Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
Rationale
2. Objectives
3. Methodology
Search Strategy and Study Selection Criteria
4. Results and Discussion
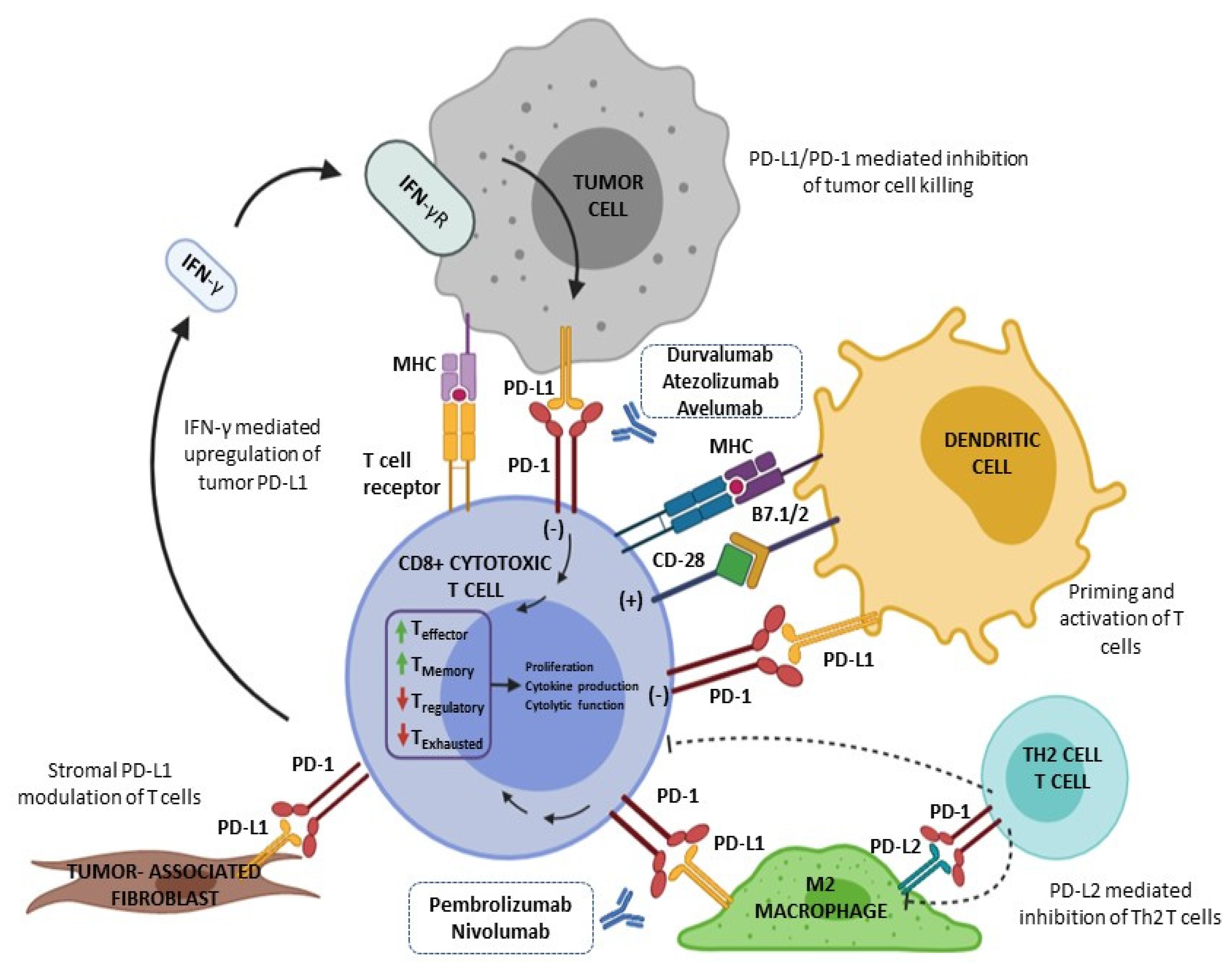
4.1. PD-L1 Pathway and PD-L1 Testing in General
4.2. PD-L1 in Breast Cancer
4.3. PD-L1 and Immunotherapy in Hormone-Receptor Positive/HER2 Negative Breast Cancer
4.4. PD-L1 and Immunotherapy in HER2 Positive Breast Cancer
4.5. PD-L1 Expression and Immunotherapy in Triple-Negative Breast Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Breast Cancer Metastasis and Drug Resistance; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019.
- Dimitrova, N.; Saz Parkinson, Z.; Bramesfeld, A.; Uluturk Tekin, A.; Bocchi, G.; Pylkkanen, L.; Lopez Alcalde, J.; Neamtiu, L.; Ambrosio, M.; Deandrea, S.; et al. European Guidelines for Breast Cancer Screening and Diagnosis—The European Breast Guidelines. EUR 28360; Publications Office of the European Union: Luxembourg, 2016; JRC104007. [Google Scholar]
- Huang, R.S.P.; Haberberger, J.; Severson, E.; Duncan, D.L.; Hemmerich, A.; Edgerly, C.; Ferguson, N.L.; Williams, E.; Elvin, J.; Vergilio, J.A.; et al. A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod. Pathol. 2021, 34, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Noske, A.; Wagner, D.-C.; Schwamborn, K.; Foersch, S.; Steiger, K.; Kiechle, M.; Karapetyan, S.; Oettler, D.; Hapfelmeier, A.; Roth, W.; et al. 13P Comparison study of different programmed death-ligand 1 (PD-L1) assays, readers and scoring methods in triple-negative breast cancer (TNBC). Ann. Oncol. 2021, 32, S26. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, N.; Park, M.-J.; Jeon, K.; Song, W. cancers Currently Used Laboratory Methodologies for Assays Detecting PD-1, PD-L1, PD-L2 and Soluble PD-L1 in Patients with Metastatic Breast Cancer. Cancers 2021, 13, 5225. [Google Scholar] [CrossRef] [PubMed]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Kim, J.W.; Kim, M.K.; Chung, B.W.; Ahn, S.K. Programmed cell death-ligand 1 expression in stromal immune cells is a marker of breast cancer outcome. J. Cancer 2020, 11, 7246–7252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, H.; Zhao, S.; Wang, Y.; Pu, H.; Zhang, Q. Expression of PD-L1 and prognosis in breast cancer: A metaanalysis. Oncotarget 2017, 8, 31347–31354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Azuma, T.; Yao, S.; Zhu, G.; Flies, A.S.; Flies, S.J.; Chen, L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 2008, 111, 3635–3643. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Immune blockade inhibition in breast cancer. Anticancer Res. 2016, 36, 5607–5622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. Oncotargets Ther. 2016, 12, 5023–5039. [Google Scholar]
- Escors, D.; Gato-Cañas, M.; Zuazo, M.; Arasanz, H.; García-Granda, M.J.; Vera, R.; Kochan, G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct. Target Ther. 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012, 2012, 656340. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.M.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Fang, J.; Chen, F.; Liu, D.; Gu, F.; Chen, Z.; Wang, Y. Prognostic value of immune checkpoint molecules in breast cancer. Biosci. Rep. 2020, 40, BSR20201054. [Google Scholar] [CrossRef] [PubMed]
- Erber, R.; Hartmann, A. Understanding PD-L1 Testing in Breast Cancer: A Practical Approach. Breast Care 2020, 15, 481–490. [Google Scholar] [CrossRef]
- Vennapusa, B.; Baker, B.; Kowanetz, M.; Boone, J.; Menzl, I.; Bruey, J.M.; Fine, G.; Mariathasan, S.; McCaffery, I.; Mocci, S.; et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl. Immunohistochem. Mol. Morphol. AIMM 2019, 27, 92–100. [Google Scholar] [CrossRef]
- Schwamborn, K.; Ammann, J.U.; Knüchel, R.; Hartmann, A.; Baretton, G.; Lasitschka, F.; Schirmacher, P.; Braunschweig, T.; Tauber, R.; Erlmeier, F.; et al. Multicentric analytical comparability study of programmed death-ligand 1 expression on tumor-infiltrating immune cells and tumor cells in urothelial bladder cancer using four clinically developed immunohistochemistry assays. Virchows Arch. 2019, 475, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Hendry, S.; Byrne, D.J.; Wright, G.M.; Young, R.J.; Sturrock, S.; Cooper, W.A.; Fox, S.B. Comparison of Four PD-L1 Immunohistochemical Assays in Lung Cancer. J. Thorac. Oncol. 2018, 13, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- Marchi, D. PD-L1 expression by Tumor Proportion Score (TPS) and Combined Positive Score (CPS) are similar in non-small cell lung cancer (NSCLC). J. Clin. Pathol. 2021, 74, 735–740. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Qian, M.; Han, W.; Tian, M.; Li, Z.; Wang, Z.; He, S.; Wu, K. PD-L1 expression and the prognostic significance in gastric cancer: A retrospective comparison of three PD-L1 antibody clones (SP142, 28–8 and E1L3N). Diagn. Pathol. 2018, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Schildhaus, H.U. Predictive value of PD-L1 diagnostics. Pathologe 2018, 39, 498–519. [Google Scholar] [CrossRef]
- Martinez-Morilla, S.; McGuire, J.; Gaule, P.; Moore, L.; Acs, B.; Cougot, D.; Gown, A.M.; Yaziji, H.; Wang, W.-L.; Cartun, R.W.; et al. Quantitative assessment of PD-L1 as an analyte in immunohistochemistry diagnostic assays using a standardized cell line tissue microarray. Lab. Investig. 2020, 100, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Villalobos, P.; Mino, B.; Rodriguez-Canales, J. Comparison of Different Antibody Clones for Immunohistochemistry Detection of Programmed Cell Death Ligand 1 (PD-L1) on Non-Small Cell Lung Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 83–93. [Google Scholar] [CrossRef]
- Tsimafeyeu, I.; Imyanitov, E.; Zavalishina, L.; Raskin, G.; Povilaitite, P.; Savelov, N.; Kharitonova, E.; Rumyantsev, A.; Pugach, I.; Andreeva, Y.; et al. Agreement between PDL1 immunohistochemistry assays and polymerase chain reaction in non-small cell lung cancer: CLOVER comparison study. Sci. Rep. 2020, 10, 3928. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Ding, Q.; Gong, Y.; Gilcrease, M.Z.; Zhao, M.; Zhao, J.; Sui, D.; Wu, Y.; Chen, H.; Liu, H.; et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. 2020, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eymerit-Morin, C.; Ilenko, A.; Gaillard, T.; Varinot, J.; Compérat, E.; Bendifallah, S.; Darai, E. PD-L1 expression with QR1 and E1L3N antibodies according to histological ovarian cancer subtype: A series of 232 cases. Eur. J. Histochem. 2021, 65, 3185. [Google Scholar] [CrossRef]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, M.S.; Kerr, K.M.; Kockx, M.; Beasley, M.B.; Borczuk, A.C.; Botling, J.; Bubendorf, L.; Chirieac, L.; Chen, G.; Chou, T.Y.; et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018, 13, 1302–1311. [Google Scholar] [CrossRef] [Green Version]
- Ilie, M.; Khambata-Ford, S.; Copie-Bergman, C.; Huang, L.; Juco, J.; Hofman, V.; Hofman, P. Use of the 22C3 anti–PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS ONE 2017, 12, e0186537. [Google Scholar]
- Rimm, D.L.; Han, G.; Taube, J.M.; Yi, E.S.; Bridge, J.A.; Flieder, D.B.; Homer, R.; West, W.W.; Wu, H.; Roden, A.C.; et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non–small cell lung cancer. JAMA Oncol. 2017, 3, 1051–1058. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Rugo, H.S.; Loi, S.; Adams, S.; Schmid, P.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Eronique Di Eras, V.; Winer, E.P.; Kockx, M.M.; et al. PD-L1 Immunohistochemistry Assay Comparison in Atezolizumab Plus nab-Paclitaxel-Treated Advanced Triple-Negative Breast Cancer. J. Natl. Cancer Inst. 2021, 113, 1733–1743. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Yan, Z.; Ma, J.; Zhu, F.; Huo, J. Prognostic value of PD-L1 expression in patients with pancreatic cancer A PRISMA-compliant meta-analysis. Medicine 2019, 98, e14006. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Li, Y.; He, M.; Zhou, Y.; Yang, C.; Wei, S.; Bian, X.; Christopher, O. The Prognostic and Clinicopathological Roles of PD-L1 Expression in Colorectal Cancer: A Systematic Review and MetaAnalysis. Front. Pharmacol. 2019, 10, 139. [Google Scholar]
- Gu, L.; Chen, M.; Guo, D.; Zhu, H.; Zhang, W.; Pan, J.; Zhong, X.; Li, X.; Qian, H.; Wang, X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0182692. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.-Y.; Huang, J.-A.; Chen, Y.; Chen, C.; Zhang, X.-G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med. Oncol. 2011, 28, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.A.; Tran, T.; Vilain, R.E.; Madore, J.; Selinger, C.I.; Kohonen-Corish, M.; Yip, P.Y.; Yu, B.; O’Toole, S.A.; McCaughan, B.C.; et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015, 89, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, J.P. Immune checkpoint blockade in cancer therapy the 2015 Lasker-Debakey clinical medical research award. JAMA J. Am. Med. Assoc. 2015, 314, 1113–1114. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.J.; Scott, M.; Scorer, P.; Barker, C. Assessment of PD-L1 mRNA and protein expression in non-small cell lung cancer, head and neck squamous cell carcinoma and urothelial carcinoma tissue specimens using RNAScope and immunohistochemistry. PLoS ONE 2019, 14, e0215393. [Google Scholar] [CrossRef] [Green Version]
- Planes-Laine, G.; Rochigneux, P.; Bertucci, F.; Chrétien, A.S.; Viens, P.; Sabatier, R.; Gonçalves, A. PD-1/PD-l1 targeting in breast cancer: The first clinical evidences are emerging:A literature review. Cancers 2019, 11, 1033. [Google Scholar] [CrossRef] [Green Version]
- Cheang, M.C.U.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 Index, HER2 Status, and Prognosis of Patients With Luminal B Breast Cancer. JNCI J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef] [Green Version]
- Litvin, I.E.; Paganella, M.P.; Wendland, E.M.; Roehe, A.V. Prognosis of PD-L1 in human breast cancer: Protocol for a systematic review and meta-Analysis. Syst. Rev. 2020, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Vigliar, E.; Malapelle, U.; Bono, F.; Fusco, N.; Cortinovis, D.; Valtorta, E.; Spyridon, A.; Bimbatti, M.; Zocchi, M.; Piva, C.; et al. The Reproducibility of the Immunohistochemical PD-L1 Testing in Non-Small-Cell Lung Cancer: A Multicentric Italian Experience. Biomed Res. Int. 2019, 2019, 6832909. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Abreu, D.R.; Felip, E.; Carcereny, E.; Gottfried, M.; Wehler, T.; Ahn, M.-J.; Dolled-Filhart, M.; Zhang, J.; Shentu, Y.; et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann. Oncol. 2016, 27 (Suppl. S6), vi363. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, H.; Zhou, Y.; Mao, F.; Lin, Y.; Pan, B.; Zhang, X.; Xu, Q.; Huang, X.; Sun, Q. Prognostic Value of PD-L1 in Breast Cancer: A Meta-Analysis. Breast J. 2017, 23, 436–443. [Google Scholar] [CrossRef]
- Humphries, M.P.; Hynes, S.; Bingham, V.; Cougot, D.; James, J.; Patel-Socha, F.; Parkes, E.E.; Blayney, J.K.; O’Rorke, M.A.; Irwin, G.W.; et al. Automated Tumour Recognition and Digital Pathology Scoring Unravels New Role for PD-L1 in Predicting Good Outcome in ER-/HER2+ Breast Cancer. J. Oncol. 2018, 2018, 2937012. [Google Scholar] [CrossRef]
- Li, Y.; Opyrchal, M.; Yao, S.; Peng, X.; Yan, L.; Jabbour, H.; Khoury, T. The role of programmed death ligand-1 and tumor-infiltrating lymphocytes in breast cancer overexpressing HER2 gene. Breast Cancer Res. Treat. 2018, 170, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Noske, A.; Möbus, V.; Weber, K.; Schmatloch, S.; Weichert, W.; Köhne, C.H.; Solbach, C.; Ingold Heppner, B.; Steiger, K.; Müller, V.; et al. Relevance of tumour-infiltrating lymphocytes, PD-1 and PD-L1 in patients with high-risk, nodal-metastasised breast cancer of the German Adjuvant Intergroup Node–positive study. Eur. J. Cancer 2019, 114, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Ali, H.R.; Viens, P.; Caldas, C.; Birnbaum, D.B.F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449–5464. [Google Scholar] [CrossRef] [Green Version]
- Van Berckelaer, C.; Rypens, C.; Van Dam, P.; Pouillon, L.; Parizel, M.; Schats, K.A.; Kockx, M.; Tjalma, W.A.A.; Vermeulen, P.; Van Laere, S.; et al. Infiltrating stromal immune cells in inflammatory breast cancer are associated with an improved outcome and increased PD-L1 expression. Breast Cancer Res. 2019, 21, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Ran, R.; Shao, B.; Li, H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2019, 178, 17–33. [Google Scholar] [CrossRef]
- Parvathareddy, S.K.; Siraj, A.K.; Ahmed, S.O.; Ghazwani, L.O.; Aldughaither, S.M.; Al-Dayel, F.; Tulbah, A.; Ajarim, D.; Al-Kuraya, K.S. PD-L1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer. Cells 2021, 10, 229. [Google Scholar] [CrossRef]
- Buisseret, L.; Garaud, S.; De Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.N.; Duvillier, H.; et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-l1 expression are linked in breast cancer. Oncoimmunology. 2017, 6, e1257452. [Google Scholar] [CrossRef]
- Dieci, M.V.; Guarneri, V.; Bisagni, G.; Tosi, A.; Musolino, A.; Spazzapan, S.; Moretti, G.; Vernaci, G.M.; Giarratano, T.; Lo Mele, M.; et al. 162MO Neoadjuvant chemotherapy and immunotherapy in Luminal B BC: Results of the phase II GIADA trial. Ann. Oncol. 2020, 31 (Suppl. S4), S303–S339. [Google Scholar] [CrossRef]
- Dirix, L.; Takács, I.; Nikolinakos, P.; Jerusalem, G.; Arkenau, H.-T.; Hamilton, E.; Von Heydebreck, A.; Grote, H.-J.; Chin, K.; Lippman, M. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase Ib JAVELIN solid tumor trial. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.M.; Gulley, J.L. Product review: Avelumab, an anti-PD-L1 antibody. Hum. Vaccines Immunother. 2019, 15, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Delord, J.P.; Im, S.A.; Ott, P.A.; Piha-Paul, S.A.; Bedard, P.L.; Sachdev, J.; Le Tourneau, C.; van Brummelen, E.M.J.; Varga, A.; et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin. Cancer Res. 2018, 24, 2804–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Liu, Z.; Yu, Q.; Wang, X.; Bian, M.; Yu, Z.; Yu, J. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci. Rep. 2019, 9, 14356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.B.; Cho, H.D.; Oh, M.H.; Lee, J.H.; Jang, S.H.; Hong, S.A.; Cho, J.; Kim, S.Y.; Han, S.W.; Lee, J.E.; et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J. Breast Cancer 2016, 19, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmanabhan, R.; Kheraldine, H.S.; Meskin, N.; Vranic, S.; Al Moustafa, A.E. Crosstalk between HER2 and PD-1/PD-L1 in breast cancer: From clinical applications to mathematical models. Cancers 2020, 12, 636. [Google Scholar] [CrossRef] [Green Version]
- Kyriazoglou, A.; Kaparelou, M.; Goumas, G.; Liontos, M.; Zakopoulou, R.; Zografos, E.; Zygogianni, A.; Dimopoulos, M.A.; Zagouri, F. Immunotherapy in HER2-Positive Breast Cancer: A Systematic Review. Breast Care 2021, 11, 53–69. [Google Scholar] [CrossRef]
- Hou, Y.; Nitta, H.; Wei, L.; Banks, P.M.; Parwani, A.V.; Li, Z. Evaluation of Immune Reaction and PD-L1 Expression Using Multiplex Immunohistochemistry in HER2-Positive Breast Cancer: The Association with Response to Anti-HER2 Neoadjuvant Therapy. Clin. Breast Cancer 2018, 18, e237–e244. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Mani, A.; Shah, J.; et al. Overall survival (OS) in KATE2, a phase II study of programmed death ligand 1 (PD-L1) inhibitor atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC). Ann. Oncol. 2019, 30 (Suppl. S5), v104. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Ghosh, J.; Chatterjee, M.; Ganguly, S.; Datta, A.; Biswas, B.; Mukherjee, G.; Agarwal, S.; Ahmed, R.; Chatterjee, S.; Dabkara, D. PDL1 expression and its correlation with outcomes in non-metastatic triple-negative breast cancer (TNBC). Ecancermedicalscience 2021, 15, 1217. [Google Scholar] [CrossRef]
- Núñez Abad, M.; Calabuig-Fariñas, S.; Lobo de Mena, M.; José Godes Sanz de Bremond, M.; García González, C.; Torres Martínez, S.; García-García, J.Á.; Iranzo González-Cruz, V.; Camps Herrero, C. Update on systemic treatment in early triple negative breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835920986749. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Huang, C.-S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Abstract GS3-04: Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Cancer Res. 2020, 80, 4. [Google Scholar]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Goodman, A. No Improved Pathologic Complete Response with Atezolizumab in Early Triple-Negative Breast Cancer. Available online: https://ascopost.com/issues/february-25-2020/no-improved-pcr-with-atezolizumab-in-early-triple-negative-breast-cancer/ (accessed on 1 January 2022).
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 tria. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib keynote-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Dieras, V.C.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. LBA16 IMpassion130: Final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann. Oncol. 2020, 31, 1148. [Google Scholar] [CrossRef]
- Miles, D.; Andre, F.; Gligorov, J.; Verma, S.; Xu, B.; Cameron, D.; Barrios, C.H.; Schneeweiss, A.; Easton, V.; Dolado, I.; et al. IMpassion131: Phase III study comparing 1L atezolizumab with paclitaxel vs placebo with paclitaxel in treatment-naive patients with inoperable locally advanced or metastatic triple negative breast cancer (mTNBC). Ann. Oncol. 2017, 28 (Suppl. S5), v105. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Mani, S.A.; Moulder, S.L. Targeting the Molecular Subtypes of Triple Negative Breast Cancer: Understanding the Diversity to Progress the Field. Oncologist 2017, 22, 1086–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; De Azambuja, E.; Demichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer 5 behalf of the ESMO Guidelines Committee. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
| PD-L1 Scores | Analysis Assessment | References |
|---|---|---|
| Tumor Cell (TC) | TC (%) = [Number of PD-L1-stained tumor cells/Total number of viable tumor cells] × 100% | [18,22,23] |
| Tumor-Proportion Score (TPS) | TPS (%) = [Number of PD-L1-stained tumor cells/Total number of viable tumor cell] × 100% | [18,22,24] |
| Immune-Cell Score (IC) | IC (%) = [Area of tumor infiltrated by PD-L1-stained immune cells/Total tumor area] × 100% | [18,22,25] |
| Immune Cells Present (ICP) | ICP (%) = Percentage of tumor area occupied by any PD-L1 positive immune cell staining. | [25] |
| Combined Positive Score (CPS) | CPS = [Number of PD-L1-stained cells (tumor cells, lymphocytes and macrophages)/Total number of viable tumor cells] × 100 | [18,22,23] |
| Antibody Clone | Platform | Scoring Method | Predictive Drug | References |
|---|---|---|---|---|
| SP142 (monoclonal, rabbit) | BenchMark Ultra | IC or TC | Atezolizumab (anti-PD-L1) | [5,6,18,27,28,29,30] |
| SP263 (monoclonal, rabbit) | BenchMark Ultra | TPS, TC or IC | Durvalumab (anti-PD-L1) | [5,6,18,27,28,29,30] |
| 22C3 (monoclonal, mouse) | Dako Autostainer Link 48 | TPS or CPS | Pembrolizumab (anti-PD-1) | [5,6,18,27,28,29,30] |
| 28-8 (monoclonal, rabbit) | Dako Autostainer Link 48 | TPS | Nivolumab (anti-PD-1) | [5,6,18,27,28,29,30] |
| E1L3N (monoclonal, rabbit) | Not linked to a specific staining platform | IC, TPS or CPS | Non-associated drug | [5,6,18,27,28,29,30,31] |
| 73-10 (monoclonal, rabbit) | Dako Autostainer Link 48 | Not established yet | Avelumab (anti-PD-L1) | [6,22] |
| Clinical Trial | Breast Cancer Subtype and Stage | Number of Patients Included | PD-L1 Expression | Antibody Clone | Immunotherapy Drug | Associated Drugs | Response Rates |
|---|---|---|---|---|---|---|---|
| GIADA | Early luminal B | 43 | Any expression | 28-8 (DAKO PharmDx) | Nivolumab | Anthracyclines plus endocrine therapy | 16.3% pCR |
| JAVELIN Solid Tumor | Pretreated and metastatic BC | 168 | Any expression | 73–10 (DAKO PharmDx) | Avelumab | No other drugs | ORR 2.8% in luminal, 0% in HER2+ and 5.2% in TNBC |
| KEYNOTE-028 | Pretreated and advanced luminal BC | 25 | CPS ≥ 1 | 22C3 (DAKO PharmDx) | Pembrolizumab | No other drugs | ORR 12% |
| KATE2 | Pretreated and advanced HER2-positive BC | 202 | Any expression | SP142 (VENTANA) | Atezolizumab | T-DM1 | Unknown ORR |
| PANACEA | Pretreated and advanced HER2-positive BC | 58 | Any expression | 22C3 (DAKO PharmDx) | Pembrolizumab | Trastuzumab | ORR 15% in PD-L1+, no ORR in PD-L1- |
| GeparNuevo | Early TNBC | 174 | Any expression | SP263 (VENTANA) | Durvalumab vs. placebo | Nab-paclitaxel, + epirubicin and CP | 53.4% of pCR in durvalumab arm vs. 44.2% in placebo arm |
| KEYNOTE-522 | Early TNBC | 602 | Any expression | 22C3 (DAKO PharmDx) | Pembrolizumab vs. placebo | Paclitaxel and carboplatin + anthracyclines and CP | 64.8% of pCR in pembrolizumab arm vs. 51.2% in placebo arm |
| NeoTRIP | Early TNBC | 280 | Any expression | SP142 (VENTANA) | Atezolizumab | Nab-paclitaxel + carboplatin | 43.5% of pCR in atezolizumab arm vs. 40.8% in placebo arm |
| IMpassion 031 | Early TNBC | 333 | Any expression | SP142 (VENTANA) | Atezolizumab | Nab-paclitaxel + doxorubicin and CP | 57.6% of pCR in atezolizumab arm vs. 41% in placebo arm |
| KEYNOTE-012 | Pretreated and advanced TNBC | 111 | ≥1% of TCs and IC by IHC | 22C3 (DAKO PharmDx) | Pembrolizumab | No other drugs | ORR 18.5% |
| KEYNOTE-119 | Pretreated and advanced TNBC | 622 | Any expression. Stratified by PD-L1 | 22C3 (DAKO PharmDx) | Pembrolizumab vs. single-drug CT | No other drugs | ORR (~9% in both arms) |
| KEYNOTE-355 | Advanced TNBC | 847 | Any expression. Stratified by PD-L1 | 22C3 (DAKO PharmDx) | Pembrolizumab vs. placebo | Chemotherapy | ORR 41% in combination arm and 35.9% in CT arm |
| IMpassion 130 | Advanced TNBC | 902 | Any expression. Stratified by PD-L1 | SP142 (VENTANA) | Atezolizumab vs. placebo | Nab-paclitaxel | ORR 56% in combination arm and 45.9% in CT arm |
| IMpassion 131 | Advanced TNBC | 651 | Any expression. Stratified by PD-L1 | SP142 (VENTANA) | Atezolizumab vs. placebo | Paclitaxel | ORR 53.6% in combination arm and 47.5% in CT arm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez Abad, M.; Calabuig-Fariñas, S.; Lobo de Mena, M.; Torres-Martínez, S.; García González, C.; García García, J.Á.; Iranzo González-Cruz, V.; Camps Herrero, C. Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer. Cancers 2022, 14, 307. https://doi.org/10.3390/cancers14020307
Núñez Abad M, Calabuig-Fariñas S, Lobo de Mena M, Torres-Martínez S, García González C, García García JÁ, Iranzo González-Cruz V, Camps Herrero C. Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer. Cancers. 2022; 14(2):307. https://doi.org/10.3390/cancers14020307
Chicago/Turabian StyleNúñez Abad, Martín, Silvia Calabuig-Fariñas, Miriam Lobo de Mena, Susana Torres-Martínez, Clara García González, José Ángel García García, Vega Iranzo González-Cruz, and Carlos Camps Herrero. 2022. "Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer" Cancers 14, no. 2: 307. https://doi.org/10.3390/cancers14020307






