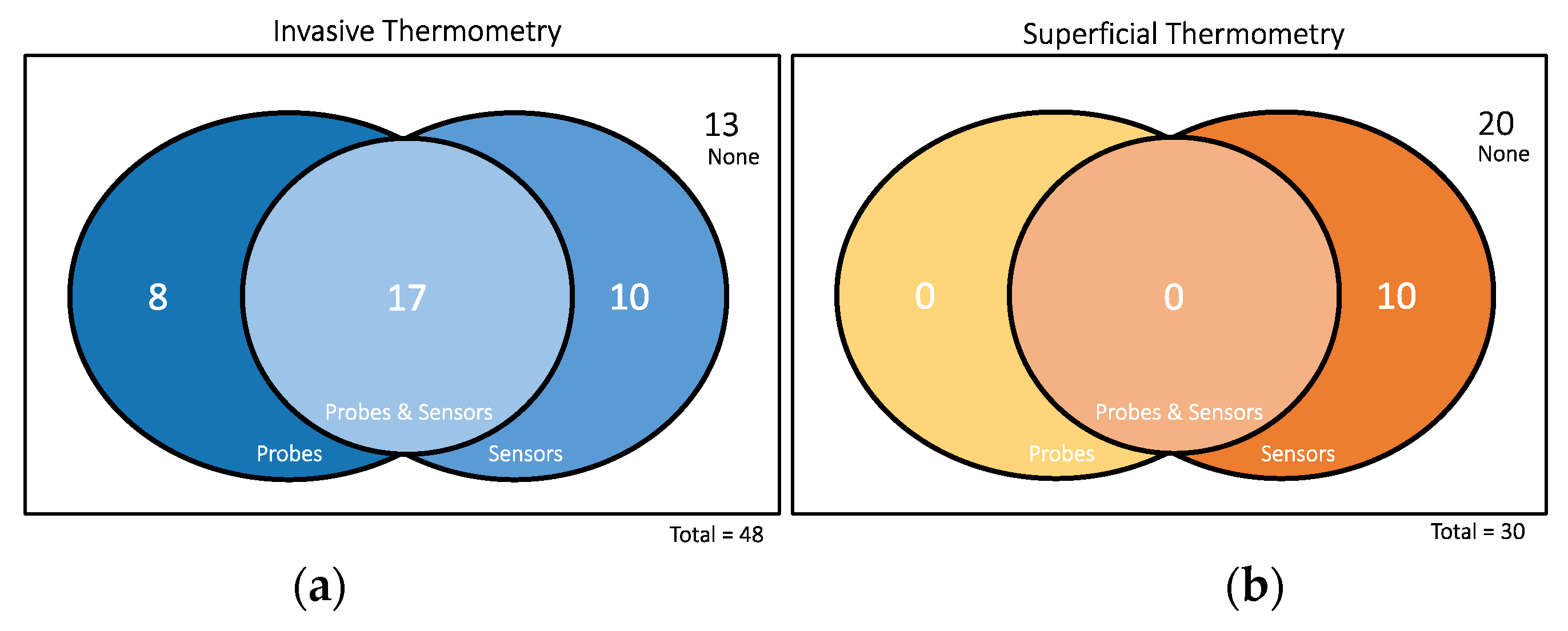
| Author, Year | Title |
|---|---|
| Luk, 1981 [31] | Clinical experiences with local microwave hyperthermia |
| Arcangeli, 1985 [32] | Tumour response to heat and radiation: prognostic variables in the treatment of neck node metastases from head and neck cancer |
| van der Zee, 1985 [33] | Clinically derived dose effect relationship for hyperthermia given in combination with low dose radiotherapy |
| Sapozink, 1986 [34] | Regional hyperthermia for clinically advanced deep-seated pelvic malignancy |
| Sapozink, 1986 [17] | Abdominal regional hyperthermia with an annular phased array |
| Arcangeli, 1987 [35] | Radiotherapy and hyperthermia. Analysis of clinical results and identification of prognostic variables |
| Gonzalez, 1988 [36] | Chestwall recurrences of breast cancer: Results of combined treatment with radiation and hyperthermia |
| Dragovic, 1989 [37] | Local superficial hyperthermia in combination with low-dose radiation therapy for palliation of locally recurrent breast carcinoma |
| Leopold, 1989 [38] | Preoperative hyperthermia and radiation for soft tissue sarcomas: advantage of two vs. one hyperthermia treatments per week |
| Sannazzari, 1989 [39] | Results of hyperthermia, alone or combined with irradiation, in chest wall recurrences of breast cancer |
| Seegenschmiedt, 1989 [40] | Superficial chest wall recurrences of breast cancer: Prognostic treatment factors for combined radiation therapy and hyperthermia |
| Myerson, 1990 [41] | Tumor control in long-term survivors following superficial hyperthermia |
| Kapp, 1990 [42] | Two or six hyperthermia treatments as an adjunct to radiation therapy yield similar tumor responses: results of a randomized trial |
| Kapp, 1991 [43] | Hyperthermia and radiation therapy of local-regional recurrent breast cancer: Prognostic factors for response and local control of diffuse or nodular tumors |
| Kapp, 1992 [44] | Thermoradiotherapy for residual microscopic cancer: elective or post-excisional hyperthermia and radiation therapy in the management of local-regional recurrent breast cancer |
| Leopold, 1992 [45] | Relationships among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas |
| Leopold, 1993 [46] | Cumulative minutes with T90 greater than tempindex is predictive of response of superficial malignancies to hyperthermia and radiation |
| Bornstein, 1993 [47] | Local hyperthermia, radiation therapy, and chemotherapy in patients with local-regional recurrence of breast carcinoma |
| Engin, 1993 [48] | Thermoradiation therapy for superficial malignant tumors |
| Engin, 1993 [49] | Randomized trial of one versus two adjuvant hyperthermia treatments per week in patients with superficial tumors |
| Engin, 1993 [50] | Hyperthermia and radiation in advanced malignant melanoma |
| Masunaga, 1994 [51] | Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer |
| Hand, 1997 [52] | Analysis of thermal parameters obtained during Phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma |
| Sherar, 1997 [53] | Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial |
| Lee, 1998 [54] | Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: Prognostic factors in 196 tumors |
| Sneed, 1998 [55] | Survival benefit of hyperthermia in a prospec- tive randomized trial of brachytherapy boost hyperthermia for glioblastoma multiforme |
| Myerson, 1999 [56] | Simultaneous superficial hyperthermia and external radiotherapy: Report of thermal dosimetry and tolerance to treatment |
| van der Zee, 1999 [57] | Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation |
| Rau, 2000 [58] | Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters |
| Maguire, 2001 [59] | A phase II trial testing the thermal dose parameter CEM43T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy |
| Hurwitz, 2002 [60] | Association of rectal toxicity with thermal dose parameters in treatment of locally advanced prostate cancer with radiation and hyperthermia |
| Li, 2004 [61] | Local hyperthermia combined with external irradiation for regional recurrent breast carcinoma |
| Hurwitz, 2005 [62] | Hyperthermia combined with radiation in treatment of locally advanced prostate cancer is associated with a favourable toxicity profile |
| Jones, 2005 [63] | Randomized trial of hyperthermia and radiation for superficial tumors |
| Franckena, 2009 [12] | Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia |
| Gabriele, 2009 [64] | Radio hyperthermia for re-treatment of superficial tumour |
| de Bruijne, 2010 [27] | Evaluation of CEM43CT90 thermal dose in superficial hyperthermia: A retrospective analysis |
| Oldenborg, 2010 [65] | Elective re-irradiation and hyperthermia following resection of persistent locoregional recurrent breast cancer: A retrospective study |
| Linthorst, 2012 [66] | The tolerance of reirradiation and hyperthermia in breast cancer patients with reconstructions |
| Varma, 2012 [67] | Simultaneous radiotherapy and superficial hyperthermia for high-risk breast carcinoma: A randomised comparison of treatment sequelae in heated versus non-heated sectors of the chest wall hyperthermia |
| Linthorst, 2013 [68] | Re-irradiation and hyperthermia after surgery for recurrent breast cancer |
| Oldenborg, 2015 [69] | Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: Size matters |
| Yahara, 2015 [70] | Definitive radiotherapy plus regional hyperthermia for high-risk and very high-risk prostate carcinoma: Thermal parameters correlated with biochemical relapse-free survival |
| Ohguri, 2018 [71] | Relationships between thermal dose parameters and the efficacy of definitive chemoradiotherapy plus regional hyperthermia in the treatment of locally advanced cervical cancer: data from a multicentre randomised clinical trial |
| Kroesen, 2019 [72] | The effect of the time interval between radiation and hyperthermia on clinical outcome in 400 locally advanced cervical carcinoma patients |
| Datta, 2021 [13] | Quantification of thermal dose in moderate clinical hyperthermia with radiotherapy: a relook using temperature–time area under the curve (AUC) |
| Nakahara, 2022 [73] | Intensity-modulated radiotherapy with regional hyperthermia for high-risk localized prostate carcinoma |
| Schem, 2022 [74] | Long-term outcome in a phase ii study of regional hyperthermia added to preoperative radiochemotherapy in locally advanced and recurrent rectal adenocarcinomas |
| Category | Count (n) | Percentage (%) | Mean, Standard Deviation |
|---|---|---|---|
| Hyperthermia and radiotherapy treatment characteristics | |||
| Number of patients | 4107 | 85.6 ± 94.1 * | |
| Radiotherapy dose (Gy) | 43.2 ± 13.8 | ||
| Chemotherapy | |||
| → no | 43 | 90 | |
| → yes | 5 | 10 | |
| HT treatment | |||
| → superficial | 33 | 69 | |
| → deep | 14 | 29 | |
| → superficial and deep | 1 | 2 | |
| HT system | |||
| → microwave | 23 | 48 | |
| → radiofrequency | 8 | 17 | |
| → capacitive | 4 | 8 | |
| → ultrasound | 3 | 6 | |
| → combination | 10 | 21 | |
| HT duration (min) | 56.5 ± 16.0 * | ||
| → superficial HT | 53.7 ± 11.7 | ||
| → deep HT | 63.0 ± 22.9 | ||
| Number of HT sessions per week | |||
| → 1 and/or 2 | 43 | 90 | |
| → 3 | 2 | 4 | |
| → not reported | 3 | 6 | |
| Number of HT sessions per patient | 5.4 ± 1.9 * | ||
| Sequence of treatments | |||
| → HT before RT/CT | 7 | 15 | |
| → HT after RT/CT | 33 | 69 | |
| → other | 3 | 6 | |
| → not reported | 5 | 10 | |
| Time interval between RT and HT treatment (min) | |||
| → 0–30 | 18 | 38 | |
| → >30–60 | 18 | 38 | |
| → >60 | 1 | 2 | |
| → not reported | 11 | 22 | |
| Temperature acquisition characteristics | |||
| Thermometry | |||
| → invasive | 48 | 100 | |
| → superficial | 30 | 64 | |
| Thermometry technique | |||
| → thermocouple | 11 | 23 | |
| → thermistor | 7 | 15 | |
| → fibreoptic | 9 | 18 | |
| → combination | 14 | 29 | |
| → not reported | 7 | 15 | |
| Invasive or minimally invasive thermometry placement | |||
| → intratumour | 25 | 52 | |
| → intraluminal | 9 | 19 | |
| → interstitial | 9 | 19 | |
| → various | 2 | 4 | |
| → not reported | 3 | 6 | |
| Temperature acquisition | |||
| → not continuous | 3 | 6 | |
| → mapping | 10 | 21 | |
| → continuous | 11 | 23 | |
| → combination | 11 | 23 | |
| → not reported | 13 | 27 | |
| Temperature acquisition rate (min) | |||
| → 0–1 | 11 | 23 | |
| → >1–5 | 11 | 23 | |
| → >5 | 6 | 12 | |
| → not reported | 20 | 42 | |
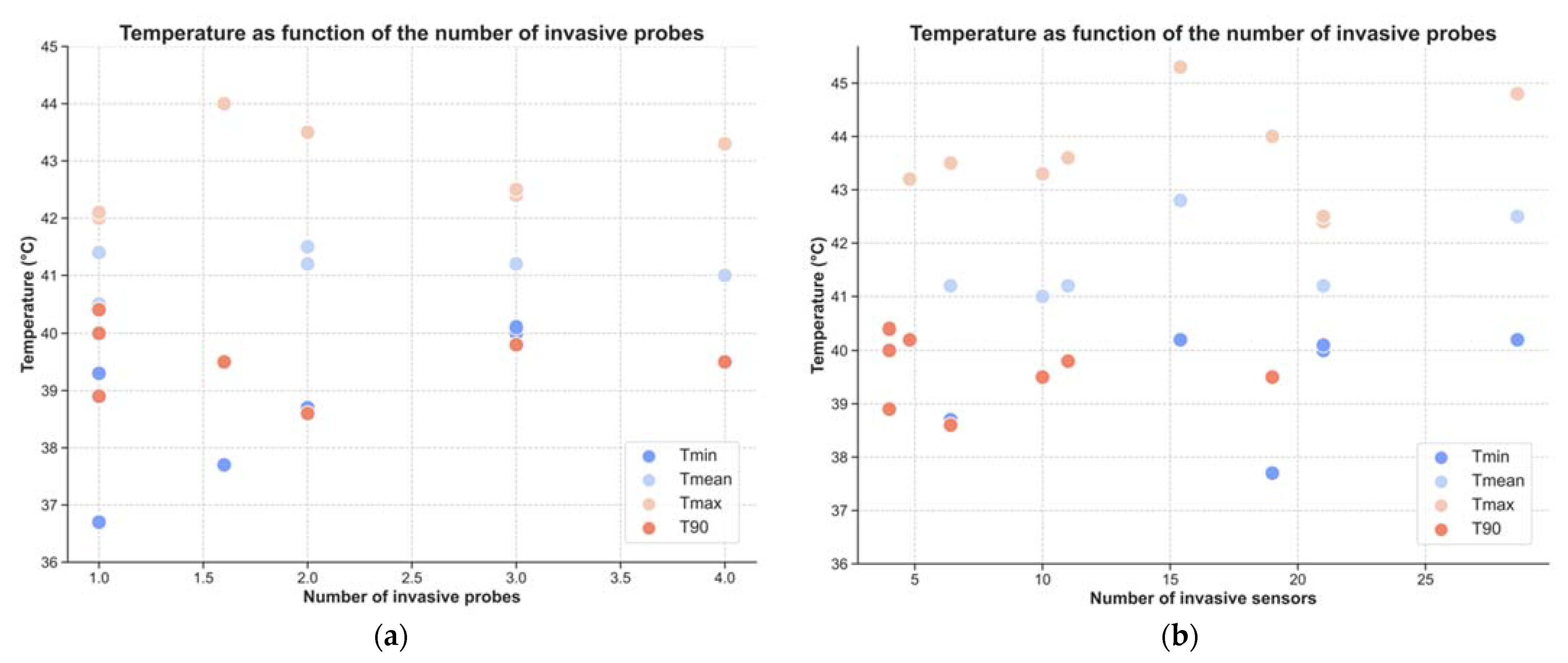
| Number of invasive probes | 2.3 ± 1.0 * | ||
| Number of invasive sensors | 9.5 ± 7.2 * | ||
| Number of superficial probes | Not reported | ||
| Number of superficial sensors | 10.9 ± 12.3 * | ||
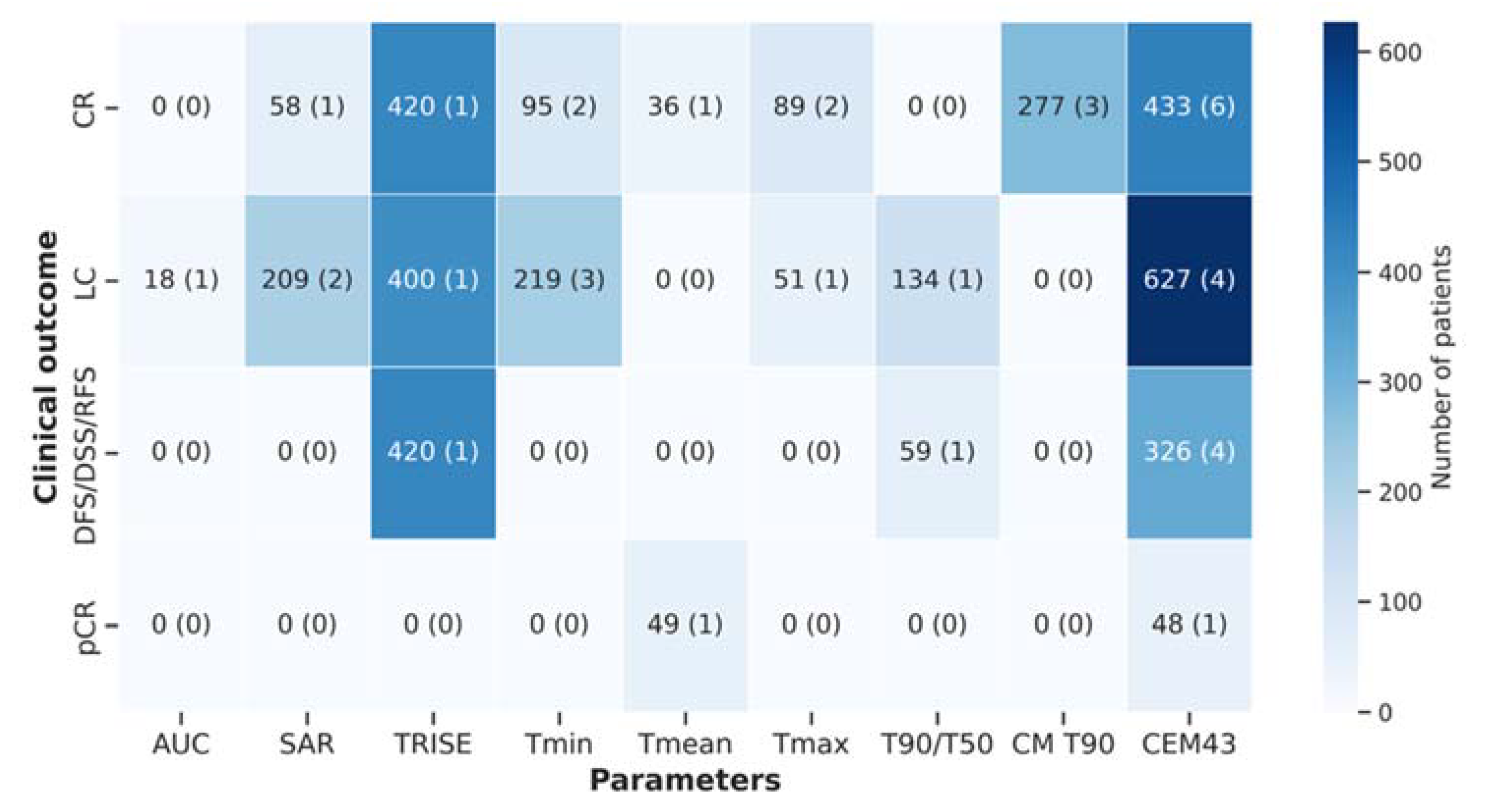
| Association of temperature descriptors with treatment outcome and toxicity | |||
| Treatment outcome | |||
| → no association | 13 | 27 | |
| → association | 30 | 63 | |
| → not reported | 5 | 10 | |
| Toxicity | |||
| → no association | 17 | 35 | |
| → association | 7 | 15 | |
| → not reported | 24 | 50 | |
| Category | Description | ||
|---|---|---|---|
| HT system (and applicator) | e.g., 8 MHz radiofrequency capacitive system [62] | ||
| Coupling method | e.g., water or mineral oil [33]; 5% NaCL [62] | ||
| Temperature of cooling liquid | e.g., mean of 39 °C | ||
| Invasive | Superficial | ||
| Thermometry system (uncertainty) | e.g., thermistor (±0.2 °C) | e.g., thermistor (±0.2 °C) | |
| Invasive thermometry placement | e.g., intraluminal | Not applicable | |
| Temperature acquisition | e.g., continuous and stationary in the bladder and rectum e.g., mapping with a step size of 1 cm and mean map length of 14 cm | e.g., skin surface of the buttocks and abdomen | |
| Temperature acquisition rate (min) | e.g., every 5 min or continuous | e.g., every 5 min or continuous | |
| Number of probes and sensors per probe | e.g., 2 probes in the rectum and bladder (each probe with 3–5 sensors) | e.g., 2 probes on the skin surface of the buttocks and 1 probe in the abdomen (each probe with 3–5 sensors) | |
| Total number of sensors | e.g., mean of 8 sensors e.g., mean of 14 sensors within mapping | e.g., mean of 8 sensors e.g., mean of 14 sensors within mapping | |
| Sampling rate | number of sensors per area/volume of target | e.g., number of sensors per area of target | |
| Invasive | Superficial | Total | |
| Temperature and thermal dose parameters | T90 Tmin Tmean Tmax CEM43 TRISE AUC | T90 Tmin Tmean Tmax | T90 Tmin Tmean Tmax CEM43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrapiço-Seabra, C.; Curto, S.; Franckena, M.; Rhoon, G.C.V. Avoiding Pitfalls in Thermal Dose Effect Relationship Studies: A Review and Guide Forward. Cancers 2022, 14, 4795. https://doi.org/10.3390/cancers14194795
Carrapiço-Seabra C, Curto S, Franckena M, Rhoon GCV. Avoiding Pitfalls in Thermal Dose Effect Relationship Studies: A Review and Guide Forward. Cancers. 2022; 14(19):4795. https://doi.org/10.3390/cancers14194795
Chicago/Turabian StyleCarrapiço-Seabra, Carolina, Sergio Curto, Martine Franckena, and Gerard C. Van Rhoon. 2022. "Avoiding Pitfalls in Thermal Dose Effect Relationship Studies: A Review and Guide Forward" Cancers 14, no. 19: 4795. https://doi.org/10.3390/cancers14194795







