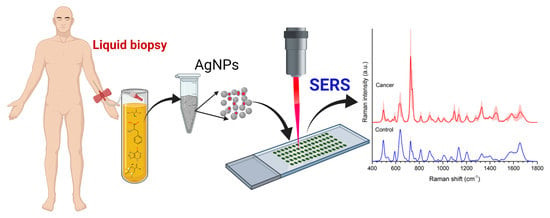
Label-Free Surface Enhanced Raman Spectroscopy for Cancer Detection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of AgNPs
2.2. Collection and Preparation of Human Blood Plasma Samples
2.3. SERS Measurements and Data Analysis Methods
3. Results
3.1. Evaluation of the Influence of Silver Colloid Concentration on the Enhancement Effect
3.2. SERS Spectra of Small Biomolecules in the Serum
3.3. Comparison between Whole Serum (WS) and Filtered Serum (FS)
3.4. Evaluation the Effects of Proteins on Serum SERS Spectra
3.5. SERS Spectra of Healthy vs. Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Balmain, A.; Barrett, J.C.; Moses, H.; Renan, M.J. How many mutations are required for tumorigenesis? Implications from human cancer data. Mol. Carcinog. 1993, 7, 139–146. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Home Page. Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer#types (accessed on 26 December 2021).
- Jafari, S.H.; Saadatpour, Z.; Salmaninejad, A.; Momeni, F.; Mokhtari, M.; Nahand, J.S.; Rahmati, M.; Mirzaei, H.; Kianmehr, M. Breast cancer diagnosis: Imaging techniques and biochemical markers. J. Cell Physiol. 2018, 233, 5200–5213. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. Precision study group collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.W.L.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, M.; Karachaliou, N.; González-Cao, M.; Altavilla, G.; Giovannetti, E.; Rosell, R. Feasibility of cell-free circulating tumor DNA testing for lung cancer. Biomark. Med. 2016, 10, 417–430. [Google Scholar] [CrossRef]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2017, 67, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan-Jansen, A.; Richards-Kortum, R.R. Raman spectroscopy for the detection of cancers and precancers. J. Biomed. Opt. 1996, 1, 31–70. [Google Scholar] [CrossRef]
- Puppels, G.J.; van Aken, M.; Wolthuis, R.; Caspers, P.J.; Schut, T.C.B.; Bruining, H.A.; Roemer, T.J.; Buschman, H.P.J.; Wach, M.L.; Robinson, J.S., Jr. In vivo tissue characterization by Raman spectroscopy. Proc. SPIE 1998, 3257, 78–83. [Google Scholar] [CrossRef]
- Shim, M.G.; Wilson, B.C.; Marple, E.; Wach, M. Study of fibre-optic probes for in vivo medical Raman spectroscopy. Appl. Spectrosc. 1999, 53, 619–625. [Google Scholar] [CrossRef]
- Feld, S.M.; Feld, M.S.; Manoharan, R.; Salenius, J.; Orenstein-Carndona, J.; Roemer, T.J.; Brennan III, J.F.; Dasari, R.R.; Wang, Y. Detection and characterization of human tissue lesions with infrared Raman spectroscopy. Proc. SPIE 1995, 2388, 99–105. [Google Scholar] [CrossRef]
- Manoharan, R.; Shafer, K.; Perelman, L.; Wu, J.; Chen, K.; Deinum, G.; Fitzmaurice, M.; Myles, J.; Crowe, J.; Dasari, R.R.; et al. Raman spectroscopy and fluorescence photon migration for breast cancer diagnosis and imaging. Photochem. Photobiol. 1998, 67, 15–22. [Google Scholar] [CrossRef]
- Alfano, R.R.; Liu, C.H.; Sha, W.L.; Zhu, H.R.; Akins, D.L.; Cleary, J.; Prudente, R.; Celmer, E. Human breast tissue studied by IR Fourier transform Raman spectroscopy. Lasers Life Sci. 1991, 4, 23–28. [Google Scholar]
- Mizuno, A.; Kitajima, H.; Kawauchi, K.; Muraishi, S.; Ozaki, Y. Near-infrared Fourier transform Raman spectroscopic study of human brain tissues and tumors. J. Raman Spectrosc. 1994, 25, 25–29. [Google Scholar] [CrossRef]
- Mahadevan-Jansen, A.; Mitchell, M.F.; Ramanujam, N.; Malpica, A.; Thomsen, S.; Utzinger, U.; Richards-Kortum, R. Near-infrared Raman spectroscopy for in vitro detection of cervical precancers. Photochem. Photobiol. 1998, 68, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Fulljames, C.; Stone, N.; Bennett, D.; Barr, H. Beyond white light endoscopy: The prospect for endoscopic optical biopsy. Ital. J. Gastroenterol. Hepatol. 1991, 31, 695–704. [Google Scholar]
- Schut, T.C.B.; Stone, N.; Kendall, C.A.; Barr, H.; Bruining, H.A.; Puppels, G.J. Progress in the detection of neoplastic progress and cancer by Raman spectroscopy. Proc. SPIE 2000, 3918, 106–113. [Google Scholar] [CrossRef]
- Gniadecka, M.; Wulf, H.C.; Nielsen, O.F.; Christensen, D.H.; Hercogova, J. Distinctive molecular abnormalities in benign and malignant skin lesions: Studies by Raman spectroscopy. Photochem. Photobiol. 1997, 66, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman electrochemistry part I. Heterocyclic, aromatic and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Culha, M.; Cullum, B.; Lavrik, N.; Klutse, C.K. Surface-enhanced Raman scattering as an emerging characterization and detection technique. J. Nanotechnol. 2012, 15. [Google Scholar] [CrossRef]
- Yilmaz, H.; Yilmaz, D.; Taskin, I.C.; Culha, M. Pharmaceutical applications of a nanospectroscopic technique: Surface-enhanced Raman spectroscopy. Adv. Drug Deliv. Rev. 2022, 184, 114184. [Google Scholar] [CrossRef]
- Darrigues, E.; Nima, Z.A.; Majeed, W.; Vang-Dings, K.B.; Dantuluri, V.; Biris, A.R.; Zharov, V.P.; Griffin, R.J.; Biris, A.S. Raman spectroscopy using plasmonic and carbon-based nanoparticles for cancer detection, diagnosis, and treatment guidance. Part 1: Diagnosis. Drug. Metab. Rev. 2017, 49, 212–252. [Google Scholar] [CrossRef]
- Vendrell, M.; Maiti, K.K.; Dhaliwal, K.; Chang, Y.T. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013, 31, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Culha, M. Raman spectroscopy for cancer diagnosis: How far have we come? Bioanalysis 2015, 7, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Cervo, S.; Sergo, V. Label-free surface-enhanced Raman spectroscopy of biofluids: Fundamental aspects and diagnostic applications. Anal. Bioanal. Chem. 2015, 407, 8265–8277. [Google Scholar] [CrossRef]
- Feng, S. Nasopharyngeal cancer detection based on blood plasma surface-enhanced Raman spectroscopy and multi-variate analysis. Biosens. Bioelectron. 2010, 25, 2414–2419. [Google Scholar] [CrossRef]
- Lin, D.; Feng, S.; Pan, J.; Chen, Y.; Lin, J.; Chen, G.; Xie, S.; Zeng, H.; Chen, R. Colorectal cancer detection by gold nanoparticle-based surface-enhanced Raman spectroscopy of blood serum and statistical analysis. Opt. Express. 2011, 19, 13565–13577. [Google Scholar] [CrossRef]
- Li, S.-X.; Zeng, Q.-Y.; Li, L.-F.; Zhang, Y.-J.; Wan, M.-M.; Liu, Z.-M.; Xiong, H.-L.; Guo, Z.-Y.; Liu, S.-H. Study of support vector machine and serum surface-enhanced Raman spectroscopy for noninvasive esophageal cancer detection. J. Biomed. Opt. 2013, 18, 27008. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, S.; Huang, H.; Chen, W.; Shi, H.; Liu, N.; Chen, L.; Chen, W.; Yu, Y.; Chen, R. Label-free detection of blood plasma using silver nanoparticle-based surface-enhanced Raman spectroscopy for esophageal cancer screening. J. Biomed. Nanotechnol. 2014, 10, 478–4784. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Xu, J.; Li, L.; Zeng, Q.; Lin, L.; Guo, Z.; Liu, Z.; Xiong, H.; Liu, S. Noninvasive prostate cancer screening based on serum surface-enhanced Raman spectroscopy and support vector machine. Appl. Phys. Lett. 2014, 105, 091104. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Li, S.; Jin, L.; Wang, D.; Guan, D.; Ding, J. Noninvasive liver diseases detection based on serum surface enhanced Raman spectroscopy and statistical analysis. Opt. Express 2015, 23, 18361–18372. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, Y.; Xu, C.; Lin, K.; Ye, Q.; Wang, X.; Xie, S.; Chen, R.; Lin, J. Label-free detection of nasopharyngeal and liver cancer using surface-enhanced Raman spectroscopy and partial lease squares combined with support vector machine. Biomed. Opt. Express 2018, 12, 6053–6066. [Google Scholar] [CrossRef]
- Chen, G.; Lin, X.; Lin, D.; Ge, X.; Feng, S.; Pan, J.; Lin, J.; Huang, Z.; Huang, X.; Chen, R. Identification of different tumor states in nasopharyngeal cancer using surface-enhanced Raman spectroscopy combined with Lasso-PLS-DA algorithm. RSC Adv. 2016, 6, 7760–7764. [Google Scholar] [CrossRef]
- Zhang, K.; Hao, C.; Huo, Y.; Man, B.; Zhang, C.; Yang, C.; Liu, M.; Chen, C. Label-free diagnosis of lung cancer with tis-sue-slice surface-enhanced Raman spectroscopy and statistical analysis. Lasers Med. Sci. 2019, 34, 1849–1855. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, X.; Rong, Z.; Xiu, B.; Yang, X.; Wang, C.; Hao, W.; Zhang, Q.; Liu, Z.; Duan, C.; et al. Non-invasive detection of hepatocellular carcinoma serum metabolic profile through surface-enhanced Raman spectroscopy. Nanomedicine 2016, 12, 2475–2484. [Google Scholar] [CrossRef]
- Bonifacio, A.; Marta, S.D.; Spizzo, R.; Cervo, S.; Steffan, A.; Colombatti, A.; Sergo, V. Surface-enhanced Raman spectroscopy of blood plasma and serum using Ag and Au nanoparticles: A systematic study. Anal. Bioanal. Chem. 2014, 406, 2355–2365. [Google Scholar] [CrossRef]
- Cervo, S.; Mansutti, E.; Mistro, G.D.; Spizzo, R.; Colombatti, A.; Steffan, A.; Sergo, V.; Bonifacio, A. SERS analysis of serum for detection of early and locally advanced breast cancer. Anal. Bioanal. Chem. 2015, 407, 7503–7509. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.; Feng, S.; Pan, J.; Zheng, X.; Su, Y.; Chen, Y.; Huang, Z.; Lin, X.; Lan, F.; et al. Label-free serum ribonucleic acid analysis for colorectal cancer detection by surface-enhanced Raman spectroscopy and multivariate analysis. J. Biomed. Opt. 2012, 17, 067003. [Google Scholar] [CrossRef] [PubMed]
- Carmicheal, J.; Hayashi, C.; Huang, X.; Liu, L.; Lu, Y.; Krasnoslobodtsev, K.; Lushnikov, A.; Kshirsagar, P.G.; Patel, A.; Jain, M.; et al. Label-free characterization of exosome via surface enhanced Raman spectroscopy for the early detection of pancreatic cancer. Nanomedicine 2019, 16, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Pan, J.; Huang, H.; Chen, G.; Qiu, S.; Shi, H.; Chen, W.; Yu, Y.; Feng, S.; Chen, R. Label-free blood plasma test based on surface-enhanced Raman scattering for tumor stages detection in nasopharyngeal cancer. Sci. Rep. 2014, 4, 4751. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, Q.; Qiu, S.; Chen, G.; Feng, S.; Chen, R.; Zeng, H. Label-free liquid biopsy based on blood circulating DNA detection using SERS-based nanotechnology for nasopharyngeal cancer screening. Nanomedicine 2019, 22, 102100. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, S.; Cui, X.; Xu, W.; Kong, C.; Zhang, Z.; Qian, W. Identifying non-muscle-invasive and muscle-invasive bladder cancer based on blood serum sur-face-enhanced Raman spectroscopy. Biomed. Opt. Express 2019, 10, 3533–3544. [Google Scholar] [CrossRef]
- Muhammad, M.; Shao, C.; Huang, Q. Label-free SERS diagnostics of radiation-induced injury via detecting the biomarker Raman signal in the serum and urine bio-samples based on Au-NPs array substrates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117282. [Google Scholar] [CrossRef]
- Moisoiu, V.; Socaciu, A.; Stefancu, A.; Iancu, S.D.; Boros, I.; Alecsa, C.D.; Rachieriu, C.; Chiorean, A.R.; Eniı, D.; Leopold, N.; et al. Breast cancer diagnosis by Surface-Enhanced Raman Scattering (SERS) of urine. Appl. Sci. 2019, 9, 806. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.G.; Lee, S.H.; Kim, W.; Bang, A.; Moon, S.W.; Song, J.; Shin, J.-H.; Yu, J.S.; Choi, S. Label-free Surface-Enhanced Raman Spectroscopy biosensor for on-site breast cancer detection using human tears. ACS Appl. Mater. Interfaces. 2020, 12, 7897–7904. [Google Scholar] [CrossRef]
- Mert, S.; Culha, M. Surface-enhanced Raman scattering-based detection of cancerous renal cells. Appl. Spectrosc. 2014, 68, 617–624. [Google Scholar] [CrossRef]
- Kuku, G.; Sarıçam, M.; Mert, S.; Çulha, M. Surface-enhanced Raman scattering from living cells: From differentiating healthy and cancerous cell to cytotoxicity assessment; International Society for Optics and Photonics. In Smart Biomedical and Physiological Sensor Technology XII; SPIE: Bellingham, WA, USA, 2015; p. 9487. [Google Scholar] [CrossRef]
- Mert, S.; Sancak, S.; Aydın, H.; Fersahoğlu, A.T.; Somay, A.; Özkan, F.; Çulha, M. Development of a SERS based cancer diagnosis approach employing cryosectioned thyroid tissue samples on PDMS. Nanomed. Nanotechnol. Biol. Med. 2022, 44, 102577. [Google Scholar] [CrossRef]
- Nargis, H.F.; Nawaz, H.; Bhatti, H.N.; Jilani, K.; Saleem, M. Comparison of surface enhanced Raman spectroscopy and Raman spectroscopy for the detection of breast cancer based on serum samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 246, 119034. [Google Scholar] [CrossRef] [PubMed]
- Moisoiu, V.; Iancu, S.D.; Stefancu, A.; Moisoiu, T.; Pardini, B.; Dragomir, M.P.; Crisan, N.; Avram, L.; Crisan, D.; Andras, I.; et al. SERS liquid biopsy: An emerging tool for medical diagnosis. Colloids Surfaces B Biointerfaces 2021, 208, 112064. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Formoso, M.; Alvarez-Puebla, R.A. Cancer diagnosis through SERS and other related techniques. Int. J. Mol. Sci. 2020, 21, 2253. [Google Scholar] [CrossRef] [PubMed]
- Avram, L.; Stefancu, A.; Crisan, D.; Leopold, N.; Donca, V.; Buzdugan, E.; Craciun, R.; Andras, D.; Coman, I. Recent advances in surface-enhanced Raman spectroscopy based liquid biopsy for colorectal cancer. Exp. Ther. Med. 2020, 20, 213. [Google Scholar] [CrossRef]
- Hong, Y.; Li, Y.; Huang, L.; He, W.; Wang, S.; Wang, C.; Zhou, G.; Chen, Y.; Zhou, X.; Huang, Y.; et al. Label-free diagnosis for colorectal cancer through coffee ring-assisted surface-enhanced Raman spectroscopy on blood serum. J. Biophotonics 2020, 13, e201960176. [Google Scholar] [CrossRef]
- Lin, X.; Lin, D.; Chen, Y.; Lin, J.; Weng, S.; Song, J.; Feng, S. High throughput blood analysis based on deep learning algorithm and self-positioning super-hydrophobic SERS platform for non-invasive multi-disease screening. Adv. Funct. Mater. 2021, 31, 2103382. [Google Scholar] [CrossRef]
- Lee, P.; Meisel, D. Adsorption and Surface-Enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Bonnier, F.; Baker, M.J.; Byrne, H.J. Vibrational spectroscopic analysis of body fluids: Avoiding molecular contamination using centrifugal filtration. Anal. Methods 2014, 6, 5155–5160. [Google Scholar] [CrossRef]
- Senger, R.; Sherr, D. Resolving complex phenotypes with Raman spectroscopy and chemometrics. Curr. Opin. Biotechnol. 2020, 66, 277–282. [Google Scholar] [CrossRef]
- Zheng, X.-S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 56–77. [Google Scholar] [CrossRef]
- Chen, X.; Tang, M.; Liu, Y.; Huang, J.; Liu, Z.; Tian, H.; Zheng, Y.; Lamy de la Chapelle, M.; Zhang, Y.; Fu, W. Surface-Enhanced Raman scattering method for the identification of methicillin-resistant Staphylococcus aureus using positively charged silver nanoparticles. Microchim. Acta 2019, 186, 102. [Google Scholar] [CrossRef] [PubMed]
- Tefas, C.; Mărginean, R.; Toma, V.; Petrushev, B.; Fischer, P.; Tanțău, M.; Știufiuc, R. Surface-Enhanced Raman scattering for the diagnosis of ulcerative colitis: Will it change the rules of the game? Anal. Bioanal. Chem. 2021, 413, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Sevilla, P.; Sánchez-Cortés, S.; García-Ramos, J.V.; Feis, A. Concentration-controlled formation of myoglobin/gold nanosphere aggregates. J. Phys. Chem. B 2014, 118, 5082–5092. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 16, e16957. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, V.; Thomas, J.K.; Marimuthu, A.; Muthusamy, B.; Radhakrishnan, A.; Sharma, R.; Khan, A.A.; Balakrishnan, L.; Sahasrabuddhe, N.A.; Kumar, S.; et al. Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 2014, 42, D959–D965. [Google Scholar] [CrossRef]
- Serum Metabolome Database Home Page. Available online: http://www.serummetabolome.ca/ (accessed on 26 December 2021).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avci, E.; Yilmaz, H.; Sahiner, N.; Tuna, B.G.; Cicekdal, M.B.; Eser, M.; Basak, K.; Altıntoprak, F.; Zengin, I.; Dogan, S.; et al. Label-Free Surface Enhanced Raman Spectroscopy for Cancer Detection. Cancers 2022, 14, 5021. https://doi.org/10.3390/cancers14205021
Avci E, Yilmaz H, Sahiner N, Tuna BG, Cicekdal MB, Eser M, Basak K, Altıntoprak F, Zengin I, Dogan S, et al. Label-Free Surface Enhanced Raman Spectroscopy for Cancer Detection. Cancers. 2022; 14(20):5021. https://doi.org/10.3390/cancers14205021
Chicago/Turabian StyleAvci, Ertug, Hulya Yilmaz, Nurettin Sahiner, Bilge Guvenc Tuna, Munevver Burcu Cicekdal, Mehmet Eser, Kayhan Basak, Fatih Altıntoprak, Ismail Zengin, Soner Dogan, and et al. 2022. "Label-Free Surface Enhanced Raman Spectroscopy for Cancer Detection" Cancers 14, no. 20: 5021. https://doi.org/10.3390/cancers14205021
APA StyleAvci, E., Yilmaz, H., Sahiner, N., Tuna, B. G., Cicekdal, M. B., Eser, M., Basak, K., Altıntoprak, F., Zengin, I., Dogan, S., & Çulha, M. (2022). Label-Free Surface Enhanced Raman Spectroscopy for Cancer Detection. Cancers, 14(20), 5021. https://doi.org/10.3390/cancers14205021