Cytotoxic Mechanism of Momilactones A and B against Acute Promyelocytic Leukemia and Multiple Myeloma Cell Lines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Viability (MTT) Assay
2.3. Cell Apoptosis (Annexin V) Assay
2.4. Cell Cycle Assay
2.5. Western Blotting Assay
2.6. Statistical Analysis
3. Results
3.1. Effects of Momilactones on Cell Viability of Non-Cancerous (MeT-5A), Acute Promyelocytic Leukemia (HL-60), and Multiple Myeloma (U266) Cell Lines
3.2. Apoptosis-Inducing Activities of Momilactones against Non-Cancerous (MeT-5A), Acute Promyelocytic Leukemia (HL-60), and Multiple Myeloma (U266) Cell Lines
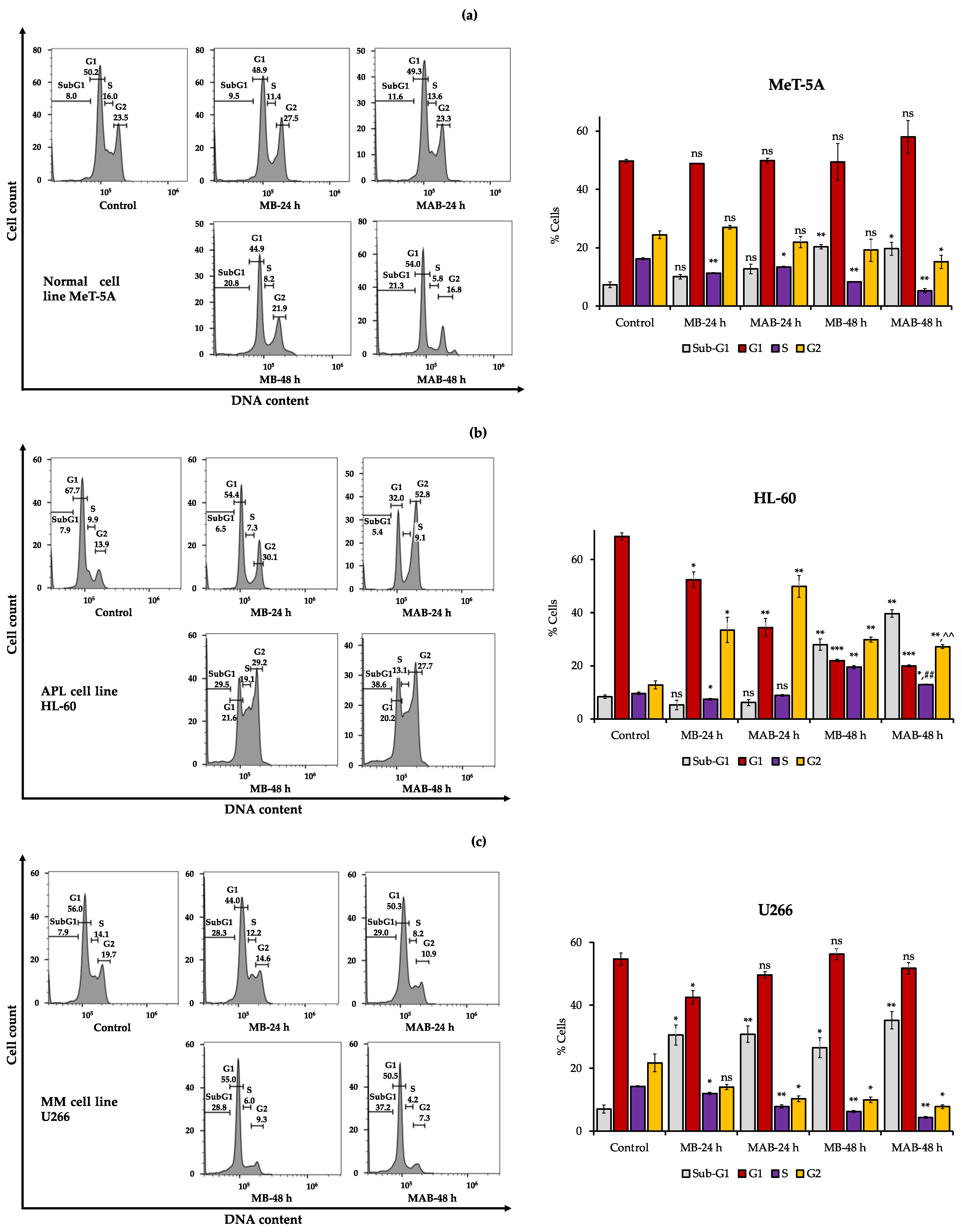
3.3. Effects of Momilactones on Inducing Cell Cycle Arrest of Non-Cancerous (MeT-5A), Acute Promyelocytic Leukemia (HL-60), and Multiple Myeloma (U266) Cell Lines
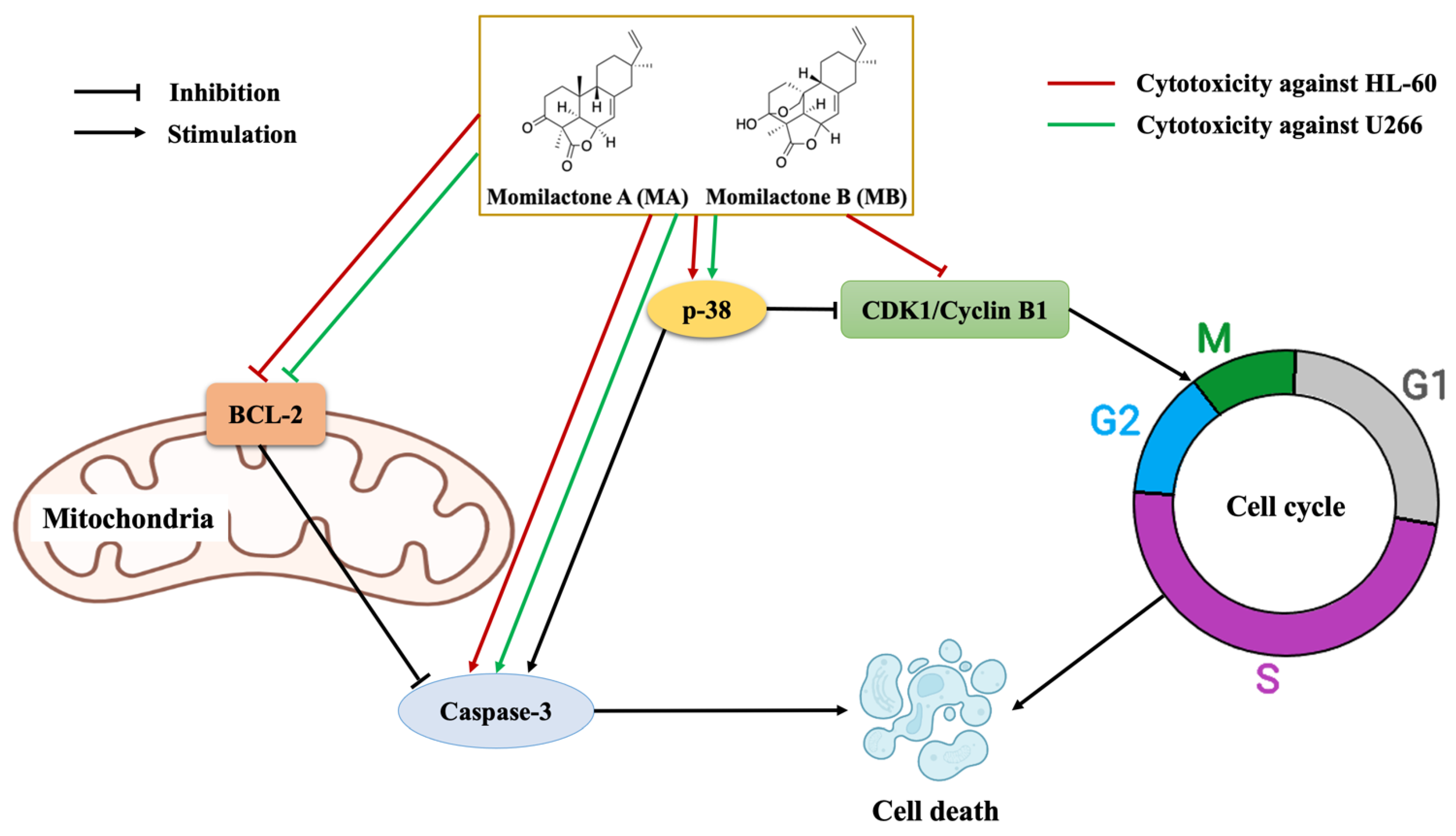
3.4. Effects of Momilactones on Expressions of Proteins Related to Apoptosis Induction and Cell Cycle Arrest of Acute Promyelocytic Leukemia (HL-60) and Multiple Myeloma (U266) Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, I. Gain/amplification of chromosome Arm 1q21 in multiple myeloma. Cancers 2021, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.; Singh, A. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, N.V.; Anh, L.H.; Lam, V.Q.; Takami, A.; Teschke, R.; Khanh, T.D.; Xuan, T.D. Anti-diabetes, anti-gout, and anti-leukemia properties of essential oils from natural spices Clausena indica, Zanthoxylum rhetsa, and Michelia tonkinensis. Molecules 2022, 27, 774. [Google Scholar] [CrossRef]
- Un, S.; Quan, N.V.; Anh, L.H.; Lam, V.Q.; Takami, A.; Khanh, T.D.; Xuan, T.D. Effects of in vitro digestion on anti-α-amylase and cytotoxic potentials of Sargassum spp. Molecules 2022, 27, 2307. [Google Scholar] [CrossRef]
- Anh, L.H.; Quan, N.V.; Lam, V.Q.; Iuchi, Y.; Takami, A.; Teschke, R.; Xuan, T.D. Antioxidant, anti-tyrosinase, anti-α-amylase, and cytotoxic potentials of the invasive weed Andropogon virginicus. Plants 2020, 10, 69. [Google Scholar] [CrossRef]
- Lam, V.Q.; Anh, L.H.; Quan, N.V.; Xuan, T.D.; Hanamura, I.; Uchino, K.; Karnan, S.; Takami, A. Cytotoxicity of Callerya speciosa fractions against myeloma and lymphoma cell lines. Molecules 2022, 27, 2322. [Google Scholar] [CrossRef]
- Teschke, R.; Xuan, T.D. Active nature based ingredients for drug discovery with pivotal role of clinical efficacy: Review and prospective. J. Mod. Med. Chem. 2020, 8, 4–18. [Google Scholar] [CrossRef]
- Ameade, E.P.K.; Ibrahim, M.; Ibrahim, H.-S.; Habib, R.H.; Gbedema, S.Y. Concurrent use of herbal and orthodox medicines among residents of Tamale, northern Ghana, who patronize hospitals and herbal clinics. Evid. Based Complement. Altern. Med. 2018, 2018, 1289125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cheng, J.; Guo, B.; Duan, J.; Che, C.-T. Momilactone and related diterpenoids as potential agricultural chemicals. J. Agric. Food Chem. 2018, 66, 7859–7872. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jeong, N.Y.; Kim, G.-Y.; Han, M.H.; Chung, I.-M.; Kim, W.-J.; Yoo, Y.H.; Choi, Y.H. Momilactone B induces apoptosis and G1 arrest of the cell cycle in human monocytic leukemia U937 cells through downregulation of pRB phosphorylation and induction of the cyclin-dependent kinase inhibitor p21Waf1/Cip1. Oncol. Rep. 2014, 31, 1653–1660. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Chung, I.-M.; Jin, Y.J.; Song, Y.S.; Seo, S.Y.; Park, B.S.; Cho, K.H.; Yoo, K.S.; Kim, T.-H.; Yee, S.-B.; et al. Momilactone B, an allelochemical of rice hulls, induces apoptosis on human lymphoma cells (Jurkat) in a micromolar concentration. Nutr. Cancer 2008, 60, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Park, H.-R.; Park, E.; Lee, S.-C. Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007, 55, 1702–1706. [Google Scholar] [CrossRef]
- Quan, N.V.; Tran, H.-D.; Xuan, T.D.; Ahmad, A.; Dat, T.D.; Khanh, T.D.; Teschke, R. Momilactones A and B are α-amylase and α-glucosidase inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef] [Green Version]
- Quan, N.V.; Xuan, T.D.; Tran, H.-D.; Ahmad, A.; Khanh, T.D.; Dat, T.D. Contribution of momilactones A and B to diabetes inhibitory potential of rice bran: Evidence from in vitro assays. Saudi Pharm. J. 2019, 27, 643–649. [Google Scholar] [CrossRef]
- Quan, N.V.; Thien, D.D.; Khanh, T.D.; Tran, H.-D.; Xuan, T.D. Momilactones A, B, and tricin in rice grain and by-products are potential skin aging inhibitors. Foods 2019, 8, 602. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.J.; et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.B.D.C.; Bomfim, L.M.; Neves, S.P.; Soares, M.B.P.; Dias, R.B.; Valverde, L.F.; Rocha, C.A.G.; Costa, E.V.; da Silva, F.M.A.; Rocha, W.C.; et al. Tingenone and 22-hydroxytingenone target oxidative stress through down-regulation of thioredoxin, leading to DNA double-strand break and JNK/p38-mediated apoptosis in acute myeloid leukemia HL-60 cells. Biomed. Pharm. 2021, 142, 112034. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, J.L.; Elbadry, M.I.; Taniwaki, M.; Harada, K.; Trung, L.Q.; Nakagawa, N.; Takami, A.; Ishiyama, K.; Yamauchi, T.; Takenaka, K.; et al. The simultaneous inhibition of the mTOR and MAPK pathways with Gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett. 2017, 400, 127–136. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hideshima, T.; Akiyama, M.; Hayashi, T.; Richardson, P.; Schlossman, R.; Chauhan, D.; Anderson, K.C. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood 2003, 101, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. The molecular response to renal injury. In Kidney Development, Disease, Repair and Regeneration; Melissa, H.L., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 367–379. [Google Scholar]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Punnoose, E.A.; Leverson, J.D.; Peale, F.; Boghaert, E.R.; Belmont, L.D.; Tan, N.; Young, A.; Mitten, M.; Ingalla, E.; Darbonne, W.C.; et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol. Cancer Ther. 2016, 15, 1132–1144. [Google Scholar] [CrossRef] [Green Version]
- Yip, K.W.; Reed, J.C. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, M.W.; Peh, J.; Hergenrother, P.J. Procaspase-3 overexpression in cancer: A paradoxical observation with therapeutic potential. ACS Chem. Biol. 2019, 14, 2335–2348. [Google Scholar] [CrossRef]
- Testa, U.; Riccioni, R. Deregulation of apoptosis in acute myeloid leukemia. Haematologica 2007, 92, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-H.; Wang, H.-S.; Soong, Y.-K. Paclitaxel-induced cell death. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.Z.; Du, G.J.; Qi, L.W.; Calway, T.; He, T.C.; Du, W.; Yuan, C.S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; Zhang, L.; Lee, W.H.; Park, K.Y. Genistein-induced G2/M arrest is associated with the inhibition of cyclin B1 and the induction of p21 in human breast carcinoma cells. Int. J. Oncol. 1998, 13, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Newell, D. Phase I clinical studies with cytotoxic drugs: Pharmacokinetic and pharmacodynamic considerations. Br. J. Cancer 1990, 61, 189–191. [Google Scholar] [CrossRef] [Green Version]
- Quan, N.V.; Xuan, T.D.; Teschke, R. Potential hepatotoxins found in herbal medicinal products: A systematic review. Int. J. Mol. Sci. 2020, 21, 5011. [Google Scholar] [CrossRef] [PubMed]
- Jaaks, P.; Coker, E.A.; Vis, D.J.; Edwards, O.; Carpenter, E.F.; Leto, S.M.; Dwane, L.; Sassi, F.; Lightfoot, H.; Barthorpe, S.; et al. Effective drug combinations in breast, colon and pancreatic cancer cells. Nature 2022, 603, 166–173. [Google Scholar] [CrossRef]
- Taşkın-Tok, T.; Gowder, S.J.T. Anticancer drug—Friend or foe. In Pharmacology and Therapeutics; Gowder, S.J.T., Ed.; IntechOpen: London, UK, 2014. [Google Scholar]
- Collins, T. Acute and chronic inflammation. In Pathologic Basis of Disease; Cotran, T., Kumar, R.S., Collins, V., Eds.; WB Saunders: Philadelphia, PA, USA, 1999; pp. 50–88. [Google Scholar]
- Kato-Noguchi, H.; Hasegawa, M.; Ino, T.; Ota, K.; Kujime, H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010, 167, 787–791. [Google Scholar] [CrossRef]
- Xuan, T.D.; Minh, T.N.; Anh, L.H.; Khanh, T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016, 36, 52. [Google Scholar] [CrossRef]
- Simpson, M.G. Diversity and classification of flowering plants. In Plant Systematics; Simpson, M.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 181–274. [Google Scholar]



| Compounds | MeT-5A (% Inhibition at 10 µM) | HL-60 IC50 (µM) | U266 IC50 (µM) |
|---|---|---|---|
| MA | 28.52 ± 2.93 c | - | - |
| MB | 38.00 ± 2.29 b | 4.49 ± 0.34 bc | 5.09 ± 0.58 a |
| MAB | 37.82 ± 3.64 b | 4.61 ± 0.10 b | 5.59 ± 0.17 a |
| Doxorubicin | 49.23 ± 6.17 a | 5.22 ± 0.15 a | 0.24 ± 0.01 b |
| ATRA | - | 3.99 ± 0.16 cd | - |
| ATRA/ATO | - | 3.67 ± 0.20 d | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, L.H.; Lam, V.Q.; Takami, A.; Khanh, T.D.; Quan, N.V.; Xuan, T.D. Cytotoxic Mechanism of Momilactones A and B against Acute Promyelocytic Leukemia and Multiple Myeloma Cell Lines. Cancers 2022, 14, 4848. https://doi.org/10.3390/cancers14194848
Anh LH, Lam VQ, Takami A, Khanh TD, Quan NV, Xuan TD. Cytotoxic Mechanism of Momilactones A and B against Acute Promyelocytic Leukemia and Multiple Myeloma Cell Lines. Cancers. 2022; 14(19):4848. https://doi.org/10.3390/cancers14194848
Chicago/Turabian StyleAnh, La Hoang, Vu Quang Lam, Akiyoshi Takami, Tran Dang Khanh, Nguyen Van Quan, and Tran Dang Xuan. 2022. "Cytotoxic Mechanism of Momilactones A and B against Acute Promyelocytic Leukemia and Multiple Myeloma Cell Lines" Cancers 14, no. 19: 4848. https://doi.org/10.3390/cancers14194848










