Percutaneous Thermal Ablation for Renal Tumors in Patients with Birt–Hogg–Dubé Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
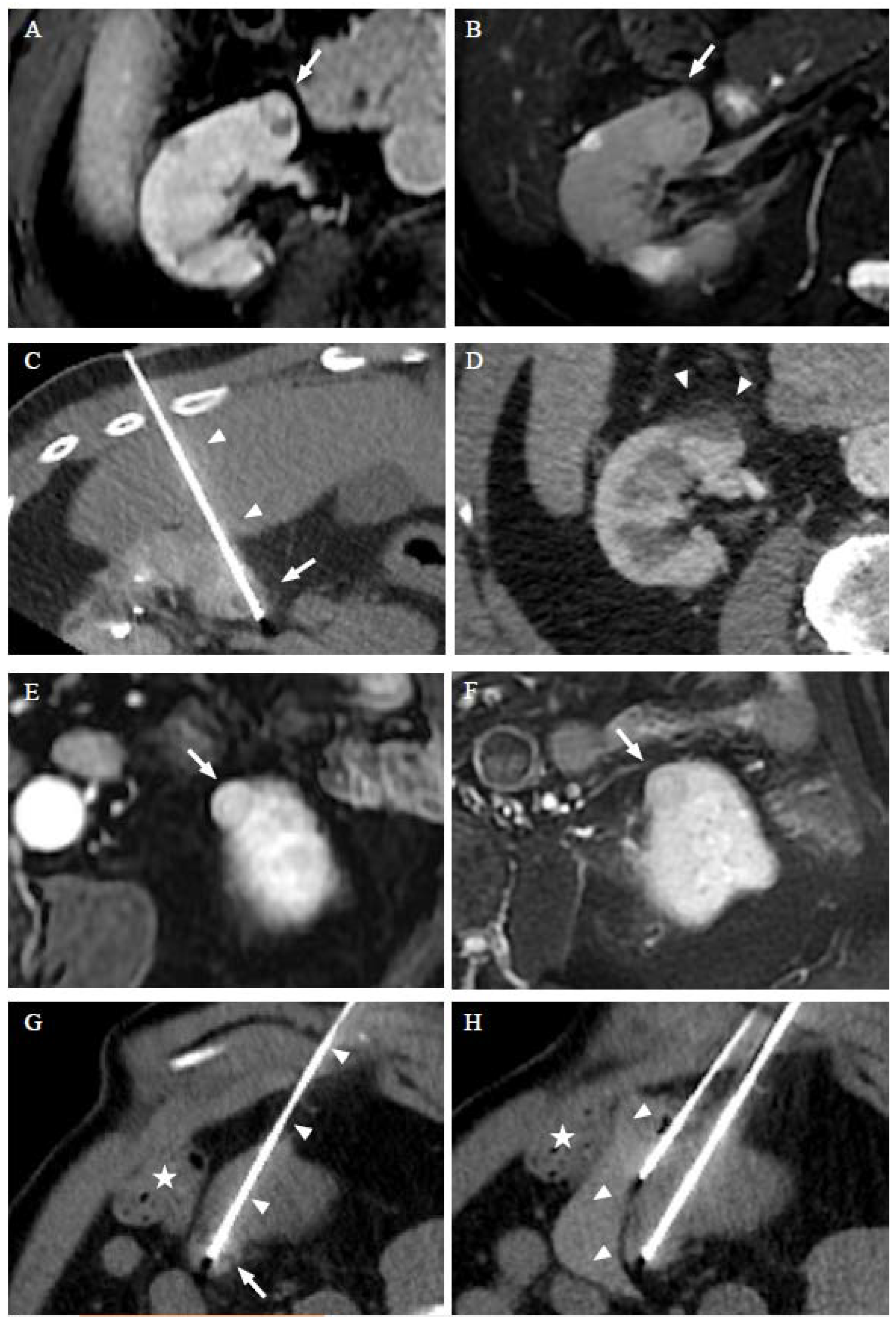
2.2. Procedure
2.3. Follow-Up
2.4. Descriptive Statistics
3. Results
3.1. Patients
3.2. Renal Tumors
3.3. Thermal Ablation Procedures
3.4. Efficiency and Adverse Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nickerson, M.L.; Warren, M.B.; Toro, J.R.; Matrosova, V.; Glenn, G.; Turner, M.L.; Duray, P.; Merino, M.; Choyke, P.; Pavlovich, C.P.; et al. Mutations in a Novel Gene Lead to Kidney Tumors, Lung Wall Defects, and Benign Tumors of the Hair Follicle in Patients with the Birt-Hogg-Dubé Syndrome. Cancer Cell 2002, 2, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Menko, F.H.; van Steensel, M.A.; Giraud, S.; Friis-Hansen, L.; Richard, S.; Ungari, S.; Nordenskjöld, M.; Hansen, T.V.; Solly, J.; Maher, E.R. Birt-Hogg-Dubé Syndrome: Diagnosis and Management. Lancet Oncol. 2009, 10, 1199–1206. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Bernhard, J.-C. Diagnostic and Interventional Radiology Is a Milestone in the Management of Renal Tumors in Birt-Hugg-Dubé Syndrome. Diagn. Interv. Imaging 2019, 100, 657–658. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Clinical Features, Genetics and Potential Therapeutic Approaches for Birt–Hogg–Dubé Syndrome. Expert Opin. Orphan Drugs 2015, 3, 15–29. [Google Scholar] [CrossRef]
- Stamatakis, L.; Metwalli, A.R.; Middelton, L.A.; Marston Linehan, W. Diagnosis and Management of BHD-Associated Kidney Cancer. Fam. Cancer 2013, 12, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Finelli, A.; Ismaila, N.; Bro, B.; Durack, J.; Eggener, S.; Evans, A.; Gill, I.; Graham, D.; Huang, W.; Jewett, M.A.S.; et al. Management of Small Renal Masses: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 668–680. [Google Scholar] [CrossRef]
- Hasumi, H.; Baba, M.; Hasumi, Y.; Furuya, M.; Yao, M. Birt–Hogg–Dubé Syndrome: Clinical and Molecular Aspects of Recently Identified Kidney Cancer Syndrome. Int. J. Urol. 2016, 23, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Vroomen, L.G.P.H.; Petre, E.N.; Cornelis, F.H.; Solomon, S.B.; Srimathveeravalli, G. Irreversible Electroporation and Thermal Ablation of Tumors in the Liver, Lung, Kidney and Bone: What Are the Differences? Diagn. Interv. Imaging 2017, 98, 609–617. [Google Scholar] [CrossRef]
- Cornelis, F.H. The Future of Percutaneous Renal Ablation. Diagn. Interv. Imaging 2017, 98, 285–286. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, C.K.; Park, S.Y.; Shen, S.-H. Percutaneous Radiofrequency Ablation of Renal Cell Carcinomas in Patients with von Hippel Lindau Disease: Indications, Techniques, Complications, and Outcomes. Acta Radiol. 2013, 54, 418–427. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Kanda, H.; Yamakado, K.; Soga, N.; Arima, K.; Takeda, K.; Sugimura, Y. Management of Renal Tumors in Von Hippel-Lindau Disease by Percutaneous CT Fluoroscopic Guided Radiofrequency Ablation: Preliminary Results. Fam. Cancer 2011, 10, 529–534. [Google Scholar] [CrossRef]
- Gobara, H.; Hiraki, T.; Iguchi, T.; Fujiwara, H.; Nasu, Y.; Kanazawa, S. Percutaneous CT-Guided Radiofrequency Ablation for Renal Cell Carcinoma in von Hippel-Lindau Disease: Midterm Results. Interv. Radiol. 2016, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Matsui, Y.; Hiraki, T.; Gobara, H.; Iguchi, T.; Tomita, K.; Uka, M.; Araki, M.; Nasu, Y.; Furuya, M.; Kanazawa, S. Percutaneous Thermal Ablation for Renal Cell Carcinoma in Patients with Birt-Hogg-Dubé Syndrome. Diagn. Interv. Imaging 2019, 100, 671–677. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; de Baère, T.; Dodd, G.D.; et al. Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria--a 10-Year Update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef]
- Tsivian, M.; Kim, C.Y.; Caso, J.R.; Rosenberg, M.D.; Nelson, R.C.; Polascik, T.J. Contrast Enhancement on Computed Tomography After Renal Cryoablation: An Evidence of Treatment Failure? J. Endourol. 2012, 26, 330–335. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. Nephrometry Score: A Comprehensive Standardized System for Quantitating Renal Tumor Size, Location and Depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Mauri, G.; Nicosia, L.; Varano, G.M.; Bonomo, G.; Della Vigna, P.; Monfardini, L.; Orsi, F. Tips and Tricks for a Safe and Effective Image-Guided Percutaneous Renal Tumour Ablation. Insights Imaging 2017, 8, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Georgiades, C.; Rodriguez, R.; Azene, E.; Weiss, C.; Chaux, A.; Gonzalez-Roibon, N.; Netto, G. Determination of the Nonlethal Margin Inside the Visible “Ice-Ball” During Percutaneous Cryoablation of Renal Tissue. Cardiovasc. Interv. Radiol. 2013, 36, 783–790. [Google Scholar] [CrossRef]
- Benusiglio, P.R.; Giraud, S.; Deveaux, S.; Méjean, A.; Correas, J.-M.; Joly, D.; Timsit, M.-O.; Ferlicot, S.; Verkarre, V.; Abadie, C.; et al. Renal Cell Tumour Characteristics in Patients with the Birt-Hogg-Dubé Cancer Susceptibility Syndrome: A Retrospective, Multicentre Study. Orphanet J. Rare Dis. 2014, 9, 163. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Walther, M.M.; Eyler, R.A.; Hewitt, S.M.; Zbar, B.; Linehan, W.M.; Merino, M.J. Renal Tumors in the Birt-Hogg-Dubé Syndrome. Am. J. Surg. Pathol. 2002, 26, 1542–1552. [Google Scholar] [CrossRef]
- Lee, E.; Sayyouh, M.; Haggerty, J.E.; Kazerooni, E.; Agarwal, P.P. Role of Radiologists in the Diagnosis of Unsuspected Birt-Hogg-Dubé Syndrome in a Tertiary Clinical Practice. Am. J. Roentgenol. 2019, 213, 792–797. [Google Scholar] [CrossRef]
- Gupta, S.; Kang, H.C.; Ganeshan, D.; Morani, A.; Gautam, R.; Choyke, P.L.; Kundra, V. The ABCs of BHD: An In-Depth Review of Birt-Hogg-Dubé Syndrome. AJR Am. J. Roentgenol. 2017, 209, 1291–1296. [Google Scholar] [CrossRef]
- Réseau National de Référence Pour Cancers Rares de l’Adulte PREDIR (PREDIspositions Aux Tumeurs Du Rein). Available online: https://predir.org/View/index.aspx (accessed on 5 July 2022).
- Marcelin, C.; Ambrosetti, D.; Bernhard, J.C.; Roy, C.; Grenier, N.; Cornelis, F.H. Percutaneous Image-Guided Biopsies of Small Renal Tumors: Current Practice and Perspectives. Diagn. Interv. Imaging 2017, 98, 589–599. [Google Scholar] [CrossRef]
- Gaillard, V.; Tricard, T.; Garnon, J.; Cazzato, R.L.; Dalili, D.; Gangi, A.; Lang, H. Repeat Ablative Therapy in Hereditary or Multifocal Renal Cancer: Functional and Oncological Outcomes. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 797.e15–797.e20. [Google Scholar] [CrossRef]
- Wile, G.E.; Leyendecker, J.R.; Krehbiel, K.A.; Dyer, R.B.; Zagoria, R.J. CT and MR Imaging after Imaging-Guided Thermal Ablation of Renal Neoplasms. Radiographics 2007, 27, 325–339. [Google Scholar] [CrossRef]
- Cornelis, F.; Tricaud, E.; Lasserre, A.S.; Petitpierre, F.; Bernhard, J.C.; Le Bras, Y.; Yacoub, M.; Bouzgarrou, M.; Ravaud, A.; Grenier, N. Routinely Performed Multiparametric Magnetic Resonance Imaging Helps to Differentiate Common Subtypes of Renal Tumours. Eur. Radiol. 2014, 24, 1068–1080. [Google Scholar] [CrossRef]
- Ganguli, S.; Brennan, D.D.; Faintuch, S.; Rayan, M.E.; Goldberg, S.N. Immediate Renal Tumor Involution after Radiofrequency Thermal Ablation. J. Vasc. Interv. Radiol. 2008, 19, 412–418. [Google Scholar] [CrossRef]
- Walther, M.M.; Choyke, P.L.; Glenn, G.; Lyne, J.C.; Rayford, W.; Venzon, D.; Linehan, W.M. Renal Cancer in Families with Hereditary Renal Cancer: Prospective Analysis Of A Tumor Size Threshold for Renal Parenchymal Sparing Surgery. J. Urol. 1999, 161, 1475–1479. [Google Scholar] [CrossRef]

| Patient # | Sex | FLCN Germline Mutation | Lung Cysts | History of Pneumothorax | History of Nephrectomy | Oral Anticoagulant | ASA | BMI | Age at Referral for Ablation (Year) | Tumor # | Histological Type of RCC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | c.1285del, p.His429Thrfs*39 | yes | yes | Partial | Yes | 3 | 26 | 76 | 1 | Chromophobe a |
| 2 | Chromophobe b | ||||||||||
| 3 | Chromophobe b | ||||||||||
| 79 | 4 | Chromophobe b | |||||||||
| 2 | M | c.1285del, p.His429Thrfs*39 | yes | yes | No | Yes | 4 | 26 | 84 | 5 | Clear cell papillary renal tumor (ccPRT) a |
| 6 | Clear cell papillary renal tumor (ccPRT) b | ||||||||||
| 3 | F | c.663dup, p.Met222Aspfs*26 | yes | yes | Total* and partial | No | 3 | 26 | 52 | 7 | Chromophobe a |
| 8 | Clear cell RCC b | ||||||||||
| 9 | Clear cell RCC b | ||||||||||
| 10 | Clear cell RCC b | ||||||||||
| 11 | Hybrid oncocytic/chromophobe tumor (HOCT) a | ||||||||||
| 4 | M | c.715C>T, p.Arg239Cys | yes | yes | Partial | No | 2 | 29 | 64 | 12 | pRCC b |
| 13 | pRCC b | ||||||||||
| 70 | 14 | pRCC b | |||||||||
| 15 | pRCC b | ||||||||||
| 5 | M | c.1300G>A, p.Glu434Lys | yes | no | Partial | No | 2 | 30 | 59 | 16 | Chromophobe a |
| 17 | Chromophobe b | ||||||||||
| 6 | M | c.1579C>T, p.Arg527* | yes | no | No | No | 2 | 24 | 68 | 17 | ccRCC a |
| 71 | 19 | ccRCC b |
| Tumor # | Renal Side | Localization | RENAL Score | Max. Diameter (mm) | Vol. (cc) | Nearness to the Collecting System or Sinus (<4 mm) | Nearness to the Digestive System | Nearness to Other Organ | Depth (mm) | Technique | Probe | Length (cm) /Exposure (mm) | Number of Probes | Number of Treated Tumors in the Same Session | Hydro- Dissection | Ureteral Stent |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Right | <50% exophytic | 7p | 25 | 7.8 | Yes | No | No | 112 | RFA | Cool-tip™ RFA Single 17 G | 15/20 | 3 | 1 | No | Yes |
| 2 | Right | entirely endophytic | 10a | 21 | 3.9 | Yes | No | No | 90 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | Yes |
| 3 | Right | entirely endophytic | 10x | 18 | 3.9 | Yes | No | No | 110 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | Yes |
| 4 | Right | < 50% exophytic | 5x | 22 | 2.9 | No | Yes | No | 107 | RFA | Cool-tip™ RFA Single 17-G | 15/30 | 2 | 1 | Yes * | No |
| 5 | Right | ≥ 50% exophytic | 4x | 37 | 24.1 | No | No | No | 70 | RFA | Cool-tip™ RFA Single 17-G | 15/30 | 3 | 1 | No | No |
| 6 | Left | ≥ 50% exophytic | 5x | 41 | 28.8 | No | No | Spleen and pancreas | 120 | RFA | Cool-tip™ RFA Single 17-G | 15/30 | 3 | 1 | No | No |
| 7 | Left | < 50% exophytic | 10a | 54 | 37.2 | Yes | No | Liver | 95 | Cryo | Galil IceSphere 1.5 17-G | 17.5/30 | 8 | 1 | No | No |
| 8 | Right | entirely endophytic | 9p | 13 | 1.1 | Yes | Yes | Liver | 120 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | No |
| 9 | Right | entirely endophytic | 9a | 13 | 1.0 | Yes | No | No | 90 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | No |
| 10 | Right | entirely endophytic | 9x | 15 | 1.0 | Yes | No | No | 95 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | No |
| 11 | Right | entirely endophytic | 9a | 14 | 0.7 | Yes | No | No | 92 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | No |
| 12 | Right | <50% exophytic | 8p | 21 | 4.4 | Yes | No | No | 92 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 1 | No | No |
| 13 | Right | <50% exophytic | 7a | 24 | 6.0 | No | Yes | No | 85 | MWA | NeuWaveTM PR Probe 17-G | 20/NA | 1 | 1 | Yes * | No |
| 14 | Right | entirely endophytic | 7p | 26 | 4.8 | No | No | No | 98 | RFA | Cool-tip™ RFA Single 17-G | 15/30 | 1 | 2 | No | No |
| 15 | Right | entirely endophytic | 6p | 15 | 1.8 | No | No | No | 86 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | No |
| 16 | Left | entirely endophytic | 9x | 20 | 2.5 | No | No | No | 126 | RFA | Cool-tip™ RFA Single 17-G | 20/20 | 1 | 2 | No | No |
| 17 | Left | entirely endophytic | 8a | 11 | 0.4 | No | Yes | No | 118 | RFA | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 2 | No | No |
| 18 | Right | entirely endophytic | 6a | 10 | 0.4 | No | No | Liver | 101 | RFA ** | Cool-tip™ RFA Single 17-G | 15/20 | 1 | 1 | No | No |
| 19 | Right | < 50% exophytic | 5a | 12 | 0.8 | No | Yes | No | 125 | RFA | Cool-tip™ RFA Single 17-G | 20/20 | 1 | 1 | Yes * | No |
| Patient # | Tumor # | Survival | Distant Metastasis | Follow-Up (Month) | Local Progression after Ablation | Complication |
|---|---|---|---|---|---|---|
| 1 | 1 | Alive | No | 169 | No | No |
| 2 | 167 | No | No | |||
| 3 | 167 | No | No | |||
| 4 | 137 | No | No | |||
| 2 | 5 | Alive | No | 49 | No | No |
| 6 | 46 | No | Subcapsular renal hemtoma | |||
| 3 | 7 | Alive | No | 84 | No | No |
| 8 | 81 | No | No | |||
| 9 | 81 | No | No | |||
| 10 | 74 | No | No | |||
| 11 | 74 | No | No | |||
| 4 | 12 | Alive | No | 75 | No | No |
| 13 | 32 | No | No | |||
| 14 | 6 | No | No | |||
| 15 | 6 | No | No | |||
| 5 | 16 | Alive | No | 22 | No | No |
| 17 | 22 | No | No | |||
| 6 | 18 | Alive | No | 62 | No | No |
| 19 | 33 | No | No |
| Patient # | Thermo-Ablation | Serum Creatinine (μmol/L) | eGFR (mL/min/1.73 m) | ΔEGFR * (mL/min/1.73 m) |
|---|---|---|---|---|
| 1 | 1st | 117 | 52 | 13 |
| 2nd and 3rd | 125 | 48 | ||
| 4th | 132 | 44 | ||
| Last follow-up | 137 | 39 | ||
| 2 | 1st | 135 | 41 | 1 |
| 2nd | 134 | 42 | ||
| Last follow-up | 135 | 40 | ||
| 3 | 1st | 85 | 67 | 22 |
| 2nd and 3rd | 96 | 58 | ||
| 4th and 5th | 112 | 48 | ||
| Last follow-up | 116 | 45 | ||
| 4 | 1st | 139 | 46 | 11 |
| 2nd | 169 | 36 | ||
| 3rd and 4th | 157 | 38 | ||
| Last follow-up | 166 | 35 | ||
| 5 | 1st and 2nd | 82 | 89 | 5 |
| Last follow-up | 85 | 84 | ||
| 6 | 1st | 130 | 49 | 5 |
| 2nd | 137 | 45 | ||
| Last follow-up | 136 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodard, S.; Boudhabhay, I.; Dariane, C.; Delavaud, C.; Guinebert, S.; Joly, D.; Timsit, M.-O.; Mejean, A.; Verkarre, V.; Hélénon, O.; et al. Percutaneous Thermal Ablation for Renal Tumors in Patients with Birt–Hogg–Dubé Syndrome. Cancers 2022, 14, 4969. https://doi.org/10.3390/cancers14204969
Bodard S, Boudhabhay I, Dariane C, Delavaud C, Guinebert S, Joly D, Timsit M-O, Mejean A, Verkarre V, Hélénon O, et al. Percutaneous Thermal Ablation for Renal Tumors in Patients with Birt–Hogg–Dubé Syndrome. Cancers. 2022; 14(20):4969. https://doi.org/10.3390/cancers14204969
Chicago/Turabian StyleBodard, Sylvain, Idris Boudhabhay, Charles Dariane, Christophe Delavaud, Sylvain Guinebert, Dominique Joly, Marc-Olivier Timsit, Arnaud Mejean, Virginie Verkarre, Olivier Hélénon, and et al. 2022. "Percutaneous Thermal Ablation for Renal Tumors in Patients with Birt–Hogg–Dubé Syndrome" Cancers 14, no. 20: 4969. https://doi.org/10.3390/cancers14204969





