A CAF-Fueled TIMP-1/CD63/ITGB1/STAT3 Feedback Loop Promotes Migration and Growth of Breast Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines/Sublines and Reagents
2.2. Western Blot Analysis
2.3. RNA Interference
2.4. Growth Assays
2.5. Migration Assays
2.6. Statistical Analyses
3. Results
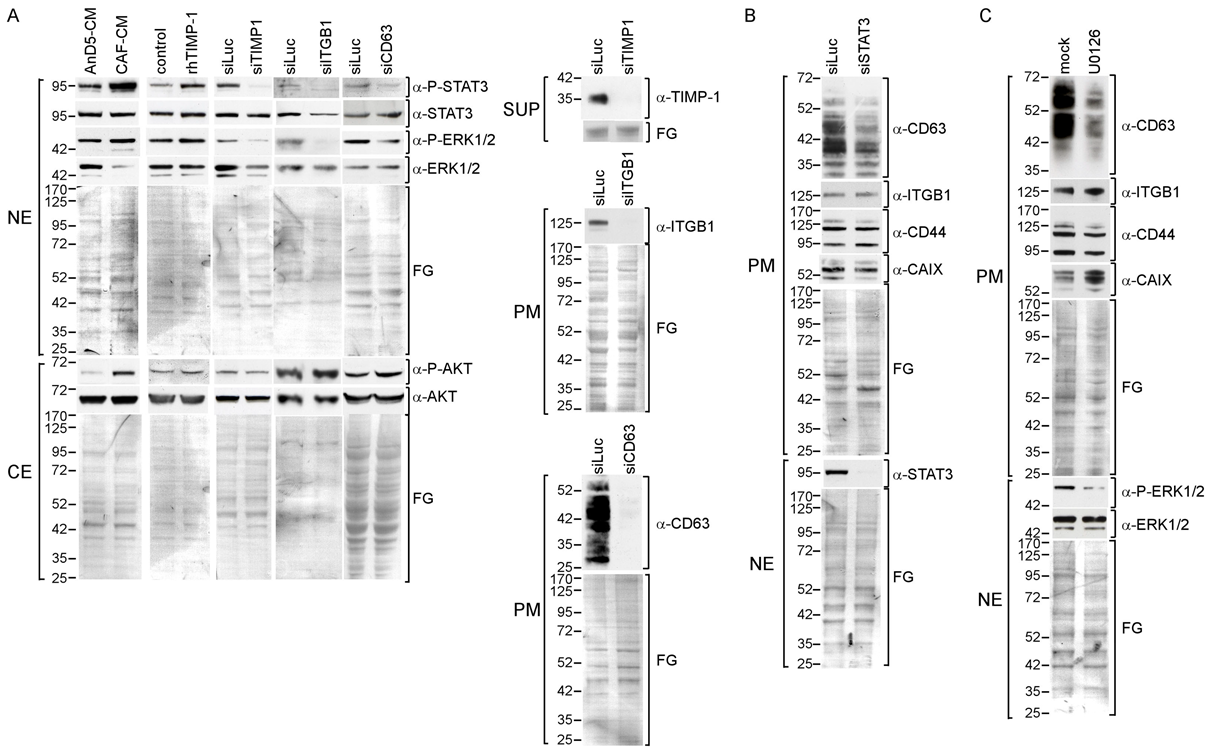
3.1. Exposure of Breast Cancer Cells to High Concentrations of Extracellular TIMP-1 Modulates the Levels of Plasma Membrane Proteins Linked to TIMP-1 Function
3.2. TIMP-1 Is Responsible for the CAF-CM-Induced Upregulation of ITGB1
3.3. CAF-CM Activates STAT3 and ERK1/2 by Stimulating the TIMP-1/CD63/ITGB1 Complex
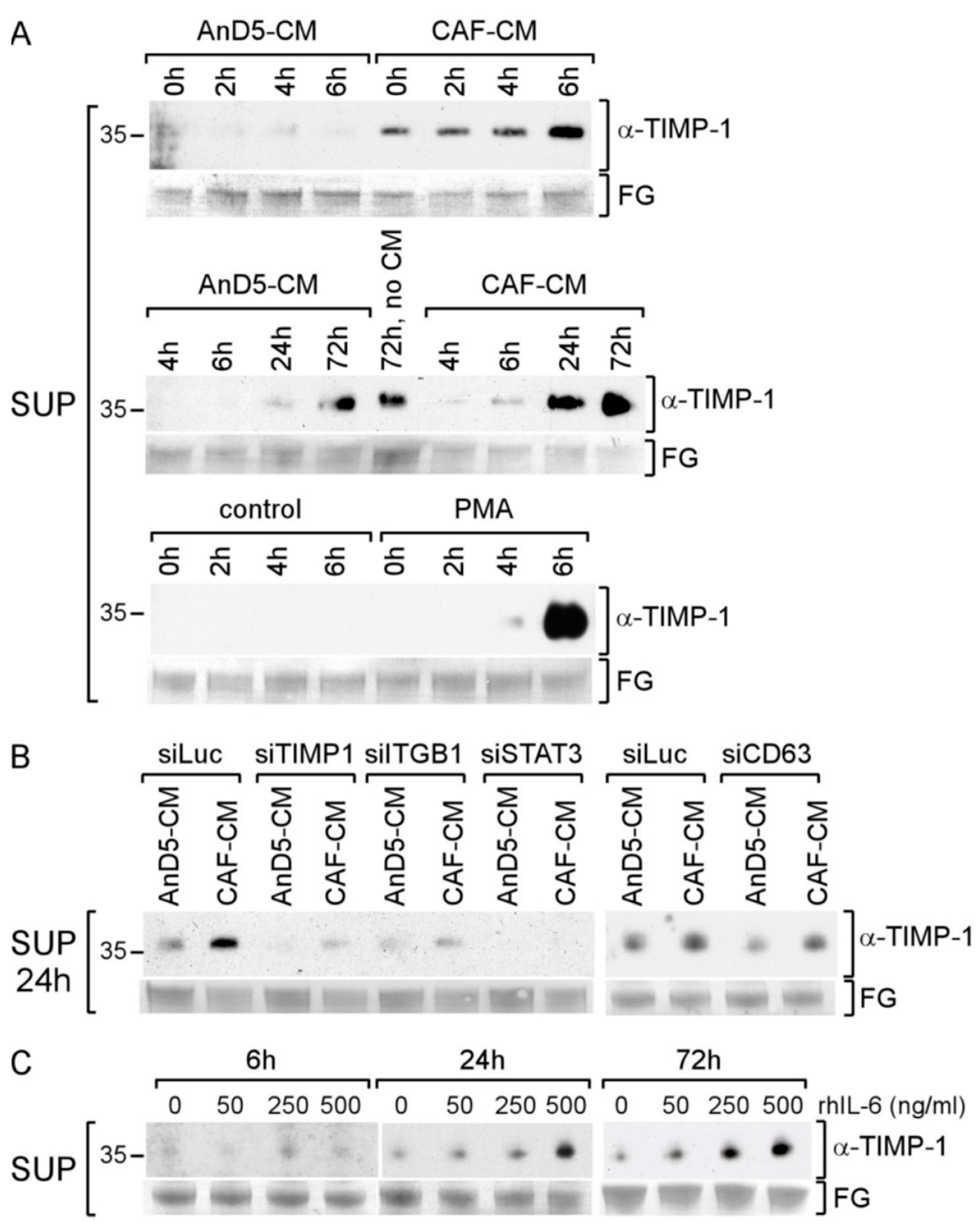
3.4. CAF-CM Stimulates AnD5 Cells to Secret More TIMP-1 by Activating STAT3
3.5. Knockdown of Any Component of the TIMP-1/CD63/ITGB1/STAT3 Pathway Affects Growth and Migration of AnD5 Cells
3.6. CAF-CM Stimulates TIMP-1 Secretion also in Other BC Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; Wang, Y.C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.M.; Carey, L.A.; McLeod, H.L. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat. Rev. Cancer 2009, 9, 576–586. [Google Scholar] [CrossRef]
- Rocca, A.; Maltoni, R.; Bravaccini, S.; Donati, C.; Andreis, D. Clinical utility of fulvestrant in the treatment of breast cancer: A report on the emerging clinical evidence. Cancer Manag. Res. 2018, 10, 3083–3099. [Google Scholar] [CrossRef]
- Dixon, J.M. Prospects of neoadjuvant aromatase inhibitor therapy in breast cancer. Expert Rev. Anticancer Ther. 2008, 8, 453–463. [Google Scholar] [CrossRef]
- Bailey, T.A.; Luan, H.; Clubb, R.J.; Naramura, M.; Band, V.; Raja, S.M.; Band, H. Mechanisms of Trastuzumab resistance in ErbB2-driven breast cancer and newer opportunities to overcome therapy resistance. J. Carcinog. 2011, 10, 28. [Google Scholar]
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef]
- Dittmer, J. Nuclear Mechanisms Involved in Endocrine Resistance. Front. Oncol. 2021, 11, 736597. [Google Scholar] [CrossRef]
- Paraiso, K.H.; Smalley, K.S. Fibroblast-mediated drug resistance in cancer. Biochem. Pharmacol. 2013, 85, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Rigiracciolo, D.C.; Belfiore, A.; Maggiolini, M.; De Francesco, E.M. Cancer associated fibroblasts: Role in breast cancer and potential as therapeutic targets. Expert Opin. Ther. Targets 2020, 24, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Leyh, B. The impact of tumor stroma on drug response in breast cancer. Semin. Cancer Biol. 2015, 31, 3–15. [Google Scholar] [CrossRef]
- Dittmer, A.; Lange, T.; Leyh, B.; Dittmer, J. Protein- and growth-modulatory effects of carcinoma-associated fibroblasts on breast cancer cells: Role of interleukin 6. Int. J. Oncol. 2020, 56, 258–272. [Google Scholar] [CrossRef]
- Morgan, M.R.; Humphries, M.J.; Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007, 8, 957–969. [Google Scholar] [CrossRef]
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, C.J.; Bissell, M.J. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006, 66, 1526–1535. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. USA 2009, 106, 10290–10295. [Google Scholar] [CrossRef]
- Yao, E.S.; Zhang, H.; Chen, Y.Y.; Lee, B.; Chew, K.; Moore, D.; Park, C. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007, 67, 659–664. [Google Scholar] [CrossRef]
- Dittmer, A.; Dittmer, J. Long-term exposure to carcinoma-associated fibroblasts makes breast cancer cells addictive to integrin β1. Oncotarget 2018, 9, 22079–22094. [Google Scholar] [CrossRef]
- Pontiggia, O.; Sampayo, R.; Raffo, D.; Motter, A.; Xu, R.; Bissell, M.J.; Joffe, E.B.; Simian, M. The tumor microenvironment modulates tamoxifen resistance in breast cancer: A role for soluble stromal factors and fibronectin through beta1 integrin. Breast Cancer Res. Treat. 2012, 133, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.K.; Liu, X.W.; Chirco, R.; Fridman, R.; Kim, H.R. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J. 2006, 25, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Justo, B.L.; Jasiulionis, M.G. Characteristics of TIMP1, CD63, and beta1-Integrin and the Functional Impact of Their Interaction in Cancer. Int. J. Mol. Sci. 2021, 22, 9319. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Würtz, S.; Schrohl, A.S.; Sørensen, N.M.; Lademann, U.; Christensen, I.J.; Mouridsen, H.; Brünner, N. Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr. Relat. Cancer 2005, 12, 215–227. [Google Scholar] [CrossRef]
- Nakai, N.; Hara, M.; Takahashi, H.; Shiga, K.; Hirokawa, T.; Maeda, Y.; Yanagita, T.; Ando, N.; Takasu, K.; Suzuki, T.; et al. Cancer cell-induced tissue inhibitor of metalloproteinase-1 secretion by cancer-associated fibroblasts promotes cancer cell migration. Oncol. Rep. 2022, 47, 112. [Google Scholar] [CrossRef]
- Dittmer, A.; Dittmer, J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis 2006, 27, 2844–2845. [Google Scholar] [CrossRef]
- Moritz, C.P. Tubulin or Not Tubulin: Heading Toward Total Protein Staining as Loading Control in Western Blots. Proteomics 2017, 17, 1600189. [Google Scholar] [CrossRef]
- Oerlecke, I.; Bauer, E.; Dittmer, A.; Leyh, B.; Dittmer, J. Cyclic AMP enhances TGFbeta responses of breast cancer cells by upregulating TGFbeta receptor I expression. PLoS ONE 2013, 8, e54261. [Google Scholar] [CrossRef]
- Leyh, B.; Dittmer, A.; Lange, T.; Martens, J.W.; Dittmer, J. Stromal cells promote anti-estrogen resistance of breast cancer cells through an insulin-like growth factor binding protein 5 (IGFBP5)/B-cell leukemia/lymphoma 3 (Bcl-3) axis. Oncotarget 2015, 6, 39307–39328. [Google Scholar] [CrossRef]
- Tominaga, N.; Hagiwara, K.; Kosaka, N.; Honma, K.; Nakagama, H.; Ochiya, T. RPN2-mediated glycosylation of tetraspanin CD63 regulates breast cancer cell malignancy. Mol. Cancer 2014, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Bjerre, C.; Vinther, L.; Belling, K.C.; Wurtz, S.O.; Yadav, R.; Lademann, U.; Rigina, O.; Do, K.N.; Ditzel, H.J.; Lykkesfeldt, A.E.; et al. TIMP1 overexpression mediates resistance of MCF-7 human breast cancer cells to fulvestrant and down-regulates progesterone receptor expression. Tumour. Biol. 2013, 34, 3839–3851. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, O.; Munk, S.; Fogh, L.; Yadav, R.; Francavilla, C.; Horn, H.; Wurtz, S.O.; Schrohl, A.S.; Damsgaard, B.; Romer, M.U.; et al. TIMP-1 increases expression and phosphorylation of proteins associated with drug resistance in breast cancer cells. J. Proteome Res. 2013, 12, 4136–4151. [Google Scholar] [CrossRef] [PubMed]
- Caterina, N.C.; Windsor, L.J.; Bodden, M.K.; Yermovsky, A.E.; Taylor, K.B.; Birkedal-Hansen, H.; Engler, J.A. Glycosylation and NH2-terminal domain mutants of the tissue inhibitor of metalloproteinases-1 (TIMP-1). Biochim. Biophys. Acta 1998, 1388, 21–34. [Google Scholar] [CrossRef]
- Cao, Z.; Li, C.; Zhu, G. Inhibitory effects of baicalin on IL-1beta- induced MMP-1/TIMP-1 and its stimulated effect on collagen-I production in human periodontal ligament cells. Eur. J. Pharmacol. 2010, 641, 1–6. [Google Scholar] [CrossRef]
- Lambert, E.; Bridoux, L.; Devy, J.; Dasse, E.; Sowa, M.L.; Duca, L.; Hornebeck, W.; Martiny, L.; Petitfrere-Charpentier, E. TIMP-1 binding to proMMP-9/CD44 complex localized at the cell surface promotes erythroid cell survival. Int. J. Biochem. Cell Biol. 2009, 41, 1102–1115. [Google Scholar] [CrossRef]
- Najy, A.J.; Jung, Y.S.; Kim, S.; Fridman, R.; Kim, H.C. Regulation of Tumor Metabolism and Extracellular Acidosis by the TIMP-10-CD63 Axis in Breast Carcinoma. Cells 2021, 10, 2721. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Zeng, C.; Liu, C.; Hao, Q.; Li, W.; Zhang, K.; Zhang, W.; Wang, S.; Zhao, H.; et al. CD63(+) Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal miR-22. Adv. Sci. 2020, 7, 2002518. [Google Scholar] [CrossRef]
- Shain, K.H.; Yarde, D.N.; Meads, M.B.; Huang, M.; Jove, R.; Hazlehurst, L.A.; Dalton, W.S. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: Implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009, 69, 1009–1015. [Google Scholar] [CrossRef]
- Bugno, M.; Graeve, L.; Gatsios, P.; Koj, A.; Heinrich, P.C.; Travis, J.; Kordula, T. Identification of the interleukin-6/oncostatin M response element in the rat tissue inhibitor of metalloproteinases-1 (TIMP-1) promoter. Nucleic. Acids Res. 1995, 23, 5041–5047. [Google Scholar] [CrossRef]
- Schaper, F.; Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015, 26, 475–487. [Google Scholar] [CrossRef] [PubMed]
- To, S.Q.; Dmello, R.S.; Richards, A.K.; Ernst, M.; Chand, A.L. STAT3 Signaling in Breast Cancer: Multicellular Actions and Therapeutic Potential. Cancers 2022, 14, 429. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, H.; Man, E.P.S.; Chau, K.M.; Khoo, U.S. Targeting the IL-6/STAT3 Signalling Cascade to Reverse Tamoxifen Resistance in Estrogen Receptor Positive Breast Cancer. Cancers 2021, 13, 1511. [Google Scholar] [CrossRef] [PubMed]
- Aaberg-Jessen, C.; Sørensen, M.D.; Matos, A.; Moreira, J.M.; Brünner, N.; Knudsen, A.; Kristensen, B.W. Co-expression of TIMP-1 and its cell surface binding partner CD63 in glioblastomas. BMC Cancer 2018, 18, 270. [Google Scholar] [CrossRef]
- Hotta, H.; Miyamoto, H.; Hara, I.; Takahashi, N.; Homma, M. Genomic structure of the ME491/CD63 antigen gene and functional analysis of the 5’-flanking regulatory sequences. Biochem. Biophys. Res. Commun. 1992, 185, 436–442. [Google Scholar] [CrossRef]
- Glauser, D.A.; Schlegel, W. Sequential actions of ERK1/2 on the AP-1 transcription factor allow temporal integration of metabolic signals in pancreatic beta cells. FASEB J. 2007, 21, 3240–3249. [Google Scholar] [CrossRef]
- Sedlakova, O.; Svastova, E.; Takacova, M.; Kopacek, J.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014, 4, 400. [Google Scholar] [CrossRef]
- Kaluz, S.; Kaluzová, M.; Stanbridge, E.J. The role of extracellular signal-regulated protein kinase in transcriptional regulation of the hypoxia marker carbonic anhydrase IX. J. Cell Biochem. 2006, 97, 207–216. [Google Scholar] [CrossRef]
- Czapiewski, P.; Cornelius, M.; Hartig, R.; Kalinski, T.; Haybaeck, J.; Dittmer, A.; Dittmer, J.; Ignatov, A.; Nass, N. BCL3 expression is strongly associated with the occurrence of breast cancer relapse under tamoxifen treatment in a retrospective cohort study. Virchows. Arch. 2022, 480, 529–541. [Google Scholar] [CrossRef]
- Chirco, R.; Liu, X.W.; Jung, K.K.; Kim, H.R. Novel functions of TIMPs in cell signaling. Cancer Metastasis. Rev. 2006, 25, 99–113. [Google Scholar] [CrossRef]
- Luparello, C.; Avanzato, G.; Carella, C.; Pucci-Minafra, I. Tissue inhibitor of metalloprotease (TIMP)-1 and proliferative behaviour of clonal breast cancer cells. Breast. Cancer Res. Treat. 1999, 54, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Charindra, D.; Shrestha, M.; Umehara, H.; Ogawa, I.; Miyauchi, M.; Takata, T. Tissue inhibitor of metalloproteinase-1 promotes cell proliferation through YAP/TAZ activation in cancer. Oncogene 2018, 37, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, T.; Zhan, S.; Qiao, N.; Zhang, X.; Zhu, Y.; Yang, N.; Sun, Y.; Zhang, X.A.; Bleich, D.; et al. TIMP-1 and CD82, a promising combined evaluation marker for PDAC. Oncotarget 2017, 8, 6496–6512. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Mannion, B.A.; Berditchevski, F.; Kraeft, S.K.; Chen, L.B.; Hemler, M.E. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29). J. Immunol. 1996, 157, 2039–2047. [Google Scholar]
- Malik, F.A.; Sanders, A.J.; Kayani, M.A.; Jiang, W.G. Effect of expressional alteration of KAI1 on breast cancer cell growth, adhesion, migration and invasion. Cancer Genom. Proteom. 2009, 6, 205–213. [Google Scholar]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e410. [Google Scholar] [CrossRef]
- Sebastian, A.; Hum, N.R.; Martin, K.A.; Gilmore, S.F.; Peran, I.; Byers, S.W.; Wheeler, E.K.; Coleman, M.A.; Loots, G.G. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers 2020, 12, 1307. [Google Scholar] [CrossRef]
- Altadill, A.; Eiro, N.; Gonzalez, L.O.; Andicoechea, A.; Fernandez-Francos, S.; Rodrigo, L.; Garcia-Muniz, J.L.; Vizoso, F.J. Relationship between Metalloprotease-7 and -14 and Tissue Inhibitor of Metalloprotease 1 Expression by Mucosal Stromal Cells and Colorectal Cancer Development in Inflammatory Bowel Disease. Biomedicines 2021, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Ito, H. IL-6 and Crohn’s disease. Curr. Drug Targets Inflamm. Allergy 2003, 2, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Zajac, E.; Juncker-Jensen, A.; Kupriyanova, T.A.; Welter, L.; Quigley, J.P. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia 2014, 16, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Pisanti, S.; Napolitano, F.; Di Somma, S.; Martinelli, R.; Portella, G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers 2020, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Prenen, H.; Mazzone, M. Tumor-associated macrophages: A short compendium. Cell Mol. Life Sci. 2019, 76, 1447–1458. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittmer, A.; Dittmer, J. A CAF-Fueled TIMP-1/CD63/ITGB1/STAT3 Feedback Loop Promotes Migration and Growth of Breast Cancer Cells. Cancers 2022, 14, 4983. https://doi.org/10.3390/cancers14204983
Dittmer A, Dittmer J. A CAF-Fueled TIMP-1/CD63/ITGB1/STAT3 Feedback Loop Promotes Migration and Growth of Breast Cancer Cells. Cancers. 2022; 14(20):4983. https://doi.org/10.3390/cancers14204983
Chicago/Turabian StyleDittmer, Angela, and Jürgen Dittmer. 2022. "A CAF-Fueled TIMP-1/CD63/ITGB1/STAT3 Feedback Loop Promotes Migration and Growth of Breast Cancer Cells" Cancers 14, no. 20: 4983. https://doi.org/10.3390/cancers14204983
APA StyleDittmer, A., & Dittmer, J. (2022). A CAF-Fueled TIMP-1/CD63/ITGB1/STAT3 Feedback Loop Promotes Migration and Growth of Breast Cancer Cells. Cancers, 14(20), 4983. https://doi.org/10.3390/cancers14204983





