Treatment of Locally Advanced Merkel Cell Carcinoma—A Multi-Center Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
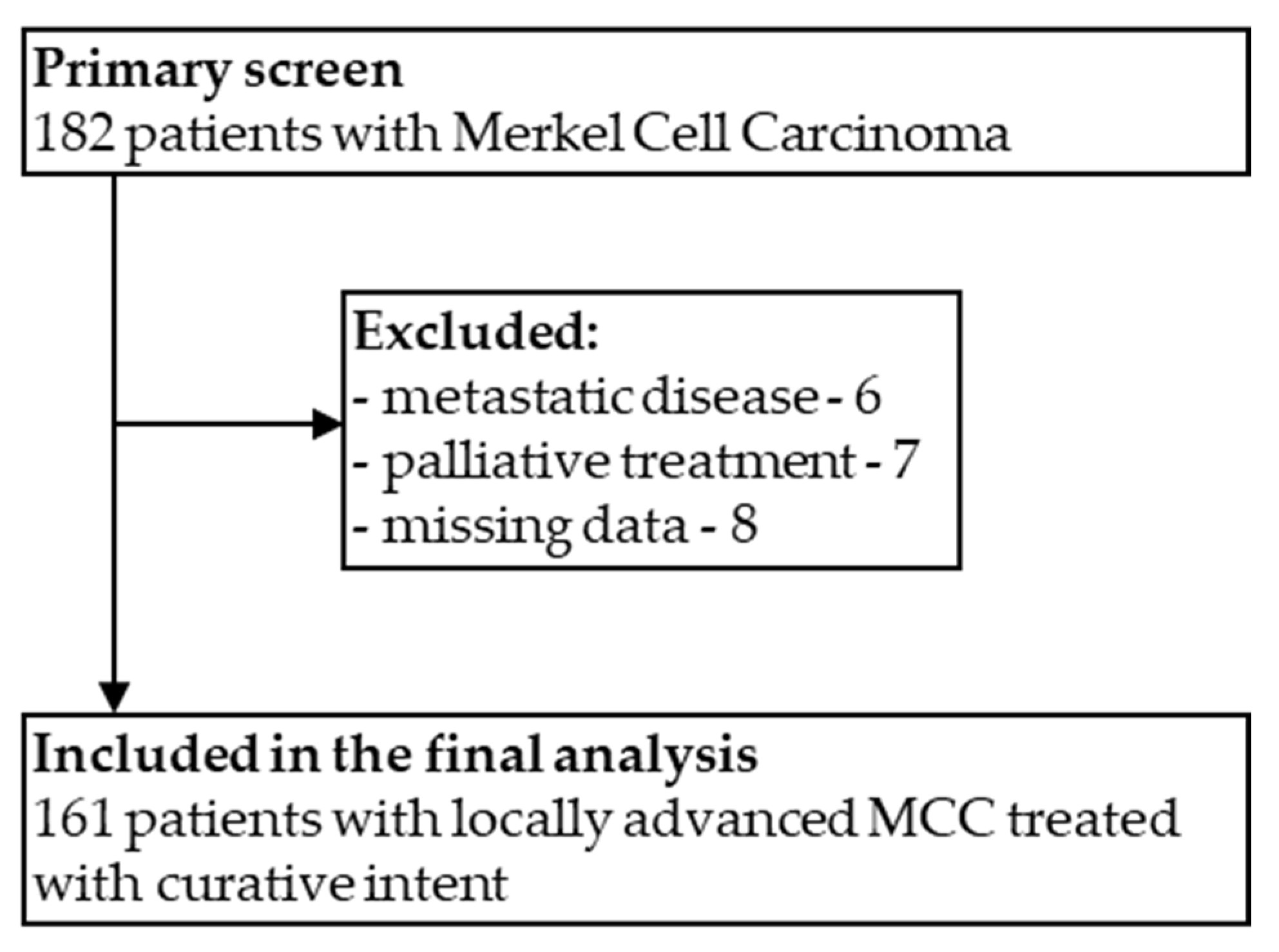
2.1. Patients Selection and Data Collection
2.2. Statistical Analyses
3. Results
3.1. Patients
3.2. Curative Treatment
3.3. Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Merkel Cell Carcinoma (Version 1.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf (accessed on 30 August 2021).
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th ed. AJCC Staging System. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef]
- Iyer, J.G.; Storer, B.E.; Paulson, K.G.; Lemos, B.; Phillips, J.L.; Bichakjian, C.K.; Zeitouni, N.; Gershenwald, J.E.; Sondak, V.; Otley, C.C.; et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J. Am. Acad. Dermatol. 2014, 70, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Allen, P.J.; Bowne, W.B.; Jaques, D.P.; Brennan, M.F.; Busam, K.; Coit, D.G. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J. Clin. Oncol. 2005, 23, 2300–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudzisz-Śledź, M.; Zdzienicki, M.; Rutkowski, P. Merkel cell carcinoma (MCC)—neuroendocrine skin cancer. Nowotwory J. Oncol. 2019, 69, 111–116. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Griffith, K.A.; Lowe, L.; Wong, S.L.; McLean, S.A.; Fullen, D.R.; Lao, C.D.; Hayman, J.A.; Bradford, C.R.; Rees, R.S.; et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J. Clin. Oncol. 2011, 29, 1036–1041. [Google Scholar] [CrossRef] [Green Version]
- Sims, J.R.; Grotz, T.E.; Pockaj, B.A.; Joseph, R.W.; Foote, R.L.; Otley, C.C.; Weaver, A.L.; Jakub, J.W.; Price, D.L. Sentinel lymph node biopsy in Merkel cell carcinoma: The Mayo Clinic experience of 150 patients. Surg. Oncol. 2018, 27, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.O.; Yue, B.; Marzban, S.S.; Walls, B.L.; Carr, M.; Jackson, R.S.; Puleo, C.A.; Padhya, T.; Cruse, C.W.; Gonzalez, R.J.; et al. Both tumor depth and diameter are predictive of sentinel lymph node status and survival in Merkel cell carcinoma. Cancer 2015, 121, 3252–3260. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, V.A.; Camp, E.R.; Lentsch, E.J. Sentinel lymph node status in Merkel cell carcinoma of the head and neck: Not a predictor of survival. Head Neck 2014, 36, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Conic, R.R.Z.; Ko, J.; Saridakis, S.; Damiani, G.; Funchain, P.; Vidimos, A.; Gastman, B.R. Sentinel lymph node biopsy in Merkel cell carcinoma: Predictors of sentinel lymph node positivity and association with overall survival. J. Am. Acad. Dermatol. 2019, 81, 364–372. [Google Scholar] [CrossRef]
- Harounian, J.A.; Molin, N.; Galloway, T.J.; Ridge, D.; Bauman, J.; Farma, J.; Reddy, S.; Lango, M.N. Effect of Sentinel Lymph Node Biopsy and LVI on Merkel Cell Carcinoma Prognosis and Treatment. Laryngoscope 2021, 131, E828–E835. [Google Scholar] [CrossRef]
- Gunaratne, D.A.; Howle, J.R.; Veness, M.J. Sentinel lymph node biopsy in Merkel cell carcinoma: A 15-year institutional experience and statistical analysis of 721 reported cases. Br. J. Dermatol. 2016, 174, 273–281. [Google Scholar] [CrossRef]
- Karunaratne, Y.G.; Gunaratne, D.A.; Veness, M.J. Systematic review of sentinel lymph node biopsy in Merkel cell carcinoma of the head and neck. Head Neck 2018, 40, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.; Kwan, W. Radiotherapy and Conservative Surgery in the Locoregional Management of Merkel Cell Carcinoma: The British Columbia Cancer Agency Experience. Ann. Surg. Oncol. 2016, 23, 573–578. [Google Scholar] [CrossRef]
- Strom, T.; Carr, M.; Zager, J.S.; Naghavi, A.; Smith, F.O.; Cruse, C.W.; Messina, J.L.; Russell, J.; Rao, N.G.; Fulp, W.; et al. Radiation Therapy is Associated with Improved Outcomes in Merkel Cell Carcinoma. Ann. Surg. Oncol. 2016, 23, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Storer, B.E.; Iyer, J.G.; Moshiri, A.; Parvathaneni, U.; Byrd, D.; Sober, A.J.; Sondak, V.K.; Gershenwald, J.E.; Nghiem, P. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases From the National Cancer Data Base. J. Natl. Cancer Inst. 2016, 108, djw042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantola, T.I.; Vallow, L.A.; Halyard, M.Y.; Weenig, R.H.; Warschaw, K.E.; Grotz, T.E.; Jakub, J.W.; Roenigk, R.K.; Brewer, J.D.; Weaver, A.L.; et al. Prognostic factors in Merkel cell carcinoma: Analysis of 240 cases. J. Am. Acad. Dermatol. 2013, 68, 425–432. [Google Scholar] [CrossRef]
- Santamaria-Barria, J.A.; Boland, G.M.; Yeap, B.Y.; Nardi, V.; Dias-Santagata, D.; Cusack, J.C., Jr. Merkel cell carcinoma: 30-year experience from a single institution. Ann. Surg. Oncol. 2013, 20, 1365–1373. [Google Scholar] [CrossRef]
- Hitchcock, C.L.; Bland, K.I.; Laney, R.G., 3rd; Franzini, D.; Harris, B.; Copeland, E.M., 3rd. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann. Surg. 1988, 207, 201–207. [Google Scholar] [CrossRef]
- Medina-Franco, H.; Urist, M.M.; Fiveash, J.; Heslin, M.J.; Bland, K.I.; Beenken, S.W. Multimodality treatment of Merkel cell carcinoma: Case series and literature review of 1024 cases. Ann. Surg. Oncol. 2001, 8, 204–208. [Google Scholar] [CrossRef]
- Akhtar, S.; Oza, K.K.; Wright, J. Merkel cell carcinoma: Report of 10 cases and review of the literature. J. Am. Acad. Dermatol. 2000, 43, 755–767. [Google Scholar] [CrossRef]
- Lemos, B.D.; Storer, B.E.; Iyer, J.G.; Phillips, J.L.; Bichakjian, C.K.; Fang, L.C.; Johnson, T.M.; Liegeois-Kwon, N.J.; Otley, C.C.; Paulson, K.G.; et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J. Am. Acad. Dermatol. 2010, 63, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Agelli, M.; Clegg, L.X. Epidemiology of primary Merkel cell carcinoma in the United States. J. Am. Acad. Dermatol. 2003, 49, 832–841. [Google Scholar] [CrossRef]
- Hodgson, N.C. Merkel cell carcinoma: Changing incidence trends. J. Surg. Oncol. 2005, 89, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.L.; Dennis, S.; Kachare, S.D.; Vohra, N.A.; Wong, J.H.; Zervos, E.E. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the United States. Am. Surg. 2015, 81, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Reichgelt, B.A.; Visser, O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993–2007. Eur. J. Cancer 2011, 47, 579–585. [Google Scholar] [CrossRef]
- Lyhne, D.; Lock-Andersen, J.; Dahlstrøm, K.; Drzewiecki, K.T.; Balslev, E.; Muhic, A.; Krarup-Hansen, A. Rising incidence of Merkel cell carcinoma. J. Plast. Surg. Hand Surg. 2011, 45, 274–280. [Google Scholar] [CrossRef]
- van Veenendaal, L.M.; van Akkooi, A.C.J.; Verhoef, C.; Grünhagen, D.J.; Klop, W.M.C.; Valk, G.D.; Tesselaar, M.E.T. Merkel cell carcinoma: Clinical outcome and prognostic factors in 351 patients. J. Surg. Oncol. 2018, 117, 1768–1775. [Google Scholar] [CrossRef]
- Mercer, D.; Brander, P.; Liddell, K. Merkel cell carcinoma: The clinical course. Ann. Plast. Surg. 1990, 25, 136–141. [Google Scholar] [CrossRef]
- Sattler, E.; Geimer, T.; Sick, I.; Flaig, M.J.; Ruzicka, T.; Berking, C.; Kunte, C. Sentinel lymph node in Merkel cell carcinoma: To biopsy or not to biopsy? J. Dermatol. 2013, 40, 374–379. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, D.; Zhao, J.; Zhu, B.; Xie, J. Clinical Features and Prognosis of Merkel Cell Carcinoma in Elderly Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e924570. [Google Scholar] [CrossRef]
- Shibayama, Y.; Imafuku, S.; Takahashi, A.; Nakayama, J. Role of sentinel lymph node biopsy in patients with Merkel cell carcinoma: Statistical analysis of 403 reported cases. Int. J. Clin. Oncol. 2015, 20, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Servy, A.; Maubec, E.; Sugier, P.E.; Grange, F.; Mansard, S.; Lesimple, T.; Marinho, E.; Couturaud, B.; Girod, A.; Albert, S.; et al. Merkel cell carcinoma: Value of sentinel lymph-node status and adjuvant radiation therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2016, 27, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Muralidhar, V.; Margalit, D.N.; Tishler, R.B.; DeCaprio, J.A.; Thakuria, M.; Rabinowits, G.; Schoenfeld, J.D. Merkel Cell Carcinoma: A Population Analysis on Survival. J. Natl. Compr. Cancer Netw. JNCCN 2016, 14, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Goldhirsch, A. Different effectiveness of anticancer immunotherapy in men and women relies on sex-dimorphism of the immune system. Oncotarget 2018, 9, 31167–31168. [Google Scholar] [CrossRef]
- Tam, M.; Luu, M.; Barker, C.A.; Gharavi, N.M.; Hamid, O.; Shiao, S.L.; Nguyen, A.T.; Lu, D.J.; Ho, A.S.; Zumsteg, Z.S. Improved survival in women versus men with merkel cell carcinoma. J. Am. Acad. Dermatol. 2021, 84, 321–329. [Google Scholar] [CrossRef]
- Chen, M.M.; Roman, S.A.; Sosa, J.A.; Judson, B.L. The role of adjuvant therapy in the management of head and neck merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.D.; Suresh, K.; Sridharan, S. Completion lymph node dissection for merkel cell carcinoma. Am. J. Surg. 2020, 220, 982–986. [Google Scholar] [CrossRef]
- Asgari, M.M.; Sokil, M.M.; Warton, E.M.; Iyer, J.; Paulson, K.G.; Nghiem, P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014, 150, 716–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAfee, W.J.; Morris, C.G.; Mendenhall, C.M.; Werning, J.W.; Mendenhall, N.P.; Mendenhall, W.M. Merkel cell carcinoma: Treatment and outcomes. Cancer 2005, 104, 1761–1764. [Google Scholar] [CrossRef]
- Harvey, J.A.; Mirza, S.A.; Erwin, P.J.; Chan, A.W.; Murad, M.H.; Brewer, J.D. Recurrence and mortality rates with different treatment approaches of Merkel cell carcinoma: A systematic review and meta-analysis. Int. J. Dermatol. 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Bhatia, S.; Amin, A.; Kudchadkar, R.R.; Sharfman, W.H.; Lebbé, C.; Delord, J.P.; Dunn, L.A.; Shinohara, M.M.; Kulikauskas, R.; et al. Neoadjuvant Nivolumab for Patients With Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020, 38, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Adjuvant Avelumab in Merkel Cell Cancer. Available online: https://ClinicalTrials.gov/show/NCT03271372 (accessed on 8 November 2021).
- Pembrolizumab Compared to Standard of Care Observation in Treating Patients with Completely Resected Stage I-III Merkel Cell Cancer, STAMP Study. Available online: https://ClinicalTrials.gov/show/NCT03712605 (accessed on 8 November 2021).
- Immunotherapy Adjuvant Trial in Patients with Stage I-III Merkel Cell Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT04291885 (accessed on 8 November 2021).



| Factor | Patients % (n) n = 161 | |
|---|---|---|
| Gender | Male | 49.7 (80) |
| Age | Median (range) [years] | 72 (30–94) |
| >70 years | 55.9 (90) | |
| Comorbidities Hypertension Diabetes Coronary artery disease Arrhythmias Neoplasm Hypothyroidism | 77.6 (125) 50.9 (82) 23.0 (37) 21.7 (35) 12.4 (20) 8.7 (14) 6.8 (11) | |
| Centre | Warsaw | 59.6 (96) |
| Cracow | 15.5 (25) | |
| Gliwice | 14.3 (23) | |
| Wrocław | 10.6 (17) | |
| Primary tumor size | Median (range) [mm] | 25.5 (4–170) |
| Missing data | 35.4 (57) | |
| Primary tumor location | Head and neck | 29.2 (47) |
| Trunk | 4.3 (7) | |
| Upper extremities | 29.2 (47) | |
| Lower Extremities | 32.9 (53) | |
| MCCUP | 4.3 (7) | |
| UV-exposed skin * | 59.6 (96) | |
| Lymph nodes involvement at diagnosis | 28.6 (46) | |
| Distant metastases at diagnosis | 0 (0) | |
| Biopsy | 28.0 (45) | |
| Treatment | Surgery +/− perioperative (neoadjuvant/adjuvant) treatment | 96.9 (156) |
| Radiotherapy alone | 3.1 (5) | |
| Factor | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Median DFS (95% CI) [Years] | p | HR (95% CI) | p | ||
| Gender | Female | NR | 0.032 | 1 | 0.029 |
| Male | 1.6 (0.8–2.3) | 1.78 (1.06–3.01) | |||
| Age | <70 | NR | 0.062 | 1 | 0.200 |
| 70+ | 1.8 (0.1–3.7) | 1.42 (0.83–2.41) | |||
| Localisation of primary tumor | Head and neck | 3.2 (NR-NR) | 0.069 | 1 | |
| Trunk | NR | 1.28 (0.26–6.18) | 0.761 | ||
| Upper extremities | NR | 0.83 (0.40–1.71) | 0.611 | ||
| Lower Extremities | 1.2 (0.4–1.9) | 1.80 (0.88–3.71) | 0.110 | ||
| MCCUP | NR | 0.26 (0.03–2.20) | 0.216 | ||
| UV exposure | Yes | NR | 0.779 | ||
| No | NR | ||||
| Lymph nodes involvement | No | NR | <0.001 | 1 | <0.001 |
| Yes | 0.6 (0.0–1.2) | 5.41 (2.39–12.26) | |||
| SLNB performed in patients without clinical nodal metastases | Yes | NR | 0.031 | 1 | <0.001 |
| No | 1.5 (0.7–2.4) | 5.45 (2.41–12.30) | |||
| Perioperative chemotherapy | Yes | 0.6 (0.0–1.4) | 0.002 | 1 | 0.170 |
| No | NR | 0.62 (0.31–1.23) | |||
| Perioperative radiotherapy | Yes | NR | 0.031 | 1 | 0.004 |
| No | 1.8 (NR-NR) | 2.19 (1.28–3.75) | |||
| Factor | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Median OS (95% CI) [Years] | p | HR (95% CI) | p | ||
| Gender | Female | 6.9 (5.8–8.0) | 0.023 | 1 | 0.012 |
| Male | 3.1 (0.8–5.4) | 1.95 (1.16–3.27) | |||
| Age | <70 | NR | 0.005 | 1 | 0.015 |
| 70+ | 3.3 (0.7–5.8) | 2.00 (1.15–3.48) | |||
| Localisation of primary tumor | Head and neck | 6.8 (4.1–9.5) | 0.851 | ||
| Trunk | NR | ||||
| Upper extremities | NR | ||||
| Lower Extremities | 8.4 (1.2–15.5) | ||||
| MCCUP | NR | ||||
| UV exposure | Yes | 6.8 (3.2–10.4) | 0.358 | ||
| no | NR | ||||
| Lymph nodes involvement | no | 10.1 (NR-NR) | 0.001 | 1 | 0.003 |
| yes | 1.9 (1.3–2.4) | 3.15 (1.49–6.68) | |||
| SLNB performed in patients without clinical nodal metastases | Yes | 8.6 (5.2–12.0) | 0.094 | 1 | 0.027 |
| no | 4.6 (0.8–8.5) | 2.30 (1.10–4.82) | |||
| Perioperative chemotherapy | Yes | 3.3 (0.0–7.5) | 0.257 | ||
| No | 6.9 (4.6–9.2) | ||||
| Perioperative radiotherapy | Yes | NR | 0.072 | 1 | 0.056 |
| No | 4.6 (1.8–7.5) | 1.67 (0.99–2.83) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudzisz-Sledz, M.; Sobczuk, P.; Kozak, K.; Switaj, T.; Kosela-Paterczyk, H.; Czarnecka, A.M.; Falkowski, S.; Rogala, P.; Morysinski, T.; Spalek, M.J.; et al. Treatment of Locally Advanced Merkel Cell Carcinoma—A Multi-Center Study. Cancers 2022, 14, 422. https://doi.org/10.3390/cancers14020422
Dudzisz-Sledz M, Sobczuk P, Kozak K, Switaj T, Kosela-Paterczyk H, Czarnecka AM, Falkowski S, Rogala P, Morysinski T, Spalek MJ, et al. Treatment of Locally Advanced Merkel Cell Carcinoma—A Multi-Center Study. Cancers. 2022; 14(2):422. https://doi.org/10.3390/cancers14020422
Chicago/Turabian StyleDudzisz-Sledz, Monika, Paweł Sobczuk, Katarzyna Kozak, Tomasz Switaj, Hanna Kosela-Paterczyk, Anna Malgorzata Czarnecka, Slawomir Falkowski, Paweł Rogala, Tadeusz Morysinski, Mateusz Jacek Spalek, and et al. 2022. "Treatment of Locally Advanced Merkel Cell Carcinoma—A Multi-Center Study" Cancers 14, no. 2: 422. https://doi.org/10.3390/cancers14020422







