LAG3-PD1 or CTLA4-PD1 Inhibition in Advanced Melanoma: Indirect Cross Comparisons of the CheckMate-067 and RELATIVITY-047 Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Selection
2.2. Data Extraction and Synthesis
2.3. Statistical Analysis
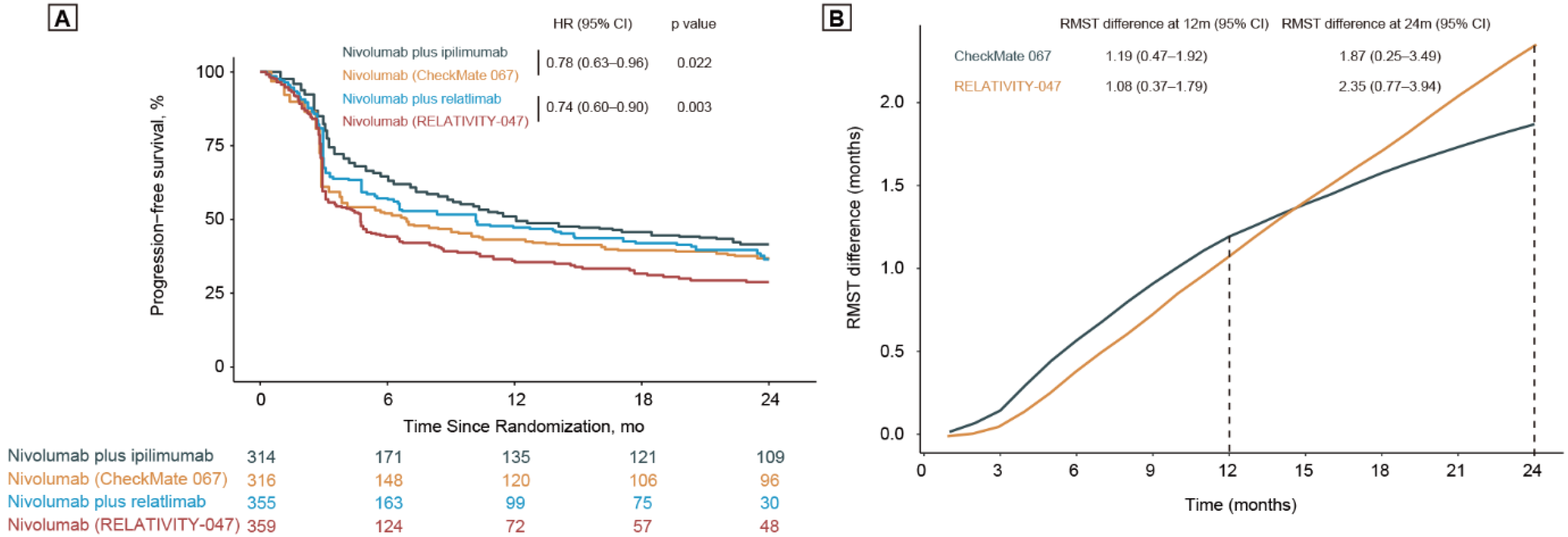
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsao, H.; Atkins, M.B.; Sober, A.J. Management of cutaneous melanoma. N. Engl. J. Med. 2004, 351, 998–1012. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Curti, B.D.; Faries, M.B. Recent Advances in the Treatment of Melanoma. N. Engl. J. Med. 2021, 384, 2229–2240. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Zhang, J.T.; Yan, L.X.; Chu, X.P.; Nie, Q.; Cui, J.X.; Yang, X.N.; Zhong, W.Z.; Wu, Y.L. Neoadjuvant immune checkpoint inhibitor plus chemotherapy in rare tracheal tumors. Cancer Commun. 2021, 41, 1243–1245. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Kisielow, M.; Kisielow, J.; Capoferri-Sollami, G.; Karjalainen, K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 2005, 35, 2081–2088. [Google Scholar] [CrossRef]
- Lythgoe, M.P.; Liu, D.S.K.; Annels, N.E.; Krell, J.; Frampton, A.E. Gene of the month: Lymphocyte-activation gene 3 (LAG-3). J. Clin. Pathol. 2021, 74, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F.; PRISMA-IPD Development Group. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Berry, D.A. Bayesian clinical trials. Nat. Rev. Drug Discov. 2006, 5, 27–36. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis, 3rd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2013. [Google Scholar]
- Royston, P.; Parmar, M.K. Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med. Res. Methodol. 2013, 13, 152. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, T.; Li, R.; Yuan, Y.; Xie, W.; Huang, X.; Wang, Q.; Chang, H.; Chen, G.; Li, Y.; et al. Outcomes and toxicities of immune checkpoint inhibitors in colorectal cancer: A real-world retrospective analysis. Cancer Commun. 2021, 41, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Wittes, J.; Fu, H.; Solomon, S.D.; Claggett, B.; Tian, S.L.; Cai, S.T.; Pfeffer, M.A.; Evans, S.R.; Wei, L.-J. Alternatives to Hazard Ratios for Comparing the Efficacy or Safety of Therapies in Noninferiority Studies. Ann. Intern. Med. 2015, 163, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley, M.L.; Enserro, D.; Hatcher, H.; Ottevanger, P.B.; Krarup-Hansen, A.; Blay, J.-Y.; Fisher, C.; Moxley, K.M.; Lele, S.B.; Lea, J.S.; et al. Adjuvant Gemcitabine Plus Docetaxel Followed by Doxorubicin Versus Observation for High-Grade Uterine Leiomyosarcoma: A Phase III NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2018, 36, 3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Aren Frontera, O.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]


| Trial, Year, Clinical Trials.Gov Identifier | No. of Countries | Main Eligibility Criteria | Intervention Groups, Dosing | PFS | BRAF Mutation | PD-L1 Expression | TRAEs | Total Included Cases |
|---|---|---|---|---|---|---|---|---|
| CheckMate-067 [2] Wolchok, 2022, NCT01844505 | 137 (Australia, Europe, Israel, New Zealand, and North America) | (1) Previously untreated unresectable stage III or IV (2) Age 18 years or older (3) ECOG PS 0-1 (4) Measurable disease as per RECIST 1.1 (5) Availability of tumor sample to assess PD-L1 status and BRAF V600 mutation | Nivolumab plus ipilimumab: nivolumab 1 mg/kg plus ipilimumab 3 mg/kg once every 3 weeks for 4 doses, followed by nivolumab 3 mg/kg once every 2 weeks for cycle 3 and beyond; Nivolumab: nivolumab 3 mg/kg once every 2 weeks (plus ipilimumab-matched placebo) | Median: 11.5 vs. 6.9 months; HR: 0.79 (0.66–0.97) | 31.9% vs. 32.2% | 23.5% vs. 21.7% | All TRAEs: 95.5% vs. 82.1%; TRAEs of Grade 3 or 4: 55.0% vs. 16.3% TRAEs leading to discontinuation: 36.4% vs. 7.7% | 630 |
| RELATIVITY-047 [11] Tawbi, 2022 NCT03470922 | 111 (North America, Central America, South America, Europe, Australia, and New Zealand) | (1) Previously untreated unresectable stage III or IV (2) Age 12 years or older (3) Measurable disease as per RECIST 1.1 (4) Availability of tumor sample to assess PD-L1 and LAG3 status | Relatlimab plus nivolumab: relatlimab 180 mg plus nivolumab 480 mg once every 4 weeks Nivolumab: nivolumab 480 mg once every 4 weeks | PFS: 10.12 vs. 4.63 months; HR: 0.75 (0.62–0.92) | 38.3% vs. 38.7% | 41.1% vs. 40.9% | All treatment-related adverse events: 81.1% vs. 69.9%; Treatment-related adverse events of Grade 3 or 4: 18.9% vs. 9.7%; TRAEs leading to discontinuation: 14.6% vs. 6.7% | 714 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.-W.; Zhang, F.-Y.; Wang, Y.; Chen, G.-M.; Nie, M.; Zhao, Z.-K.; Chen, X.-J.; Jiang, K.-M.; Nie, R.-C.; Chen, Y.-B. LAG3-PD1 or CTLA4-PD1 Inhibition in Advanced Melanoma: Indirect Cross Comparisons of the CheckMate-067 and RELATIVITY-047 Trials. Cancers 2022, 14, 4975. https://doi.org/10.3390/cancers14204975
Zhao B-W, Zhang F-Y, Wang Y, Chen G-M, Nie M, Zhao Z-K, Chen X-J, Jiang K-M, Nie R-C, Chen Y-B. LAG3-PD1 or CTLA4-PD1 Inhibition in Advanced Melanoma: Indirect Cross Comparisons of the CheckMate-067 and RELATIVITY-047 Trials. Cancers. 2022; 14(20):4975. https://doi.org/10.3390/cancers14204975
Chicago/Turabian StyleZhao, Bai-Wei, Fei-Yang Zhang, Yun Wang, Guo-Ming Chen, Man Nie, Zhou-Kai Zhao, Xiao-Jiang Chen, Kai-Ming Jiang, Run-Cong Nie, and Ying-Bo Chen. 2022. "LAG3-PD1 or CTLA4-PD1 Inhibition in Advanced Melanoma: Indirect Cross Comparisons of the CheckMate-067 and RELATIVITY-047 Trials" Cancers 14, no. 20: 4975. https://doi.org/10.3390/cancers14204975
APA StyleZhao, B.-W., Zhang, F.-Y., Wang, Y., Chen, G.-M., Nie, M., Zhao, Z.-K., Chen, X.-J., Jiang, K.-M., Nie, R.-C., & Chen, Y.-B. (2022). LAG3-PD1 or CTLA4-PD1 Inhibition in Advanced Melanoma: Indirect Cross Comparisons of the CheckMate-067 and RELATIVITY-047 Trials. Cancers, 14(20), 4975. https://doi.org/10.3390/cancers14204975






