Intensity-Modulated Radiotherapy with Regional Hyperthermia for High-Risk Localized Prostate Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
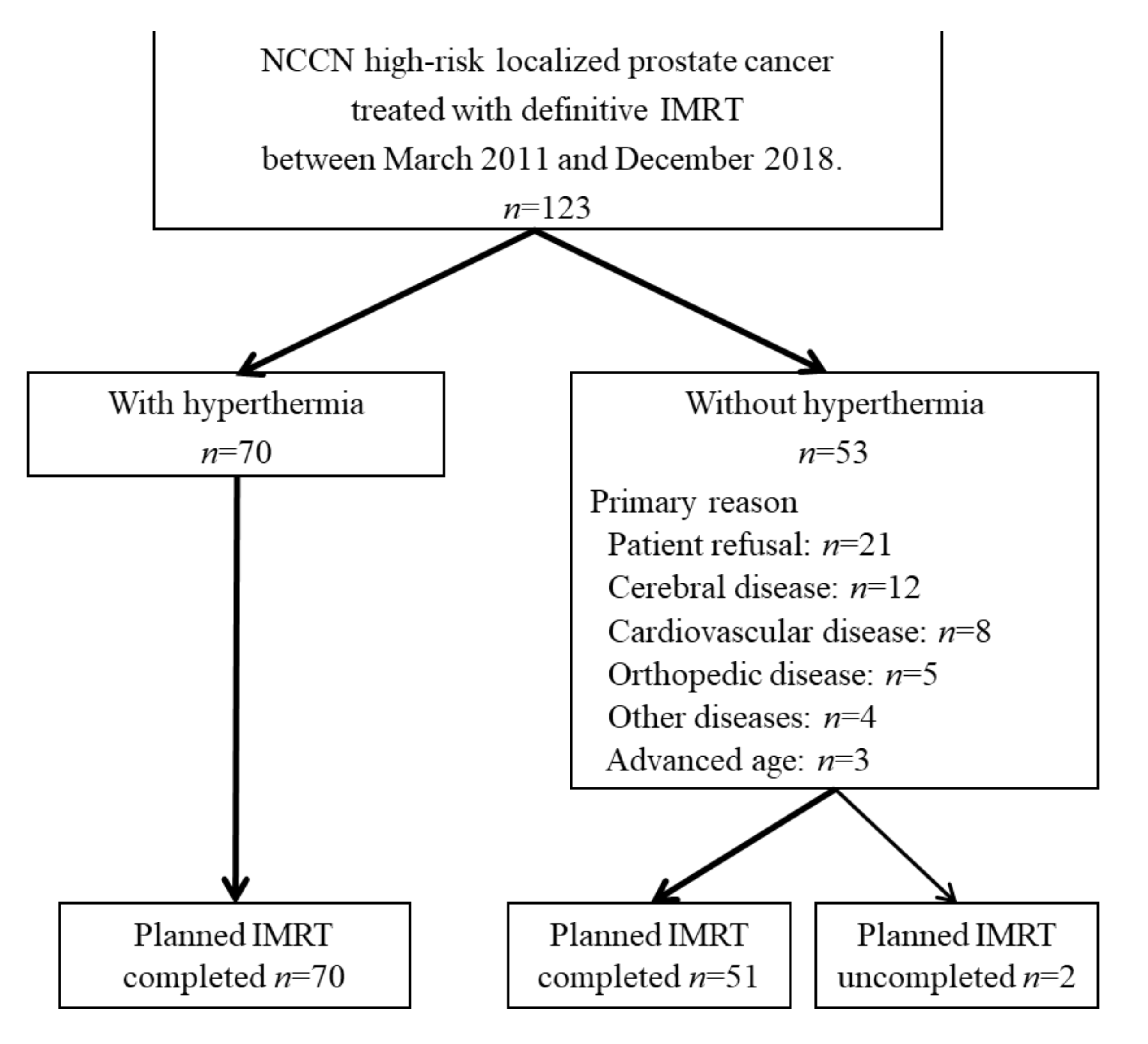
2.1. Patients
2.2. IMRT
2.3. Hyperthermia
2.4. Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Thermal Data
3.2. Efficacy and Prognostic Factors
3.3. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dal Pra, A.; Souhami, L. Prostate cancer radiation therapy: A physicians’ perspective. Phys. Med. 2016, 32, 438–445. [Google Scholar] [CrossRef]
- Grimm, P.; Billiet, I.; Bostwick, D.; Dicker, A.; Frank, S.; Immerzeel, J.; Keyes, M.; Kupelian, P.; Lee, W.R.; Machtens, S.; et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012, 109 (Supplement S1), 22–29. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Ordonez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Cihoric, N.; Tsikkinis, A.; van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis, S.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int. J. Hyperth. 2015, 31, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; Samulski, T.V.; Vujaskovic, Z.; Leonard, R.P.; Dewhirst, M.W. Hyperthermia. Principles and Practice of Radiation Oncology, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Anscher, M.S.; Samulski, T.V.; Leopold, K.A.; Oleson, J.R. Phase I/II study of external radio frequency phased array hyperthermia and external beam radiotherapy in the treatment of prostate cancer: Technique and results of intraprostatic temperature measurements. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 489–495. [Google Scholar] [CrossRef]
- Anscher, M.S.; Samulski, T.V.; Dodge, R.; Prosnitz, L.R.; Dewhirst, M.W. Combined external beam irradiation and external regional hyperthermia for locally advanced adenocarcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 1059–1065. [Google Scholar] [CrossRef]
- Van Vulpen, M.; De Leeuw, A.A.; Raaymakers, B.W.; Van Moorselaar, R.J.; Hofman, P.; Lagendijk, J.J.; Battermann, J.J. Radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate cancer: Preliminary results. BJU Int. 2004, 93, 36–41. [Google Scholar] [CrossRef]
- Tilly, W.; Gellermann, J.; Graf, R.; Hildebrandt, B.; Weissbach, L.; Budach, V.; Felix, R.; Wust, P. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol. 2005, 181, 35–41. [Google Scholar] [CrossRef]
- Maluta, S.; Dall’Oglio, S.; Romano, M.; Marciai, N.; Pioli, F.; Giri, M.G.; Benecchi, P.L.; Comunale, L.; Porcaro, A.B. Conformal radiotherapy plus local hyperthermia in patients affected by locally advanced high risk prostate cancer: Preliminary results of a prospective phase II study. Int. J. Hyperth. 2007, 23, 451–456. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Hansen, J.L.; Prokopios-Davos, S.; Manola, J.; Wang, Q.; Bornstein, B.A.; Hynynen, K.; Kaplan, I.D. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: Long-term results from Dana-Farber Cancer Institute study 94-153. Cancer 2011, 117, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Yahara, K.; Ohguri, T.; Yamaguchi, S.; Imada, H.; Narisada, H.; Ota, S.; Tomura, K.; Sakagami, M.; Fujimoto, N.; Korogi, Y. Definitive radiotherapy plus regional hyperthermia for high-risk and very high-risk prostate carcinoma: Thermal parameters correlated with biochemical relapse-free survival. Int. J. Hyperth. 2015, 3, 600–608. [Google Scholar]
- Abe, M.; Hiraoka, M.; Takahashi, M.; Egawa, S.; Matsuda, C.; Onoyama, Y.; Morita, K.; Kakehi, M.; Sugahara, T. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer 1986, 58, 1589–1595. [Google Scholar] [CrossRef]
- Hiraoka, M.; Jo, S.; Akuta, K.; Nishimura, Y.; Takahashi, M.; Abe, M. Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer 1987, 60, 121–127. [Google Scholar] [CrossRef]
- Kakehi, M.; Ueda, K.; Mukojima, T.; Hiraoka, M.; Seto, O.; Akanuma, A.; Nakatsugawa, S. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int. J. Hyperth. 1990, 6, 719–740. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M.; Nishimura, Y.; Nagata, Y.; Mitsumori, M.; Okuno, Y.; Li, P.Y.; Abe, M.; Takahashi, M.; Masunaga, S.; Akuta, K.; et al. Site-specific phase I, II trials of hyperthermia at Kyoto University. Int. J. Hyperth. 1994, 10, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, S.I.; Hiraoka, M.; Akuta, K.; Nishimura, Y.; Nagata, Y.; Jo, S.; Takahashi, M.; Abe, M.; Terachi, T.; Oishi, K.; et al. Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int. J. Hyperth. 1994, 10, 31–40. [Google Scholar] [CrossRef]
- Song, C.W.; Rhee, J.G.; Lee, C.K.; Levitt, S.H. Capacitive heating of phantom and human tumors with an 8 MHz radiofrequency applicator (Thermotron RF-8). Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 365–372. [Google Scholar] [CrossRef]
- Tomura, K.; Ohguri, T.; Mulder, H.T.; Murakami, M.; Nakahara, S.; Yahara, K.; Korogi, Y. The usefulness of mobile insulator sheets for the optimisation of deep heating area for regional hyperthermia using a capacitively coupled heating method: Phantom, simulation and clinical prospective studies. Int. J. Hyperth. 2018, 34, 1092–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleson, J.R.; Samulski, T.V.; Leopold, K.A.; Clegg, S.T.; Dewhirst, M.W.; Dodge, R.K.; George, S.L. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 289–297. [Google Scholar] [CrossRef]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized trial of hyperthermia and radiation for superficial tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef] [Green Version]
- Dewhirst, M.W.; Viglianti, B.L.; Lora-Michiels, M.; Hanson, M.; Hoopes, P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth. 2003, 19, 267–294. [Google Scholar] [CrossRef]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Torrecilla, J.; Pastor-Peidro, J.; Vicedo-Gonzalez, A.; Gonzalez-Sanchis, D.; Hernandez-Machancoses, A.; Almendros-Blanco, P.; García-Miragall, E.; Gordo-Partearroyo, J.C.; García-Hernández, T.; Brualla-González, L.; et al. Patterns of treatment failure in patients with prostate cancer treated with 76-80 Gy radiotherapy to the prostate and seminal vesicles +/− hormonotherapy. Clin. Transl. Oncol. 2021, 23, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, D.; Yamazaki, H.; Nishimura, T.; Aibe, N.; Okabe, H. Long-term Tumor Control and Late Toxicity in Patients with Prostate Cancer Receiving Hypofractionated (2.2 Gy) Soft-tissue-matched Image-guided Intensity-modulated Radiotherapy. Anticancer. Res. 2017, 37, 5829–5835. [Google Scholar] [PubMed]
- Marvaso, G.; Riva, G.; Ciardo, D.; Gandini, S.; Fodor, C.I.; Zerini, D.; Colangione, S.P.; Timon, G.; Comi, S.; Cambria, R.; et al. “Give me five” ultra-hypofractionated radiotherapy for localized prostate cancer: Non-invasive ablative approach. Med. Oncol. 2018, 35, 96. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Fuks, Z.; Hunt, M.; Yamada, Y.; Marion, C.; Ling, C.; Amols, H.; Venkatraman, E.; A Leibel, S. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1111–1116. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Kaplan, I.D.; Hansen, J.L.; Prokopios-Davos, S.; Topulos, G.P.; Wishnow, K.; Manola, J.; Bornstein, B.A.; Hynynen, K. Hyperthermia combined with radiation in treatment of locally advanced prostate cancer is associated with a favourable toxicity profile. Int. J. Hyperth. 2005, 21, 649–656. [Google Scholar] [CrossRef]
- Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, D.; Whaley, J.B.; van der Zee, J.; van Putten, W.L.; van Rhoon, G.C.; van Dijk, J.D.; Gonzales Gonzales, D.; et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar]
- Overgaard, J.; Gonzalez Gonzalez, D.; Hulshof, M.C.; Arcangeli, G.; Dahl, O.; Mella, O.; Bentzen, S.M. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Eur. Soc. Hyperthermic Oncol. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef]
- Sherar, M.; Liu, F.F.; Pintilie, M.; Levin, W.; Hunt, J.; Hill, R.; Hand, J.; Vernon, C.; van Rhoon, G.; van der Zee, J.; et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Overgaard, J.; Gonzalez Gonzalez, D.; Hulshof, M.C.; Arcangeli, G.; Dahl, O.; Mella, O.; Bentzen, S.M. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. Eur. Soc. Hyperthermic Oncol. Int. J. Hyperth. 1996, 12, 3–20. [Google Scholar] [CrossRef]
- Franckena, M.; Fatehi, D.; De Bruijne, M.; Canters, R.A.M.; Van Norden, Y.; Mens, J.W.; Van Rhoon, G.C.; Van Der Zee, J. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Wust, P.; Tilly, W.; Gellermann, J.; Harder, C.; Riess, H.; Budach, V.; Felix, R.; Schlag, P.M. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 381–391. [Google Scholar] [CrossRef]
- Van Rhoon, G.C. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int. J. Hyperth. 2016, 32, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.; Wust, P.; Stauffer, P.; Bardati, F.; van Rhoon, G.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Canters, R.A.M.; Paulides, M.; Franckena, M.F.; Van Der Zee, J.; Van Rhoon, G.C. Implementation of treatment planning in the routine clinical procedure of regional hyperthermia treatment of cervical cancer: An overview and the Rotterdam experience. Int. J. Hyperth. 2012, 28, 570–581. [Google Scholar] [CrossRef]
- VilasBoas-Ribeiro, I.; Van Rhoon, G.C.; Drizdal, T.; Franckena, M.; Paulides, M.M. Impact of Number of Segmented Tissues on SAR Prediction Accuracy in Deep Pelvic Hyperthermia Treatment Planning. Cancers (Basel) 2020, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Ohguri, T.; Kuroda, K.; Yahara, K.; Nakahara, S.; Kakinouchi, S.; Itamura, H.; Morisaki, T.; Korogi, Y. Optimization of the clinical setting using numerical simulations of the electromagnetic field in an obese patient model for deep regional hyperthermia of an 8 MHz radiofrequency capacitively coupled device in the pelvis. Cancers (Basel) 2021, 13, 979. [Google Scholar] [CrossRef] [PubMed]
- Rodda, S.; Tyldesley, S.; Morris, W.J.; Keyes, M.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; et al. ASCENDE-RT: An analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 286–295. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | With Hyperthermia | Without Hyperthermia | p |
|---|---|---|---|
| n = 70 (%) | n = 51 (%) | ||
| Age (median, range) | 72 (54–80) | 71 (54–83) | 0.3381 |
| Performance status | 0.1948 | ||
| 0 | 41 (59) | 25 (49) | |
| 1 | 29 (41) | 23 (45) | |
| 2 | 0 | 2 (4) | |
| 3 | 0 | 1 (2) | |
| T stage | 0.8000 | ||
| T1 | 25 (36) | 18 (35) | |
| T2 | 31 (44) | 25 (49) | |
| T3a | 14 (20) | 8 (16) | |
| N stage | |||
| N0 | 72 (100) | 51 (100) | |
| Gleason score | 0.4774 | ||
| ≤7 | 17 (24) | 14 (28) | |
| 8 | 25 (36) | 22 (43) | |
| 9–10 | 28 (40) | 15 (29) | |
| Pretreatment PSA (ng/mL) | 0.6095 | ||
| <10 | 20 (29) | 17 (33) | |
| 10–20 | 19 (27) | 16 (31) | |
| >20 | 31 (44) | 18 (35) | |
| IMRT | |||
| 76 Gy, 38 fractions | 72 (100) | 51 (100) | |
| Total ADT duration | 0.2296 | ||
| <6 months | 2 (3) | 0 (0) | |
| 6–11 months | 46 (66) | 29 (57) | |
| ≥12 months | 22 (31) | 22 (43) | |
| Hyperthermia | |||
| Number of sessions | |||
| 1 | 1 (1) | - | |
| 2 | 1 (1) | - | |
| 3 | 3 (4) | - | |
| 4 | 2 (3) | - | |
| 5 | 49 (70) | - | |
| 6 | 12 (17) | - | |
| 7 | 2 (3) | - |
| Variation | Patients (n) | 5-y (%) | p (Log-Rank Test) | Hazard Ratio * (95% Confidence Interval) |
|---|---|---|---|---|
| T stage | ||||
| T1–T2 | 99 | 87.4 | 0.6978 | 0.777 (0.217–2.786) |
| T3a | 22 | 84.0 | ||
| Gleason score | ||||
| ≤8 | 78 | 86.8 | 0.8710 | 0.913 (0.306–2.726) |
| ≥9 | 43 | 87.2 | ||
| Pretreatment PSA (ng/mL) | ||||
| ≤20 | 72 | 88.4 | 0.4478 | 0.668 (0.234–1.905) |
| >20 | 49 | 84.8 | ||
| Total ADT (months) | ||||
| ≤10 | 70 | 84.0 | 0.3344 | 0.569 (0.178–1.815) |
| >10 | 51 | 91.3 | ||
| Hyperthermia | ||||
| Yes | 70 | 89.8 | 0.2170 | 0.519 (0.180–1.497) |
| None | 51 | 82.9 | ||
| Hyperthermia | ||||
| CEM43T90 > 7 | 39 | 96.4 | 0.0296 | 0.144 (0.019–1.099) |
| None or CEM43T90 ≤ 7 | 82 | 82.4 |
| Variation | Patients (n) | 5-y (%) | p (Log-Rank Test) | Hazard Ratio * (95% Confidence Interval) |
|---|---|---|---|---|
| T stage | ||||
| T1–T2 | 56 | 89.0 | 0.8403 | 0.802 (0.094–6.869) |
| T3a | 14 | 92.9 | ||
| Gleason score | ||||
| ≤8 | 42 | 91.2 | 0.5298 | 0.602 (0.121–2.984) |
| ≥9 | 28 | 87.7 | ||
| Pretreatment PSA (ng/mL) | ||||
| ≤20 | 39 | 89.7 | 0.784 | 0.800 (0.161–3.964) |
| >20 | 31 | 89.7 | ||
| Total ADT (months) | ||||
| ≤10 | 42 | 86.3 | 0.2986 | 0.338 (0.039–2.894) |
| >10 | 28 | 96.3 | ||
| Hyperthermia | ||||
| CEM43T90 (min) | ||||
| ≤7 | 31 | 81.5 | 0.0316 | 0.134 (0.016–1.152) |
| >7 | 39 | 96.4 |
| Variation | Patients (n) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| 5-y (%) | p * | Hazard Ratio ** (95% CI) | p | Hazard Ratio (95% CI) | ||
| T stage | ||||||
| T1–T2 | 99 | 94.2 | 0.5391 | 0.601 (0.116–3.107) | 0.564 | 0.600 (0.106–3.403) |
| T3a | 22 | 93.3 | ||||
| Gleason score | ||||||
| ≤8 | 78 | 95.7 | 0.5723 | 0.651 (0.145–2.920) | 0.317 | 0.455 (0.097–2.125) |
| ≥9 | 43 | 90.3 | ||||
| Pretreatment PSA (ng/mL) | ||||||
| ≤20 | 72 | 92.8 | 0.5504 | 0.610 (0.118–3.144) | 0.597 | 0.612 (0.100–3.766) |
| >20 | 49 | 95.5 | ||||
| Total ADT (months) | ||||||
| ≤10 | 70 | 91.5 | 0.1592 | 0.246 (0.030–2.043) | 0.121 | 0.170 (0.018–1.599) |
| >10 | 51 | 97.4 | ||||
| Hyperthermia | ||||||
| Yes | 70 | 98.0 | 0.0229 | 0.126 (0.015–1.049) | 0.035 | 0.099 (0.000–0.852) |
| None | 51 | 88.6 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakahara, S.; Ohguri, T.; Kakinouchi, S.; Itamura, H.; Morisaki, T.; Tani, S.; Yahara, K.; Fujimoto, N. Intensity-Modulated Radiotherapy with Regional Hyperthermia for High-Risk Localized Prostate Carcinoma. Cancers 2022, 14, 400. https://doi.org/10.3390/cancers14020400
Nakahara S, Ohguri T, Kakinouchi S, Itamura H, Morisaki T, Tani S, Yahara K, Fujimoto N. Intensity-Modulated Radiotherapy with Regional Hyperthermia for High-Risk Localized Prostate Carcinoma. Cancers. 2022; 14(2):400. https://doi.org/10.3390/cancers14020400
Chicago/Turabian StyleNakahara, Sota, Takayuki Ohguri, Sho Kakinouchi, Hirohide Itamura, Takahiro Morisaki, Subaru Tani, Katuya Yahara, and Naohiro Fujimoto. 2022. "Intensity-Modulated Radiotherapy with Regional Hyperthermia for High-Risk Localized Prostate Carcinoma" Cancers 14, no. 2: 400. https://doi.org/10.3390/cancers14020400
APA StyleNakahara, S., Ohguri, T., Kakinouchi, S., Itamura, H., Morisaki, T., Tani, S., Yahara, K., & Fujimoto, N. (2022). Intensity-Modulated Radiotherapy with Regional Hyperthermia for High-Risk Localized Prostate Carcinoma. Cancers, 14(2), 400. https://doi.org/10.3390/cancers14020400






