Development of a High-Affinity Antibody against the Tumor-Specific and Hyperactive 611-p95HER2 Isoform
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Carcinoma Material
2.2. Antibodies
2.3. Animals
2.4. Cell Culture
2.5. Retroviral Production and Transduction
2.6. Generation of Oslo-2 mAb
2.7. Immunoblotting
2.8. Flow Cytometry
2.9. Immunofluorescence Staining and Fluorescence Confocal Microscopy
2.10. Immunohistochemistry
2.11. Surface Plasmon Resonance (SPR)
2.12. Epitope Mapping
2.13. Hydrogen Deuterium Exchange (HDX)
2.14. Liquid Chromatography and Mass Spectrometry
2.15. HDX-MS Data Analysis
2.16. Modeling of p95HER2 Protein and Oslo-2 mAb
2.17. Computational Docking
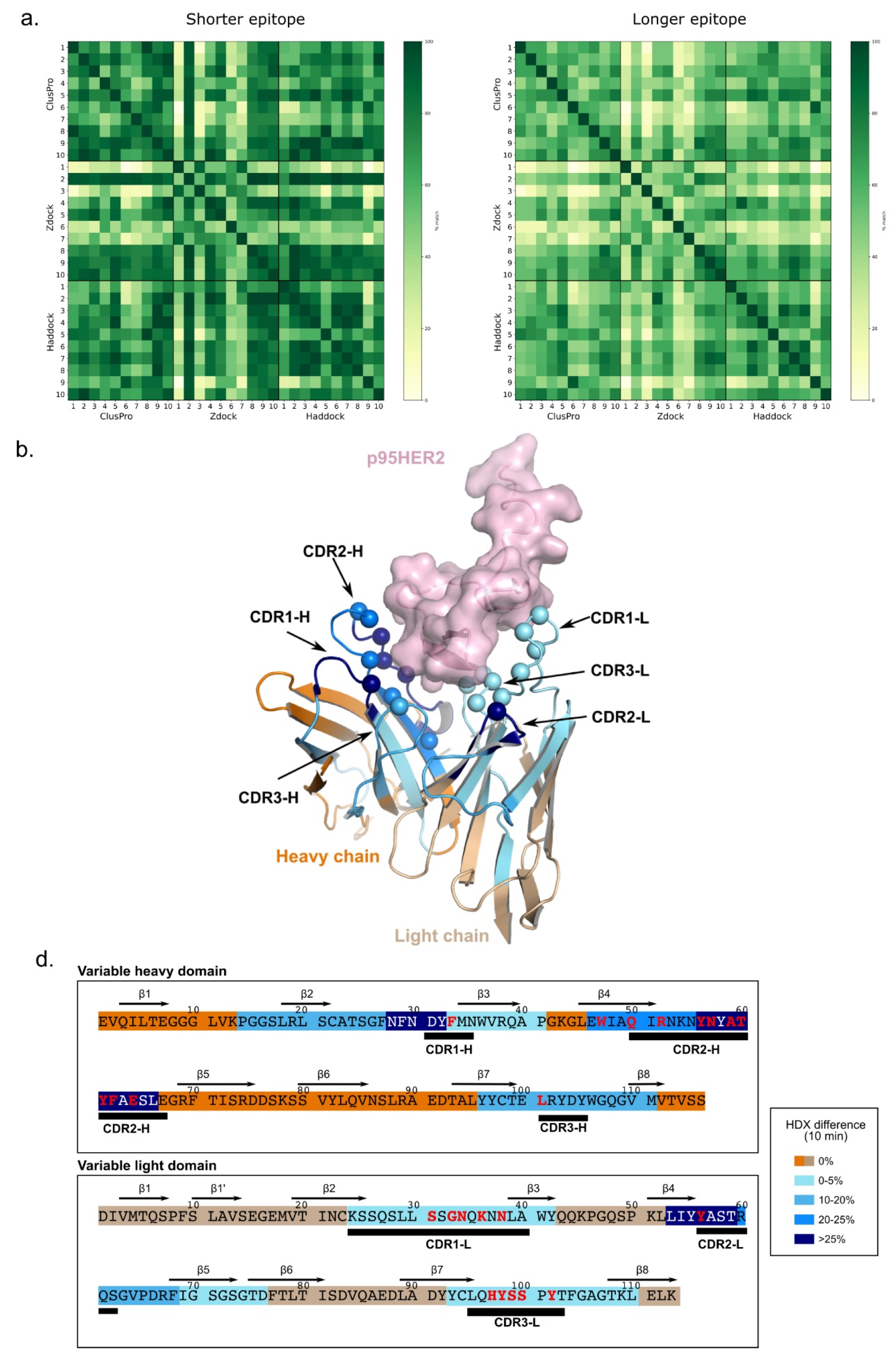
3. Results
3.1. Murine Immunization and Hybridoma Screening
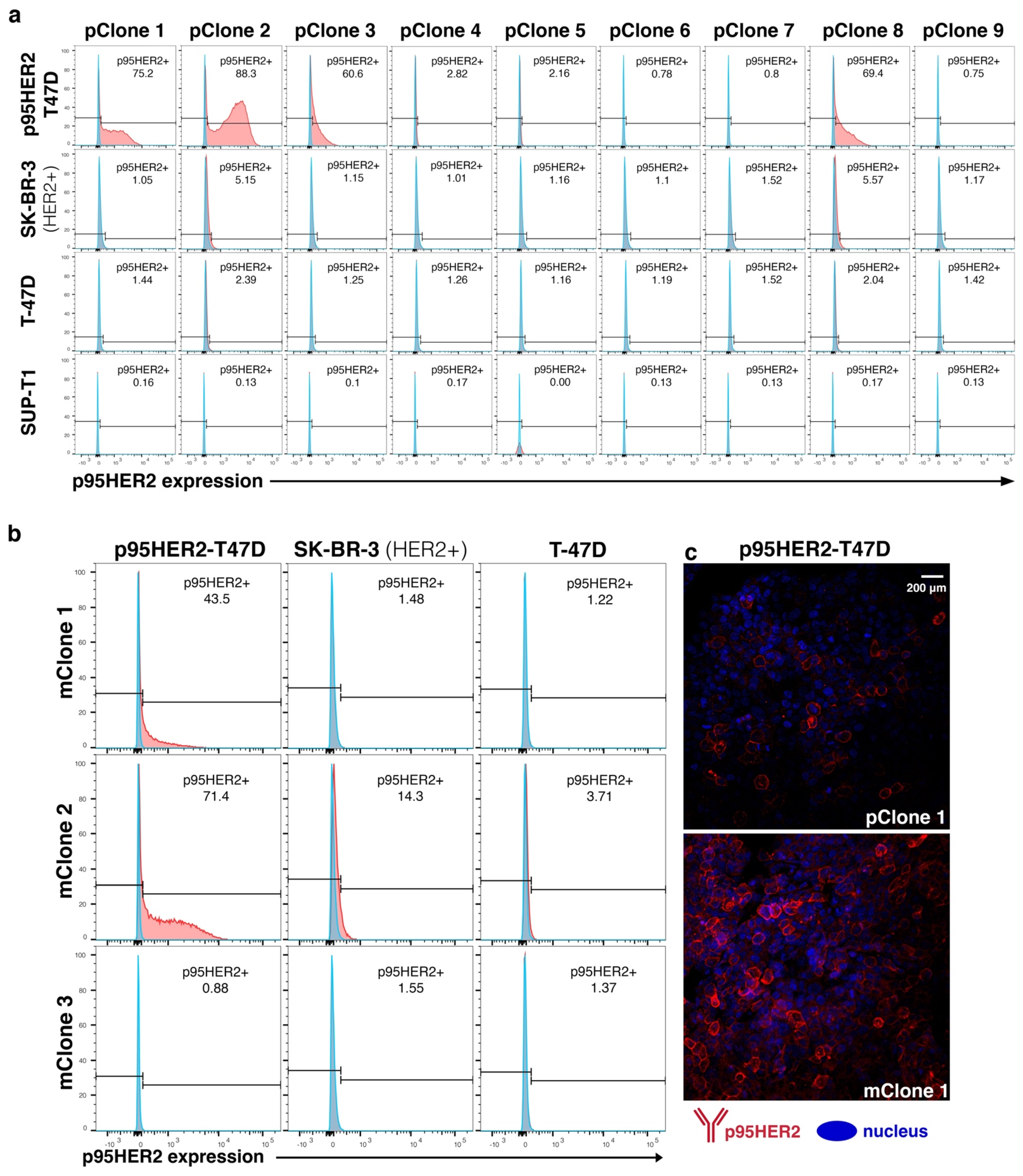
3.2. Subcloning and Evaluation of Monoclonal Hybridoma
3.3. Generation of Oslo-2 mAb and Flow Cytometry Evaluation Using Cell Line Panel
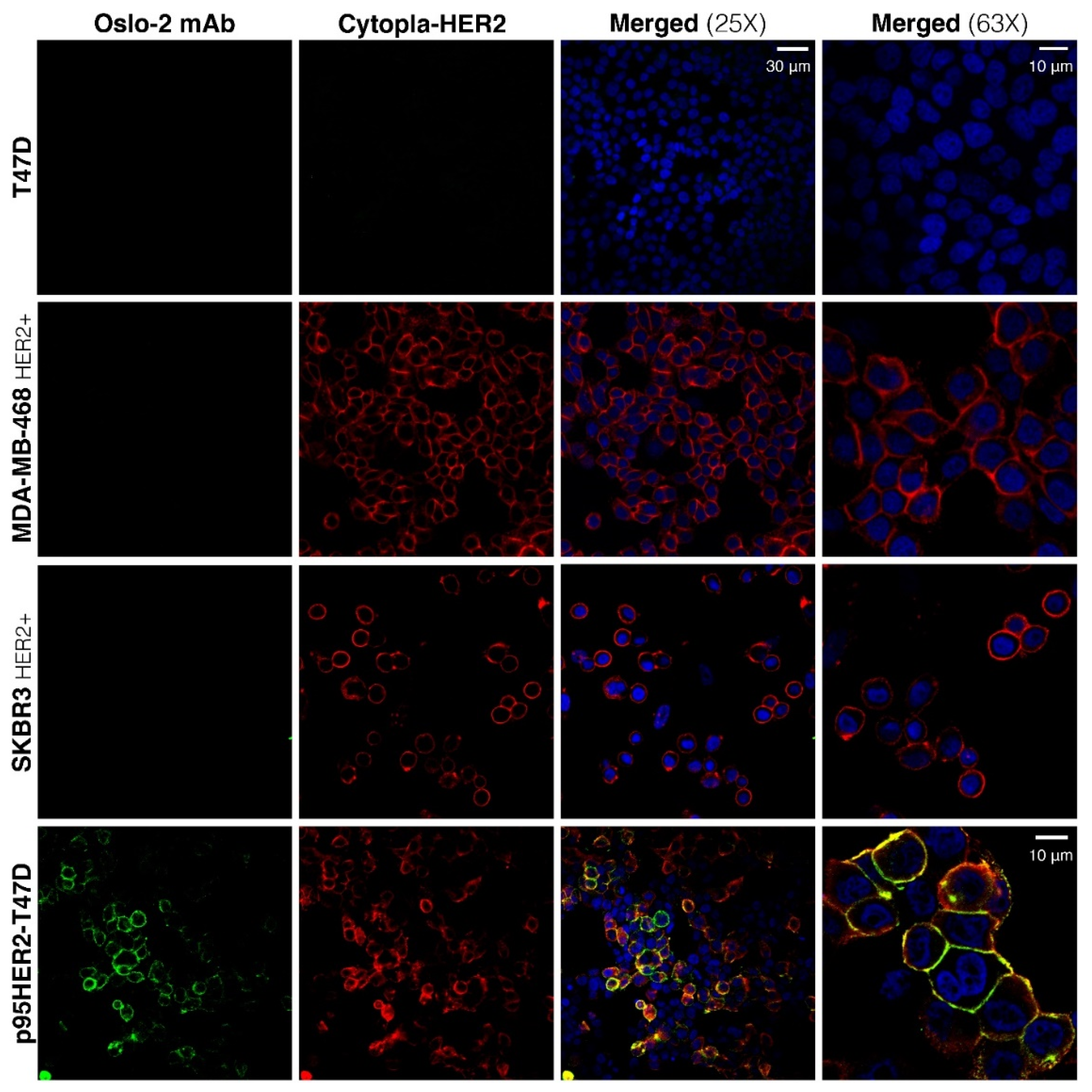
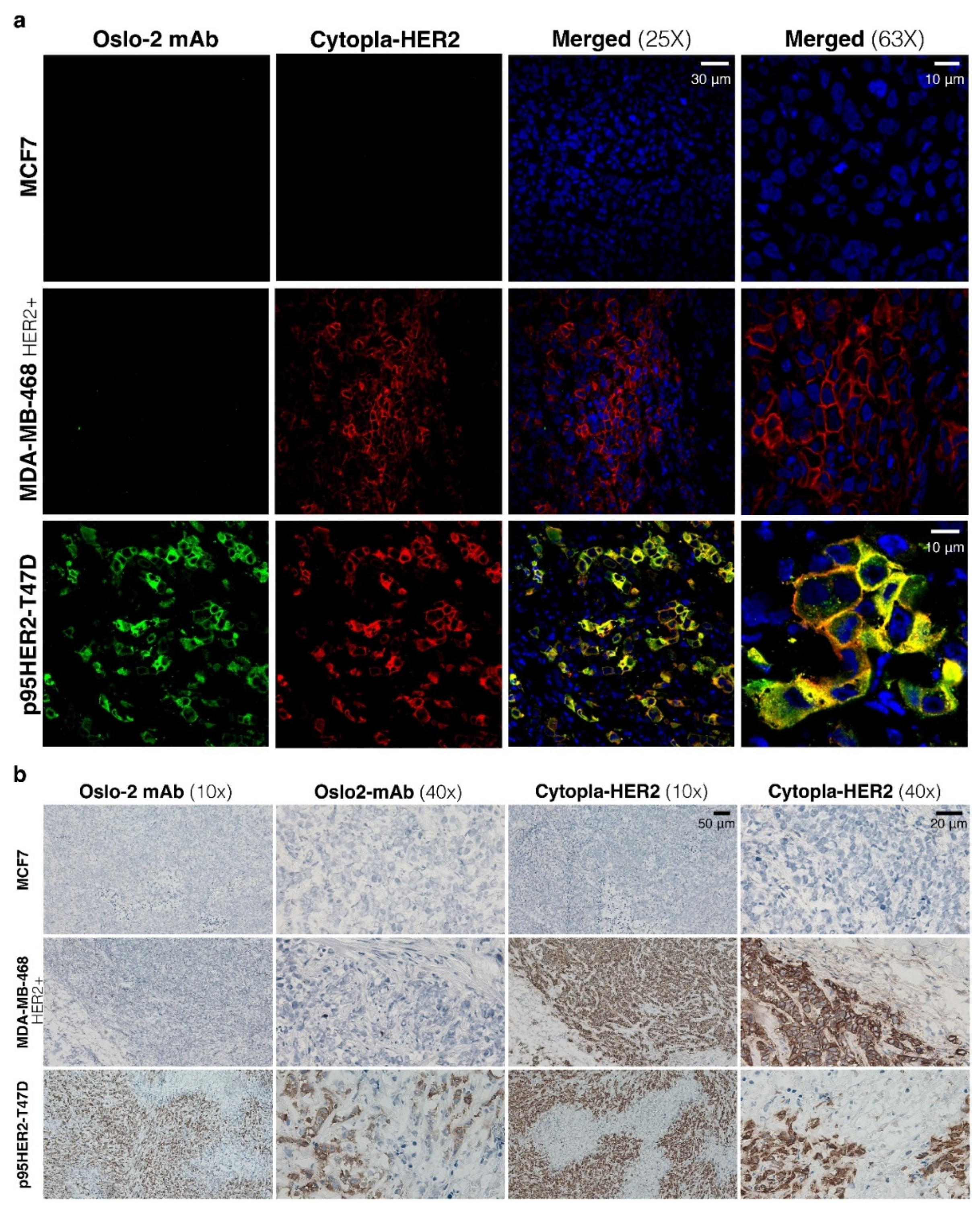
3.4. Immunofluorescence and Immunohistochemistry Evaluation of Oslo-2 mAb
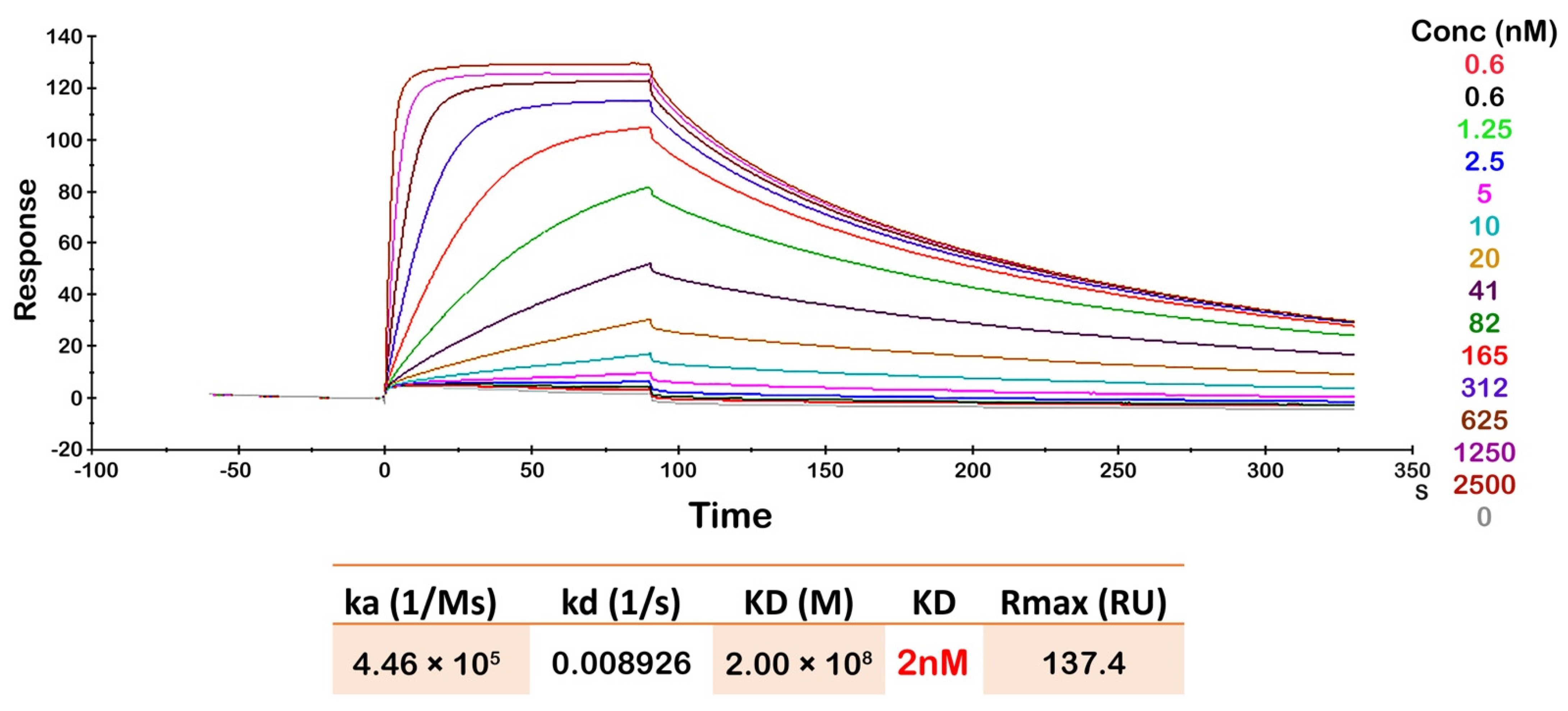
3.5. Oslo-2 mAb Affinity and Epitope Mapping
3.6. Oslo-2 mAb Paratope Mapping by Hydrogen Deuterium Exchange Mass Spectrometry
3.7. Docking Study of Oslo-2 mAb
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAR | chimeric antigen receptor |
| CDR | complementarity-determining regions |
| CTF | carboxy-terminal fragment |
| cytopla-HER2 | cytoplasmic domain of HER2 |
| EGFR | epidermal growth factor receptor |
| FF | fresh-frozen |
| HDX | hydrogen deuterium exchange |
| HER2+ | human epidermal growth factor 2 positive |
| IF | immunofluorescence |
| IHC | immunohistochemistry |
| mAb | monoclonal Ab |
| mBC | metastatic breast cancer |
| mClone | monoclonal hybridoma culture |
| MS | mass spectrometry |
| p95HER2 | 611-CTF p95HER2 isoform |
| pClone | polyclonal hybridoma culture |
| scFv | single-chain variable fragment |
| SPR | surface plasmon resonance |
| RT | room temperature |
References
- Baselga, J.; Norton, L. Focus on breast cancer. Cancer Cell 2002, 1, 319–322. [Google Scholar] [CrossRef][Green Version]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Caswell-Jin, J.L.; Lorenz, C.; Curtis, C. Molecular Heterogeneity and Evolution in Breast Cancer. Annu. Rev. Cancer Biol. 2020, 5, 79–94. [Google Scholar] [CrossRef]
- Owens, M.A.; Horten, B.C.; Da Silva, M.M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer 2004, 5, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef]
- Pupa, S.M.; Ligorio, F.; Cancila, V.; Franceschini, A.; Tripodo, C.; Vernieri, C.; Castagnoli, L. HER2 Signaling and Breast Cancer Stem Cells: The Bridge behind HER2-Positive Breast Cancer Aggressiveness and Therapy Refractoriness. Cancers 2021, 13, 4778. [Google Scholar] [CrossRef]
- Van Der Geer, P.; Hunter, T.; Lindberg, R.A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell Biol. 1994, 10, 251–337. [Google Scholar] [CrossRef]
- Schulze, W.X.; Deng, L.; Mann, M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol. Syst. Biol. 2005, 1, 2005-0008. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF-ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Christianson, T.A.; Doherty, J.K.; Lin, Y.J.; Ramsey, E.E.; Holmes, R.; Keenan, E.J.; Clinton, G.M. NH2-terminally truncated HER-2/neu protein: Relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998, 58, 5123–5129. [Google Scholar] [PubMed]
- Anido, J.; Scaltriti, M.; Bech Serra, J.J.; Josefat, B.S.; Rojo Todo, F.; Baselga, J.; Arribas, J. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006, 25, 3234–3244. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, L.H.; Ho, P.; Spector, N.L. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene 2004, 23, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Angelini, P.-D.; Laos, S.; Bach-Faig, A.; Cunningham, M.P.; Ferrer-Ramón, C.; Luque-García, A.; García-Castillo, J.; Parra-Palau, J.L.; Scaltriti, M.; et al. A Naturally Occurring HER2 Carboxy-Terminal Fragment Promotes Mammary Tumor Growth and Metastasis. Mol. Cell. Biol. 2009, 29, 3319–3331. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Clinton, G.M. A soluble protein related to the HER-2 proto-oncogene product is released from human breast carcinoma cells. Oncogene 1991, 6, 639–643. [Google Scholar]
- Zabrecky, J.R.; Lam, T.; McKenzie, S.J.; Carney, W. The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J. Biol. Chem. 1991, 266, 1716–1720. [Google Scholar] [CrossRef]
- Kang, C.-C.; Ward, T.M.; Bockhorn, J.; Schiffman, C.; Huang, H.; Pegram, M.D.; Herr, A.E. Electrophoretic cytopathology resolves ERBB2 forms with single-cell resolution. Npj Precis. Oncol. 2018, 2, 10. [Google Scholar] [CrossRef]
- Arribas, J.; Parra-Palau, J.L.; Pedersen, K. HER2 fragmentation and breast cancer stratification. Clin. Cancer Res. 2010, 16, 4071–4073. [Google Scholar] [CrossRef][Green Version]
- Parra-Palau, J.L.; Pedersen, K.; Peg, V.; Scaltriti, M.; Angelini, P.D.; Escorihuela, M.; Mancilla, S.; Sanchez Pla, A.; Ramon, Y.C.S.; Baselga, J.; et al. A major role of p95/611-CTF, a carboxy-terminal fragment of HER2, in the down-modulation of the estrogen receptor in HER2-positive breast cancers. Cancer Res. 2010, 70, 8537–8546. [Google Scholar] [CrossRef]
- Martín-Pérez, R.; Yerbes, R.; Mora-Molina, R.; Cano-González, A.; Arribas, J.; Mazzone, M.; López-Rivas, A.; Palacios, C. Oncogenic p95HER2/611CTF primes human breast epithelial cells for metabolic stress-induced down-regulation of FLIP and activation of TRAIL-R/Caspase-8-dependent apoptosis. Oncotarget 2017, 8, 93688–93703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sperinde, J.; Jin, X.; Banerjee, J.; Penuel, E.; Saha, A.; Diedrich, G.; Huang, W.; Leitzel, K.; Weidler, J.; Ali, S.M.; et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin. Cancer Res. 2010, 16, 4226–4235. [Google Scholar] [CrossRef] [PubMed]
- Sáez, R.; Molina, M.A.; Ramsey, E.E.; Rojo, F.; Keenan, E.J.; Albanell, J.; Lluch, A.; Garcia-Conde, J.; Baselga, J.; Clinton, G.M. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin. Cancer Res. 2006, 12, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Rojo, F.; Ocana, A.; Anido, J.; Guzman, M.; Cortes, J.; Di Cosimo, S.; Matias-Guiu, X.; Ramon y Cajal, S.; Arribas, J.; et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 Receptor and Breast Cancer: Ten Years of Targeted Anti–HER-2 Therapy and Personalized Medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef] [PubMed]
- Kocar, M.; Bozkurtlar, E.; Telli, F.; Yumuk, F.; Kaya, H.; Kocar, H.; Turhal, N.S. P95-HER2 and trastuzumab resistance in metastatic breast cancer; is immunohistochemistry appropriate? J. BUON 2014, 19, 245–249. [Google Scholar]
- Eliyatkin, N.O.; Aktas, S.; Ozgur, H.; Ercetin, P.; Kupelioglu, A. The role of p95HER2 in trastuzumab resistance in breast cancer. J. BUON 2016, 21, 382–389. [Google Scholar]
- Sperinde, J.; Huang, W.; Vehtari, A.; Chenna, A.; Kellokumpu-Lehtinen, P.L.; Winslow, J.; Bono, P.; Lie, Y.S.; Petropoulos, C.J.; Weidler, J.; et al. P95HER2 methionine 611 carboxy-terminal fragment is predictive of trastuzumab adjuvant treatment benefit in the fin her trial. Clin. Cancer Res. 2018, 24, 3046–3052. [Google Scholar] [CrossRef]
- Scaltriti, M.; Chandarlapaty, S.; Prudkin, L.; Aura, C.; Jimenez, J.; Angelini, P.D.; Sanchez, G.; Guzman, M.; Parra, J.L.; Ellis, C.; et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin. Cancer Res. 2010, 16, 2688–2695. [Google Scholar] [CrossRef]
- Parra-Palau, J.L.; Morancho, B.; Peg, V.; Escorihuela, M.; Scaltriti, M.; Vicario, R.; Zacarias-Fluck, M.; Pedersen, K.; Pandiella, A.; Nuciforo, P.; et al. Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. J. Natl. Cancer Inst. 2014, 106, dju291. [Google Scholar] [CrossRef]
- Jin, Y.; Lorvik, K.B.; Jin, Y.; Beck, C.; Sike, A.; Persiconi, I.; Kvaloy, E.; Saatcioglu, F.; Dunn, C.; Kyte, J.A. Development of STEAP1 targeting chimeric antigen receptor for adoptive cell therapy against cancer. Mol. Ther. Oncolytics 2022, 26, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Esmaeil Dorraji, S.; Hovd, A.M.K.; Kanapathippillai, P.; Bakland, G.; Eilertsen, G.Ø.; Figenschau, S.L.; Fenton, K.A. Mesenchymal stem cells and T cells in the formation of Tertiary Lymphoid Structures in Lupus Nephritis. Sci. Rep. 2018, 8, 7861. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Andreu, D. Direct kinetic assay of interactions between small peptides and immobilized antibodies using a surface plasmon resonance biosensor. J. Immunol Methods 2002, 259, 217–230. [Google Scholar] [CrossRef][Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Leem, J.; Dunbar, J.; Georges, G.; Shi, J.; Deane, C.M. ABodyBuilder: Automated antibody structure prediction with data–driven accuracy estimation. mAbs 2016, 8, 1259–1268. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Honorato, R.V.; Koukos, P.I.; Jiménez-García, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M.J.J. Structural Biology in the Clouds: The WeNMR-EOSC Ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef]
- Brenke, R.; Hall, D.R.; Chuang, G.Y.; Comeau, S.R.; Bohnuud, T.; Beglov, D.; Schueler-Furman, O.; Vajda, S.; Kozakov, D. Application of asymmetric statistical potentials to antibody-protein docking. Bioinformatics 2012, 28, 2608–2614. [Google Scholar] [CrossRef]
- Mayne, L. Hydrogen Exchange Mass Spectrometry. Methods Enzymol. 2016, 566, 335–356. [Google Scholar]
- Zhang, M.M.; Huang, R.Y.C.; Beno, B.R.; Deyanova, E.G.; Li, J.; Chen, G.; Gross, M.L. Epitope and Paratope Mapping of PD-1/Nivolumab by Mass Spectrometry-Based Hydrogen–Deuterium Exchange, Cross-linking, and Molecular Docking. Anal. Chem. 2020, 92, 9086–9094. [Google Scholar] [CrossRef]
- Grauslund, L.R.; Calvaresi, V.; Pansegrau, W.; Norais, N.; Rand, K.D. Epitope and Paratope Mapping by HDX-MS Combined with SPR Elucidates the Difference in Bactericidal Activity of Two Anti-NadA Monoclonal Antibodies. J. Am. Soc. Mass Spectrom. 2021, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Kabat, E.A.; Wu, T.T. Attempts to locate complementarity-determining residues in the variable positions of light and heavy chains. Ann. N. Y. Acad. Sci. 1971, 190, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Te Wu, T.; Kabat, E.A. An analysis of the sequences of the variable regions of bence jones proteins and myeloma light chains and their implications for antibody complementarity. J. Exp. Med. 1970, 132, 211–250. [Google Scholar]
- Rawat, P.; Sharma, D.; Srivastava, A.; Janakiraman, V.; Gromiha, M.M. Exploring antibody repurposing for COVID-19: Beyond presumed roles of therapeutic antibodies. Sci. Rep. 2021, 11, 10220. [Google Scholar] [CrossRef]
- Ruiz, I.R.; Vicario, R.; Morancho, B.; Morales, C.B.; Arenas, E.J.; Herter, S.; Freimoser-Grundschober, A.; Somandin, J.; Sam, J.; Ast, O.; et al. P95HER2–T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med. 2018, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: State of the art and future directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Spector, N.L.; Blackwell, K.L. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2009, 27, 5838–5847. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Ohnstad, H.O.; Borgen, E.; Falk, R.S.; Lien, T.G.; Aaserud, M.; Sveli, M.A.T.; Kyte, J.A.; Kristensen, V.N.; Geitvik, G.A.; Schlichting, E.; et al. Prognostic value of PAM50 and risk of recurrence score in patients with early-stage breast cancer with long-term follow-up. Breast Cancer Res. 2017, 19, 120. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorraji, E.; Borgen, E.; Segura-Peña, D.; Rawat, P.; Smorodina, E.; Dunn, C.; Greiff, V.; Sekulić, N.; Russnes, H.; Kyte, J.A. Development of a High-Affinity Antibody against the Tumor-Specific and Hyperactive 611-p95HER2 Isoform. Cancers 2022, 14, 4859. https://doi.org/10.3390/cancers14194859
Dorraji E, Borgen E, Segura-Peña D, Rawat P, Smorodina E, Dunn C, Greiff V, Sekulić N, Russnes H, Kyte JA. Development of a High-Affinity Antibody against the Tumor-Specific and Hyperactive 611-p95HER2 Isoform. Cancers. 2022; 14(19):4859. https://doi.org/10.3390/cancers14194859
Chicago/Turabian StyleDorraji, Esmaeil, Elin Borgen, Dario Segura-Peña, Puneet Rawat, Eva Smorodina, Claire Dunn, Victor Greiff, Nikolina Sekulić, Hege Russnes, and Jon Amund Kyte. 2022. "Development of a High-Affinity Antibody against the Tumor-Specific and Hyperactive 611-p95HER2 Isoform" Cancers 14, no. 19: 4859. https://doi.org/10.3390/cancers14194859
APA StyleDorraji, E., Borgen, E., Segura-Peña, D., Rawat, P., Smorodina, E., Dunn, C., Greiff, V., Sekulić, N., Russnes, H., & Kyte, J. A. (2022). Development of a High-Affinity Antibody against the Tumor-Specific and Hyperactive 611-p95HER2 Isoform. Cancers, 14(19), 4859. https://doi.org/10.3390/cancers14194859






