Advances in Preclinical/Clinical Glioblastoma Treatment: Can Nanoparticles Be of Help?
Abstract
:Simple Summary
Abstract
1. Glioblastoma Treatment: State-of-the-Art
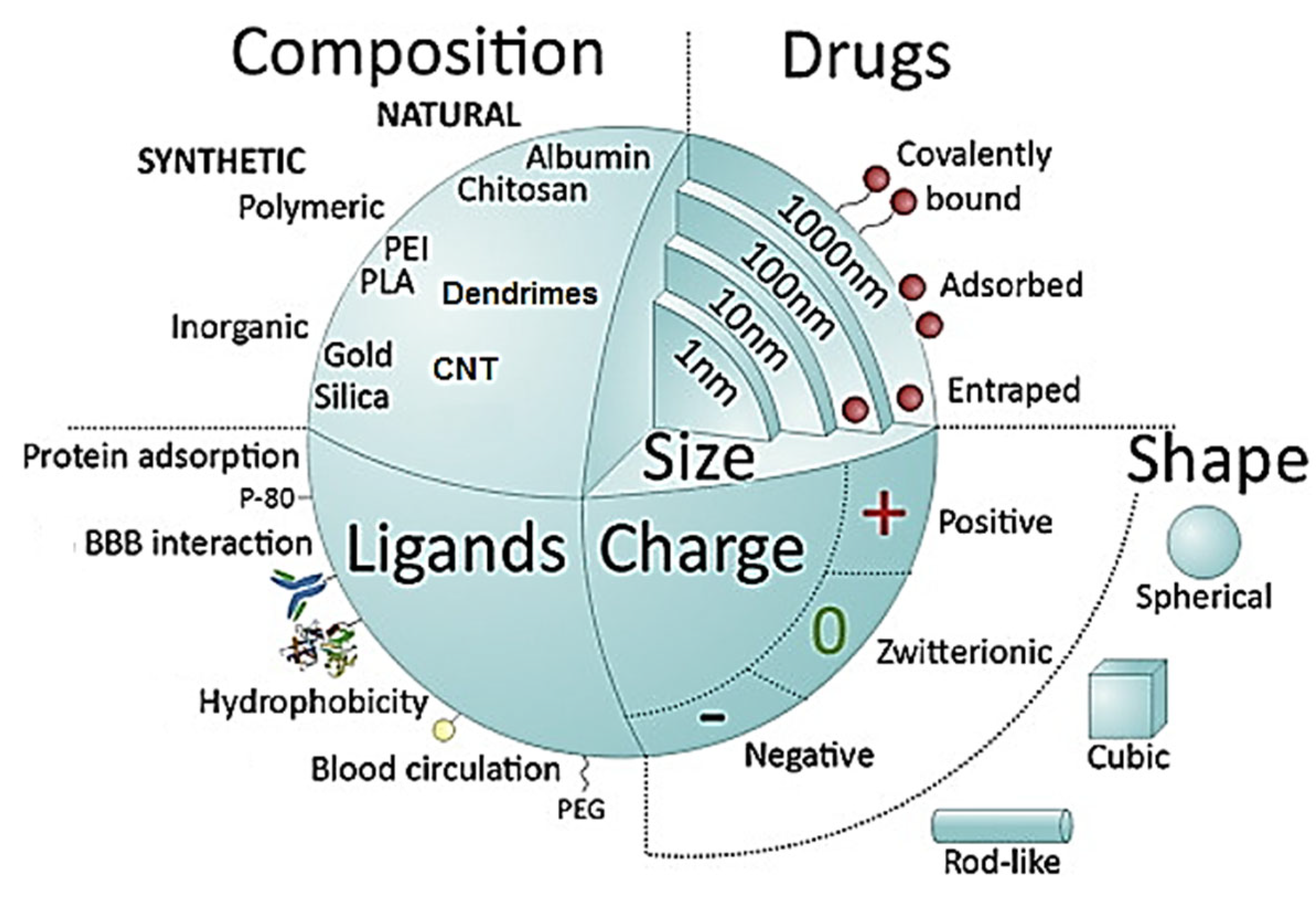
2. Nanoparticles for Glioblastoma
- They can incorporate additional fluorescent/MRI/radioactive compounds that allow the non-invasive monitoring of its biodistribution [58];
- They confer chemical protection to the drug and a theoretical control over the release upon activation with a stimulus that minimize undesired side effects [59];
- They can combine different additional therapeutic approaches, such as, not exclusively, radiotherapy sensitization, immune cells stimulation, or induction of heat/radical oxygen species (ROS) [65];
- They can benefit from the well-known enhanced permeability and retention effect (EPR) to access (and remain on) tumor tissues;
- Nanocapsulation increases the half-life activity, for instance in the case of TMZ-loaded chitosan NPs from 1.8 to 13.4 h [66].
3. Preclinical Studies
- Orthotopic GB studies. Glial tumors are characterized by their heterogeneity and their immunosuppressive tumor microenvironment, which can be hardly replicated in a heterotopic model (e.g., subcutaneous). Such “niches”, comprising all components of a tumor as well as its interaction with tumor microenvironment, must be considered as it might play a role in the therapeutic efficacy [97]. Thus, useful translational studies of relevance in subsequent clinical cases should replicate as faithfully as possible the human situation.
- Animal and gender model. Regarding species, circa two-thirds of the studies were performed in mice, while the remaining ones have used rats as experimental subjects. With respect to gender of the preclinical subjects, it is worth mentioning that it was not detailed in almost one-third of the studies, and only one of the mentioned papers included representation of both sexes. As for the rest, males are slightly more represented than females, but it is still quite balanced. Overall, it was reported that glioblastoma growth and aggressiveness may vary between males and females [98], so a lack of this information in part of the published studies can lead to a biased information [99].
- Administration schedule. Administration schedule and methodology used is quite different along the studies shown in Table 1. Regarding the therapy starting point, circa one-third of the studies have started therapy ranging 1–6 days post cell inoculation. The remaining studies are distributed equally around days 7–10 post cell inoculation or later time points. The administration schedule was probably the most variable, both from the interval point of view and the final number of administrations (in general, intravenous). It was already shown that the administration protocol may strongly influence outcomes [100,101]. Thus, discrepancy in this factor may help to explain the differences in the results obtained.
- Immune system. Undesirable interactions between the immune system and nanoparticles can take place, due to either immunostimulation or immunosuppression [102], removing at least part of the administered nanoparticles before their delivery to the target area. Thus, selection of immunocompetent (i.e., mice/rats bearing gliomas originating from their same species) versus immune-deprived (i.e., PDX models or xenograft inoculated with cell lines from human origin) models represents an important step. Moreover, specific pathogen free (SPF) husbandry is a common practice applied in laboratories conducting preclinical experiments, and SPF mice have an immature immune system when compared with wild strains [103]. Therefore, the immune system of SPF mice does not adequately reflect that of clinical subjects, neither do the results [104]. Immunocompetent, syngeneic/isogeneic murine models may be of help, but even in this case, certain cell targets or metabolic pathways may be different from human counterparts, since they will bear murine tumors. The most advisable model would be humanized PDX, although those are more complex and challenging than the already-established murine models [105,106]
- Animal age. This is a relevant factor that governs baseline immunity and affects the hormone levels (sexual maturity) of the individuals; both impact the disease evolution. However, in most cases indicated in Table 1, the age of individuals is not given, so it was inferred from the standard growth charts. Having this limitation in mind, circa 50% were within 4–6 weeks of age, and the remaining ones were around 7–10 weeks of age, with one study going above (14–18 weeks). These values would rather correspond to early adolescence [107], while glioblastoma incidence increases with age, with circa 50% of the cases diagnosed in patients equal or more than 65 years old [108].
- Tumor volume. This is an essential parameter, since preclinical tumors are usually variable even when experiments are performed by experienced researchers. This value was reported in circa 60% of the studies included in this review, while it is unclear whether it was not performed or not reported in the remaining studies. Additionally, it is definitely not the same for any therapeutic agent to fight a large established or an early, low-volume, starting tumor [109]. Moreover, based on our experience, we should not assume that the whole cohort will have tumors with comparable volumes.
- Therapeutic efficacy. Some articles report euthanization of mice and postmortem evaluation of tumors, which may definitely inform about the immediate action of the agent, but ideal situations may imply non-invasive assessment of tumor disappearance or growth arrest at long-term follow-up procedures.
| Drug(s) | Type of NPs | Animal Model | Age | Immunocompetent/Immunodeprived | Dose and Administration Route | Administration Schedule | Therapy Starting Point | Tumor Volume/Presence Estimated at Starting Point | Targeting | Evaluation of Antitumor Effect/Site Arrival | Refs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TMZ | Liposomes | U87-TL-bearing BALB/c male nude mice | 4–6 wk | immuno-deprived | 5–10 mg/kg intravenous | Every 3 days, 5 times in total | Day 1 p.i. | yes | Angiopep-2 + anti-CD133 mAb | In vivo bioluminiscence | [110] |
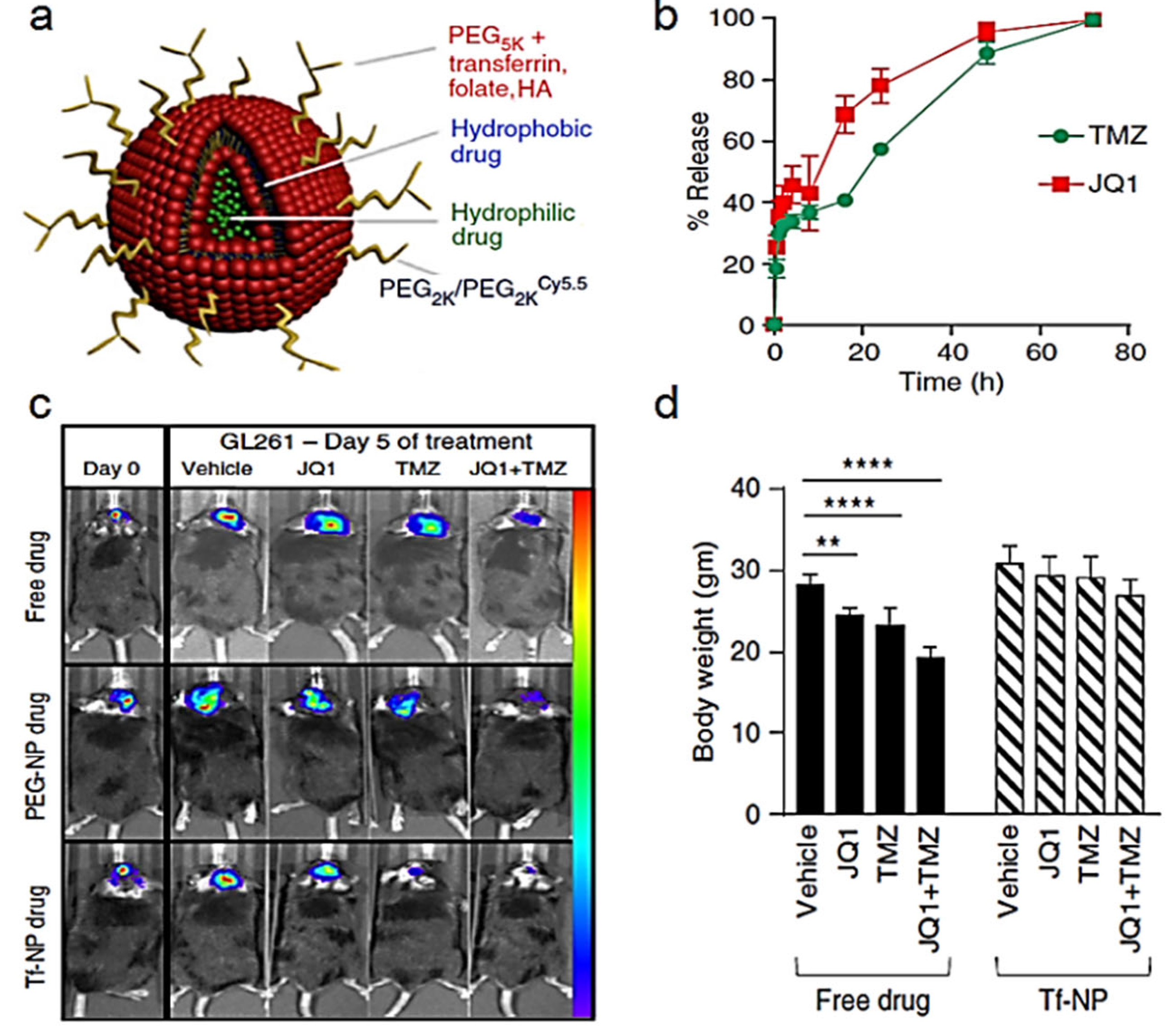
| TMZ + JQ1 | Liposomes | U87-bearing NCR nude mice/GL261-bearing C57/BL6 male mice | 6 wk | Both | 100 µL intravenous | Every day during 5 days | Day 14 p.i. | yes | Transferrin | In vivo bioluminiscence | [111] |
| TMZ + ART | Liposomes | TMZ-resistant U251-TR GB nude mice | 5–6 wk | immune-competent | 5–10 mg/kg intravenous | Every 3 days, 5 times in total | Day 8 p.i. | no | ApoE peptide | In vivo bioluminiscence | [112] |
| TMZ | Albumin NP | C6-bearing BALB/c and KM mice | 5–6 wk | Both | 10 mg/kg intravenous | Every 2 days, 8 times in total | Day 5 p.i. | no | Sinapic acid | Histopathology at endpoint | [113] |
| TMZ | Lactoferrin NP | GL261-bearing C57/BL6 mice | 5 to 10 wk * | immune-competent | 5 mg/kg intravenous | Every 2 days, 4 times in total | Day 3 p.i. | no | Lactoferrin | Histopathology at endpoint | [114] |
| TMZ + siTGF | β Polymer-lipid hybrid NP | GL261-bearing C57/BL6 male mice | n.d. | immune-competent | 10 mg/kg intravenous | Every 2 days, 3 times in total | Day 8 p.i. | no | Angiopep-2 | T2w MRI/ Prussian staining | [115] |
| TMZ + OTX015 | erythrocytemembrane camouflaged nanoparticle | C57BL/6 mice bearing orthotopic GL261-Luc tumor | 3–4 wk | Immune-competent | 5 mg/kg intravenous | Every 2 days 5 times in total | Day 20 p.i. | no | ApoE peptide | In vivo and ex vivo fluorescence | [116] |
| Carmustine + O6-Benzylguanine | Polymeric NP | F98-bearing Fischer 344 male rats | 7 wk * | immune-competent | 6.43–19.29 mg/kg intravenous | Every 4 days, 3 times in total | Day 5 p.i. | no | ----------- | T1 and T2w MRI/histopathology at endpoint | [117] |
| Carmustine + O6-Benzylguanine | Polymeric NP | F98-bearing ICR male mice and F98-bearing male nude mice | 5–6 wk * | Both | 6.43–19.29 mg/kg intravenous | Every 4 days, 3 times in total | Day 5 p.i. | no | iRGD | Overall survival/In vivo fluorescence | [17] |
| Carmustine | Magnetic NP | C6-bearing Sprague-Dawley male rats | 14–18 wk | immune-competent | 0.5–13 mg/kg intravenous, via jugularvein | Single dose | Day 17 p.i. | yes | Magnetic targeting + transient ultrasound-mediated BBB disruption | T1 and T2 * w MRI/Histology | [118] |
| Carmustine | Micelles | U87-bearing BALB/c male nude mice | 5–6 wk * | immune-deprived | 1 mg/kg intravenous | Single dose | Day 14 p.i. | yes | T7 peptide | In vivo fluorescence/postmortem brain fluorescence | [119] |
| Carmustine | Micelles | BT325-bearing BALB/c nude mice | n.d. | immune-deprived | 2 mg/kg intravenous | Every 3 days, 5 times in total | Day 14 p.i. | yes | Pep-1 + borneol | In vivo bioluminiscence | [120] |
| Lomustine | Nanocapsules | U87-bearing female CD-1 nude mice | 5–6 wk * | Immuno-deprived | 1.2–13 mg/kg intravenous | 10 consecutive days | Day 7 p.i. | yes | ------- | T2w MRI | [121] |
| Cisplatin, Oxaliplatin | Liposomes | F98-bearing male Fischer rats | n.d. | Immune-competent | 3–5 mg (calculated to body surface area), intracarotid | Single dose | Day 10 p.i. | no | ------- | Overall survival/ICP-MS | [122] |
| Cisplatin | PMAA-PEG Nanogel | 101/8-bearing female Wistar rats | 9–10 wk * | Immune-competent | 5 mg/kg, intravenous (femoral) | Every 5 days, 3 times in total | Day 5 p.i. | yes | mAb anti-Cx43 + mAb anti-BSAT1 | T2w MRI | [123] |
| Cisplatin | PAA-PEG NP | F98-bearing female Fischer344 rats/9L-bearing Sprague-Dawleyrats | 8–9 wk * | Immune-competent | 2–5 mg/kg, intravenous | Every 7 days, 3 times in total | Day 14 p.i. | yes | - - -- | T1w MRI | [124] |
| Cilengitide | Gelatin-heparin NP | C6-bearing Sprague Dawley male rats | 8–10 wk * | Immune-competent | 2 mg/kg, intravenous | Every 2–3 days, 8 times in total | Day 7 p.i. | yes | Transient ultrasound-mediated BBB disruption | T1 and T2w MRI | [27] |
| Cilengitide | Liposomes | C6-bearing Sprague Dawley male rats | 8–10 wk * | Immune-competent | 2 mg/kg, intravenous | Twice a week, 8 times in total | Day 7 p.i. | yes | Magnetic targeting + transient ultrasound-mediated BBB disruption | T2w MRI and fluorescence imaging/Histology | [125] |
| Erlotinib + DOX | Liposomes | U87-bearing nude female and male mice | n.d. | Immune-deprived | 15.2 µmoles/kg, intravenous | Every 2 days, 3 times in total | Day 10 p.i. | no | Transferrin + Penetratin | Overall survival/Histopathology | [29] |
| Lapatinib | Albumin NP | U87-bearing BALB/C mice | 4–6 wk | Immune-deprived | 10–100 mg/kg, intravenous | 2–4 times a week, for 2 weeks | Day 8 p.i. | no | - - - | Histopathology | [30] |
| Nimotuzumab | Methacrylamide NP | U87-EGFRwt-bearing female mice | 5 wk | Immune-deprived | 5 mg/kg, intravenous | Every other day, 9 times in total | Day 3 p.i. | yes | Choline analogues | In vivo bioluminiscence | [126] |
| Regorafenib +Disulfiram/cooper | Albumin NP | U87-bearing nude mice GL261-bearing C57/BL6 mice | 4–6 wk | Both | 1.5 mg/kg, intravenous | Not specified, 5 times total | Day 10 p.i. | yes | Peptide T12 + mannose | In vivo bioluminiscence | [127] |
| Cediranib +Paclitaxel | PEG-bilirrubin NP | C6-bearing male Balb/c mice | n.d. | Immune-deprived | 1.7–3.6 mg/kg, intravenous | Every 2 days, 6 times in total | Day 10 p.i. | no | D-T7 peptide | Histopathology | [128] |
| Camptothecin | Polymeric NP | U87-bearing athymic nude mice | 8 wk | Immune-deprived | 4 or 10 mg/kg, intravenous | Every 3 days or every 5 days, 3 times in total | Day 3 or day 5 p.i. | no | Adenosine | Overall survival | [129] |
| Camptothecin | Polymeric NP | GL261-bearing C57 albino mice | 10 wk | Immuno-competent | 10–20 mg/kg intravenous, | Every 7 days, 3 times in total | Day 8 p.i. | yes | - - - | In vivo bioluminiscence | [130] |
| Topotecan | Liposomes | U87, GBM43, or GBM6-bearingFemale athymic mice | 6 wk | Immune-deprived | 1 mg/kg, intravenous | Twice a week, up to 6 times in total | Day 6–8 pi.i | yes | - - - | In vivo bioluminiscence | [131] |
| Irinotecan | Liposomes | U251-bearing Rag2 female mice | 7–10 wk | “non-leaky” immune-deprived | 25–100 mg/kg | Every 7 to 14 days | Day 21 p.i. | no | - - - | Overall survival, histopathology | [132] |
| Irinotecan + TMZ | Liposomes | U251-bearing NOD.CB17-SCIDfemale mice | 7–10 wk | Immuno-deprived | 25–50 mg/kg, intravenous | Every 7 days, 3 times in total | Day 14 p.i. | yes | - - - | In vivo fluorescence, overall survival, histopathology | [133] |
| Irinotecan | Liposomes | U87-bearing male nude rats | 7–9 wk * | Immune-deprived | 50 mg/kg | Twice a week, 4 times in total | Day 5 p.i. | no | - - - | Overall survival, histopathology | [134] |
| Irinotecan | Liposomes | GS2-bearing male athymic rats | 6 wk | Immune-deprived | 3.5 mg, intranasal 0.01 to 1 mg, CED 30 mg/kg, intravenous | Every 7 days, 3 times in total | Unclear15–30 p.i. | yes | - - - | In vivo bioluminiscence | [135] |
| Irinotecan + Cetuximab | Liposomes | U87-bearing Balb/c nude mice | 6–8 wk | Immune-deprived | 30 mg/kg, intravenous | Every 3 days, 3 times in total | Day 11 p.i. | yes | Cetuximab + Magnetic targeting | In vivo bioluminiscence | [136] |
| DOX | Liposomes | U87-bearing male Balb/c nude mice | n.d. | Immune-deprived | 2 mg/kg, intravenous | Every 3 days, 5 times in total | Day 6 or day 15 p.i. | no | MC + DA7R | Ex vivo (postmortem) bioluminiscence | [137] |
| DOX | Liposomes | U87-bearing nude mice | n.d. | Immune-deprived | 100 μL with a concentration of 0.01 μM (total dose 10 mg/kg), intravenous | Every 3 days, 5 times in total | Day 10 p.i. | no | CB5005 peptide | Overall survival, Ex vivo (postmortem) bioluminiscence | [138] |
| DOX + Curcumin | pH-sensitive coreshell NP | C6-bearing male Sprague-DawleyRats | 8–10 wk * | Immune-competent | 0.33–1 mg/kg, intravenous | Unclear schedule | Day 7 p.i. | yes | ---- | T1w MRI | [139] |
| DOX +1-MT i | MSNs | GL261luc-bearing C57BL/6 female mice | 6 wk | Immune-competent | 2.5 mg/kg | Every 3 days, 5 times in total | Day 5 p.i. | yes | iRGD | In vivo bioluminiscence, MRI | [140] |
| DOX + HCQ | Legumain responsive gold NP | C6-bearing mice | n.d. | unclear | 2.5–15 mg/kg | Every 2 days, 5 times in total | Day 10 p.i. | No | ---- | Overall survival | [141] |
3.1. Representative Examples
3.2. Targeting and Theranostic
4. Clinical Studies
5. Summary and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stavrovskaya, A.A.; Shushanov, S.S.; Rybalkina, E.Y. Problems of Glioblastoma Multiforme Drug Resistance. Biochemistry 2016, 81, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Cbtrus Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef] [Green Version]
- Han, S.J.; Englot, D.J.; Birk, H.; Molinaro, A.M.; Chang, S.M.; Clarke, J.L.; Prados, M.D.; Taylor, J.W.; Berger, M.S.; Butowski, N.A. Impact of Timing of Concurrent Chemoradiation for Newly Diagnosed Glioblastoma: A Critical Review of Current Evidence. Neurosurgery 2015, 62, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Dietrich, P.-Y.; Kraljevic, S.O.; Pica, A.; Maillard, I.; Maeder, P.; Meuli, R.; Janzer, R.; Pizzolato, G.; Miralbell, R.; et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J. Clin. Oncol. 2002, 20, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cai, S.; Ernstberger, A.; Bailey, B.J.; Wang, M.Z.; Cai, W.; Goebel, W.S.; Czader, M.B.; Crean, C.; Suvannasankha, A.; et al. Temozolomide-Mediated DNA Methylation in Human Myeloid Precursor Cells: Differential Involvement of Intrinsic and Extrinsic Apoptotic Pathways. Clin. Cancer Res. 2013, 19, 2699–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, H.; Baisch, T.; Fitzel, R.; Schilberg, K.; Siegelin, M.D.; Karpel-Massler, G.; Debatin, K.M.; Westhoff, M.A. Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-G.; Kim, C.-H.; Park, J.-S.; Park, S.-D.; Kim, C.-K.; Chung, D.-S.; Hong, Y.-K. Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma mode. Clin. Vaccine Immunol. 2010, 17, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, V.A.; Patel, S.P.; Hwu, W.-J. The safety of temozolomide in the treatment of malignancies. Expert Opin. Drug Saf. 2009, 8, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, S. Temozolomide/PLGA microparticles and antitumor activity against Glioma C6 cancer cells in vitro. Int. J. Pharm. 2007, 329, 122–128. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide Vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, K.J.; Wagner, M.; You, S.J.; Franz, K.; Harter, P.N.; Burger, M.C.; Voss, M.; Ronellenfitsch, M.W.; Fokas, E.; Steinbach, J.P.; et al. Bevacizumab as a Last-Line Treatment for Glioblastoma Following Failure of Radiotherapy, Temozolomide and Lomustine. Oncol. Lett. 2017, 14, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase Ii Trial of Single-Agent Bevacizumab Followed by Bevacizumab Plus Irinotecan at Tumor Progression in Recurrent Glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef]
- Carrillo, J.A.; Munoz, C.A. Alternative Chemotherapeutic Agents: Nitrosoureas, Cisplatin, Irinotecan. Neurosurg. Clin. N. Am. 2012, 23, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Sen, Y.; Li, X.Q.; Feng, W.; Jiang, Y.Y. iRGD-mediated core-shell nanoparticles loading carmustine and O-6-benzylguanine for glioma therapy. J. Drug Target. 2017, 25, 235–246. [Google Scholar]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA Drug Approval Summary: Bevacizumab (Avastin®) as Treatment of Recurrent Glioblastoma Multiforme. Oncol. 2009, 14, 1131–1138. [Google Scholar] [CrossRef]
- Carter, T.C.; Medina-Flores, R.; Lawler, B.E. Glioblastoma Treatment with Temozolomide and Bevacizumab, Overall Survival in a Rural Tertiary Healthcare Practice. BioMed Res. Int. 2018, 2018, 6204676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahm, C.G.; van Linde, M.E.; Enting, R.H.; Schuur, M.; Otten, R.H.; Heymans, M.W.; Verheul, H.M.; Walenkamp, A.M. The Current Status of Immune Checkpoint Inhibitors in Neuro-Oncology: A Systematic Review. Cancers 2020, 12, 586. [Google Scholar] [CrossRef] [Green Version]
- Roberts, N.B.; Wadajkar, A.S.; Winkles, J.A.; Davila, E.; Kim, A.J.; Woodworth, G.F. Repurposing platinum-based chemotherapies for multi-modal treatment of glioblastoma. OncoImmunology 2016, 5, e1208876. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J. Neuro-Oncol. 2013, 115, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Eiseman, J.L.; Beumer, J.H.; Rigatti, L.H.; Strychor, S.; Meyers, K.; Dienel, S.; Horn, C.C. Plasma pharmacokinetics and tissue and brain distribution of cisplatin in musk shrews. Cancer Chemother. Pharmacol. 2015, 75, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckner, J.C.; Ballman, K.V.; Michalak, J.C.; Burton, G.V.; Cascino, T.L.; Schomberg, P.J.; Hawkins, R.B.; Scheithauer, B.W.; Sandler, H.M.; Marks, R.S.; et al. Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93-72-52 and Southwest Oncology Group 9503 Trials. J. Clin. Oncol. 2006, 24, 3871–3879. [Google Scholar]
- Zhao, Y.Z.; Lin, Q.; Wong, H.L.; Shen, X.T.; Yang, W.; Xu, H.L.; Mao, K.L.; Tian, F.R.; Yang, J.J.; Xu, J.; et al. Glioma-targeted therapy using Cilengitide nanoparticles combined with UTMD enhanced delivery. J. Control. Release 2016, 224, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Fink, K.L.; Mikkelsen, T.; Cloughesy, T.F.; O'Neill, A.; Plotkin, S.; Glantz, M.; Ravin, P.; Raizer, J.J.; Rich, K.M.; et al. Randomized Phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J. Clin. Oncol. 2008, 26, 5610–5617. [Google Scholar] [CrossRef]
- Gao, H.L.; Wang, Y.C.; Chen, C.; Chen, J.; Wei, Y.; Cao, S.L.; Jiang, X.G. Incorporation of lapatinib into core-shell nanoparticles improves both the solubility and anti-glioma effects of the drug. Int. J. Pharm. 2014, 461, 478–488. [Google Scholar] [CrossRef]
- Lakkadwala, S.; dos Santos Rodrigues, B.; Sun, C.; Singh, J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release 2019, 307, 247–260. [Google Scholar] [CrossRef]
- Hasselbalch, B.; Lassen, U.; Hansen, S.; Holmberg, M.; Sorensen, M.; Kosteljanetz, M.; Broholm, H.; Stockhausen, M.T.; Poulsen, H.S. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro-Oncol. 2010, 12, 508–516. [Google Scholar] [PubMed] [Green Version]
- Westphal, M.; Heese, O.; Steinbach, J.P.; Schnell, O.; Schackert, G.; Mehdorn, M.; Schulz, D.; Simon, M.; Schlegel, U.; Senft, C.; et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur. J. Cancer 2015, 51, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein, J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.I.; Burks, S.R.; Frank, J.A. Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics 2018, 8, 2245–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.M.; Patel, B.M. Crossing the blood-brain barrier: Recent advances in drug delivery to the brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Sharabi, S.; Bresler, Y.; Ravid, O.; Shemesh, C.; Atrakchi, D.; Schnaider-Beeri, M.; Gosselet, F.; Dehouck, L.; Last, D.; Guez, D.; et al. Transient blood–brain barrier disruption is induced by low pulsed electrical fields in vitro: An analysis of permeability and trans-endothelial electric resistivity. Drug Deliv. 2019, 26, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Sheleg, S.; Korotkevich, E.; Zhavrid, E.; Muravskaya, G.; Smeyanovich, A.; Shanko, Y.; Yurkshtovich, T.; Bychkovsky, P.; Belyaev, S. Local chemotherapy with cisplatin-depot for glioblastoma multiforme. J. Neuro-Oncol. 2002, 60, 53–59. [Google Scholar] [CrossRef]
- Minghan, S.; Sanche, L. Convection-Enhanced Delivery in Malignant Gliomas: A Review of Toxicity and Efficacy. J. Oncol. 2019, 2019, 9342796. [Google Scholar]
- Vogelbaum, M.A.; Aghi, M.K. Convection-enhanced delivery for the treatment of glioblastoma. Neuro-Oncol. 2015, 17, ii3–ii8. [Google Scholar] [CrossRef] [Green Version]
- Juratli, T.A.; Schackert, G.; Krex, D. Current Status of Local Therapy in Malignant Gliomas--a Clinical Review of Three Selected Approaches. Pharmacol. Ther. 2013, 139, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Graham-Gurysh, E.; Moore, K.M.; Satterlee, A.B.; Sheets, K.T.; Lin, F.C.; Bachelder, E.M.; Miller, C.R.; Hingtgen, S.D.; Ainslie, K.M. Sustained Delivery of Doxorubicin Via Acetalated Dextran Scaffold Prevents Glioblastoma Recurrence after Surgical Resection. Mol. Pharm. 2018, 15, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Tabet, A.; Jensen, M.P.; Parkins, C.C.; Patil, P.G.; Watts, C.; Scherman, O.A. Designing Next-Generation Local Drug Delivery Vehicles for Glioblastoma Adjuvant Chemotherapy: Lessons from the Clinic. Adv. Healthc. Mater. 2019, 8, e1801391. [Google Scholar] [CrossRef] [PubMed]
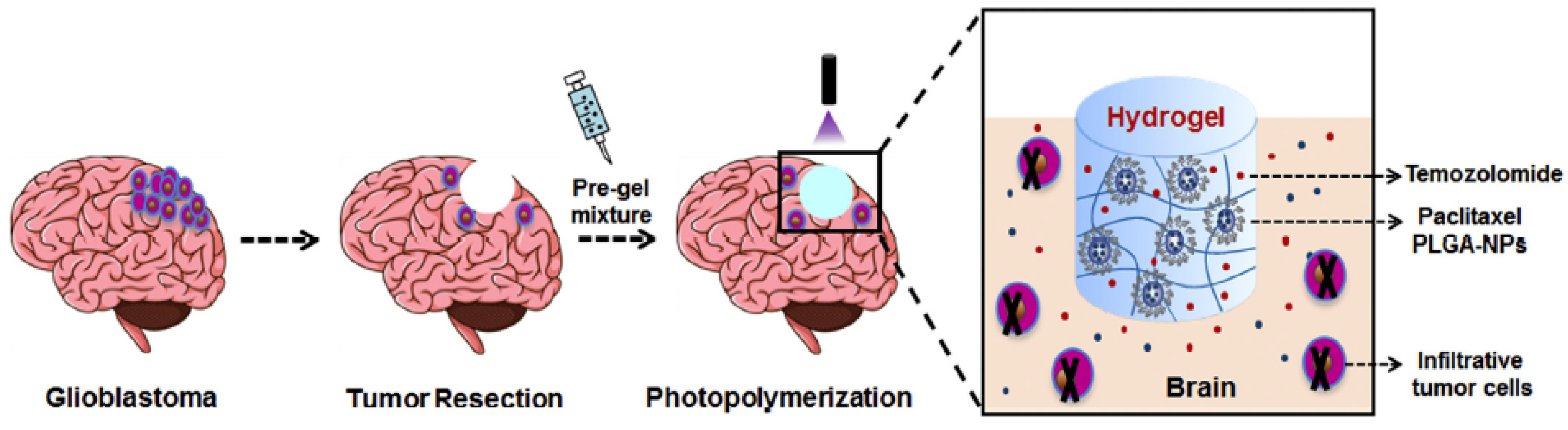
- Bastiancich, C.; Bianco, J.; Vanvarenberg, K.; Ucakar, B.; Joudiou, N.; Gallez, B.; Bastiat, G.; Lagarce, F.; Préat, V.; Danhier, F. Injectable Nanomedicine Hydrogel for Local Chemotherapy of Glioblastoma after Surgical Resection. J. Control. Release 2017, 264, 45–54. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Yao, Q.; Zhang, Y.; Zhu, X.; Xia, T.; Wang, J.; Li, G.; Li, X.; Ni, S. Local in Vitro Delivery of Rapamycin from Electrospun Peo/Pdlla Nanofibers for Glioblastoma Treatment. Biomed. Pharmacother. 2016, 83, 1345–1352. [Google Scholar]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Préat, V. Codelivery of Paclitaxel and Temozolomide through a Photopolymerizable Hydrogel Prevents Glioblastoma Recurrence after Surgical Resection. J. Control. Release 2019, 309, 72–81. [Google Scholar]
- Pallud, J.; Audureau, E.; Noel, G.; Corns, R.; Lechapt-Zalcman, E.; Duntze, J.; Pavlov, V.; Guyotat, J.; Hieu, P.D.; Le, R.; et al. Long-Term Results of Carmustine Wafer Implantation for Newly Diagnosed Glioblastomas: A Controlled Propensity-Matched Analysis of a French Multicenter Cohort. Neuro-Oncol. 2015, 17, 1609–1619. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, S.A.; Ryken, T.; Newton, H.B. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: A meta-analysis. J. Neuro-Oncol. 2015, 122, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Michael, J.S.; Lee, B.-S.; Zhang, M.; Yu, J.S. Nanotechnology for treatment of glioblastoma multiforme. J. Transl. Intern. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar]
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug Delivery Challenges and Future of Chemotherapeutic Nanomedicine for Glioblastoma Treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. Int. J. Mol. Sci. 2022, 23, 1711. [Google Scholar] [CrossRef] [PubMed]
- Harder, B.G.; Blomquist, M.R.; Wang, J.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A.; Loftus, J.C.; Tran, N.L. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, K.; Kawakami, M.; Puri, R.K. Specifically targeted killing of interleukin-13 (IL-13) receptor-expressing breast cancer by IL-13 fusion cytotoxin in animal model of human disease. Mol. Cancer Ther. 2004, 3, 137–147. [Google Scholar] [CrossRef]
- Joshi, B.H.; Leland, P.; Asher, A.; Prayson, R.A.; Varricchio, F.; Puri, R.K. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001, 61, 8058–8061. [Google Scholar]
- Wadajkar, A.S.; Dancy, J.G.; Hersha, D.S.; Anastasiadis, P.; Tran, N.L.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Tumor-targeted Nanotherapeutics: Overcoming Treatment Barriers for Glioblastoma, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1439. [Google Scholar]
- Gonawala, S.; Ali, M.M. Application of Dendrimer-based Nanoparticles in Glioma Imaging. J. Nanomed. Nanotechnol. 2017, 8, 444. [Google Scholar]
- Schneider, C.S.; Perez, J.G.; Cheng, E.; Zhang, C.; Mastorakos, P.; Hanes, J.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Minimizing the non-specific binding of nanoparticles to the brain enables active targeting of Fn14-positive glioblastoma cells. Biomaterials 2015, 42, 42–51. [Google Scholar] [CrossRef]
- Alphandéry, E. Nano-Therapies for Glioblastoma Treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef] [Green Version]
- Hersh, D.; Wadajkar, A.S.; Roberts, N.B.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyautdin, R.; Khalin, I.; Nafeeza, M.I.; Haron, M.H.; Kuznetsov, D. Nanoscale drug delivery systems and the blood-brain barrier. Int. J. Nanomed. 2014, 9, 795–811. [Google Scholar]
- McCarthy, D.J.; Malhotra, M.A.; O’Mahony, M.; Cryan, J.F.; O’Driscoll, C.M. Nanoparticles and the Blood-Brain Barrier: Advancing from In-Vitro Models Towards Therapeutic Significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brainbarrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for Glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Fang, C.; Wang, K.; Stephen, Z.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.; Zhang, M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yang, H.; Yang, W.; Chen, X.; Gao, J.; Gong, X.; Wang, H.; Duan, Y.; Wei, D.; Chang, J. Nanoparticle-Based Diagnostic and Therapeutic Systems for Brain Tumors. J. Mater. Chem. B 2019, 7, 4734–4750. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Shi, Z.; Liu, R.; Wu, Y.; Zhang, X. Combined-Therapeutic Strategies Synergistically Potentiate Glioblastoma Multiforme Treatment Via Nanotechnology. Theranostics 2020, 10, 3223–3239. [Google Scholar]
- Mahmoud, A.S.; AlAmri, A.H.; McConville, C. Polymeric Nanoparticles for the Treatment of Malignant Gliomas. Cancers 2020, 12, 175. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood−Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Han, L.; Kong, D.K.; Zheng, M.-Q.; Murikinati, S.; Ma, C.; Yuan, P.; Li, L.; Tian, D.; Cai, Q.; Ye, C.; et al. Increased Nanoparticle Delivery to Brain Tumors by Autocatalytic Priming for Improved Treatment and Imaging. ACS Nano 2016, 10, 4209–4218. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Liu, Y.; Yang, Z.; Zhang, D.; Lu, Y.; Zheng, M.; Xue, X.; Geng, J.; Chung, R.; Shiet, B. Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv. Mater. 2018, 30, 1803717. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Pi, Z.; Yan, F.; Yeh, C.-K.; Zeng, X.; Diao, X.; Hu, Y.; Chen, S.; Chen, X.; Zheng, H. Enhanced delivery of paclitaxel liposomes using focused ultrasound with microbubbles for treating nude mice bearing intracranial glioblastoma xenografts. Int. J. Nanomed. 2017, 12, 5613–5629. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.W.; Ang, J.; Mahakian, L.M.; Tam, S.; Fite, B.; Ingham, E.S.; Beyer, J.; Forsayeth, J.; Bankiewicz, K.S.; Xu, T.; et al. Self-assembled 20-nm 64Cu-micelles enhance accumulation in rat glioblastoma. J. Control. Release 2015, 220, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Chaudhari, K.; Dantuluri, P.; Murthy, R.; Das, S. Paclitaxel-loaded PLGA nanoparticles surface modified with transferrin and Pluronic® P85, an in vitro cell line and in vivo biodistribution studies on rat model. J. Drug Target. 2009, 17, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [Green Version]
- Madhankumar, A.; Slagle-Webb, B.; Mintz, A.; Sheehan, J.M.; Connor, J.R. Interleukin-13 receptor–targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol. Cancer Ther. 2006, 5, 3162–3169. [Google Scholar] [CrossRef] [Green Version]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef]
- Rehman, M.; Madni, A.; Shi, D.; Ihsan, A.; Tahir, N.; Chang, K.R.; Javed, I.; Webster, T.J. Enhanced blood brain barrier permeability and glioblastoma cell targeting via thermoresponsive lipid nanoparticles. Nanoscale 2017, 9, 15434–15440. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wang, Y.; Xu, H.; Li, X.; Yan, H.; Tang, H.; Wen, C.; Li, Y. The construction of the multifunctional targeting ursolic acids liposomes and its apoptosis effects to C6 glioma stem cells. Oncotarget 2017, 8, 64129–64142. [Google Scholar] [CrossRef] [PubMed]
- Singleton, W.G.; Collins, A.M.; Bienemann, A.S.; Killick-Cole, C.L.; Haynes, H.R.; Asby, D.J.; Butts, C.P.; Wyatt, M.J.; Barua, N.U.; Gill, S.S. Convection enhanced delivery of panobinostat (LBH589)-loaded pluronic nano-micelles prolongs survival in the F98 rat glioma model. Int. J. Nanomed. 2017, 12, 1385–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Choi, K.; Kim, D.-H.; Oh, B.K.; Yim, H.; Jo, S.; Choi, C. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics 2022, 14, 672. [Google Scholar] [CrossRef]
- Tapeinos, C.; Marino, A.; Battaglini, M.; Migliorin, S.; Brescia, R.; Scarpellini, A.; Fernández, C.D.J.; Prato, M.; Dragog, F.; Ciofani, G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale 2019, 11, 1–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Zhang, X.; Wen, Z.; Tan, Y.; Huang, N.; Cheng, S.; Zheng, H.; Cheng, Y. Asn-Gly-Arg-modified polydopamine-coated nanoparticles for dual-targeting therapy of brain glioma in rats. Oncotarget 2016, 7, 73681–73696. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Liu, T.; Li, Y.; Lau, J.; Yang, Z.; Fan, W.; Zhou, Z.; Shi, C.; Ke, C.; Bregadze, V.E.; et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano 2018, 12, 11355–11365. [Google Scholar] [CrossRef]
- Lee, C.; Hwang, H.S.; Lee, S.; Kim, B.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.-G.; Youn, Y.S. Rabies Virus-Inspired Silica-Coated Gold Nanorods as a Photothermal Therapeutic Platform for Treating Brain Tumors. Adv. Mater. 2017, 29, 1605563. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Du, Y.; Wang, S.; Li, C.; Jiang, H.; Shi, W.; Chen, J.; Wang, Y.; Wagner, E.; Huang, R. Highly Crystalline Multicolor Carbon Nanodots for Dual-Modal Imaging-Guided Photothermal Therapy of Glioma. ACS Appl. Mater. Interfaces 2018, 10, 4031–4040. [Google Scholar] [CrossRef]
- Ramachandran, R.; Junnuthula, V.R.; Gowd, G.S.; Ashokan, A.; Thomas, J.; Peethambaran, R.; Thomas, A.; Unni, A.K.K.; Panikar, D.; Nair, S.V.; et al. Theranostic 3-Dimensional nano brain-implant for prolonged and localized treatment of recurrent glioma. Sci. Rep. 2017, 7, 43271. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Han, S.; Zheng, H.; Ma, R.; Ping, Y.; Zou, J.; Tang, H.; Zhang, Y.; Xu, X.; Li, F. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int. J. Nanomed. 2018, 13, 5937–5952. [Google Scholar] [CrossRef] [Green Version]
- Yeini, E.; Ofek, P.; Albeck, N.; Rodriguez Ajamil, D.; Neufeld, L.; Eldar-Boock, A.; Kleiner, R.; Vaskovich, D.; Koshrovski-Michael, S.; Israeli Dangooret, S. Targeting Glioblastoma: Advances in Drug Delivery and Novel Therapeutic Approaches. Adv. Therap. 2021, 4, 2000124. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Sanz-Arriazu, L.; Lorenzoni, R.; Blanco-Prieto, M.J. Glioblastoma chemotherapeutic agents used in the clinical setting and in clinical trials: Nanomedicine approaches to improve their efficacy. Int. J. Pharm. 2020, 581, 119283. [Google Scholar] [PubMed]
- Amaral, M.; Cruz, N.; Rosa, A.; Nogueira, B.; Costa, D.; Santos, F.; Brazão, M.; Policarpo, P.; Mateus, R.; Kobozev, Y.; et al. An update of advanced nanoplatforms for Glioblastoma Multiforme Management. EXCLI J. 2021, 20, 1544–1570. [Google Scholar] [CrossRef] [PubMed]
- Rabha, B.; Bharadwaj, K.K.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Kari, Z.A.; Edinur, H.A.; Baishya, D.; Atanase, L.I. Development of Polymer-Based Nanoformulations for Glioblastoma Brain Cancer Therapy and Diagnosis: An Update. Polymers 2021, 13, 4114. [Google Scholar]
- Ortiz, R.; Cabeza, L.; Perazzoli, G.; Jimenez-Lopez, J.; García-Pinel, B.; Melguizo, C.; Prados, J. Nanoformulations for glioblastoma multiforme: A new hope for treatment. Future Med. Chem. 2019, 11, 2459–2480. [Google Scholar] [CrossRef] [PubMed]
- Malyala, P.; Singh, M. Endotoxin limits in formulations for preclinical research. J. Pharm. Sci. 2008, 97, 2041–2044. [Google Scholar]
- Hetze, S.; Sure, U.; Schedlowski, M.; Hadamitzky, M.; Barthel, L. Rodent Models to Analyze the Glioma Microenvironment. ASN Neuro 2021, 13, 1–12. [Google Scholar]
- Pérez-Carro, R.; Cauli, O.; López-Larrubia, P. Multiparametric magnetic resonance in the assessment of the gender differences in a high-grade glioma rat model. EJNMMI Res. 2014, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Warrington, N.M.; Taylor, S.J.; Whitmire, P.; Carrasco, E.; Singleton, K.W.; Wu, N.; Lathia, J.D.; Berens, M.E.; Kim, A.H.; et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019, 11, eaao5253. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Calero-Pérez, P.; Villamañan, L.; Arias-Ramos, N.; Pumarola, M.; Ortega-Martorell, S.; Julià-Sapé, M.; Arús, C.; Candiota, A.P. Anti-tumour immune response in GL261 glioblastoma generated by Temozolomide Immune-Enhancing Metronomic Schedule monitored with MRSI-based nosological images. NMR Biomed. 2020, 33, e4229. [Google Scholar]
- Wu, J.; Waxman, D.J. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8+ T-cell responses and immune memory. Oncoimmunology 2015, 4, e1005521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.E.; Banerjee, S.K. The Impact of Nanoparticles on the Immune System: A GrayZone of Nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Beura, L.K.; Hamilton, S.E.; Bi, K.; Schenkel, J.M.; Odumade, O.A.; Casey, K.A.; Thompson, E.A.; Fraser, K.A.; Rosato, P.C.; Filali-Mouhim, A.; et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016, 532, 512–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Wang, Y.; Wargo, J.A.; Lang, F.F.; Kim, B.Y.S. Considerations for designing preclinical cancer immune nanomedicine studies. Nat. Nanotechnol. 2021, 16, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, X.J.; Chen, D.X.; Liu, X.N.; Wang, X.J. Humanized mouse model: A review on preclinical applications for cancer immunotherapy. Am. J. Cancer. Res. 2020, 10, 4568–4584. [Google Scholar]
- Semenkow, S.; Li, S.; Kahlert, U.D.; Raabe, E.H.; Xu, J.; Arnold, A.; Janowski, M.; Oh, B.C.; Brandacher, G.; Bulte, J.W.; et al. An immunocompetent mouse model of human glioblastoma. Oncotarget 2017, 8, 61072–61082. [Google Scholar] [CrossRef]
- Ladomersky, E.; Zhai, L.; Lauing, K.L.; Bell, A.; Xu, J.; Kocherginsky, M.; Zhang, B.; Wu, J.D.; Podojil, J.R.; Platanias, L.C.; et al. Advanced Age Increases Immunosuppression in the Brain and Decreases Immunotherapeutic Efficacy in Subjects with Glioblastoma. Clin. Cancer Res. 2020, 26, 5232–5245. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Tritz, Z.P.; Ayasoufi, K.; Johnson, A.J. Anti-PD-1 checkpoint blockade monotherapy in the orthotopic GL261 glioma model: The devil is in the detail. Neuro-oncol. Adv. 2021, 3, vdab066. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, D.H.; Kim, J.S. Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells. J. Control. Release 2018, 269, 245–257. [Google Scholar] [CrossRef]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Han, T.L.V.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, M.; Yang, W.; Li, Y.; Chai, T.; Zhang, D.; Du, Q.; Muhammad, P.; Hanif, S.; Zheng, M.; Shi, B. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials 2022, 287, 121608. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, P.; Lv, M.M.; Tong, G.S.; Jin, X.; Zhu, X.Y. Mustard-inspired delivery shuttle for enhanced blood-brain barrier penetration and effective drug delivery in glioma therapy. Biomater. Sci. 2017, 5, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [Green Version]
- Qiao, C.; Yang, J.; Shen, Q.; Liu, R.; Li, Y.; Shi, Y.; Chen, J.; Shen, Y.; Xiao, Z.; Weng, J.; et al. Traceable Nanoparticles with Dual Targeting and ROS Response for RNAi-Based Immunochemotherapy of Intracranial Glioblastoma Treatment. Adv. Mater. 2018, 30, e1705054. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zhang, D.; Sun, Y.; Li, F.; Zheng, M.; Shi, B. Brain co-delivery of first-line chemotherapy drug and epigenetic bromodomain inhibitor for multidimensional enhanced synergistic glioblastoma therapy. Exploration 2022, 2, 20210274. [Google Scholar] [CrossRef]
- Qian, L.L.; Zheng, J.J.; Wang, K.; Tang, Y.; Zhang, X.F.; Zhang, H.S.; Huang, F.P.; Pei, Y.Y.; Jiang, Y.Y. Cationic core-shell nanoparticles with carmustine contained within O-6-benzylguanine shell for glioma therapy. Biomaterials 2013, 34, 8968–8978. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Liu, H.-L.; Hua, M.-Y.; Yang, H.-W.; Huang, C.-Y.; Chu, P.-C.; Lyu, L.-A.; Tseng, I.-C.; Feng, L.-Y.; Tsai, H.-C.; et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro-Oncol. 2010, 12, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Liu, L.; Lu, Y.; Sun, T.; Shen, C.; Chen, X.; Chen, Q.; An, S.; He, X.; Ruan, C.; et al. T7 Peptide-Functionalized PEG-PLGA Micelles Loaded with Carmustine for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2016, 8, 27465–27473. [Google Scholar] [CrossRef]
- Guo, X.Y.; Wu, G.J.; Wang, H.; Chen, L.K. Pep-1 & borneol-Bifunctionalized Carmustine-Loaded Micelles Enhance Anti-Glioma Efficacy Through Tumor- Targeting and BBB-Penetrating. J. Pharm. Sci. 2019, 108, 1726–1735. [Google Scholar]
- Fisusi, F.A.; Siew, A.; Chooi, K.W.; Okubanjo, O.; Garrett, N.; Lalatsa, K.; Serrano, D.; Summers, I.; Moger, J.; Stapleton, P.; et al. Lomustine Nanoparticles Enable Both Bone Marrow Sparing and High Brain Drug Levels–A Strategy for Brain Cancer Treatments. Pharm. Res. 2016, 33, 1289–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Glioblastoma treatment: Bypassing the toxicity of platinum compounds by using liposomal formulation and increasing treatment efficiency with concomitant radiotherapy. Oncol. Biol. Phys. 2012, 84, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baklaushev, V.P.; Nukolova, N.N.; Khalansky, A.S.; Gurina, O.I.; Yusubalieva, G.M.; Grinenko, N.P.; Gubskiy, I.L.; Melnikov, P.A.; Kardashova, K.S.; Kabanov, A.V.; et al. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT1. Drug Deliv. 2015, 22, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Miller, G.W.; Suk, J.S.; Hanes, J.; Price, R.J. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Yang, J.J.; Zhuge, D.L.; Lin, M.T.; Zhu, Q.Y.; Jin, B.H.; Tong, M.Q.; Shen, B.X.; Xiao, J.; Zhao, Y.Z. Glioma-Targeted Delivery of a Theranostic Liposome Integrated with Quantum Dots, Superparamagnetic Iron Oxide, and Cilengitide for Dual-Imaging Guiding Cancer Surgery. Adv. Healthc. Mater. 2018, 7, 1701130. [Google Scholar] [CrossRef]
- Han, L.; Liu, C.Y.; Qi, H.Z.; Zhou, J.H.; Wen, J.; Wu, D.; Xu, D.; Qin, M.; Ren, J.; Wang, Q.X.; et al. Systemic Delivery of monoclonal antibodies to the central nervous system for brain tumor therapy. Adv. Mater. 2019, 31, 1805697. [Google Scholar] [CrossRef]
- Zhao, P.F.; Wang, Y.H.; Kang, X.J.; Wu, A.H.; Yin, W.M.; Tang, Y.S.; Wang, J.Y.; Zhang, M.; Duan, Y.F.; Huang, Y.Z. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophagemediated immunotherapy. Chem. Sci. 2018, 9, 2674–2689. [Google Scholar] [CrossRef]
- Yu, M.A.; Su, D.Y.; Yang, Y.Y.; Qin, L.; Hu, C.; Liu, R.; Zhou, Y.; Yang, C.Y.; Yang, X.T.; Wang, G.L.; et al. D-T7 Peptide-Modified PEGylated Bilirubin Nanoparticles Loaded with Cediranib and Paclitaxel for Antiangiogenesis and Chemotherapy of Glioma. ACS Appl. Mater. Interfaces 2019, 11, 176–186. [Google Scholar] [CrossRef]
- Saucier-Sawyer, J.K.; Deng, Y.; Seo, Y.E.; Cheng, C.J.; Zhang, J.W.; Quijano, E.; Saltzman, W.M. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J. Drug Target. 2015, 23, 736–749. [Google Scholar] [CrossRef] [Green Version]
- Householder, K.T.; DiPerna, D.M.; Chung, E.P.; Wohlleb, G.M.; Dhruv, H.D.; Berens, M.E.; Sirianni, R.W. Intravenous delivery of camptothecin-loaded PLGA nanoparticles for the treatment of intracranial glioma. Int. J. Pharm. 2015, 479, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Serwer, L.P.; Noble, C.O.; Michaud, K.; Drummond, D.C.; Kirpotin, D.B.; Ozawa, T.; Prados, M.D.; Park, J.W.; James, C.D. Investigation of intravenous delivery of nanoliposomal topotecan for activity against orthotopic glioblastoma xenografts. Neuro-Oncol. 2011, 13, 1288–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verreault, M.; Strutt, D.; Masin, D.; Anantha, M.; Waterhouse, D.; Yapp, D.T.; Bally, M.B. Irinophore C (TM), a lipid-based nanoparticulate formulation of irinotecan, is more effective than free irinotecan when used to treat an orthotopic glioblastoma model. J. Control. Release 2012, 158, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Verreault, M.; Wehbe, M.; Strutt, D.; Masin, D.; Anantha, M.; Walker, D.; Chu, F.; Backstrom, I.; Kalra, J.; Waterhouse, D.; et al. Determination of an optimal dosing schedule for combining Irinophore C (TM) and temozolomide in an orthotopic model of glioblastoma. J. Control. Release 2015, 220, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.; Krauze, M.T.; Drummond, D.C.; Forsayeth, J.; E Hayes, M.; Beyer, J.; Hadaczek, P.; Berger, M.S.; Kirpotin, D.B.; Bankiewicz, K.S.; et al. Pharmacokinetics, tumor accumulation and antitumor activity of nanoliposomal irinotecan following systemic treatment of intracranial tumors. Nanomedicine 2014, 9, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Louis, N.; Liu, S.R.; He, X.Y.; Drummond, D.C.; Noble, C.O.; Goldman, S.; Mueller, S.; Bankiewicz, K.; Gupta, N.; Hashizume, R. New therapeutic approaches for brainstem tumors: A comparison of delivery routes using nanoliposomal irinotecan in an animal model. J. Neuro-Oncol. 2018, 136, 475–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.J.; Chuang, E.Y.; Cheng, Y.H.; Anilkumar, T.S.; Chen, H.A.; Chen, J.P. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. Chem. Eng. J. 2019, 373, 720–733. [Google Scholar] [CrossRef]
- Ying, M.; Wang, S.L.; Zhang, M.F.; Wang, R.F.; Zhu, H.C.; Ruan, H.T.; Ran, D.N.; Chai, Z.L.; Wang, X.Y.; Lu, W.Y.; et al. Myristic Acid-Modified (D)A7R Peptide for Whole-Process Glioma-Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 19473–19482. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, L.; Hu, Y.; Jiang, K.; Li, Z.Q.; Lin, Y.Z.; Wei, G.; Lu, W.Y. Cellpermeable NF-kappa B inhibitor-conjugated liposomes for treatment of glioma. J. Control. Release 2018, 289, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Fan, Z.L.; ZhuGe, D.L.; Tong, M.Q.; Shen, B.X.; Lin, M.T.; Zhu, Q.Y.; Jin, B.H.; Sohawon, Y.; Yao, Q.; et al. Ratiometric delivery of two therapeutic candidates with inherently dissimilar physicochemical property through pH-sensitive core-shell nanoparticles targeting the heterogeneous tumor cells of glioma. Drug Deliv. 2018, 25, 1302–1318. [Google Scholar] [CrossRef] [Green Version]
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.P.; Tao, J.; Liu, C.J.; He, X.H.; Zhang, X.Z. iRGD Modified Chemo-immunotherapeutic Nanoparticles for Enhanced Immunotherapy against Glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025. [Google Scholar] [CrossRef]
- Ruan, S.B.; Xie, R.; Qin, L.; Yu, M.N.; Xiao, W.; Hu, C.; Yu, W.Q.; Qian, Z.Y.; Ouyang, L.; He, Q.; et al. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with Anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 2019, 19, 8318–8332. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.J.; Lee, K.J.; Peng, I.C.; Hsu, C.H.; Lin, J.H.; Chen, K.H.; Tien, Y.W.; Hsieh, P.C.H. Inducing a transient increase in blood-brain barrier permeability for improved liposomal drug therapy of glioblastoma multiforme. ACS Nano 2019, 13, 97–113. [Google Scholar] [CrossRef]
- Ismail, M.; Yang, W.; Li, Y.; Wang, Y.; He, W.; Wang, J.; Muhammad, P.; Chaston, T.B.; Rehman, F.U.; Zheng, M.; et al. Biomimetic Dp44mT-nanoparticles selectively induce apoptosis in Cu-loaded glioblastoma resulting in potent growth inhibition. Biomaterials 2022, 289, 121760. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, Y.; Cui, G.; Zhong, A.; Deng, C. Brain delivery of Plk1 inhibitor via chimaeric polypeptide polymersomes for safe and superb treatment of orthotopic glioblastoma. J. Control. Release 2021, 329, 1139–1149. [Google Scholar] [CrossRef]
- Xue, X.; Qu, H.; Li, Y. Stimuli-responsive crosslinked nanomedicine for cancer treatment. Exploration 2022, 2, 20210134. [Google Scholar] [CrossRef]
- López, T.; Recillas, S.; Guevara, P.; Sotelo, J.; Alvarez, M.; Odriozola, J.A. Pt/TiO2 brain biocompatible nanoparticles: GBM treatment using the C6 model in Wistar rats. Acta Biomater. 2008, 4, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Kaushik, N.; Khan, F.; Kaushik, N.K.; Choi, E.H.; Musarrat, J.; Al-Khedhairy, A.A. Self-Styled ZnO Nanostructures promotes the cancer cell damage and supresses the epithelial phenotype of glioblastoma. Sci. Rep. 2015, 2016, 1–13. [Google Scholar] [CrossRef]
- Alle, M.; Kim, T.H.; Park, S.H.; Lee, S.H.; Kim, J.C. Doxorubicin-carboxymethyl xanthan gum capped gold nanoparticles: Microwave synthesis, characterization, and anti-cancer activity. Carbohydr. Polym. 2019, 229, 115511. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomedicine 2016, 11, 5003–5014. [Google Scholar] [CrossRef] [Green Version]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyo, M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; He, L.; Ma, B.; Chen, T. Tailoring particle size of mesoporous silica nanosystem to antagonize glioblastoma and overcome blood-brain barrier. ACS Appl. Mater. Interfaces 2016, 8, 6811–6825. [Google Scholar] [CrossRef]
- You, Y.; Yang, L.; He, L.; Chen, T. Tailored mesoporous silica nanosystem with enhanced permeability of the blood-brain barrier to antagonize glioblastoma. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 5980–5990. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Liu, Y.; Wang, X.Q.; Liu, L.H.; Hu, J.J.; Luo, G.F.; Chen, W.H.; Rong, L.; Zhang, X.Z. One-pot construction of functional mesoporous silica nanoparticles for the tumor-acidity-activated synergistic chemotherapy of glioblastoma. ACS Appl. Mater. Interfaces. 2013, 5, 7995–8001. [Google Scholar] [CrossRef]
- Salazar, A.; Pérez-de la Cruz, V.; Muñoz-Sandoval, E.; Chavarria, V.; García Morales, M.L.; Espinosa-Bonilla, A.; Pineda, B. Potential use of nitrogen-doped carbon nanotube sponges as payload carriers against malignant glioma. Nanomaterials 2021, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Surhland, C.; Sanchez, Z.; Chaudhary, P.; Kumar, M.S.; Lee, S.; Peña, L.A.; Waring, M.; Sitharaman, B.; Naidu, M. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine: Nanotechnol. Biol. Med. 2014, 11, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, T.L.; Grimes, S.W.; Lewis, R.L.; Alexis, F. Multilayered polymer-coated carbon nanotubes to deliver dasatinib. Mol. Pharm. 2014, 11, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Wang, X.; Tang, Q.; Chen, H.; Zhang, Q.; Jiang, H.; Wang, Z. Functionalized graphene oxide against U251 glioma cells and its molecular mechanism. Mater. Sci. Eng. C 2020, 116, 111187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Liu, J.-Y.; Sui, L.; Zhao, P.-H.; Ma, H.-D.; Wei, Z.; Wang, Y.-L. Folate-modified Graphene Oxide as the Drug Delivery System to Load Temozolomide. Curr. Pharm. Biotechnol. 2020, 21, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, H.; Zhang, X. Modification of graphene oxide by angiopep-2 enhances anti-glioma efficiency of the nanoscaled delivery system for doxorubicin. Aging 2020, 12, 10506–10516. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Qian, M.; Jiang, H.; Shi, W.; Chen, J.; Lächelt, U.; Wagner, E.; Lu, W.; Wang, Y.; et al. Augmented glioma-targeted theranostics using multifunctional polymer-coated carbon nanodots. Biomaterials 2017, 141, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, P.Y.; Zhou, Y.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Vanni, S.; Leblanc, R.M. Pediatric glioblastoma target-specific efficient delivery of gemcitabine across the blood-brain barrier via carbon nitride dots. Nanoscale 2020, 12, 7927–7938. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle Drug Delivery System for Glioma and Its Efficacy Improvement Strategies: A Comprehensive Review. Int. J. Nanomed. 2020, 15, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Cartera, D.; Liua, X.; Tockarya, T.A.; Dirisalaa, A.; Toha, K.; Anrakua, Y.; Kataoka, K. Targeting nanoparticles to the brain by exploiting the blood–brain barrier impermeability to selectively label the brain endothelium. Proc. Natl. Acad. Sci. USA. 2020, 117, 19141–19150. [Google Scholar] [CrossRef] [PubMed]
- Chekhonin, V.P.; Baklaushev, V.P.; Yusubalieva, G.M.; Belorusova, A.E.; Gulyaev, M.V.; Tsitrin, E.B.; Grinenko, N.F.; Gurina, O.I.; Pirogov, Y.A. Targeted delivery of liposomal nanocontainers to the peritumoral zone of glioma by means of monoclonal antibodies against GFAP and the extracellular loop of Cx43. Nanomedicine 2012, 8, 63–70. [Google Scholar] [CrossRef]
- Kim, S.S.; Rait, A.; Kim, E.; DeMarco, J.; Pirollo, K.F.; Chang, E.H. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anticancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett. 2015, 369, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Rait, A.; Kim, E.; Pirollo, K.F.; Nishida, M.; Farkas, N.; Dagata, J.A.; Chang, E.H. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano 2014, 8, 5494–5514. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.A.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L.; et al. Blood-brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef]
- Lee, B.S.; Amano, T.; Wang, H.Q.; Pantoja, J.L.; Yoon, C.W.; Hanson, C.J.; Amatya, R.; Yen, A.; Black, K.L.; Yu, J.S. Reactive oxygen species responsive nanoprodrug to treat intracranial glioblastoma. ACS Nano 2013, 7, 3061–3077. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Wang, K.; Ozawa, T.; Tarudji, A.W.; Silber, J.R.; Holland, E.C.; Ellenbogen, R.G.; Zhang, M. Nanoparticle-mediated knockdown of DNA repair sensitizes cells to radiotherapy and extends survival in a genetic mouse model of glioblastoma. Nanomedicine 2017, 13, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Lin, G.; Patton, V.K.; Wang, K.; Press, O.W.; Zhang, M. Gemcitabine and Chlorotoxin Conjugated Iron Oxide Nanoparticles for Glioblastoma Therapy. J. Mater. Chem. B 2016, 4, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Jeon, M.; Hsiao, M.H.; Patton, V.K.; Wang, K.; Press, O.W.; Xhang, M. Stable and efficient Paclitaxel nanoparticles for targeted glioblastoma therapy. Adv. Healthc. Mater. 2015, 4, 1236–1245. [Google Scholar] [CrossRef] [Green Version]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Zucchetti, M.; Boiardi, A.; Silvani, A.; Parisi, I.; Piccolrovazzi, S.; D'Incalci, M. Distribution of daunorubicin and daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother. Pharmacol. 1999, 44, 173–176. [Google Scholar] [CrossRef]
- Chastagner, P.; Devictor, B.; Geoerger, B.; Aerts, I.; Leblond, P.; Frappaz, D.; Gentet, J.C.; Bracard, S.; André, N. Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother. Pharmacol. 2015, 76, 425–432. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef] [PubMed]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: Pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma—A phase II study. BMC Cancer 2009, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Ananda, S.; Nowak, A.K.; Cher, L.; Dowling, A.; Brown, C.; Simes, J.; Rosenthal, M.A. Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J. Clin. Neurosci. 2011, 18, 1444–1448. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Wu, S.; Calero-Pérez, P.; Candiota, A.P.; Alfonso, P.; Bruna, J.; Yuste, V.Y.; Lorenzo, J.; Novio, F.; Ruiz-Molina, D. Synthesis and Validation of a Bioinspired Catechol-Functionalized Pt(IV) Prodrug for Preclinical Intranasal Glioblastoma Treatment. Cancers 2022, 14, 410. [Google Scholar] [CrossRef]
- Mao, X.; Calero-Pérez, P.; Montpeyó, D.; Bruna, J.; Yuste, V.Y.; Candiota, A.P.; Lorenzo, J.; Novio, F.; Ruiz-Molina, D. Intranasal Administration of Catechol-Based Pt(IV) Coordination Polymer Nanoparticles for Glioblastoma Therapy. Nanomaterials 2022, 12, 1221. [Google Scholar] [CrossRef]


| Name | Drug | Particle Type | Targeting Moieties | Clinical Trials.Gov Identifier |
|---|---|---|---|---|
| Onyvide® | Irinotecan | PEGylated liposomes | - - - | NCT03119064 |
| NL CPT-11 | Irinotecan | PEGylated liposomes | - - - | NCT00734682 |
| Caelix® | DOX (combined with prolonged TMZ) | PEGylated liposomes | - - - | NCT00944801 |
| 2B3-101 | DOX | PEGylated liposomes | Glutathione | NCT01386580 |
| C225-ILs-Dox | DOX | Liposomes | Cetuximab | NCT03603379 |
| Nanotherm® | - - - | Iron oxide nanoparticles | - - - | Magforce, Inc. (Berlin, Germany) (Approv. 2013) |
| SGT-53 | P53 plasmid (combined with oral TMZ) | cationic liposomes | anti-TfR antibody | NCT02340156 NCT03554707 |
| SGT94-01 | RB94 plasmid | Liposomes | anti-TfR antibody | NCT01517464 |
| (NU-0129) | - - - | gold nanoparticles | nucleic acids targeting BCL2L12 gene | NCT03020017 |
| DaunoXome® | - - - | Liposomes | - - - | (Zucchetti et al.) [176] |
| Myocet® | - - - | Liposomes | - - - | NCT02861222 (Chastagner et al.) [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Molina, D.; Mao, X.; Alfonso-Triguero, P.; Lorenzo, J.; Bruna, J.; Yuste, V.J.; Candiota, A.P.; Novio, F. Advances in Preclinical/Clinical Glioblastoma Treatment: Can Nanoparticles Be of Help? Cancers 2022, 14, 4960. https://doi.org/10.3390/cancers14194960
Ruiz-Molina D, Mao X, Alfonso-Triguero P, Lorenzo J, Bruna J, Yuste VJ, Candiota AP, Novio F. Advances in Preclinical/Clinical Glioblastoma Treatment: Can Nanoparticles Be of Help? Cancers. 2022; 14(19):4960. https://doi.org/10.3390/cancers14194960
Chicago/Turabian StyleRuiz-Molina, Daniel, Xiaoman Mao, Paula Alfonso-Triguero, Julia Lorenzo, Jordi Bruna, Victor J. Yuste, Ana Paula Candiota, and Fernando Novio. 2022. "Advances in Preclinical/Clinical Glioblastoma Treatment: Can Nanoparticles Be of Help?" Cancers 14, no. 19: 4960. https://doi.org/10.3390/cancers14194960
APA StyleRuiz-Molina, D., Mao, X., Alfonso-Triguero, P., Lorenzo, J., Bruna, J., Yuste, V. J., Candiota, A. P., & Novio, F. (2022). Advances in Preclinical/Clinical Glioblastoma Treatment: Can Nanoparticles Be of Help? Cancers, 14(19), 4960. https://doi.org/10.3390/cancers14194960






