Simple Summary
Although surgery has been recognized as the cornerstone of treatment for patients with resectable thymic epithelial tumors, the role of postoperative radiotherapy remains controversial. We performed this SEER-based propensity-matched analysis to investigate the prognostic value of postoperative radiotherapy in thymoma and thymic carcinoma. The results showed that postoperative radiotherapy improved both overall survival and cancer-specific survival in patients with Masaoka-Koga stage IIB–IV thymoma. This study is the first to demonstrate the prognostic value of postoperative radiotherapy in stage IIB thymic carcinoma. This large, up-to-date population-based longitudinal study may provide guidance on the use of postoperative radiotherapy for a thymoma or thymic carcinoma.
Abstract
(1) Objectives: The effect of postoperative radiotherapy (PORT) for thymoma and thymic carcinoma remains controversial. This study aimed to investigate the prognostic value of PORT for thymoma and thymic carcinoma in a population-based registry. (2) Methods: This retrospective study used the Surveillance, Epidemiology, and End Results (SEER) database to identify patients diagnosed with thymoma and thymic carcinoma between 2010 and 2019. Propensity score matching was performed to adjust statistical influences between the PORT and non-PORT groups. (3) Results: A total of 2558 patients with thymoma (n = 2138) or thymic carcinoma (n = 420) were included. In the multivariate analysis, PORT was an independent prognostic factor for OS (overall survival; p < 0.001) and CSS (cancer-specific survival; p = 0.001) in thymoma and an independent prognostic factor for OS in thymic carcinoma (p = 0.018). Subgroup analyses revealed that PORT was beneficial to OS and CSS in patients with Masaoka-Koga stage IIB-IV thymoma (OS: IIB, p < 0.001; III-IV, p = 0.005; CSS: IIB, p = 0.015; III-IV, p = 0.002) and stage IIB thymic carcinoma (OS: p = 0.012; CSS: p = 0.029). (4) Conclusion: This propensity-matched analysis identified the prognostic value of PORT in thymoma and thymic carcinoma based on the SEER database. For patients with stage IIB-IV thymoma and stage IIB thymic carcinoma, PORT was associated with improved OS and CSS. A more positive attitude towards the use of PORT for nonlocalized thymoma and thymic carcinoma may be appropriate.
1. Introduction
Thymoma and thymic carcinoma are the most common anterior mediastinal thymic epithelial tumors, although they are still relatively rare in general [1]. Before the World Health Organization (WHO) Consensus Committee published the distinction between the diagnosis and histological features of thymoma and thymic carcinoma in 1999, they were often confused [2]. Thymoma is a potentially malignant disease that can invade mediastinal organs and is associated with many autoimmune paraneoplastic diseases [3,4,5]. Thymic carcinoma is more advanced than thymoma and has a worse prognosis, with lymphatic or hematogenous metastasis in about 30% of cases [6,7].
The Masaoka staging system was first proposed by Masaoka et al. in 1981 [8]. In 1994, Koga et al. modified this system and proposed the Masaoka-Koga staging system [9], which is now widely accepted as the clinical staging standard for thymic epithelial tumors. Thymic epithelial tumors are classified into stages I to IV based on the local extension of the primary tumor and the degree of involvement of the surrounding organs [10]. When feasible, surgical resection is the recommended treatment for thymoma and thymic carcinoma, and the extent of resection proved to be an independent predictor of survival [11]. In addition, postoperative radiotherapy (PORT) is also considered an important component of treating thymic epithelial tumors. Still, there is no consensus on its optimal use, especially for Masaoka-Koga stage II patients [12,13,14]. According to the guidelines of the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (EMSO), PORT is recommended for incompletely resected thymoma and thymic carcinoma and for Masaoka-Koga stage III-IV completely resected thymoma and thymic carcinoma [15,16]. The low incidence of thymic epithelial tumors made randomized trials difficult to conduct and hindered the development of evidence-based recommendations.
The National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) database is a nationwide cancer dataset that tracks demographic and clinical data for nearly one-third of the United States population. This study investigated the prognostic value of PORT on patients with thymoma and thymic carcinoma using the SEER database. Considering the selection bias for the receipt of PORT in the database, propensity score matching was performed to balance the distribution of baseline clinicopathological variables.
2. Methods
2.1. Database
This retrospective study analyzed the SEER 18-Registry maintained by the NCI (1975–2019; dataset submitted in November 2021; www.seer.cancer.gov (accessed on 16 August 2022)). The SEER*Stat software (version 8.3.9; National Institutes of Health, Bethesda, MD, USA) was used to extract clinicopathologic and survival information from the database. Permission to access the research data file in the SEER registry was received from the NCI, USA (reference No. 16521-Nov 2021).
2.2. Study Population
Patients with a primary site of the thymus (C37.9) between 1975 and 2019 were initially identified. The inclusion criteria were as follows: (1) histologic types of thymoma (8580–8585) and thymic carcinoma (8023, 8033, 8070, 8082, 8123, 8140, 8200, 8260, 8310, 8430, 8480, 8560, 8576, 8586, 8588, and 8589) with the malignant behavior code (/3) according to the International Classification of Disease for Oncology, Third Edition (ICD-O-3) [17]; (2) age >18 years; (3) diagnosis between 2010 and 2019; (4) receipt of cancer-directed surgery with or without PORT. Demographic features and clinicopathological characteristics of these patients were collected, such as the age of diagnosis, gender, race, year of diagnosis, other malignancies, time from diagnosis to treatment, tumor size, lymph node dissection, the extent of surgery, WHO classification, and histological grade. Due to a lack of information on the Masaoka-Koga stages in SEER, we inferred stages based on the primary tumor extension: stage I-IIA (localized; confined to the gland of origin, not otherwise specified), stage IIB (regional; invasion to the adjacent connective tissue), stage III-IV (distant; invasion to the adjacent organs/structures or pleural/pericardial implants and metastases), and unknown (unknown extent of disease). This approach was previously used by Fernandes et al. [18] and Mou et al. [19] to assign the Masaoka-Koga stages, but stages I and IIA or III and IV could not be distinguished based on the SEER data.
2.3. Study Outcomes
The outcomes of the present study were overall survival (OS) and cancer-specific survival (CSS). OS was measured from the date of diagnosis to the date of death from any cause. CSS was measured from the date of diagnosis to the date of death directly or indirectly from thymic epithelial tumors. Survival status was shown as “Vital Status” in the SEER database.
2.4. Propensity Score Matching
Selection bias due to baseline characteristics in the database may affect the receipt of PORT. A propensity score, the probability of being assigned to the PORT or non-PORT groups given the clinicopathological baseline, was performed to minimize selection bias. The propensity scores were developed from the non-parsimonious logistic regression model with baseline covariates consisting of age of diagnosis, gender, race, year of diagnosis, other malignancies, time from diagnosis to treatment, Masaoka-Koga stage, tumor size, lymph node dissection, the extent of surgery, WHO classification, and histological grade. The PORT groups were matched to non-PORT groups using 1:1 matching based on the nearest neighbor method with a caliper width of 0.02.
2.5. Statistical Analysis
Categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test, and continuous variables were analyzed using Student’s t-test. The Kaplan–Meier method was used to calculate the OS and CSS curves before and after propensity score matching between PORT and non-PORT groups. The log-rank test was performed to determine statistical significance. Univariate Cox regression analysis was used to determine variables associated with the OS and CSS of the matched population, and variables with a p-value less than 0.1 were selected for the multivariate Cox regression model. Hazard ratios (HR) with 95% confidence intervals (CI) were reported, and two-sided p-values less than 0.05 indicated statistical significance. All statistical analyses were performed using SPSS (version 27.0, SPSS Inc. Chicago, IL, USA).
3. Results
3.1. Baseline Characteristics
A total of 2558 patients with thymoma (n = 2138) or thymic carcinoma (n = 420) were identified from the SEER database based on the eligibility criteria. Baseline characteristics are summarized in Table 1. Among the study population, the mean age ± standard deviation (SD) for patients with thymoma was 59.7 ± 14.1 years (range, 19–94), and that for patients with thymic carcinoma was 63.1 ± 12.9 years (range, 19–92). The median tumor size was 6.5 cm for thymoma and 6.0 cm for thymic carcinoma. Postoperative radiotherapy was performed in 963 (45.0%) thymoma and 168 (40.0%) thymic carcinoma patients. Based on the staging method (described in the Study Population), 909 (35.5%), 1181 (46.2%), 394 (15.4%), and 74 (2.9%) patients were classified as Masaoka-Koga stage I-IIA, IIB, III-IV and unknown, respectively. The vast majority of patients were in the localized (I-IIA) or regional (IIB) stage, and thymic carcinoma had a higher proportion of the distant stage (III-IV) than thymoma (20.7 and 14.4%, respectively). In addition, thymic carcinoma had a higher proportion of patients histologically graded poor or undifferentiated than thymoma (25.0 and 8.9%, respectively), and more than half (56.2%) underwent lymph node dissection. More patients with thymoma underwent total or radical resection than patients with thymic carcinoma (58.3 and 51.9%, respectively).

Table 1.
Characteristics of thymoma and thymic carcinoma in the SEER database.
3.2. Survival Outcomes before and after Propensity Score Matching
Table 2 and Table 3 show the balances of each variable before and after propensity score matching in thymoma and thymic carcinoma. In the matched cohort, there were 783 thymoma patients each in the PORT group and non-PORT group and 156 thymic carcinoma patients each in the PORT group and non-PORT group. The p-values for all covariates after matching were more than 0.1, indicating that the propensity score matching minimized potential selection bias in PORT reception.

Table 2.
Characteristics of thymoma patients before and after propensity score matching.

Table 3.
Characteristics of thymic carcinoma patients before and after propensity score matching.
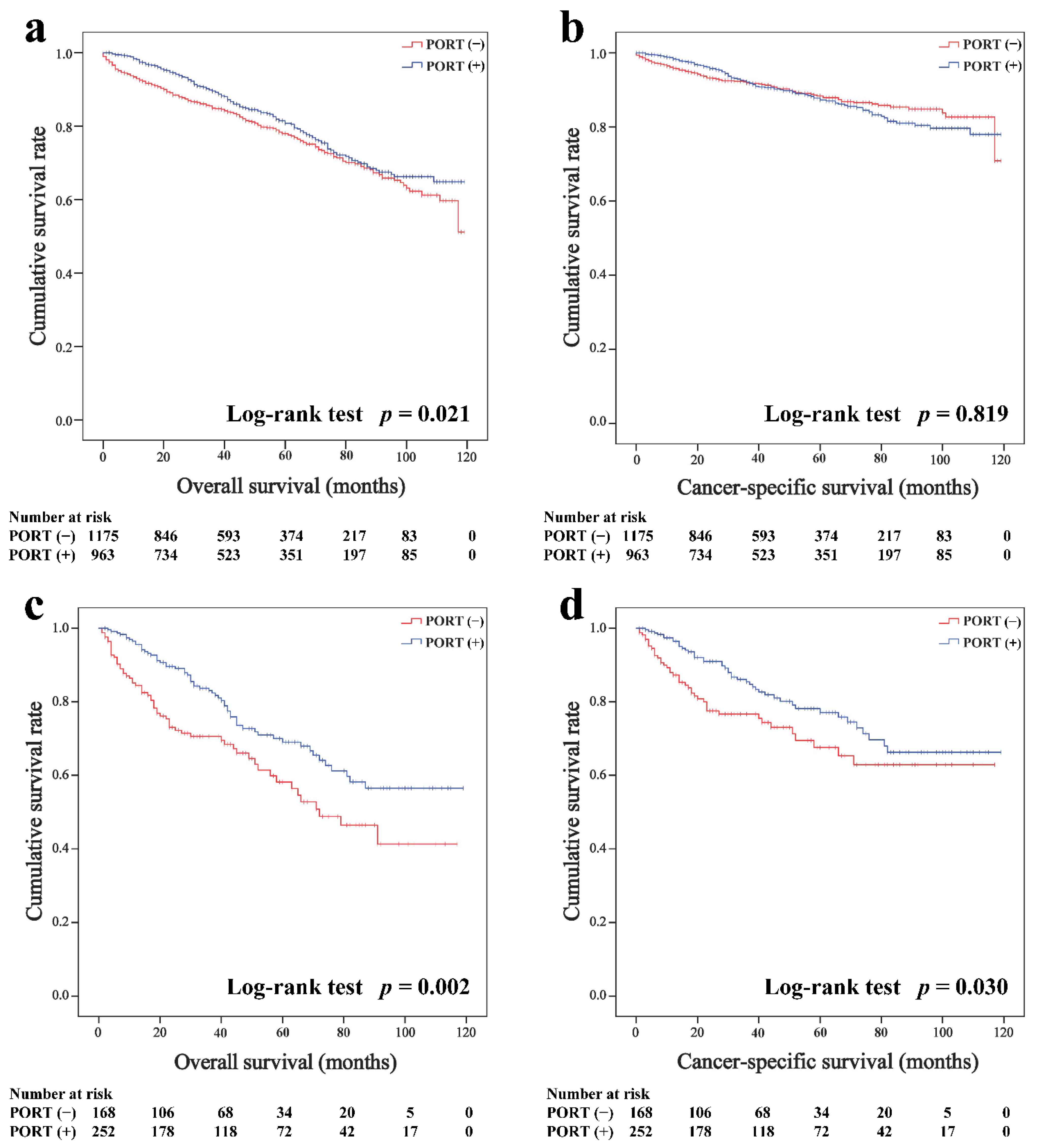
In the overall cohort, the average follow-up periods were 94.1 months (95% CI, 92.1–96.4 months, Kaplan–Meier estimate) for thymoma and 80.8 months (95% CI, 75.4–86.1 months) for thymic carcinoma. The Kaplan–Meier survival curves of the overall cohort according to the receipt of PORT are shown in Figure 1. For patients with thymoma, the PORT group had a better OS than the non-PORT group (five-year survival rates were 80.8 and 78.0%, p = 0.021, respectively; Figure 1a, but PORT was not associated with CSS before matching (87.3 and 88.4%, p = 0.819; Figure 1b. For patients with thymic carcinoma, both the OS (69.0 and 58.2%, p = 0.002; Figure 1c) and CSS (77.1 and 67.6%, p = 0.030; Figure 1d) of the PORT group were better than the non-PORT group.
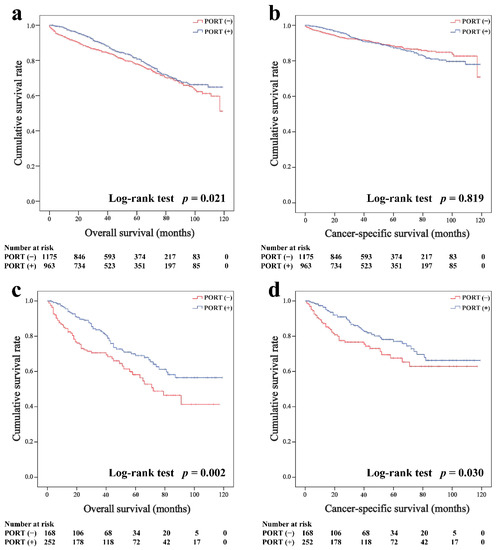
Figure 1.
Overall and cancer-specific survival in patients with thymoma (a,b) and thymic carcinoma (c,d) before matched.
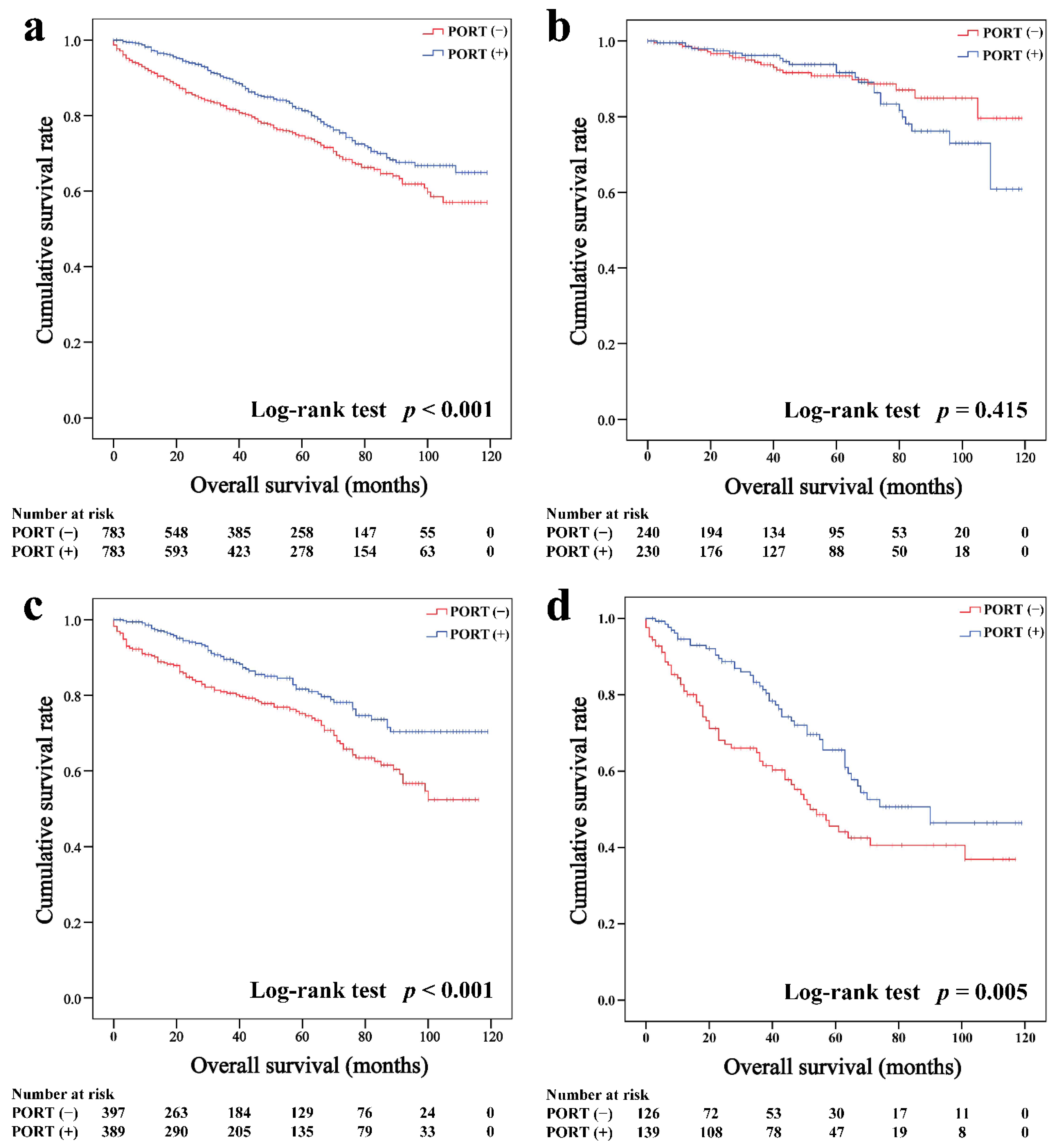
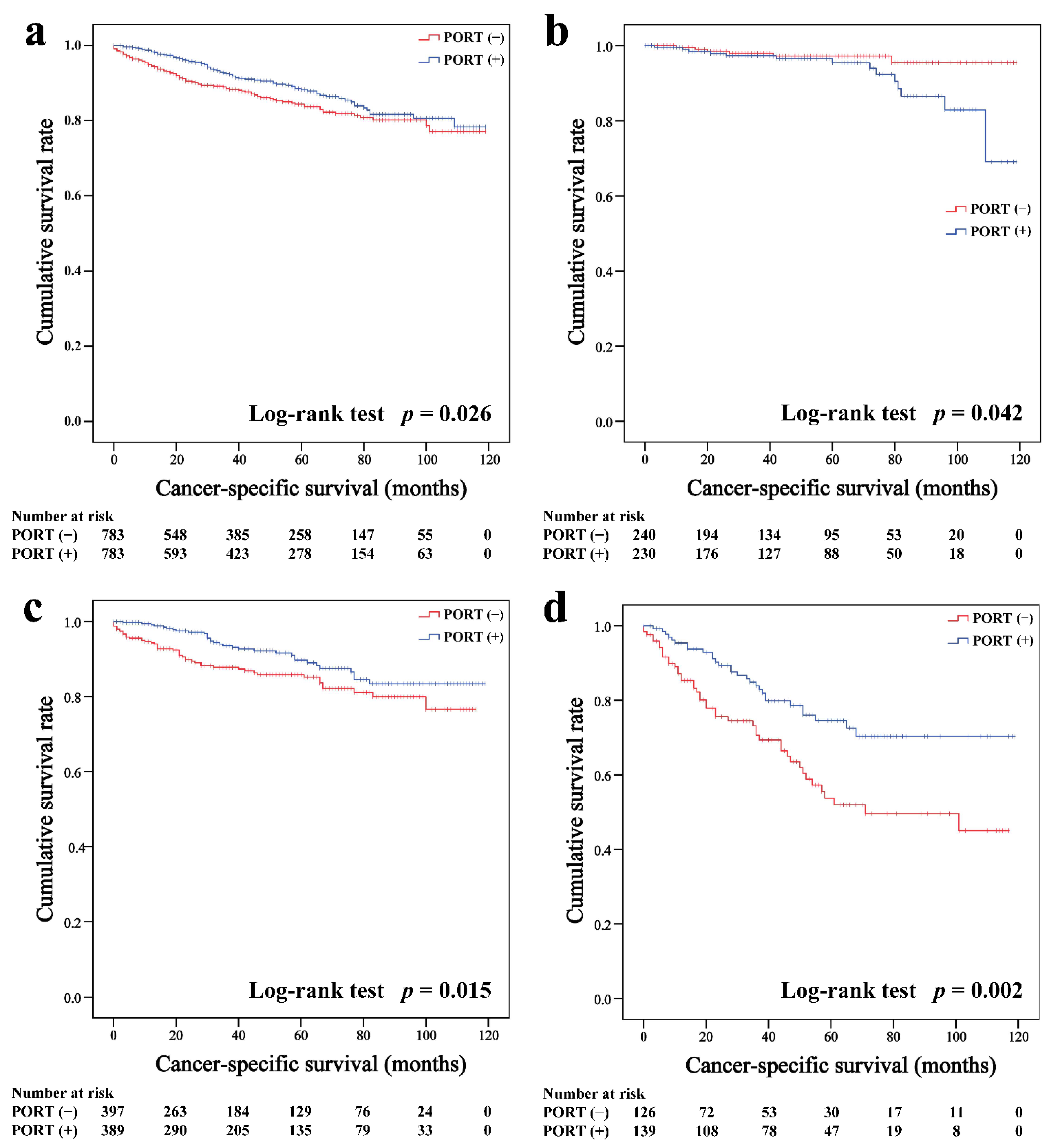
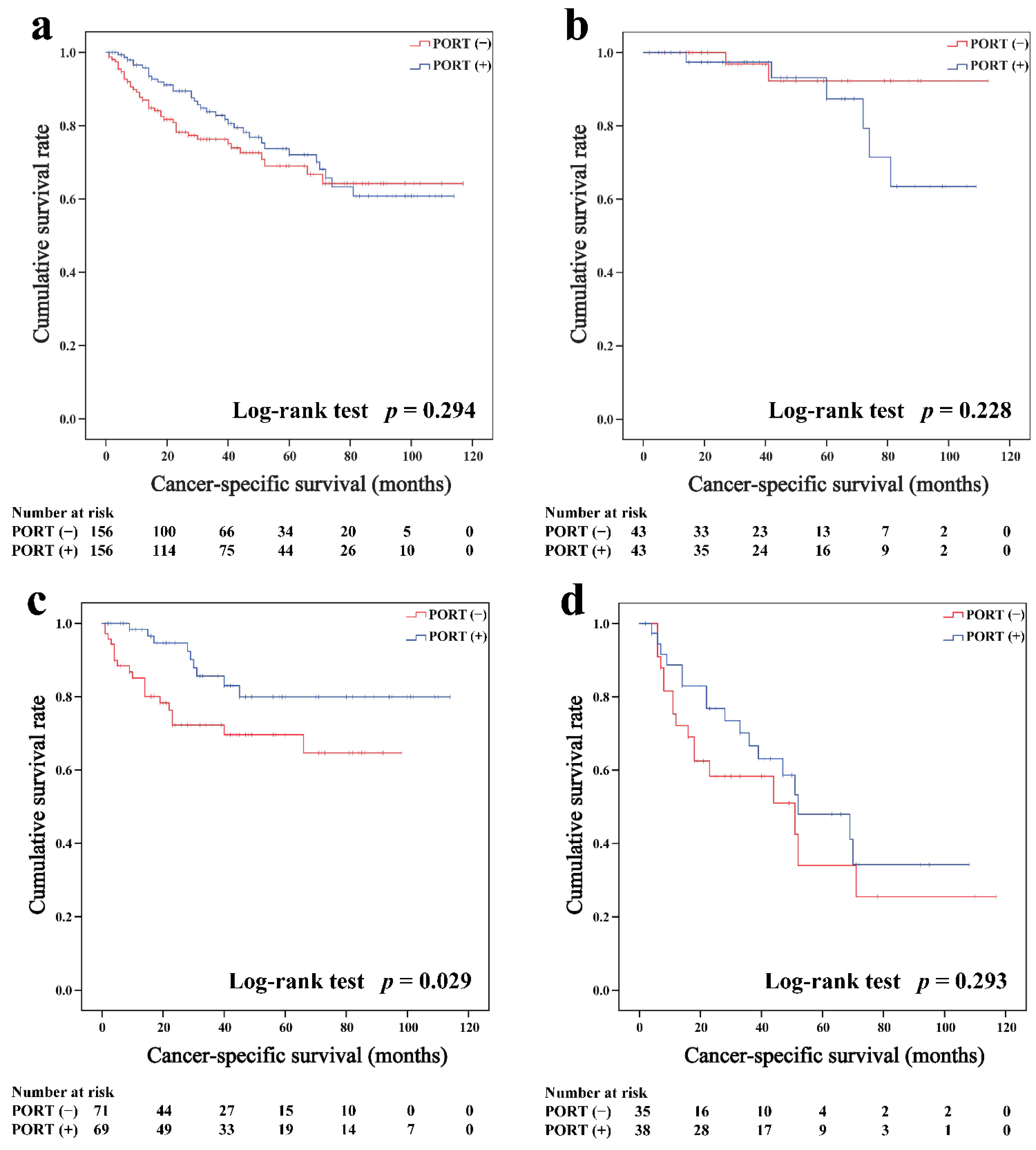
In the matched cohort, the Kaplan–Meier survival curves of the OS and CSS for thymoma stratified by PORT or non-PORT are shown in Figure 2a and Figure 3a, respectively. Both the OS (81.3 and 74.7%, p < 0.001) and CSS (88.2 and 84.4%, p = 0.026) of the PORT group were better than the non-PORT group after matching. The Kaplan–Meier survival curves of the OS and CSS for thymic carcinoma are shown in Figure 4a and Figure 5a, respectively. There were no significant differences in the OS and CSS between the PORT and non-PORT groups in thymic carcinomas (p > 0.05).
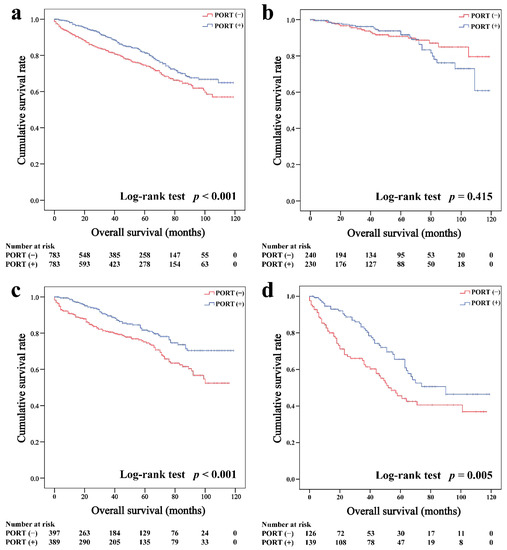
Figure 2.
Overall survival in all (a), Masaoka–Koga stage I-IIA (b), IIB (c), and III-IV (d) thymoma patients.
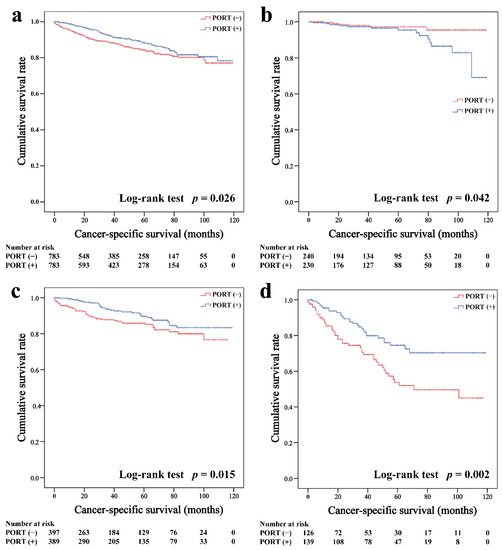
Figure 3.
Cancer-specific survival in all (a), Masaoka–Koga stage I-IIA (b), IIB (c), and III-IV (d) thymoma patients.
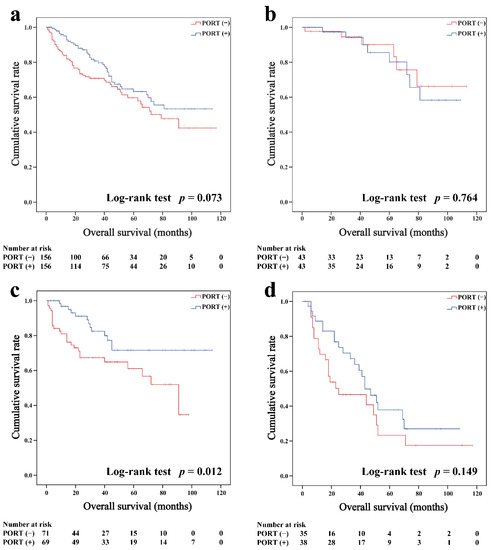
Figure 4.
Overall survival in all (a), Masaoka–Koga stage I-IIA (b), IIB (c), and III-IV (d) thymic carcinoma patients.
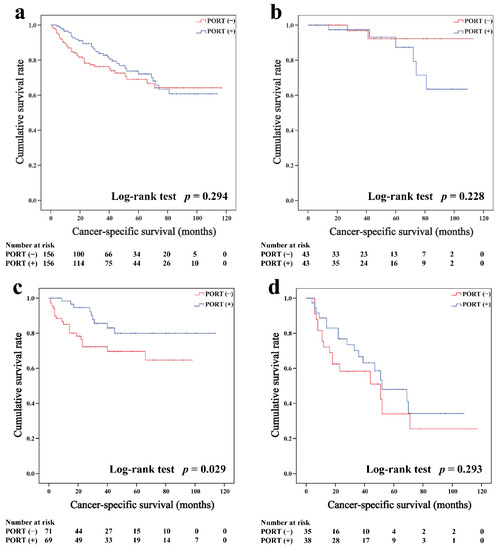
Figure 5.
Cancer-specific survival in all (a), Masaoka–Koga stage I-IIA (b), IIB (c), and III-IV (d) thymic carcinoma patients.
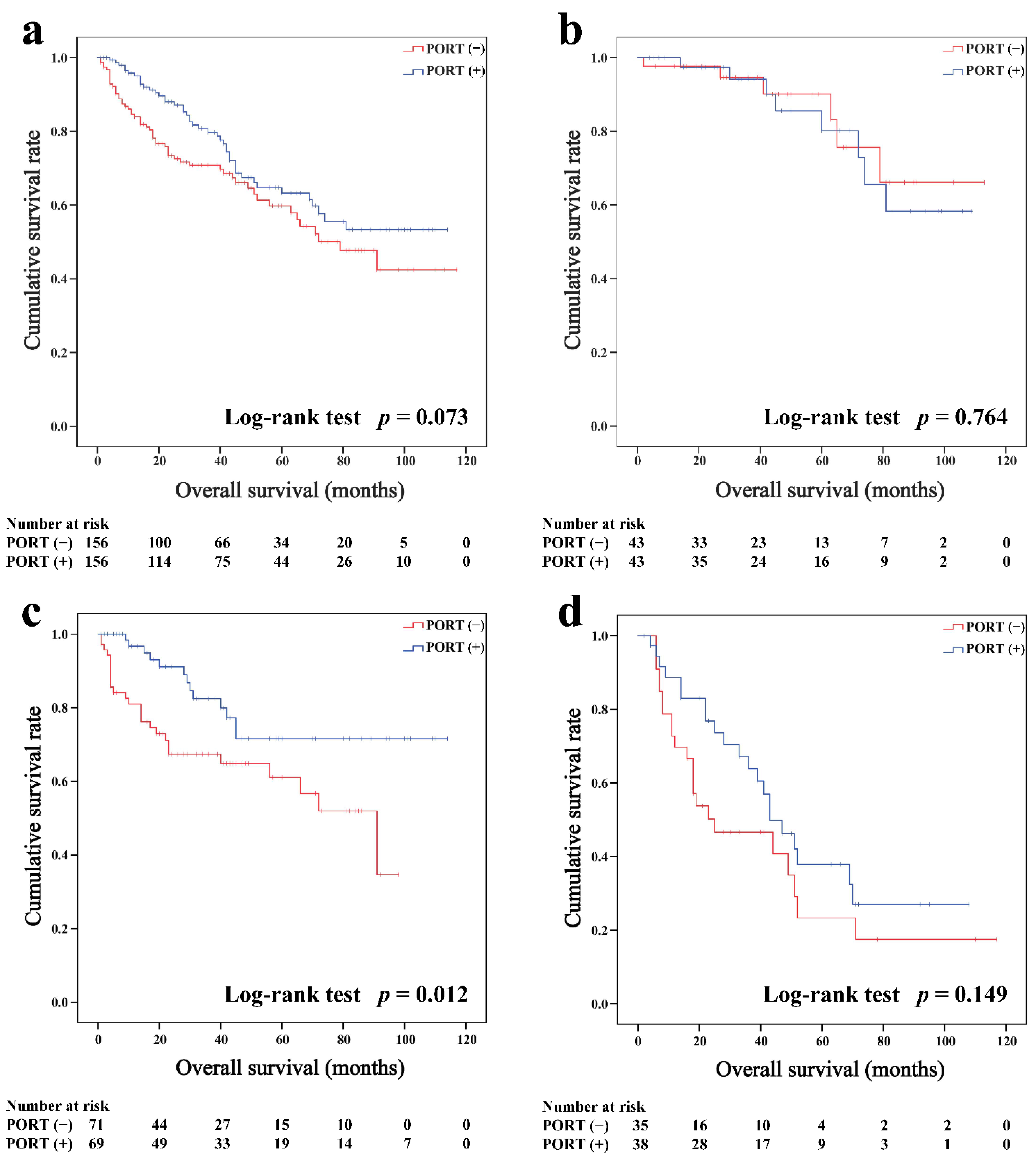
Subgroup survival analysis of the OS and CSS was performed stratified by the Masaoka-Koga stage (Figure 2b–d, Figure 3b–d, Figure 4b–d and Figure 5b–d). Among the patients with stage IIB and III/IV thymoma, PORT was associated with a better OS (IIB: 81.6 and 75.2%, p < 0.001; III/IV: 65.5 and 45.6%, p = 0.005, respectively) and CSS (IIB: 89.7 and 85.8%, p = 0.015; III/IV: 74.6 and 53.7%, p = 0.002, respectively) than non-PORT. However, for patients with stage I/IIA thymoma, PORT had no benefit on the OS (91.6% vs. 90.8%, p = 0.415) and even had a negative effect on the CSS (95.4% vs. 97.2%, p = 0.042). PORT was beneficial only for the stage IIB thymic carcinoma (OS: 71.5% vs. 61.0%, p = 0.012; CSS: 79.9% vs. 69.6%, p = 0.029), but not for other stages (p > 0.05). For patients with stage III-IV thymic carcinoma, PORT did not significantly improve their survival (OS: 37.9% vs. 23.3%, p = 0.149; CSS: 48.0% vs. 34.0%, p = 0.293).
3.3. Cox Regression Analysis
Table 4 and Table 5 list 11 variables included in the univariate Cox regression model after propensity score matching for thymoma and thymic carcinoma, respectively. Variables with a univariate analysis p < 0.1 were selected for the multivariate Cox regression models. The results of the multivariate Cox regression analysis showed that PORT was an independent prognostic factor for both the OS (p < 0.001) and CSS (p = 0.001) in patients with thymoma. Besides, age, Masaoka–Koga stage, tumor size, and histological grade were also independent prognostic factors for both the OS and CSS. Gender was an independent predictor for OS (p = 0.011) but not CSS (p = 0.109). For thymic carcinoma, the Masaoka–Koga stage and extent of surgery were independent prognostic factors for both the OS and CSS. PORT was an independent prognostic factor for OS (p = 0.018), and tumor size was an independent prognostic factor for CSS (p = 0.021).

Table 4.
Univariate and Multivariate Cox regression analysis of clinical characteristics for overall survival and cancer-specific survival rate in thymoma patients in the matched population.

Table 5.
Univariate and Multivariate Cox regression analysis of clinical characteristics for overall survival and cancer-specific survival rate in thymic carcinoma patients in the matched population.
3.4. Subgroup Analysis by Forest Plot
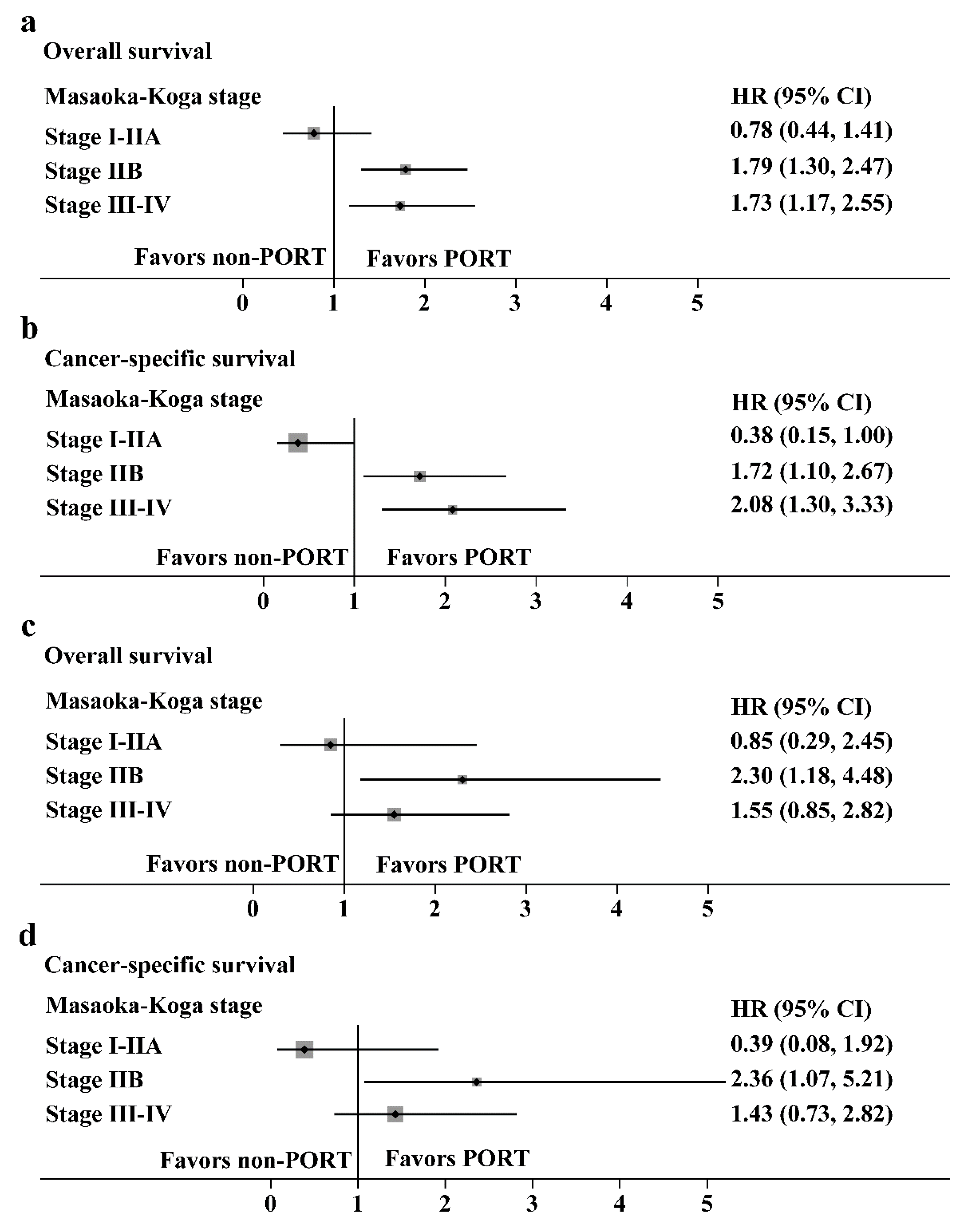
Hazard ratios with 95% CIs for the OS and CSS in the prespecified subgroups are shown in Figure 6 (thymoma, Figure 6a,b; thymic carcinoma, Figure 6c,d). For patients with Masaoka-Koga stage IIB and III/IV thymoma, the PORT was a favorable factor for the OS (HR: 1.79, 95% CI: 1.30–2.47 and HR: 1.73, 95% CI: 1.17–2.55, respectively) or CSS (HR: 1.72, 95% CI: 1.10–2.67 and HR: 2.08, 95% CI: 1.30–3.33, respectively). However, for patients with stage I/IIA thymoma, PORT had no benefit on the OS (HR: 0.78, 95% CI: 0.44–1.41) and was an unfavorable factor for CSS (HR: 0.38, 95% CI: 0.15–1.00). For patients with Masaoka-Koga stage IIB thymic carcinoma, PORT was a favorable factor for the OS (HR: 2.30, 95% CI: 1.18–4.48) or CSS (HR: 2.36, 95% CI: 1.07–5.21). PORT had no benefit on the OS and CSS in patients with stage I-IIA and III-IV thymic carcinoma.
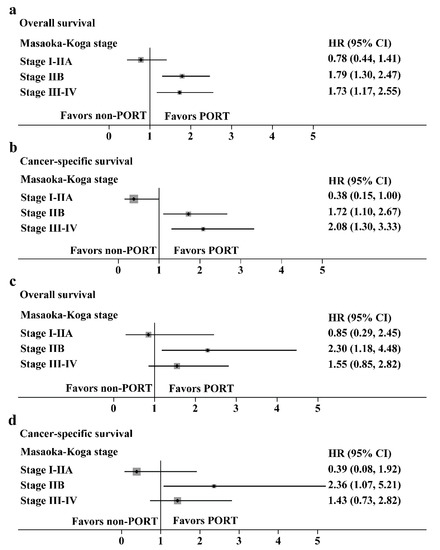
Figure 6.
Subgroup analysis with the Cox regression model. (a,b) Hazard ratios with 95% CI for the overall survival and cancer-specific survival in thymoma stratified by the Masaoka-Koga stage. (c,d) Hazard ratios with 95% CI for the overall survival and cancer-specific survival in thymic carcinoma stratified by the Masaoka-Koga stage.
4. Discussion
Although surgery has been recognized as the cornerstone of treatment for patients with resectable thymic epithelial tumors, the role of PORT remains controversial [12,20]. In this population-based study, we analyzed the survival outcomes of thymoma and thymic carcinoma patients over the last decade using data from the SEER database. We found that PORT was an independent prognostic factor for both OS and CSS in patients with thymoma and an independent prognostic factor for OS in patients with thymic carcinoma after propensity score matching. Patients with thymoma and thymic carcinoma who received PORT had better OS before and after matching. In subgroup analysis, PORT improved both OS and CSS in patients with Masaoka-Koga stage IIB–IV thymoma and Masaoka-Koga stage IIB thymic carcinoma.
For stage I thymoma, several studies have demonstrated excellent local control rates with surgery. Forquer et al. [21] divided patients into localized (stage I) and regional (stage II-III) groups, and results showed that PORT had no advantage in patients with stage I thymoma and thymic carcinoma (5-year CSS rate: 91% vs. 98%, p = 0.03). A randomized trial of 29 patients with stage I thymoma by Zhang et al. [22] showed no difference in outcomes between PORT and surgery alone. Whereas a study based on the International Thymic Malignancy Interest Group (ITMIG) and the European Society of Thoracic Surgeons (ESTS) found that PORT improved the OS and RFS in patients with stage I thymic carcinoma [6], some studies showed no benefit from PORT in this setting [23,24]. Our results showed that both the OS and CSS were shorter in the PORT group for patients with stage I/IIA thymoma or thymic carcinoma. Due to a lack of information in the SEER database, stage I and IIA could not be distinguished, so it was impossible to conclude whether stage IIA patients would benefit from PORT. Nonetheless, we noticed that the results seemed to support the view that complete resection was sufficient for stage I thymoma and thymic carcinoma patients.
The use of PORT is most controversial in stage II thymoma and thymic carcinoma. According to the ESMO guidelines [15], PORT is not recommended after complete resection of stage II thymoma but can be considered in the setting of aggressive histology (type B2, B3) or extensive transcapsular invasion (stage IIB). For stage II thymic carcinoma, the ESMO guidelines indicate that PORT should be considered. A study based on the National Cancer Data Base (NCDB) by Jackson et al. [24] reported that PORT improves OS in patients with stage IIB thymoma (HR = 0.61, p = 0.035). Still, no difference was observed in patients with stage I-IIA thymoma or thymic carcinoma. A recent meta-analysis including 4746 patients revealed that PORT was associated with a significantly better OS for patients with stage II (HR = 0.63, 95% CI: 0.44–0.91, p = 0.01) and stage III (HR = 0.72, 95% CI: 0.55–0.95, p = 0.02) thymoma [25]. This study is the first to demonstrate the prognostic value of PORT in stage IIB thymic carcinoma. We found that patients with stage IIB thymic carcinoma undergoing PORT had better OS (71.5% vs. 61.0%, p = 0.012) and CSS (79.9% vs. 69.6%, p = 0.029) than surgery alone.
It is now generally accepted that PORT is of great value in improving the prognosis of patients with stage III-IV thymoma and thymic carcinoma, with or without complete resection [6,7,15,26]. Our study also confirmed the positive effect of PORT on the OS and CSS in patients with stage III-IV thymoma. However, for patients with stage III-IV thymic carcinoma, there was no significant difference in survival between the PORT and non-PORT groups (OS: p = 0.149; CSS: p = 0.293).
In addition to PORT, we also found that age, gender, Masaoka-Koga stage, tumor size, and histological grade were independent prognostic factors for thymoma. Multi-institutional studies have confirmed the significant impact of the Masaoka-Koga stage on thymoma prognosis [7,27,28]. It is worth mentioning that female patients had a better prognosis than male patients (HR = 0.759, p = 0.015), contrary to the previous SEER-based study of thymic neuroendocrine tumors (TNETs) [29]. One explanation may be that the proportion of male patients with Masaoka stage IIB or III-IV was more in this study. In addition, many studies reported a slight predominance of women with type A, AB, and B1, which might be one of the reasons [15]. Our study also found that the extent of resection was an independent prognostic factor for patients with thymic carcinoma. Many studies have confirmed that complete resection is an important prognostic factor for resectable thymic carcinoma [30,31].
Previously, several SEER-based studies investigated the prognostic value of PORT in patients with thymoma or thymic carcinoma [19,32,33,34]. However, we differed from previous studies in that (1) propensity matching was performed to minimize selection bias, (2) the latest data from 2010 to 2019 were included, (3) subgroup analysis was performed stratified by the Masaoka-Koga stage, and (4) simultaneous study of thymoma and thymic carcinoma. This large, up-to-date population-based longitudinal study may provide guidance on the use of PORT for thymoma or thymic carcinoma.
This study had several limitations. First, there is a lack of information about Masaoka-Koga stages and radiotherapy regimens in the SEER database. Second, selection bias cannot be completely avoided in this retrospective study. Third, due to the ethnic diversity of the SEER database, our results may not apply to regions with high levels of homogeneity.
5. Conclusions
In conclusion, this propensity-matched analysis identified the prognostic value of PORT in thymoma and thymic carcinoma based on the SEER database (2010–2019). For patients with Masaoka-Koga stage IIB-IV thymoma and stage IIB thymic carcinoma, PORT was associated with improved OS and CSS. Further randomized controlled trials are needed to determine the efficacy and indications of PORT for thymoma and thymic carcinoma. A more positive attitude towards the use of PORT for nonlocalized thymoma and thymic carcinoma may be appropriate.
Author Contributions
Conceptualization, C.Z. and Q.W.; methodology, C.Z., Z.Z. and X.L.; software, C.Z. and C.L.; validation, C.Z., L.H., B.Q., Y.S. and Y.Q.; formal analysis, F.X. and Z.C.; investigation, X.L. and C.Z.; resources, C.Z. and X.L.; data curation, C.Z. and X.L.; writing—original draft preparation, C.Z.; writing—review and editing, C.Z., X.L., Y.S., Z.Z., C.L., B.Q. and F.X.; visualization, C.Z. and X.L.; supervision, C.Z., Y.S. and L.H.; project administration, X.L., Y.S. and L.H.; funding acquisition, Y.S. and L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [81702444] and the Natural Science Foundation of Jiangsu Province [BK20181239].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in this study can be obtained from the public SEER database (www.seer.cancer.gov (accessed on 16 August 2022)). Permission to access the research data file in the SEER registry was received from the NCI, USA (reference No. 16521-Nov 2021).
Acknowledgments
The authors would like to acknowledge the support of all colleagues in the department of Cardiothoracic Surgery at Jingling Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Engels, E.A. Epidemiology of thymoma and associated malignancies. J. Thorac. Oncol. 2010, 5, S260–S265. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Yoshizawa, K.; Tsuyuguchi, M.; Kimura, S.; Sumitomo, M.; Morita, J.; Miyoshi, T.; Sakiyama, S.; Mukai, K.; Monden, Y. WHO histologic classification is a prognostic indicator in thymoma. Ann. Thorac. Surg. 2004, 77, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Frih, H.; Pasquet, F.; Kerever, S.; Jamilloux, Y.; Tronc, F.; Guibert, B.; Isaac, S.; Devouassoux, M.; Chalabreysse, L.; et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun. Rev. 2016, 15, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.S.; Im, J.G.; Lee, B.H.; Kim, K.H.; Oh, Y.W.; Chin, S.Y.; Zo, J.I.; Jang, J.J. CT findings in malignant tumors of thymic epithelium. J. Comput. Assist. Tomogr. 1995, 19, 192–197. [Google Scholar] [CrossRef]
- Jung, K.J.; Lee, K.S.; Han, J.; Kim, J.; Kim, T.S.; Kim, E.A. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am. J. Roentgenol. 2001, 176, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.; Yao, X.; Detterbeck, F.; Huang, J.; Antonicelli, A.; Filosso, P.L.; Ruffini, E.; Travis, W.; Jones, D.R.; Zhan, Y.; et al. Thymic carcinoma outcomes and prognosis: Results of an international analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 95–101.e2. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Detterbeck, F.; Van Raemdonck, D.; Rocco, G.; Thomas, P.; Weder, W.; Brunelli, A.; Guerrera, F.; Keshavjee, S.; Altorki, N.; et al. Thymic carcinoma: A cohort study of patients from the European society of thoracic surgeons database. J. Thorac. Oncol. 2014, 9, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, A.; Monden, Y.; Nakahara, K.; Tanioka, T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981, 48, 2485–2492. [Google Scholar] [CrossRef]
- Koga, K.; Matsuno, Y.; Noguchi, M.; Mukai, K.; Asamura, H.; Goya, T.; Shimosato, Y. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol. Int. 1994, 44, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, A. Staging system of thymoma. J. Thorac. Oncol. 2010, 5, S304–S312. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, D.; Port, J.L.; Weksler, B.; Delgado, R.; Rosai, J.; Bains, M.S.; Ginsberg, R.J.; Martini, N.; McCormack, P.M.; Rusch, V.; et al. Thymoma: A multivariate analysis of factors predicting survival. Ann. Thorac. Surg. 1995, 60, 908–914. [Google Scholar] [CrossRef]
- Girard, N.; Mornex, F. The role of radiotherapy in the management of thymic tumors. Thorac. Surg. Clin. 2011, 21, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lombe, D.C.; Jeremic, B. A Review of the Place and Role of Radiotherapy in Thymoma. Clin. Lung Cancer 2015, 16, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.D.; Housman, D.M.; Thomas, C.R. Radiotherapy for thymoma and thymic carcinoma. Hematol. Oncol. Clin. N. Am. 2008, 22, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S.; Committee, E.G. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015, 26 (Suppl. 5), v40–v55. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Riely, G.J.; Akerley, W.; Borghaei, H.; Chang, A.C.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Govindan, R.; et al. Thymomas and thymic carcinomas: Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2013, 11, 562–576. [Google Scholar] [CrossRef]
- Fritz, A.G. International Classification of Diseases for Oncology: ICD-O, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000; Volume vii, 240p. [Google Scholar]
- Fernandes, A.T.; Shinohara, E.T.; Guo, M.; Mitra, N.; Wilson, L.D.; Rengan, R.; Metz, J.M. The role of radiation therapy in malignant thymoma: A Surveillance, Epidemiology, and End Results database analysis. J. Thorac. Oncol. 2010, 5, 1454–1460. [Google Scholar] [CrossRef]
- Mou, H.; Liao, Q.; Hou, X.; Chen, T.; Zhu, Y. Clinical characteristics, risk factors, and outcomes after adjuvant radiotherapy for patients with thymoma in the United States: Analysis of the Surveillance, Epidemiology, and End Results (SEER) Registry (1988-2013). Int. J. Radiat. Biol. 2018, 94, 495–502. [Google Scholar] [CrossRef]
- Maggi, G.; Casadio, C.; Cavallo, A.; Cianci, R.; Molinatti, M.; Ruffini, E. Thymoma: Results of 241 operated cases. Ann. Thorac. Surg. 1991, 51, 152–156. [Google Scholar] [CrossRef]
- Forquer, J.A.; Rong, N.; Fakiris, A.J.; Loehrer, P.J., Sr.; Johnstone, P.A. Postoperative radiotherapy after surgical resection of thymoma: Differing roles in localized and regional disease. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, N.; Wang, M.; Gu, X.; Zhang, D. Postoperative radiotherapy for stage I thymoma: A prospective randomized trial in 29 cases. Chin. Med. J. 1999, 112, 136–138. [Google Scholar] [PubMed]
- Sakai, M.; Onuki, T.; Inagaki, M.; Yamaoka, M.; Kitazawa, S.; Kobayashi, K.; Iguchi, K.; Kikuchi, S.; Goto, Y.; Onizuka, M.; et al. Early-stage thymic carcinoma: Is adjuvant therapy required? J. Thorac. Dis. 2013, 5, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.W.; Palma, D.A.; Camidge, D.R.; Jones, B.L.; Robin, T.P.; Sher, D.J.; Koshy, M.; Kavanagh, B.D.; Gaspar, L.E.; Rusthoven, C.G. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2017, 12, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, Y.; Horita, N.; Namkoong, H.; Enomoto, T.; Takeda, A.; Kaneko, T. Postoperative Radiotherapy for Completely Resected Masaoka/Masaoka-Koga Stage II/III Thymoma Improves Overall Survival: An Updated Meta-Analysis of 4746 Patients. J. Thorac. Oncol. 2021, 16, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.; Shende, M.; Nason, K.S.; Gallagher, A.; Ferson, P.F.; Pennathur, A. The role of adjuvant radiation therapy for resected stage III thymoma: A population-based study. Ann. Thorac. Surg. 2012, 93, 1822–1828, discussion 1828–1829. [Google Scholar] [CrossRef]
- Omasa, M.; Date, H.; Sozu, T.; Sato, T.; Nagai, K.; Yokoi, K.; Okamoto, T.; Ikeda, N.; Tanaka, F.; Maniwa, Y.; et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: The Japanese Association for Research on the Thymus Database Study. Cancer 2015, 121, 1008–1016. [Google Scholar] [CrossRef]
- Weksler, B.; Dhupar, R.; Parikh, V.; Nason, K.S.; Pennathur, A.; Ferson, P.F. Thymic carcinoma: A multivariate analysis of factors predictive of survival in 290 patients. Ann. Thorac. Surg. 2013, 95, 299–303. [Google Scholar] [CrossRef]
- Wen, J.; Chen, J.; Chen, D.; Liu, D.; Xu, X.; Huang, L.; Cao, J.; Zhang, J.; Gu, Y.; Fan, M.; et al. Evaluation of the prognostic value of surgery and postoperative radiotherapy for patients with thymic neuroendocrine tumors: A propensity-matched study based on the SEER database. Thorac. Cancer 2018, 9, 1603–1613. [Google Scholar] [CrossRef]
- Kondo, K.; Monden, Y. Therapy for thymic epithelial tumors: A clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003, 76, 878–884. [Google Scholar] [CrossRef]
- Bruni, A.; Stefani, A.; Perna, M.; Borghetti, P.; Giaj Levra, N.; D’Angelo, E.; D’Onofrio, A.; Rubino, L.; Frassinelli, L.; Salvestrini, V.; et al. The role of postoperative radiotherapy for thymomas: A multicentric retrospective evaluation from three Italian centers and review of the literature. J. Thorac. Dis. 2020, 12, 7518–7530. [Google Scholar] [CrossRef]
- Lim, Y.J.; Song, C.; Kim, J.S. Improved survival with postoperative radiotherapy in thymic carcinoma: A propensity-matched analysis of Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2017, 108, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Kong, Y.; Wu, Y.; Wu, Y.; Yu, L. Effect of Postoperative Radiotherapy in Thymoma Patients: A SEER-Based Study. Oncol. Res. Treat. 2021, 44, 28–35. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, H.J.; Wu, H.G. Role of Postoperative Radiotherapy in Nonlocalized Thymoma: Propensity-Matched Analysis of Surveillance, Epidemiology, and End Results Database. J. Thorac. Oncol. 2015, 10, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).