Simple Summary
Non-muscle-invasive bladder cancer is associated with its high rates of progression and recurrence, the proper diagnosis and management can save lives. Bladder cancer is the tenth most common type of cancer in the world. This review discusses the current markers used in the recurrence prediction of non-muscle-invasive bladder cancer and future trends, published in the last decade, in addition to the limitations and future prospects in the field of AI-based prediction systems.
Abstract
Bladder cancer (BC) is the 10th most common cancer globally and has a high mortality rate if not detected early and treated promptly. Non-muscle-invasive BC (NMIBC) is a subclassification of BC associated with high rates of recurrence and progression. Current tools for predicting recurrence and progression on NMIBC use scoring systems based on clinical and histopathological markers. These exclude other potentially useful biomarkers which could provide a more accurate personalized risk assessment. Future trends are likely to use artificial intelligence (AI) to enhance the prediction of recurrence in patients with NMIBC and decrease the use of standard clinical protocols such as cystoscopy and cytology. Here, we provide a comprehensive survey of the most recent studies from the last decade (N = 70 studies), focused on the prediction of patient outcomes in NMIBC, particularly recurrence, using biomarkers such as radiomics, histopathology, clinical, and genomics. The value of individual and combined biomarkers is discussed in detail with the goal of identifying future trends that will lead to the personalized management of NMIBC.
1. Introduction
According to GLOBOCAN 2020, bladder cancer (BC) has the 10th highest incidence among cancers globally [1]. By 2022, in the United States, two out of ten patients with BC are expected to die from the disease [2,3]. Bladder cancer is usually classified into two main categories, non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). The majority of bladder tumors are non-muscle-invasive and include the following pathological stages: papillary tumors confined to bladder mucosa (Ta) which account for most NMIBC cases, invasion of the subepithelial connective tissue (stage T1), and tumor in situ (Tis), which is a high-grade, non-invasive urothelial carcinoma. Approximately 50% of patients with NMIBC will progress to MIBC if they go untreated, and the recurrence rate after treatment is approximately 70%–80% [4,5]. Bladder cancer accounts for almost USD 3.7 billion in direct costs in the US [6]. The treatment costs for NMIBC range from USD 5594 to USD 9554 per patient [7]. The cost of care varies considerably depending on the stage at first diagnosis and the success of initial treatment. The global burden of the disease is rising drastically [8,9] as the availability of some treatments, such as BCG instillation, is decreasing [10]. This reinforces the need for accurate tools which can predict treatment success at early stages.
Several diagnostic techniques can effectively detect and predict the recurrence of NMIBC. Such techniques can also suggest an optimal treatment plan for patients at first diagnosis. Current surveillance relies on the gold standard methods of cystoscopy, urine cytology, imaging, and biopsy (usually via the transurethral resection of bladder tumor or TURBT). Newer endoscopic techniques, such as white light cystoscopy, promise to improve the detection of first or recurrent NMIBC [11]. Imaging modalities as multi-slice spiral CT, MRI, transurethral ultrasound of the bladder, and positron emission tomography/computed tomography (PET/CT) can increase the diagnostic accuracy and provide useful pre-procedural information before TURBT is performed [12,13,14]. FDG PET/CT, in particular, has a high prognostic value in assessing patients with suspected recurrent BC [15,16]. MRI has recently become an important tool in the management of BC, giving rise to VI-RADS (Vesical Imaging-Reporting and Data System), a standardized reporting system aiming to improve the management of BC. [17,18]. Biopsy and histology are valuable tools for the initial diagnosis of NMIBC or the evaluation of recurrence and can improve management by determining the tumor stage and grade [3]. Recently, combinations of radiomic, clinical, pathological, and genomic markers have been used for the prediction of recurrence and to improve risk stratification [19,20].
To personalize the management of the disease, efforts have been directed toward the development of more effective tools for risk stratification, the prediction of recurrence, and the selection of optimal treatment. Previous risk assessment tools, including WHO 1973 and WHO 2004/2016 classification systems, European Organization for Research and Treatment Club (EORTC), and the Club Urologico Español de Tratamiento Oncologico (CEUTO), used clinical and histopathological markers to stratify patients into low, intermediate, and high risks of progression or recurrence [21]. The mainstay treatment for high- and intermediate-risk patients identified following TURBT is the local instillation of Bacillus Calmette–Guerin (BCG) immunotherapy [4]. For low-risk tumors (low-grade Ta), the treatment is TURBT optionally followed by intravesical chemotherapy with mitomycin C, epirubicin (Ellence), or doxorubicin (Adriamycin). For patients who do not respond to BCG immunotherapy or certain patients with high-risk disease, treatment may require radical cystectomy followed by chemotherapy and radiation [4,8,22,23]. Proper grading and staging are mandatory for the selection of initial treatment, however, current predictive tools have poor accuracy for predicting recurrence. They may underestimate the recurrence and progression in low-risk NMIBC but overestimate recurrence and progression in high-risk disease [24]. In addition, several questions remain unanswered: what is the recurrence probability cut-off that justifies a certain procedure such as cystectomy or BCG treatment? Can the type and dose of intravesical treatment be optimized based on a more precise determination of the recurrence risk? To answer such questions, new methods are imperative.
AI in BC Management
The current generation of predictive tools for NMIBC risk assessment are based on statistical methods. In recent years, artificial intelligence (AI) has shown superior accuracy in the prediction of disease recurrence and progression compared to statistical methods. AI, a broad category of computational methods designed to mimic human intelligence, has been widely used in the medical field, usually for computer-aided diagnosis (CAD) or, in our case, computer-aided predictive (CAP) systems. A major branch of AI, machine learning (ML), was developed to solve problems in the field of medicine [25,26]. The training of an ML model or algorithm is usually divided into three steps: training, validating, and testing. During training and validation, the ML model adapts to input data and provides the outputs used for classification or regression. Both approaches fall under the umbrella of supervised learning, where the input data have been previously labeled into desired categories, representing the “ground truth”. Most medical ML applications, including those applied to NMIBC, are designed for classification using supervised learning [27].
As the management of NMIBC heavily depends on accurate diagnosis risk assessment, many CAD and CAP systems have been developed using different ML algorithms such as support vector machines (SVM), random forest (RF), artificial neural network (ANN), and deep learning (DL) [28]. The CAD systems aid in detecting BC tumors [29,30,31], bladder segmentation [32], and the identification of NMIBC [33,34]. In addition, tumor staging and grading are important factors in the personalized management of NMIBC, and have been approached using AI [35,36,37,38]. The prediction of survival rates [39], response to certain chemotherapies [40,41,42], and recurrence rates [43,44,45] are examples of CAP systems. To improve the prediction of recurrence and risk stratification, combinations of radiomic, clinical, pathological, imaging, and genomic markers have been used [19,20] to develop ML algorithms.
Here, we provide a comprehensive survey of studies from the last decade that used a wide range of markers coupled with artificial intelligence and machine learning algorithms to predict the recurrence of NMIBC at an early stage—namely clinical, radiomic, histopathological, genomic, and/or combinations of the these markers—using search engines including Google Scholar, PubMed, and ResearchGate.
We identified studies specifically related to the recurrence of NMIBC published in highly ranking journals and conferences in the last decade from 2012 to 2022. We used the following keywords individually or combined in our search: Bladder Cancer, NMIBC, Prediction, Artificial Intelligence, Machine Learning, Computer-Aided Prediction, Radiomic Markers, Clinical Markers, Histopathological Markers, Genomics, Genetic Markers, Prognostic, Recurrence, Outcomes, Surveillance, Predictor, etc., resulting in a total of 69 studies that met our inclusion criteria. Priority was given to the studies that met the aforementioned inclusion criteria and used AI or ML. To our knowledge, there is no reliable AI algorithm that can precisely predict NMIBC recurrence and the improve management of NMIBC through a combination of the aforementioned markers. We hope that, by reviewing the aforementioned studies, we open the pathway for researchers to develop highly accurate AI-based NMIBC recurrence prediction systems and provide optimal personalized management of early-stage NMIBC.
Below, we summarize the studies that utilized different types of predictive markers coupled with AI and ML to develop a computer-aided prediction (CAP) system (shown in Figure 1 to predict the recurrence of NMIBC. Due to the low number of studies that use AI or ML, we also considered statistical models with high accuracy. These summaries are tabulated in Tables 1–5, in which Table 1 summarizes four studies where radiomic markers were extracted from different imaging modalities; Table 2 summarizes seven studies that used histopathological slides to extract pathological markers; Table 3 summarizes 14 studies that used different clinical markers. Table 4 summarizes 25 studies where genomic markers were extracted; and Table 5 summarizes 19 studies that used combinations of the aforementioned markers.
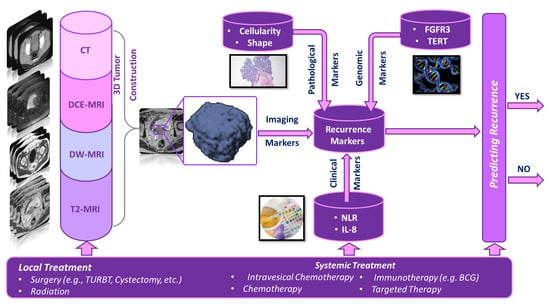
Figure 1.
A typical pipeline for a computer-aided prediction (CAP) system that can predict the recurrence of bladder cancer at an early stage.
2. Radiomics Markers
Several studies, as shown in Table 1, differentiate tumor from other tissues through radiologic imaging to predict recurrence. Wang et al. [46] and El-Assmy et al. [47] assessed the usefulness of different MRI sequences to predict the presence of recurrent tumors. Both studies concluded that diffusion-weighted imaging (DWI) provides the best results with an accuracy of 0.926 and 0.915, respectively. Yang et al. [48] and Alongi et al. [15] used FDG PET/CT images to predict tumor recurrence but found a lower accuracy of 0.886 for [48] and 0.90 for [15] when compared to the MRI-based studies. All four of these studies heavily relied on the experience of the physician interpreting the images and used statistical methods as opposed to AI.

Table 1.
Literature review on the use of radiomic markers to predict recurrence in NMIBC.
The studies included in Table 1 were only concerned with differentiating recurrent NMIBC from other tissues and did not explore the power of radiomic markers as predictors of recurrence after initial treatment. To date, no AI algorithms have used only radiomic markers as the input. The four studies used the MRI signal intensity or FDG uptake on PET-CT as the most significant marker, with an accuracy ranging from 0.915 to 0.926 and from 0.886 to 0.90, respectively. Moreover, these were retrospective studies with a small number of patients. Additional markers that have the potential to improve prediction performance are illustrated below.
3. Histopathological Markers
Many of the studies shown in Table 2 used similar pathological parameters and immunohistochemical (IHC) markers to predict recurrence, specifically features related to tumor stage and grade. Chen et al. [49] used Ki67, a nuclear protein indicating the extent of cell proliferation, and vascular endothelial growth factor (VEGF) immunoactivity as indicators of tumor grade. Specifically, high values for both Ki67 and VEGF indicated a higher tumor grade and higher risk of recurrence. Studies by Li et al. [50], Xu et al. [51], and Zhao et al. [52] found histological variants that were predictors of poor prognosis, including squamous differentiation, glandular differentiation, and lymphovascular invasion (LVI). Chamie et al. [53] found that the tumor stage correlated with prognosis. The aforementioned studies relied on statistical regression analysis where all pathological data were pathologist-dependent and subject to interobserver variability. To address these limitations, several studies have used AI to extract features from histological slides and predict prognosis. The study by Urdal et al. [54] constructed a RUSBoost classifier with an accuracy of 0.72. Tokuyama et al. [44] compared RF and SVM-based models, finding the highest accuracy (0.90) with the SVM. The relatively higher accuracy of this algorithm may be due to a larger dataset, more textural markers, and more complete images compared to Urdal et al. [54].

Table 2.
Literature review on using histopathological parameters and IHC markers to predict recurrence in NMIBC.
Although there are few AI studies using pathological parameters and IHC markers only, they have shown that it is possible to extract textural features without the need for time-consuming human segmentation and classification [44,54] and achieve promising results with an accuracy of up to 0.90. Other studies using statistical methods [50,51,52] have identified an important variant histology which predicts poor response to intravesical therapy and suggests that earlier cystectomy could improve survival in such patients. Tumor multiplicity, tumor size, tumor grade, and tumor stage are attributed to higher morbidity and poor response to treatment [50,51,52,53]. Although Ki67 and VEFG were found to predict recurrence, the value of those predictors remains unclear since these findings have not been validated [49]. All of the studies in Table 2 included patients who underwent TURBT followed by intravesical chemotherapy, however, some of them also included other treatments, such as BCG, which could represent a confounding factor when directly comparing the results between studies. All of these studies were performed as retrospective data analyses, some with small datasets.
4. Clinical Markers
Clinical markers are important in current risk stratification models and in the selection of the proper treatment strategy. Pretreatment markers can help determine the need for cystectomy in high-risk patients or BCG intravesical therapy in intermediate-risk patients. Mano et al. [55] studied high-risk NMIBC patients using statistical methods to find clinical markers correlated with tumor recurrence. They found that a neutrophil-to-lymphocyte ratio (NLR) > 2.43 was associated with a high tumor grade and stage, implying a high risk of recurrence. This study was limited by an uneven distribution of stage Ta and T1 tumors in the patient groups. Rubinstein et al. [56] developed a decision tree (DT) algorithm to predict the tumor recurrence in high-grade T1 patients treated with BCG using age and NLR as independent predictors. Different accuracies were obtained from two individual cohorts and from a combination of both. The highest accuracy was found in cohort 1, with the DT model suggesting an NLR > 2.5 as the decision node, then NLR < 2.3, and lastly age > 78. Albayrak [57] suggested adjusting the age before considering NLR as a predictor. The population of this study was comprised of NMIBC patients in a very early phase, limiting the generalizability of the results. The study by Ferro et al. [58] evaluated NLR, the erythrocyte sedimentation rate (ESR), and modified Glasgow prognostic score (mGPS) as predictors of recurrence. The mGPS scoring system is classified according to C-reactive protein (CRP) levels as follows: score 0 for patients with CRP <10 mg/L without high-serum albumin levels, score 1 for patients with CRP (>10 mg/L), and score 2 for CRP (>10 mg/L) with hypoalbuminemia (<3.5 g/dL). They found that ESR, NLR, and score 1 mGPS to be predictors for the recurrence of high-grade stage T1 patients. This study was limited by its retrospective design, and all of the above studies were limited by a lack of standardized treatment.
Several urinary biomarkers have been studied as predictors of disease recurrence. Srougi et al. [59] prospectively evaluated PAI-1 and IL-8 as diagnostic markers and predictive markers of recurrence, respectively. Optimized cut-off values found using the Youden index were PAI-1 < 0.266 and IL-8 < 0.047. The analysis using logistic regression found the stability of urinary biomarker levels regardless of the use of intravesical BCG. Although the accuracy in predicting recurrence was 0.793, the specificity was poor, suggesting that the model could falsely predict lower recurrence rates than expected. Another limitation is that data were only collected early in the treatment, and so, the correlation of serial biomarkers with recurrence was not studied. Rosser et al. [60] studied 10 biomarkers including IL8, MMP9, MMP10, SERPINA1, VEGFA, ANG, CA9, APOE, SERPINE1, and SDC1. The authors developed 11 ROC models using the Wilcoxon rank-sum test to identify the association between each individual biomarker and the presence of recurrence. The best resulting model included 10 combined biomarkers with an accuracy of 0.84. The model only using the SER-PINA1 marker had the best individual marker model with an accuracy of 0.78. Limitations of this study were a small dataset and heterogeneous data with relatively few low-grade tumors.
Chevalie et al. [61] was the first prospective study to show the relationship between immunobiomarkers and recurrence in patients undergoing BCG therapy. Specifically, T-cell and monocytic myeloid-derived suppressor cells (M-MDSCs) levels were assessed. A ratio between both markers indicative of type 2 immunity was found to be a potential predictor of recurrence and predict the response to BCG therapy. The study had a small number of patients, and the results needed further validation. Alberice [62] evaluated different metabolite urinary markers: N, N-trimethyllysine, N-acetyltryptophan, dopaquinone, leucine, and hypoxanthine. Elevated levels of dopaquinone, leucine, and hypoxanthine were associated with an increased risk of recurrence for high-risk patients (TaG3 and T1G2/3). N, N-trimethyllysine and N-acetyltryptophan were associated with an increased risk of recurrence for low-risk patients Ta/G1/2. This study was limited by a small dataset and inhomogeneous patient distribution.
Several studies demonstrated the influence of cystoscopic methods on patient outcomes. For example, Naselli et al. [63] found that the use of narrow-band imaging during initial transurethral resection can decrease the risk of recurrence by at least 10% compared to white-light imaging. The primary limitation of this study was that the patients were not randomized. Work by Sfakianos et al. [64] suggested that a second restaging TURBT performed prior to BCG therapy is associated with lower rates of 5-year recurrence for high-risk patients. This study was limited by a lack of randomization. A recent study by Culpan et al. [65] evaluated the impact of delayed follow-up cystoscopy on tumor recurrence for NMIBC patients after TURBT due to the influence of the global COVID-19 pandemic. Multivariate logistic regression analysis confirmed that a 2–5 month delay is a significant risk factor in all EAU risk categories. The total number of recurrences and cystoscopy delay time were also significant risk factors for progression. Notably, no survival analysis was performed due to the limited follow-up.
A recent network meta-analysis study by Lu et al. [66] evaluated the superiority of various intravesical monotherapies in reducing recurrence in intermediate-to-high-risk NMIBC. Using a Bayesian model, they found gemcitabine to be the most effective, followed by interferon and BCG. A random-effect meta-analysis by Uhling et al. [67] found a higher recurrence rate in females compared to males, and a poor response to BCG therapy in high-risk female patients. The limitations of this study include heterogeneous data and the inclusion of only NMIBC patients receiving local treatment. A prospective study by van Osch [68] studied the effect of smoking cessation on the risk of NMIBC recurrence and found that smoking cessation was not associated with a reduced risk of recurrence. However, the treatment (smoking cessation) was not randomized and only a small proportion of patients quit during the follow-up period.
Briefly, the 22 distinct clinical markers in Table 3 were studied with the aim of predicting bladder cancer recurrence. NLR was evaluated in four studies [55,56,57,58], one of which used an AI in the form of a DT algorithm, with accuracies ranging from 0.638 to 0.923. It was also found that the age may affect the accuracy of NLR [57], and age-correction may be necessary when using NLR as a predictor of recurrence. The mGPS score and ESR [58], type of intervesical monotherapy [66], and gender [67] were found to correlate with an increased recurrence risk. Furthermore, a total of 12 urinary biomarkers were evaluated [59,60,61] with the combination of IL8, MMP9, MMP10, SERPINA1, VEGFA, ANG, CA9, APOE, SERPINE1, and SDC1 biomarkers [60] considered to be the best predictive model of the three studies. Four markers related to cystoscopy and surgical techniques were evaluated in three studies [63,64,65], including narrow-band versus white-light TURBT, restaging TURBT prior to BCG, and delays in surveillance cystoscopy. It should be noted that the included patient risk categories were highly variable between these studies, and most were retrospective, non-randomized analyses. The paucity of studies using AI suggests the potential of further personalizing patient treatment using such models. Genomic markers have shown greater success in improving NMIBC management. In the following Table 4, we illustrate a number of examples with promising results.

Table 3.
Literature review on the use of clinical markers to predict recurrence in NMIBC.

Table 4.
Literature review on genomics biomarkers to predict NMIBC recurrence.
5. Genomics Markers
Currently, cystoscopy and cytology are standard tools used to monitor NMIBC, but cost-effective non-invasive tests have been developed with the aim of reducing treatment costs and improve patient follow-up. The following tests were primarily designed to detect promoter genes in urine or serum. To illustrate this, Kinde et al. [69] investigated a telomerase reverse transcriptase (TERT) promoter mutation as a significant marker for an increased risk of recurrence in NMIBC. The study showed high sensitivity compared to specificity with an accuracy of 0.933. However, the study included a very small number of patients. Likewise, Rachakonda et al. [70] found that TERT and rs2853669 polymorphisms were associated with tumor recurrence but were not statistically analyzed for accuracy, sensitivity, or specificity, despite the relatively large number of subjects. The TERT mutation, fibroblast growth factor receptor (FGFR3) gene, and OTX1 genes were evaluated as markers of increased recurrence risk by Beuker et al. [71]. However, the results were dependent on the tumor grade. Specificity was better for predicting the recurrences of high-grade than low-grade NMIBC due to a correlation between FGFR3 and tumor grade. No relation was found between the TERT mutation and tumor grade in any of the aforementioned studies [69,70,71]. Kandimalla et al. [72] studied mutations in eight genes detected in urine samples and found the highest sensitivity (74%) in a combination of OTX1, ONECUT2, and OSR1. The addition of FGFR3 increased the sensitivity to 79%. A urine-based test called UroMonitor developed by Batista [73] and colleagues detected TERT and FGFR3 mutations to detect the recurrence of NMIBC. When combined with cystoscopy, the test can detect recurrence with an accuracy of 0.90 and high specificity/sensitivity. One study that deserves mentioning is a retrospective study by Park et al. [74], which found no significant utility for FGFR3 in managing T1G3 NMIBC.
Several mRNA urine-based tests have also been developed. The CXbladder test created by Kavalieris et al. [75] analyzed the expressions of five genes: IGFBP5, HOXA13, MDK, CDK1, and CXCR2. The test result was calculated through a logistic regression model using the most recent tumor status (primary or recurrent), time of last tumor (RFS), and the five mRNA genes. The test-negative rate of this test is 0.34 with 0.93 sensitivity. The results are robust to the effects of BCG, making the test good for ruling out recurrence in intermediate-to-high-risk patients undergoing BCG therapy. An additional mRNA urine-based test, Xpert Monitor, detects ABL1, ANXA10, UPK1B, CRH, and IGF2 mRNA markers and has been validated in two studies [76,77]. Van Valenberg et al. [76] found the Xpert Monitor to have the highest sensitivity in detecting low-grade tumors and Ta recurrent tumors compared to urine cytology and UroVysion (a FISH-based urine test discussed below). Using linear discriminate analysis (LDA), an optimal accuracy of 0.79 and sensitivity of 0.74 were achieved. However, the study did not a have long-term follow-up to further validate the results. Elsawy et al. [77] verified the superiority of Xpert Monitor over urine cytology in high-grade tumors, finding a sensitivity of 100%. They also found Xpert Monitor to be an independent predictor of recurrence in patients with negative cystoscopy findings. The small number of recurrent high-grade tumors in the study (9 out of 181 patients) indicates that further testing on a larger set of patients is required to confirm the validity of the Xpert Monitor. The utility of RNA genomes was evaluated by Bi et al. [78]. They found a high correlation between low circular RNA (circRNA) expression and the risk of recurrence as well as an association with the tumor stage and grade. Lian et al. [79] found eight long non-encoding RNA (lnc-RNA) sequences (APCDD1L-AS1, FAM225B, LINC00626, LINC00958, LOC100996694, LOC441601, LOC101928111, and ZSWIM8-AS1) to be highly correlated with tumor recurrence. These studies included both MIBC and NMIBC cases, and did not report measures of test accuracy [78,79].
Additional urine-based tests include the UroVysion, a multi-target test which uses fluorescence in situ hybridization (FISH) to predict recurrence in intermediate- and high-risk patients undergoing BCG therapy. Liem et al. [45] evaluated UroVysion in three different time intervals: pre-BCG, 6 weeks post-TURBT, 3 months post-TURBT. They found a significant correlation between the recurrence and positive UroVysion test in the 3 month post-TURBT time interval with an accuracy of 0.77 and sensitivity of 0.59, noting a limited number of subjects for this interval. Kojima et al. conducted a prospective study [80], and found that two consecutive UroVysion tests predicted recurrence better than urine cytology, with an accuracy of 0.703 and 0.50 sensitivity for a single test. The UroVysion test, however, is expensive and has a high false-positive rate which could lead to increased follow-up cystoscopy and a further increased cost. Another urine-based DNA genome test, EpiCheck (BE), detected 15 DNA methylation biomarkers (Witje et al. [81]). The test had an accuracy of 0.883 and sensitivity of 0.971 when the low-grade tumors were excluded. Test accuracy was not affected by current or previous treatments. In a prospective study, Roupret et al. [82] evaluated the ADXBLADDER urine-based which uses the MCM5 DNA gene status as a single marker and found an accuracy of 0.688, a sensitivity of 0.449, and an NPV of 0.99. A urine-based NMP22 gene immunoassay (Önal et al. [83]) was found to be superior to urine cytology in the overall cohort and in low-grade tumors, but with lower sensitivity and specificity than urine cytology for high-grade tumors. The overall sensitivity and specificity of the NMP22 assay were 0.854 and 0.765, respectively. The author concludes that using NMP22 with cytology is an optimum predictive solution for recurrence.
The levels of DNA methylation in specific markers can also be used to detect the recurrence of NMIBC. Three DNA methylation markers, including SOX1, IRAK3, and L1-MET, were tested by Su et al. [84]. These markers outperformed cytology and cystoscopy in early recurrence detection with a sensitivity of 0.86 and a specificity of 0.80. Shindo et al. [85] evaluated four miRNA methylation markers (iR-9-3, miR-124-2, miR-124-3, and miR-137) collected from urine samples at the time of recurrence and during a follow-up period. Using the number of methylated genes (M-score), they found a sensitivity of 0.62 and a specificity of 0.74 for current recurrence. An M-score was correlated with worse recurrence-free survival. A study of five DNA methylation markers by Reinert et al. [86] included HOXA9, POU4F2, TWIST1, VIM, and ZNF154. VIM had the highest sensitivity (0.89) and specificity (1.0). The authors noted that combining FGFR3 mutation analysis with DNA methylation analysis could increase the sensitivity for the detection of recurrence. Maldonado et al. [87] analyzed promoter methylation in the CCND2, CCNA1, and CALCA genes in urine samples and found statistically significant differences between patients with recurrent and non-recurrent tumors as well as significant differences between patients with BC and controls. Bellmunt et al. [88] evaluated nine markers (RHOB, ARID1A and TP53 mutations, CDKN2A deletion, and focal gain in CCNE1, PVRL4, YWHAZ, E2F3-SOX4, and PPARG genes) for association with recurrence and progression in patients with high-grade T1 NMIBC. They found significant correlations with disease progression, recurrence, as well as good outcomes among the various markers. A study by Kobayashi et al. [89] found significant correlations between human leukocyte antigen (HLA) genotypes in serum samples and intravesical recurrence after BCG therapy. Specifically, the combination of HLA-B07 and HLA-B44 homozygosity with CUETO is significantly correlated with intravesical recurrence. A meta-analysis by Galeshoot [90] searched for single nucleotide polymorphisms (SNPs) correlated with recurrence-free and progression-free survival in NMIBC. They found that lead SNP rs12885353 on chromosome 14 was associated with an increased expression of SCFD1 and associated with recurrence-free survival. The heterogeneous cohort was a limitation of this study.
To the best of our knowledge, only two studies have used AI to analyze genetic markers. Frantzi et al. [91] used an SVM to detect primary and recurrent tumors using 106 peptide genome markers. The study included intermediate- and high-risk NMIBC patients, and achieved a sensitivity and specificity of 0.88 and 0.51, respectively. The author suggested that higher performance could be obtained when combined with cytology, yielding 100% sensitivity. Unfortunately, there was not enough data to show a correlation between the clinical and genetic markers. In a study by Bartsch et al. [92], genetic programming (GP) was used to discover mathematical models to predict recurrence over 5 years of follow-up based on using the whole genome profiling of the bladder tumor specimens. The highest performance was achieved with a three-gene rule which predicted recurrence with a specificity of 0.71 and a specificity of 0.67 in a test set. Both studies were limited by the lack of an external dataset for validation.
In summary, based on the data collected on Table 4, the TERT and FGFR3 genomes were the most widely tested markers among the included studies. They were evaluated in six studies ([69,70,71,72,73,74]) and associated with accuracies ranging from 0.877 to 0.933 and a sensitivity ranging from 0.57 to 100%. The best results were found by Batista et al. [73] using the Uromonitor urine-based test which included both TERT and FGFR3 genomic markers combined with cytology. Notably, the sensitivity of any test including FGFR3 is higher in higher-grade tumors, unlike TERT. Studies assessing the Xpert urine-based test [76,77] found an accuracy of 0.79 and a sensitivity of 0.80. Other tests discussed included Cxbladder [75], EpiCheck [81], and ADXBLADDER [82]. The highest accuracy of 0.856 was found with EpiCheck [75], while the best sensitivity of 0.93 was found for Cxbladder [75]. The three tests had a higher sensitivity for high-grade tumors. The UroVysion test evaluated in two studies [45,80] predicted recurrence in patients undergoing intervesical BCG treatment with an accuracy ranging from 0.703 to 0.77 and a sensitivity from 0.5 to 0.59. Studies evaluating the genetic markers such as NMP22 [83] and DNA methylation [84,85,86] have good results with a sensitivity ranging from 0.615 to 0.94, the highest found in [86] for ZNF154 methylation. Eight statistical studies [70,74,78,79,87,88,89,90] found a correlation of multiple genetic markers with recurrence but did not evaluate test performance characteristics. Some of the markers were strongly correlated with the tumor stage and grade, such as Circ-ZKSCAN1 [78]. Maldonado et al. [87] correlated multiple markers with recurrence in low-grade T0 NMIBC. A study by Lian et al. [79] also showed a correlation between the high-tumor grade and gender with their markers in predicting recurrence. A study correlating HLA genotypes [89] with recurrence rates, while two studies [70,90] suggested the use of SNPs for predicting tumor recurrence. Two AI algorithms were developed genetic markers, an SVM and rule-based ensemble algorithms developed using genetic programming (GP) [91,92]. The SVM had a higher sensitivity of 0.88, however, a three-gene rule constructed using GP had a higher specificity of 0.69. There were high NPVs for the majority of these tests, indicating that they minimize the use of cystoscopy for follow-up. However, a high false positive rate for some of the tests could lead to unnecessary cystoscopy and biopsy. Most of the studies, however, need validation to widely mandate their clinical use. Adequate prospective studies with a long follow-up are required to confirm the impact of these tests on disease management. In the following Table 5, we explore studies using combined classes of markers.

Table 5.
Literature review on using combined markers to predict recurrence in NMIBC.
6. Combined Markers
Recently, efforts have been directed towards using combinations of markers from different classes to enhance the prediction of MIBC recurrence and progression. These are summarized in Table 5. Only one study combined radiological data with clinical data (Xu et al. [93]). Radiomic features were extracted from multiparametric MRI images using an SVM, achieving an accuracy of 0.755. A combination of radiomic and statistically selected clinical markers yielded an accuracy of 0.809 and a better accuracy for the prediction of recurrence over 2 years. The use of a small number of clinical markers limited this study. The author suggested that genomic markers could be used to increase the overall performance.
More than half of the combined studies used clinical and pathological markers in their models, and many of these studies used machine learning as their core methodology. Starting with Borgi et al. [94], they developed a classifier based on association rules (CBA) with 24 attributes. The most common markers were age, gender, smoking history, and tumor-related attributes (such as stage and of multiplicity). Their model achieved an accuracy of 0.51 in predicting intravesical recurrence in patients who received BCG. The results are limited due to the retrospective nature of the study, heterogeneous and incomplete data, and imbalanced classes between no-recurrence and recurrence cases. SVM models were used by many authors. Lee and other colleagues [95], for example, show that the presence of intravesical prostate protusion (IPP) and other clinicopathological markers in an SVM model yielded accuracies of 0.754 to 0.803. Despite their promising results, further studies are necessary to confirm the utility of IPP as a predictor. Hasnain et al. [96] used an SVM for the prediction of NMIBC and MIBC recurrence at 1, 3, and 5 years using 52 pathological, radiological, and clinical markers. They found the superiority of a 1 year predictive model vs. 3 and 5 years, using a metaclassifier algorithm consisting of SVM, bagged SVM, KNN, AdaBoost, RF, and gradient-boosted trees for NMIBC and MIBC. Regarding deep learning, Lucas and Jobczyk [97,98] used such a model for recurrence prediction. Lucas constructed two models in their study [97] that predicted recurrence within 1 and 5 years using 204 and 200 histopathological markers extracted from histopathological slides with four and three clinical markers including any previous malignancies, tumor stage (Ta vs. other), intravesical chemotherapy, and smoking history, respectively. The best accuracy was obtained from a combined 1-year recurrence prediction model. Hence, the author concluded that markers extracted from digital histopathological images combined with other markers could be useful for recurrence prediction. Jobczyk et al. [98] used Cox proportional-hazards (CPH) deep neural networks to predict recurrence for up to 10 years. The study combined both EORTC and CUETO scores with other markers including gender, age, and the type of intravesical treatment. The main performance metric for the model was the C-index, derived by scoring different results relative to the type of treatment. They found a different C-index for chemotherapy (0.666) and TURBT and BCG immunotherapy (0.651). Despite the large cohort of this study, the author is discrete in using their model in high-risk patients that did not receive any intravesical therapy. EORTC and CUETO scores were also used by Vedder et al. [99] and validated in different cohorts to predict the NMIBC recurrence, however, without using AI. Vedder used statistical methodology, finding a C-index ranging from 0.55 to 0.61, suggesting the superiority of ML in prediction. Getzler et al. [100] found an increased performance with the addition of the EORTC score to NLR. Cambier and colleagues [101] studied intervesical BCG recurrence within 1–5 years with nomograms and found a prior recurrence rate and number of tumors as significant prognostic factors for recurrence after using EORTC to stratify patients into intermediate- and high-risk categories. They achieved a C-index of 0.56 and found the highest recurrence rate in T1G3 patients. However, the high recurrence risk may be influenced by the lack of re-TURBT and exclusion of CIS patients. Kim et al. [102] studied Korean patients using a nomogram with similar markers to those used in Jobcyzk [98], although excluding age and gender while including gross hematuria and previous or concomitant upper urinary tract cancer in their analysis. The nomogram resulted in an almost identical C-index despite there being insufficient data for stratifying models according to each intervesical treatment as Jobcykz did. The same markers were examined by Ali-El-Dien et al. [103] with the addition tumor stage and intervesical therapy. They also used nomograms, finding a C-index of 0.694 in predicting 5-year recurrence. However, a low number of BCG-treated cases limited the validity of the results. Evaluating patients with multiple low-grade-T0 tumors, Nerli’s study [104] correlated the use of tobacco and absence of intravesical BCG as significant predictors of tumor recurrence. It should be noted that many studies neglected low-grade categories, making this study a unique. Another unique study by Zgao et al. [105] evaluated the controlling nutritional status (CONUT) score (a score designed to screen for undernutrition), using a cut-off score of 1 and other markers such as age, smoking history, as well as tumor stage and grade. They found a sensitivity of 0.8486 and a C-index of 0.851 using a nomogram model. Notably, this was the best C-score among the aforementioned studies, although the study was comprised of a small number of subjects and was retrospective. An additional two studies included surgical parameters. Suarez-Ibarrola et al. [106] used a surgical checklist with eight elements that should be used in a high-quality TURBT. Two of the elements were significant predictors for recurrence over 3 years, including the number and location of the tumors. Li et al. [107] evaluated different operative methods as predictors of the decreased recurrence rate. They concluded that pin-ERBT (a method using a pin-shaped electrode for tumor resection) was the most effective procedure for reducing recurrence. In addition to the operative method, age, smoking, and tumor grade were statistically correlated with recurrence. A notable drawback of this study is that multiple larger tumors (more than 3 cm) could not be completely resected using pin-ERBT, and few samples had such characteristics.
Four combined studies [108,109,110,111] used genomic markers. Ajili et al. [108] used an ANN with clinicopathological markers and a single genomic marker (CD34 gene) to predict the intravesical recurrence. The model had an accuracy of 0.957. The model was trained on a small dataset, and validation using a larger external dataset would be needed to confirm the utility of this model. Two additional studies [109,110] evaluated the use of protein panels for recurrence prediction. Zhan et al. [109] used MALAT1, PCAT-1, and SPRY4-IT1 biomarkers, and found a sensitivity of 0.625. Additionally, the tumor stage was statistically correlated as a predictor and PCAT-1 was found to be an independent predictor. Gogalic et al. [110] used common clinicopathological markers with ECadh, IL8, MMP9, EN2, and VEGF biomarkers. A model using a combination of these markers achieved an AUC of 0.84. Finally, Lopez et al. [111] used 171,295 SNP to examine the role of those markers in predicting recurrence. Despite the prospective, detailed data and long follow-up, the author did not find any correlation of SNP in recurrence predictability.
As discussed in Table 5, many authors used combined markers and AI to predict tumor recurrence. The most used AI algorithm was SVM, which was used in three studies [93,95,96] with radiomic, clinical, and pathological markers, achieving an overall accuracy of approximately 0.75 and a sensitivity between 0.593 and 0.774. Deep learning algorithms were used in three studies [96,97,98,108] with clinical, pathological, and genomics markers yielding accuracies between 0.65 and 0.975. The classifier based on association rules (CBA) was also used by a single study [94], with an accuracy of 0.51. Statistical methods were used in most studies [99,100,101,102,103,104,105,106,107,109,110,111]. A unique study [98] combined statistical and ML methods, developing a CPH deep neural network. Many of the markers used were consistent among these studies. To illustrate, tumor characteristics such as stage, grade, size, and multiplicity were frequently used. Likewise, intervesical treatment with either BCG or chemotherapy were common clinical markers. Furthermore, the age, gender, smoking history, and previous recurrence were used in more than five studies. Studies using genomic markers still need a further investigation since only four such studies were found [106,108,109,110]. Other studies included unique clinical markers such as CONUT score [105], IPP [95], and NLR [100]. Two studies [106,107] assessed the quality of TURBT as an influence on recurrence risk rates. Common limitations found were the need for more detailed patient history and retrospective design which is inherently vulnerable to bias. Despite the limitations, these studies challenge the validated recurrence prediction systems as EORTC and CEUTO, and have the potential to reduce bias and interobserver variability.
6.1. Limitations and Strengths
Despite the adequate number of studies that show promising results in predicting NMIBC recurrence, major limitations were found in data that were retrospective, nonhomogeneous, the lack of interobservers’ consistency and small number to validate such studies. Additionally, many of them only used statistical methods, treating the problem of prediction as one of linear regression. However, AI techniques can successfully solve both linear and non-linear problems as well as both classification and regression. Hence, AI models are more robust and well-generalized with the potential to predict new unseen cases, revealing that AI strength can assist in developing more accurate personalized management systems that are objective and diminish any biases due to subjectivity.
6.2. Conclusion and Future Trends
NMIBC recurrences are common in BC, with recurrence rates ranging from 70% to 80%. In this survey, we covered several techniques as radiomics, histopathological, clinical, genomics, and any other combinations of them that can predict NMIBC recurrence and help manage and individualize management of this disease. From approximately 70 studies, our conclusions show initial results for radiomics using intensity markers in MRI or CT images that had an accuracy range of 0.886–0.926 using statistical methods and not AI. Histopathological markers yielded accuracies ranging from 0.72 to 0.90 using AI approaches. Common histopathological markers used were tumor multiplicity, tumor size, tumor grade, and tumor stage. Furthermore, an AI model using an NLR had a range of accuracies from 0.638 to 0.923. This marker was used in several studies using clinical markers and other studies using additional markers. Commonly used genetic markers were TERT and FGFR3 with accuracies ranging from 0.877 to 0.9233. In addition, many genomic studies used urine and serum tests such as Cxbladder, EpiCheck, Xpert monitor, and many others to enhance the prediction of recurrence. Studies using combinations of markers from different classes achieved high accuracies in predicting tumor recurrence. Most of the AI-based studies used SVM. Other AI techniques were used to analyze the combinations of markers. The future of disease management in NMIBC and many other diseases will use AI-based models to reduce bias and interobserver variability, but this will require wide-scale effort to develop the large, high-quality datasets needed to train such models. Despite the increased power of AI and its added value in the personalized medicine field assessing recurrence risk, its ability to predict recurrences with such medical markers cannot yet be treated as a gold standard. Our review investigated the usage of AI-models regardless of their possibility to fail, so intense studies should be investigated to assure its benefit.
Author Contributions
Conceptualization, A.T.S., M.S., E.V.B., K.M.A., A.A., A.M., E.M.E.-G., M.A.M., G.A.G., S.C. and A.E.-B.; Project administration, A.E.-B.; Supervision, E.M.E.-G., M.A.M. and A.E.-B.; Writing—original draft, A.T.S., M.S., E.V.B., K.M.A., A.A., A.M., E.M.E.-G., M.A.M., G.A.G., S.C. and A.E.-B.; Writing—review and editing, A.T.S., M.S., E.V.B., K.M.A., A.A., A.M., E.M.E.-G., M.A.M., G.A.G., S.C. and A.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Prim. 2017, 3, 17022. [Google Scholar] [CrossRef]
- DeGeorge, K.C.; Holt, H.R.; Hodges, S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician 2017, 96, 507–514. [Google Scholar] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Botteman, M.F.; Pashos, C.L.; Redaelli, A.; Laskin, B.; Hauser, R. The health economics of bladder cancer. Pharmacoeconomic 2003, 21, 1315–1330. [Google Scholar] [CrossRef]
- Skolarus, T.A.; Ye, Z.; Zhang, S.; Hollenbeck, B.K. Regional Differences in Early Stage Bladder Cancer Care and Outcomes. Urology 2010, 76, 391–396. [Google Scholar] [CrossRef]
- Tan, W.S.; Rodney, S.; Lamb, B.; Feneley, M.; Kelly, J. Management of non-muscle invasive bladder cancer: A comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat. Rev. 2016, 47, 22–31. [Google Scholar] [CrossRef]
- Packiam, V.T.; Werntz, R.P.; Steinberg, G.D. Current Clinical Trials in Non-muscle-Invasive Bladder Cancer: Heightened Need in an Era of Chronic BCG Shortage. Curr. Urol. Rep. 2019, 20, 84. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2019, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Palou, J.; Hernández, C.; Solsona, E.; Abascal, R.; Burgués, J.P.; Rioja, C.; Cabrera, J.A.; Gutiérrez, C.; Rodríguez, O.; Iborra, I.; et al. Effectiveness of hexaminolevulinate fluorescence cystoscopy for the diagnosis of non-muscle- invasive bladder cancer in daily clinical practice: A Spanish multicentre observational study. BJU Int. 2015, 116, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Oktem, G.C.; Kocaaslan, R.; Karadag, M.A.; Bagcioglu, M.; Demir, A.; Cecen, K.; Unluer, E. The role of transcavitary ultrasonography in diagnosis and staging of nonmuscle-invasive bladder cancer: A prospective non-randomized clinical study. Springerplus 2014, 3, 519. [Google Scholar] [CrossRef][Green Version]
- Su, H.; Jiang, H.; Tao, T.; Kang, X.; Zhang, X.; Kang, D.; Li, S.; Li, C.; Wang, H.; Yang, Z.; et al. Hope and challenge: Precision medicine in bladder cancer. Cancer Med. 2019, 8, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European association of urology guidelines on non–muscle-invasive bladder cancer (ta, T1, and carcinoma in situ). Eur. Urol. 2021, 81, 75–94. [Google Scholar] [CrossRef]
- Alongi, P.; Caobelli, F.; Gentile, R.; Stefano, A.; Russo, G.; Albano, D.; Baldari, S.; Gilardi, M.C.; Midiri, M. Recurrent bladder carcinoma: Clinical and prognostic role of 18 F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imag. 2016, 44, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Golijanin, J.; Amin, A.; Moshnikova, A.; Brito, J.M.; Tran, T.Y.; Adochite, R.-C.; Andreev, G.O.; Crawford, T.; Engelman, D.M.; Andreev, O.A.; et al. Targeted imaging of urothelium carcinoma in human bladders by an ICG pHLIP peptide ex vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 11829–11834. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Duran, C.; Williams, S.B. Vesical imaging reporting and data system (VI-RADS) and impact on identifying depth of invasion with subsequent management in bladder cancer patients: Ready for prime time? Transl. Androl. Urol. 2020, 9, 2467. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Panebianco, V.; Narumi, Y.; Del Giudice, F.; Muglia, V.F.; Takeuchi, M.; Ghafoor, S.; Bochner, B.H.; Goh, A.C.; Hricak, H.; et al. Diagnostic performance of vesical imaging reporting and data system for the prediction of muscle-invasive bladder cancer: A systematic review and meta-analysis. Eur. Urol. Oncol. 2020, 3, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, S.; Moschini, M.; Simeone, C.; Antonelli, A.; Mattei, A.; Baumeister, P.; Xylinas, E.; Hakenberg, O.W.; Aziz, A. Prediction tools in non-muscle invasive bladder cancer. Transl. Androl. Urol. 2019, 8, 39–45. [Google Scholar] [CrossRef]
- Youssef, R.F.; Lotan, Y. Predictors of Outcome of Non–Muscle-Invasive and Muscle-Invasive Bladder Cancer. Sci. World J. 2011, 11, 369–381. [Google Scholar] [CrossRef]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Orsola, A.; Maldonado, X.; Kataja, V. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii40–iii48. [Google Scholar] [CrossRef] [PubMed]
- Matulay, J.T.; Kamat, A.M. Advances in risk stratification of bladder cancer to guide personalized medicine. F1000Research 2018, 7, 1137. [Google Scholar] [CrossRef]
- Xylinas, E.; Kent, M.; Kluth, L.; Pycha, A.; Comploj, E.; Svatek, R.S.; Lotan, Y.; Trinh, Q.-D.; Karakiewicz, P.I.; Holmang, S.; et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in nonmuscle-invasive urothelial carcinoma of the bladder. Br. J. Cancer 2014, 109, 1460–1466. [Google Scholar] [CrossRef]
- Kononenko, I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif. Intell. Med. 2001, 23, 89–109. [Google Scholar] [CrossRef]
- El Naqa, I.; Murphy, M.J. What Are Machine and Deep Learning? In Machine and Deep Learning in Oncology, Medical Physics and Radiology; Murphy, M.J., Ed.; Springer: Cham, Switzerland, 2022; pp. 3–15. [Google Scholar] [CrossRef]
- Borhani, S.; Borhani, R.; Kajdacsy-Balla, A. Artificial intelligence: A promising frontier in bladder cancer diagnosis and outcome prediction. Crit. Rev. Oncol. 2022, 171, 103601. [Google Scholar] [CrossRef]
- Gandi, C.; Vaccarella, L.; Bientinesi, R.; Racioppi, M.; Pierconti, F.; Sacco, E. Bladder cancer in the time of machine learning: Intelligent tools for diagnosis and management. Urol. J. 2021, 88, 94–102. [Google Scholar] [CrossRef]
- Shkolyar, E.; Jia, X.; Chang, T.C.; Trivedi, D.; Mach, K.E.; Meng, M.Q.-H.; Xing, L.; Liao, J.C. Augmented Bladder Tumor Detection Using Deep Learning. Eur. Urol. 2019, 76, 714–718. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, L.; Zheng, X.; Shao, J.; Wang, T.; Zhang, E.; Gao, F.; Wang, X.; Zheng, J. Clinical use of machine learning-based pathomics signature for diagnosis and survival prediction of bladder cancer. Cancer Sci. 2021, 112, 2905–2914. [Google Scholar] [CrossRef]
- Tsai, I.-J.; Shen, W.-C.; Lee, C.-L.; Wang, H.-D.; Lin, C.-Y. Machine Learning in Prediction of Bladder Cancer on Clinical Laboratory Data. Diagnostics 2022, 12, 203. [Google Scholar] [CrossRef]
- Dolz, J.; Xu, X.; Rony, J.; Yuan, J.; Liu, Y.; Granger, E.; Desrosiers, C.; Zhang, X.; Ben Ayed, I.; Lu, H. Multiregion segmentation of bladder cancer structures in MRI with progressive dilated convolutional networks. Med. Phys. 2018, 45, 5482–5493. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.-N.; KC, K.; Wei, S.; Yu, Q.; Li, R.; Haake, A.R.; Miyamoto, H.; Cui, F. Histopathological distinction of non-invasive and invasive bladder cancers using machine learning approaches. BMC Med. Inform. Decis. Mak. 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zou, X.; Wang, Y.; Ma, X. Application of deep learning as a noninvasive tool to differentiate muscle-invasive bladder cancer and non–muscle-invasive bladder cancer with CT. Eur. J. Radiol. 2021, 139, 109666. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wen, R.; Wen, D.; Huang, J.; Qin, H.; Li, X.; Wang, X.; He, Y.; Yang, H. Radiomics Analysis Based on Ultrasound Images to Distinguish the Tumor Stage and Pathological Grade of Bladder Cancer. J. Ultrasound Med. 2021, 40, 2685–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, L.; Zhao, L.; Mao, L.; Li, X.; Jin, Z.; Sun, H. CT-based radiomics to predict the pathological grade of bladder cancer. Eur. Radiol. 2020, 30, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, D.; Yao, H.; Chen, M.; Li, S.; Chen, H.; Luo, J.; Feng, Y.; Guo, Y. Radiomics analysis of multiparametric MRI for the preoperative evaluation of pathological grade in bladder cancer tumors. Eur. Radiol. 2019, 29, 6182–6190. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Wang, J. The effect of CT high-resolution imaging diagnosis based on deep residual network on the pathology of bladder cancer classification and staging. Comput. Methods Programs Biomed. 2022, 215, 106635. [Google Scholar] [CrossRef]
- Bhambhvani, H.P.; Zamora, A.; Shkolyar, E.; Prado, K.; Greenberg, D.R.; Kasman, A.M.; Liao, J.; Shah, S.; Srinivas, S.; Skinner, E.C.; et al. Development of robust artificial neural networks for prediction of 5-year survival in bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 193.e7–193.e12. [Google Scholar] [CrossRef]
- Wu, E.; Hadjiiski, L.M.; Samala, R.K.; Chan, H.-P.; Cha, K.H.; Richter, C.; Cohan, R.H.; Caoili, E.M.; Paramagul, C.; Alva, A.; et al. Deep Learning Approach for Assessment of Bladder Cancer Treatment Response. Tomography 2019, 5, 201–208. [Google Scholar] [CrossRef]
- Cha, K.H.; Hadjiiski, L.; Chan, H.-P.; Weizer, A.Z.; Alva, A.; Cohan, R.H.; Caoili, E.M.; Paramagul, C.; Samala, R.K. Bladder Cancer Treatment Response Assessment in CT using Radiomics with Deep-Learning. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Cha, K.H.; Hadjiiski, L.M.; Samala, R.K.; Chan, H.-P.; Cohan, R.H.; Caoili, E.M.; Paramagul, C.; Alva, A.; Weizer, A.Z. Bladder Cancer Segmentation in CT for Treatment Response Assessment: Application of Deep-Learning Convolution Neural Network—A Pilot Study. Tomography 2016, 2, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.N.; Naguib, R.N.G.; Hamdy, F.C.; Neal, D.E.; Mellon, J.K. Neural network analysis of clinicopathological and molecular markers in bladder cancer. J. Urol. 2000, 163, 630–633. [Google Scholar] [CrossRef]
- Tokuyama, N.; Saito, A.; Muraoka, R.; Matsubara, S.; Hashimoto, T.; Satake, N.; Matsubayashi, J.; Nagao, T.; Mirza, A.H.; Graf, H.-P.; et al. Prediction of non-muscle invasive bladder cancer recurrence using machine learning of quantitative nuclear features. Mod. Pathol. 2022, 35, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Liem, E.I.M.L.; Baard, J.; Cauberg, E.C.C.; Bus, M.T.J.; de Bruin, D.M.; Laguna Pes, M.P.; de la Rosette, J.J.M.C.H.; de Reijke, T.M. Fluorescence in situ hybridization as prognostic predictor of tumor recurrence during treatment with Bacillus Calmette–Guérin therapy for intermediate- and high-risk non-muscle-invasive bladder cancer. Med. Oncol. 2017, 34, 172. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pui, M.H.; Guo, Y.; Yang, D.; Pan, B.; Zhou, X. Diffusion-weighted MRI in bladder carcinoma: The differentiation between tumor recurrence and benign changes after resection. Abdom. Imag. 2013, 39, 135–141. [Google Scholar] [CrossRef]
- El-Assmy, A.; Abou-El-Ghar, M.E.; Refaie, H.F.; Mosbah, A.; El-Diasty, T. Diffusion-weighted magnetic resonance imaging in follow-up of superficial urinary bladder carcinoma after transurethral resection: Initial experience. BJU Int. 2012, 110, E622–E627. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cheng, J.; Pan, L.; Hu, S.; Xu, J.; Zhang, Y.; Wang, M.; Zhang, J.; Ye, D.; Zhang, Y. Is whole-body fluorine-18 fluorodeoxyglucose PET/CT plus additional pelvic images (oral hydration–voiding–refilling) useful for detecting recurrent bladder cancer? Ann. Nucl. Med. 2012, 26, 571–577. [Google Scholar] [CrossRef]
- Chen, J.-X.; Deng, N.; Chen, X.; Chen, L.-W.; Qiu, S.-P.; Li, X.-F.; Li, J.-P. A Novel Molecular Grading Model: Combination of Ki67 and VEGF in Predicting Tumor Recurrence and Progression in Non-invasive Urothelial Bladder Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 2229–2234. [Google Scholar] [CrossRef]
- Li, G.; Hu, J.; Niu, Y. Squamous differentiation in pT1 bladder urothelial carcinoma predicts poor response for intravesical chemotherapy. Oncotarget 2017, 9, 217–223. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Liu, X.; Zhang, Y.; Shen, Z.; Chen, T.; Qiu, X.; Sha, N.; Xing, C.; Wu, Z.; et al. Impact of squamous and/or glandular differentiation on recurrence and progression following transurethral resection for non-muscle invasive urothelial carcinoma of bladder. Oncol. Lett. 2017, 14, 3522–3528. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, C.; Tang, Y.; Liu, X.; Liu, Z.; Li, G.; Mei, Y. Glandular differentiation in pT1 urothelial carcinoma of bladder predicts poor prognosis. Sci. Rep. 2019, 9, 5323. [Google Scholar] [CrossRef] [PubMed]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Urdal, J.; Engan, K.; Kvikstad, V.; Janssen, E.A.M. Prognostic prediction of histopathological images by local binary patterns and RUSBoost. In Proceedings of the 25th European Signal Processing Conference (EUSIPCO), Kos, Greece, 28 August–2 September 2017. [Google Scholar] [CrossRef]
- Mano, R.; Baniel, J.; Shoshany, O.; Margel, D.; Bar-On, T.; Nativ, O.; Rubinstein, J.; Halachmi, S. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 67.e1–67.e7. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, J.; Bar-On, T.; Bahouth, Z.; Mano, R.; Shoshany, O.; Baniel, J.; Nativ, O.; Halachmi, S. A mathematical model for predicting tumor recurrence within 24 months following surgery in patients with T1 high-grade bladder cancer treated with BCG immunotherapy. Bladder 2015, 2, 18. [Google Scholar] [CrossRef]
- Albayrak, S.; Zengin, K.; Tanik, S.; Atar, M.; Unal, S.H.; Imamoglu, M.A.; Gurdal, M. Can the neutrophil-to-lymphocyte ratio be used to predict recurrence and progression of non-muscle-invasive bladder cancer? Kaohsiung J. Med. Sci. 2016, 32, 327–333. [Google Scholar] [CrossRef]
- Ferro, M.; Tătaru, O.S.; Musi, G.; Lucarelli, G.; Abu Farhan, A.R.; Cantiello, F.; Damiano, R.; Hurle, R.; Contieri, R.; Busetto, G.M.; et al. Modified Glasgow Prognostic Score as a Predictor of Recurrence in Patients with High Grade Non-Muscle Invasive Bladder Cancer Undergoing Intravesical Bacillus Calmette–Guerin Immunotherapy. Diagnostics 2022, 12, 586. [Google Scholar] [CrossRef]
- Srougi, V.; Reis, S.T.; Viana, N.; Gallucci, F.P.; Leite, K.R.; Srougi, M.; Nahas, W.C. Prospective evaluation of a urinary biomarker panel to detect and predict recurrence of non-muscle-invasive bladder cancer. World J. Urol. 2020, 39, 453–459. [Google Scholar] [CrossRef]
- Rosser, C.J.; Chang, M.; Dai, Y.; Ross, S.; Mengual, L.; Alcaraz, A.; Goodison, S. Urinary Protein Biomarker Panel for the Detection of Recurrent Bladder Cancer. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1340–1345. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.-S.; et al. ILC2-modulated T cell–to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef]
- Alberice, J.V.; Amaral, A.F.S.; Armitage, E.G.; Lorente, J.A.; Algaba, F.; Carrilho, E.; Márquez, M.; García, A.; Malats, N.; Barbas, C. Searching for urine biomarkers of bladder cancer recurrence using a liquid chromatography–mass spectrometry and capillary electrophoresis–mass spectrometry metabolomics approach. J. Chromatogr. 2013, 1318, 163–170. [Google Scholar] [CrossRef]
- Naselli, A.; Introini, C.; Timossi, L.; Spina, B.; Fontana, V.; Pezzi, R.; Germinale, F.; Bertolotto, F.; Puppo, P. A Randomized Prospective Trial to Assess the Impact of Transurethral Resection in Narrow Band Imaging Modality on Non–Muscle-Invasive Bladder Cancer Recurrence. Eur. Urol. 2012, 61, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Sfakianos, J.P.; Kim, P.H.; Hakimi, A.A.; Herr, H.W. The Effect of Restaging Transurethral Resection on Recurrence and Progression Rates in Patients with Nonmuscle Invasive Bladder Cancer Treated with Intravesical Bacillus Calmette-Guérin. J. Urol. 2014, 191, 341–345. [Google Scholar] [CrossRef]
- Culpan, M.; Keser, F.; Acar, H.C.; Otunctemur, A.; Kucuk, E.V.; Erdem, S.; Ozer, M.; Sen, U.T.; Degirmenci, E.; Ergul, R.; et al. Impact of delay in cystoscopic surveillance on recurrence and progression rates in patients with non-muscle-invasive bladder cancer during the COVID-19 pandemic. Int. J. Clin. Pract. 2021, 75, e14490. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xia, Q.; Lu, Y.; Liu, Z.; Zhou, P.; Hu, H.; Wang, S. Efficacy of intravesical therapies on the prevention of recurrence and progression of non-muscle-invasive bladder cancer: A systematic review and network meta-analysis. Cancer Med. 2020, 9, 7800–7809. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, A.; Strauss, A.; Seif Amir Hosseini, A.; Lotz, J.; Trojan, L.; Schmid, M.; Uhlig, J. Gender-specific Differences in Recurrence of Non-muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, F.H.M.; Jochems, S.H.J.; Reulen, R.C.; Pirrie, S.J.; Nekeman, D.; Wesselius, A.; James, N.D.; Wallace, D.M.A.; Cheng, K.K.; van Schooten, F.J.; et al. The association between smoking cessation before and after diagnosis and non-muscle-invasive bladder cancer recurrence: A prospective cohort study. Cancer Causes Control 2018, 29, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kinde, I.; Munari, E.; Faraj, S.F.; Hruban, R.H.; Schoenberg, M.; Bivalacqua, T.; Allaf, M.; Springer, S.; Wang, Y.; Diaz, L.A.; et al. TERT Promoter Mutations Occur Early in Urothelial Neoplasia and Are Biomarkers of Early Disease and Disease Recurrence in Urine. Cancer Res. 2013, 73, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Beukers, W.; van der Keur, K.A.; Kandimalla, R.; Vergouwe, Y.; Steyerberg, E.W.; Boormans, J.L.; Jensen, J.B.; Lorente, J.A.; Real, F.X.; Segersten, U.; et al. FGFR3, TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. J. Urol. 2017, 197, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Masius, R.; Beukers, W.; Bangma, C.H.; Orntoft, T.F.; Dyrskjot, L.; van Leeuwen, N.; Lingsma, H.; van Tilborg, A.A.G.; Zwarthoff, E.C. A 3-Plex Methylation Assay Combined with the FGFR3 Mutation Assay Sensitively Detects Recurrent Bladder Cancer in Voided Urine. Clin. Cancer Res. 2013, 19, 4760–4769. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Vinagre, J.; Prazeres, H.; Sampaio, C.; Peralta, P.; Conição, P.; Sismeiro, A.; Leao, R.; Gomes, A.; Furriel, F.; et al. Validation of a Novel, Sensitive, and Specific Urine-Based Test for Recurrence Surveillance of Patients With Non-Muscle-Invasive Bladder Cancer in a Comprehensive Multicenter Study. Front. Genet. 2019, 10, 1237. [Google Scholar] [CrossRef]
- Park, J.; Song, C.; Shin, E.; Hong, J.H.; Kim, C.-S.; Ahn, H. Do molecular biomarkers have prognostic value in primary T1G3 bladder cancer treated with bacillus Calmette-Guerin intravesical therapy? Urol. Oncol. Semin. Orig. Investig. 2013, 31, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kavalieris, L.; O’Sullivan, P.; Frampton, C.; Guilford, P.; Darling, D.; Jacobson, E.; Suttie, J.; Raman, J.; Shariat, S.; Lotan, Y. Performance Characteristics of a Multigene Urine Biomarker Test for Monitoring for Recurrent Urothelial Carcinoma in a Multicenter Study. J. Urol. 2017, 196, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- van Valenberg, F.J.P.; Hiar, A.M.; Wallace, E.; Bridge, J.A.; Mayne, D.J.; Beqaj, S.; Sexton, W.J.; Lotan, Y.; Weizer, A.Z.; Jansz, G.K.; et al. Prospective Validation of an mRNA-based Urine Test for Surveillance of Patients with Bladder Cancer. Eur. Urol. 2019, 75, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, A.A.; Awadalla, A.; Elsayed, A.; Abdullateef, M.; Abol-Enein, H. Prospective Validation of Clinical Usefulness of a Novel mRNA-based Urine Test (Xpert® Bladder Cancer Monitor) for surveillance in Non Muscle Invasive Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 77.e9–77.e16. [Google Scholar] [CrossRef]
- Bi, J.; Liu, H.; Dong, W.; Xie, W.; He, Q.; Cai, Z.; Huang, J.; Lin, T. Circular RNA circ-ZKSCAN1 inhibits bladder cancer progression through miR-1178-3p/p21 axis and acts as a prognostic factor of recurrence. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Wang, Q.; Zhao, Y.; Chen, C.; Sun, X.; Li, H.; Deng, J.; Zhang, H.; Ji, Z.; Zhang, X.; et al. An eight-long non-coding RNA signature as a candidate prognostic biomarker for bladder cancer. Aging 2019, 11, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Nishiyama, H.; Ozono, S.; Hinotsu, S.; Keino, N.; Yamaguchi, A.; Sakai, H.; Enomoto, Y.; Horie, S.; Fujimoto, K.; et al. Clinical evaluation of two consecutive UroVysion fluorescence in situ hybridization tests to detect intravesical recurrence of bladder cancer: A prospective blinded comparative study in Japan. Int. J. Clin. Oncol. 2018, 23, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y.; et al. Performance of the Bladder EpiCheck™ Methylation Test for Patients Under Surveillance for Non–muscle-invasive Bladder Cancer: Results of a Multicenter, Prospective, Blinded Clinical Trial. Eur. Urol. Oncol. 2018, 1, 307–313. [Google Scholar] [CrossRef]
- Roupret, M.; Gontero, P.; McCracken, S.R.C.; Dudderidge, T.; Stockley, J.; Kennedy, A.; Rodriguez, O.; Sieverink, C.; Vanié, F.; Allasia, M.; et al. Diagnostic Accuracy of MCM5 for the Detection of Recurrence in Nonmuscle Invasive Bladder Cancer Followup: A Blinded, Prospective Cohort, Multicenter European Study. J. Urol. 2020, 204, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Önal, B.; Han, Ü.; Yilmaz, S.; Köybasioglu, F.; Altuğ, U. The use of urinary nuclear matrix protein 22 (NMP22) as a diagnostic adjunct to urine cytology for monitoring of recurrent bladder cancer-institutional experience and review. Diagn. Cytopathol. 2014, 43, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-F.; de Castro Abreu, A.L.; Chihara, Y.; Tsai, Y.; Andreu-Vieyra, C.; Daneshmand, S.; Skinner, E.C.; Jones, P.A.; Siegmund, K.D.; Liang, G. A Panel of Three Markers Hyper- and Hypomethylated in Urine Sediments Accurately Predicts Bladder Cancer Recurrence. Clin. Cancer Res. 2014, 20, 1978–1989. [Google Scholar] [CrossRef]
- Shindo, T.; Shimizu, T.; Nojima, M.; Niinuma, T.; Maruyama, R.; Kitajima, H.; Kai, M.; Itoh, N.; Suzuki, H.; Masumori, N. Evaluation of Urinary DNA Methylation as a Marker for Recurrent Bladder Cancer: A 2-Center Prospective Study. Urology 2018, 113, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Borre, M.; Christiansen, A.; Hermann, G.G.; Orntoft, T.F.; Dyrskjot, L. Diagnosis of Bladder Cancer Recurrence Based on Urinary Levels of EOMES, HOXA9, POU4F2, TWIST1, VIM, and ZNF154 Hypermethylation B. C. Christensen, ed. PLoS ONE 2012, 7, e46297. [Google Scholar] [CrossRef]
- Maldonado, L.; Brait, M.; Michailidi, C.; Munari, E.; Driscoll, T.; Schultz, L.; Bivalacqua, T.; Schoenberg, M.; Sidransky, D.; Netto, G.J.; et al. An epigenetic marker panel for recurrence risk prediction of low grade papillary urothelial cell carcinoma (LGPUCC) and its potential use for surveillance after transurethral resection using urine. Oncotarget 2014, 5, 5218–5233. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Kim, J.; Mullane, S.A.; Reardon, B.; Orsola, A.; VanAllen, E.; Getz, G.; Kwiatkowski, D.J. Genomic predictors of recurrence (R) or progression (P) in high grade T1 (HGT1) non-muscle invasive (NMI) bladder cancer. J. Clin. Oncol. 2016, 4539. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fujiyama, N.; Tanegashima, T.; Narita, S.; Yamamoto, Y.; Fujimoto, N.; Ueda, S.; Takeuchi, A.; Numakura, K.; Habuchi, T.; et al. Effect of HLA genotype on intravesical recurrence after bacillus Calmette–Guérin therapy for non-muscle-invasive bladder cancer. Cancer Immunol. Immunother. 2021, 71, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Galesloot, T.E.; Grotenhuis, A.J.; Kolev, D.; Aben, K.K.; Bryan, R.T.; Catto, J.W.F.; Cheng, K.K.; Conroy, S.; Dyrskjøt, L.; Fleshner, N.E.; et al. Genome-wide Meta-analysis Identifies Novel Genes Associated with Recurrence and Progression in Non–muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Frantzi, M.; van Kessel, K.E.; Zwarthoff, E.C.; Marquez, M.; Rava, M.; Malats, N.; Merseburger, A.S.; Katafigiotis, I.; Stravodimos, K.; Mullen, W.; et al. Development and Validation of Urine-based Peptide Biomarker Panels for Detecting Bladder Cancer in a Multi-center Study. Clin. Cancer Res. 2016, 22, 4077–4086. [Google Scholar] [CrossRef]
- Bartsch, G.; Mitra, A.P.; Mitra, S.A.; Almal, A.A.; Steven, K.E.; Skinner, D.G.; Fry, D.W.; Lenehan, P.F.; Worzel, W.P.; Cote, R.J. Use of Artificial Intelligence and Machine Learning Algorithms with Gene Expression Profiling to Predict Recurrent Nonmuscle Invasive Urothelial Carcinoma of the Bladder. J. Urol. 2016, 195, 493–498. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Du, P.; Zhang, F.; Li, S.; Zhang, Z.; Yuan, J.; Liang, Z.; Zhang, X.; Guo, Y.; et al. A predictive nomogram for individualized recurrence stratification of bladder cancer using multiparametric MRI and clinical risk factors. J. Magn. Reson. Imag. 2019, 50, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Borgi, A.; Ounallah, S.; Stambouli, N.; Selami, S.; Ben Ammar Elgaaied, A. Diagnosic system for predicting bladder cancer recurrence using association rules. In Proceedings of the IEEE SAI Intelligent Systems Conference (IntelliSys), London, UK, 10–11 November 2015; pp. 93–99. [Google Scholar] [CrossRef]
- Lee, J.; Choo, M.S.; Yoo, S.; Cho, M.C.; Son, H.; Jeong, H. Intravesical Prostatic Protrusion and Prognosis of Non-Muscle Invasive Bladder Cancer: Analysis of Long-Term Data over 5 Years with Machine-Learning Algorithms. J. Clin. Med. 2021, 10, 4263. [Google Scholar] [CrossRef]
- Hasnain, Z.; Mason, J.; Gill, K.; Miranda, G.; Gill, I.S.; Kuhn, P.; Newton, P.K. Machine learning models for predicting post-cystectomy recurrence and survival in bladder cancer patients. PLoS ONE 2019, 14, e0210976. [Google Scholar] [CrossRef]
- Lucas, M.; Jansen, I.; van Leeuwen, T.G.; Oddens, J.R.; de Bruin, D.M.; Marquering, H.A. Deep Learning–based Recurrence Prediction in Patients with Non–muscle-invasive Bladder Cancer. Eur. Urol. Focus 2022, 8, 165–172. [Google Scholar] [CrossRef]
- Jobczyk, M.; Stawiski, K.; Kaszkowiak, M.; Rajwa, P.; Różański, W.; Soria, F.; Shariat, S.F.; Fendler, W. Deep Learning-based Recalibration of the CUETO and EORTC Prediction Tools for Recurrence and Progression of Non–muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Vedder, M.M.; Márquez, M.; de Bekker-Grob, E.W.; Calle, M.L.; Dyrskjøt, L.; Kogevinas, M.; Segersten, U.; Malmström, P.-U.; Algaba, F.; Beukers, W.; et al. Risk Prediction Scores for Recurrence and Progression of Non-Muscle Invasive Bladder Cancer: An International Validation in Primary Tumours G. PLoS ONE 2014, 9, e96849. [Google Scholar] [CrossRef] [PubMed]
- Getzler, I.; Bahouth, Z.; Nativ, O.; Rubinstein, J.; Halachmi, S. Preoperative neutrophil to lymphocyte ratio improves recurrence prediction of non-muscle invasive bladder cancer. BMC Urol. 2018, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; van Andel, G.; Kirkels, W.J.; Silva, F.C.D.; Oosterlinck, W.; Prescott, S.; et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non–Muscle-invasive Stage Ta–T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Novel nomograms to predict recurrence and progression in primary non-muscle-invasive bladder cancer: Validation of predictive efficacy in comparison with European Organization of Research and Treatment of Cancer scoring system. World J. Urol. 2018, 37, 1867–1877. [Google Scholar] [CrossRef]
- Ali-El-Dein, B.; Sooriakumaran, P.; Trinh, Q.-D.; Barakat, T.S.; Nabeeh, A.; Ibrahiem, E.-H.I. Construction of predictive models for recurrence and progression in >1000 patients with non-muscle-invasive bladder cancer (NMIBC) from a single centre. BJU Int. 2013, 111, E331–E341. [Google Scholar] [CrossRef]
- Nerli, R.B.; Ghagane, S.C.; Shankar, K.; Sanikop, A.C.; Hiremath, M.B.; Dixit, N.S.; Magadum, L. Low-Grade, Multiple, Ta Non-muscle-Invasive Bladder Tumors: Tumor Recurrence and Worsening Progression. Indian J. Surg. Oncol. 2018, 9, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, J.; Sun, J.; Wang, K.; Tai, S.; Hua, R.; Yu, Y.; Fan, Y. Development of a new recurrence-free survival prediction nomogram for patients with primary non-muscle-invasive bladder cancer based on preoperative controlling nutritional status score. Cancer Manag. Res. 2021, 13, 6473. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ibarrola, R.; Soria, F.; Abufaraj, M.; D’Andrea, D.; Preto, M.; Gust, K.M.; Briganti, A.; Shariat, S.F.; Gontero, P. Surgical checklist impact on recurrence-free survival of patients with non-muscle-invasive bladder cancer undergoing transurethral resection of bladder tumour. BJU Int. 2018, 123, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jia, Y.; Yu, C.; Xiao, H.; Guo, L.; Sun, F.; Wei, D.; Zhang, P.; Li, J.; Liu, J. Influences of Different Operative Methods on the Recurrence Rate of Non-Muscle-Invasive Bladder Cancer. Urol. J. 2020, 18, 411–416. [Google Scholar] [CrossRef]
- Ajili, F.; Issam, B.M.; Kourda, N.; Darouiche, A.; Chebil, M.; Boubaker, S. Prognostic Value of Artificial Neural Network in Predicting Bladder Cancer Recurrence after BCG Immunotherapy. J. Cytol. Histol. 2014, 5, 226. [Google Scholar] [CrossRef]
- Zhan, Y.; Du, L.; Wang, L.; Jiang, X.; Zhang, S.; Li, J.; Yan, K.; Duan, W.; Zhao, Y.; Wang, L.; et al. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol. Cancer 2018, 17, 142. [Google Scholar] [CrossRef]
- Gogalic, S.; Sauer, U.; Doppler, S.; Heinzel, A.; Perco, P.; Lukas, A.; Simpson, G.; Pandha, H.; Horvath, A.; Preininger, C. Validation of a protein panel for the noninvasive detection of recurrent non-muscle invasive bladder cancer. Biomarkers 2017, 22, 674–681. [Google Scholar] [CrossRef]
- López de Maturana, E.; Picornell, A.; Masson-Lecomte, A.; Kogevinas, M.; Márquez, M.; Carrato, A.; Tardón, A.; Lloreta, J.; García-Closas, M.; Silverman, D.; et al. Prediction of non-muscle invasive bladder cancer outcomes assessed by innovative multimarker prognostic models. BMC Cancer 2016, 16, 351. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).