Effects of Weight Status and Related Metabolic Disorders on Fertility-Sparing Treatment Outcomes in Endometrial Atypical Hyperplasia and Endometrial Cancer: A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
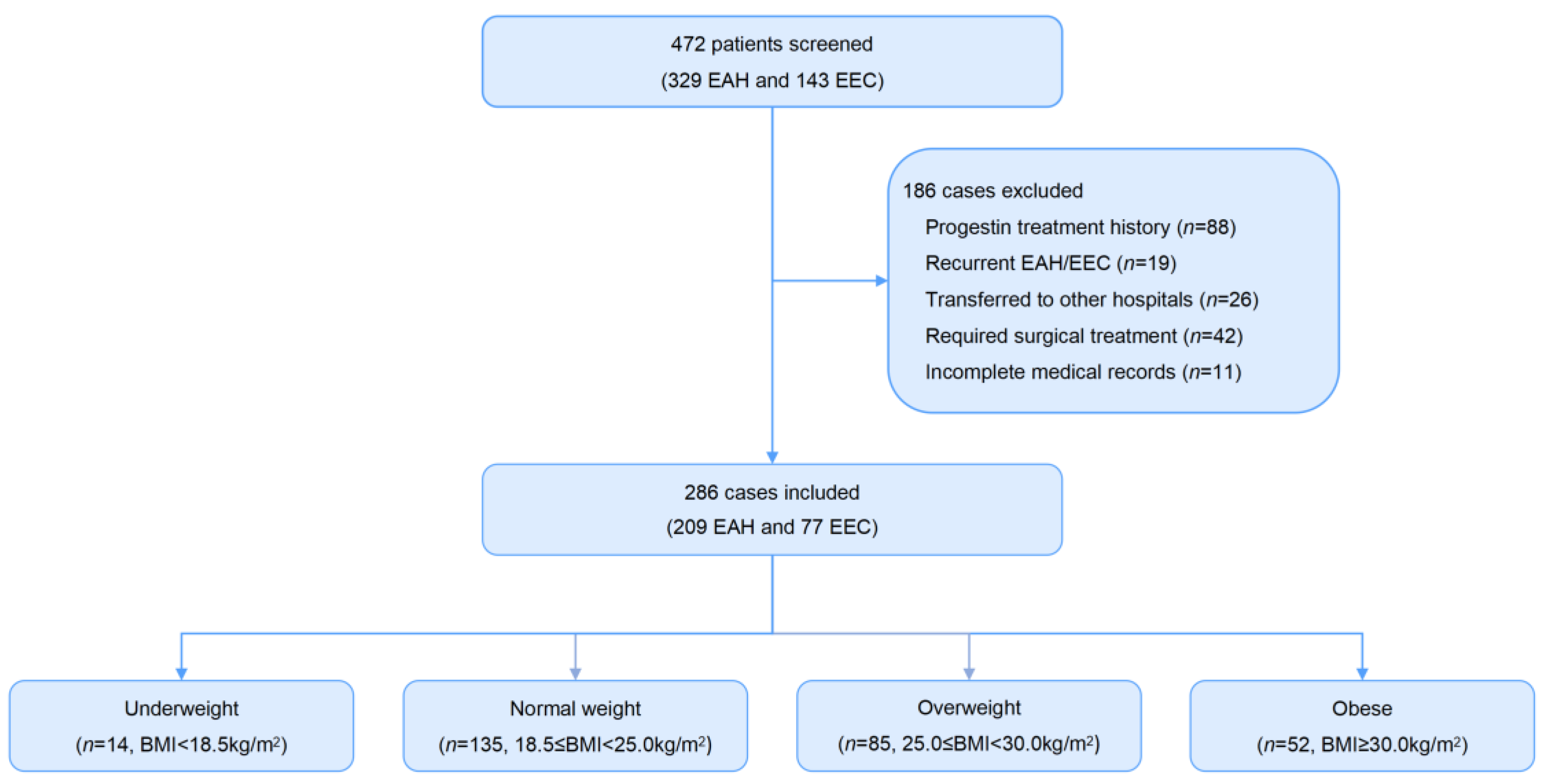
2.1. Study Population
2.2. Diagnosis and Assessment
2.3. Treatment and Evaluation
2.4. Fertility and Follow-Up
2.5. Statistics Analysis
3. Results
3.1. Correlation between Weight Status and Metabolic Disorders
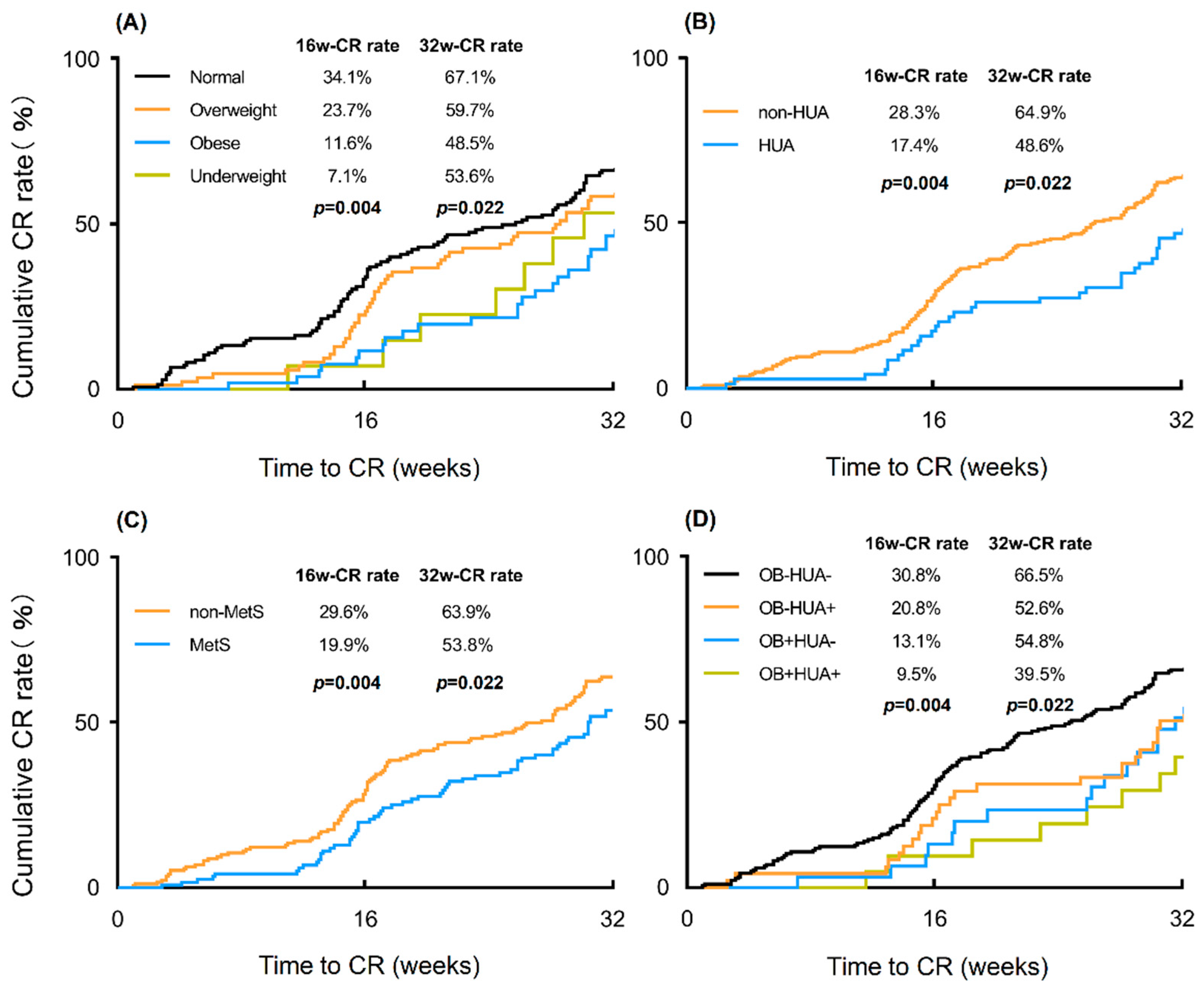
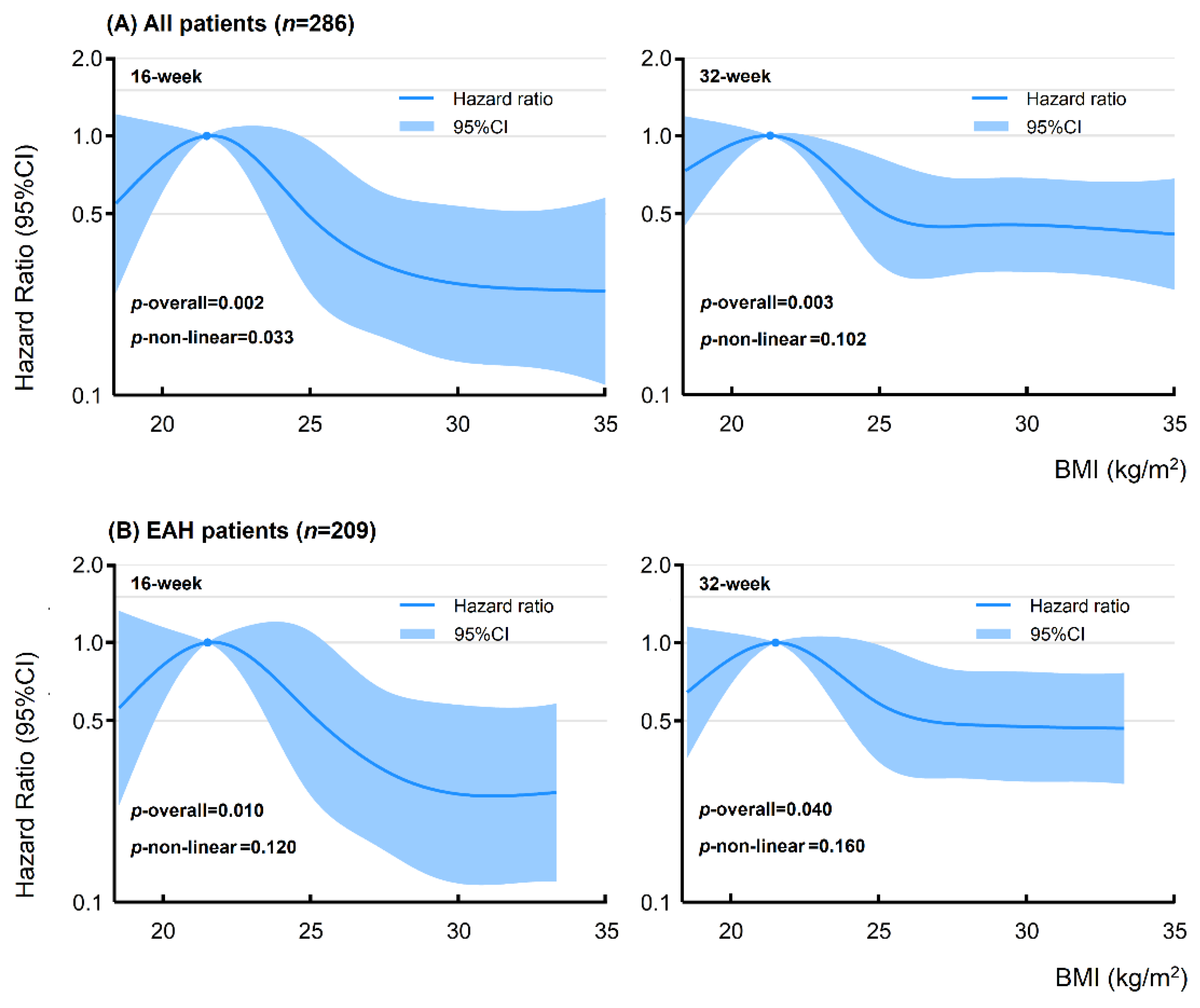
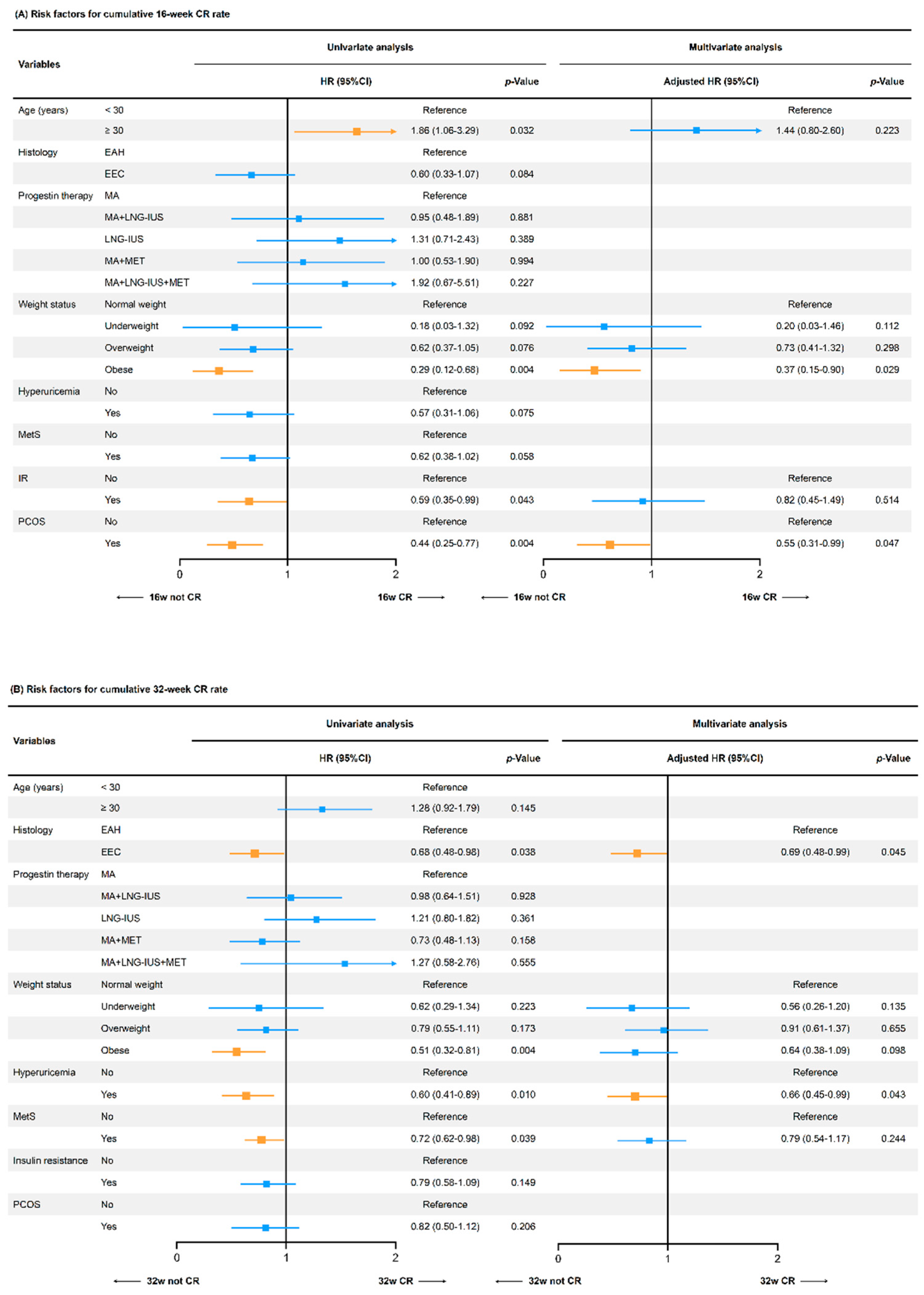
3.2. Effects of Weight Status on Oncological and Reproductive Treatment Outcomes
3.3. Effects of Related Metabolic Factors on Treatment Outcomes
4. Discussion
4.1. Effects of Weight Status on Fertility-Sparing Treatment
4.2. Effects of Metabolic Disorders on Fertility-Sparing Treatment
4.3. Strengths and Limitations
4.4. Implications for Clinical Practice and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| BMI (kg/m2) | All Patients (n = 286) | EAH Patients (n = 209) | ||
|---|---|---|---|---|
| 16-Week | 32-Week | 16-Week | 32-Week | |
| 18.0 | 0.54 (0.24–1.22) | 0.72 (0.44–1.19) | - * | - * |
| 19.0 | 0.65 (0.35–1.18) | 0.81 (0.56–1.16) | 0.64 (0.33–1.26) | 0.72 (0.46–1.13) |
| 20.0 | 0.83 (0.61–1.11) | 0.93 (0.78–1.10) | 0.82 (0.58–1.15) | 0.87 (0.69–1.09) |
| 21.0 | Reference | Reference | Reference | Reference |
| 22.0 | 0.99 (0.93–1.06) | 0.97 (0.91–1.03) | 0.99 (0.92–1.08) | 0.98 (0.93–1.04) |
| 23.0 | 0.85 (0.66–1.09) | 0.81 (0.68–0.98) | 0.86 (0.64–1.17) | 0.85 (0.69–1.06) |
| 24.0 | 0.65 (0.39–1.07) | 0.63 (0.45–0.90) | 0.69 (0.39–1.20) | 0.70 (0.47–1.04) |
| 25.0 | 0.48 (0.25–0.95) | 0.51 (0.32–0.82) | 0.52 (0.25–1.09) | 0.58 (0.34–0.98) |
| 26.0 | 0.39 (0.19–0.78) | 0.46 (0.28–0.70) | 0.42 (0.20–0.88) | 0.52 (0.30–0.89) |
| 28.0 | 0.30 (0.16–0.58) | 0.44 (0.29–0.68) | 0.30 (0.15–0.62) | 0.48 (0.29–0.78) |
| 30.0 | 0.27 (0.13–0.54) | 0.45 (0.30–0.69) | 0.26 (0.12–0.58) | 0.47 (0.29–0.77) |
| 32.0 | 0.26 (0.13–0.51) | 0.44 (0.29–0.67) | 0.26 (0.12–0.56) | 0.47 (0.29–0.76) |
| 34.0 | 0.25 (0.12–0.54) | 0.42 (0.27–0.67) | - * | - * |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Clarke, M.A.; Long, B.J.; Sherman, M.E.; Lemens, M.A.; Podratz, K.C.; Hopkins, M.R.; Ahlberg, L.J.; Mc Guire, L.J.; Laughlin-Tommaso, S.K.; Wentzensen, N.; et al. A prospective clinical cohort study of women at increased risk for endometrial cancer. Gynecol. Oncol. 2020, 156, 169–177. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Uehara, T.; Hanawa, S.; Shozu, M. Prospective evaluation of abnormal glucose metabolism and insulin resistance in patients with atypical endometrial hyperplasia and endometrial cancer. Support. Care Cancer 2017, 25, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.S.; Kabat, G.C.; Kim, M.Y.; Wild, R.A.; Shadyab, A.H.; Wactawski-Wende, J.; Ho, G.Y.F.; Reeves, K.W.; Kuller, L.H.; Luo, J.; et al. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: A prospective study. Cancer Causes Control 2019, 30, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, W.; Han, C.; Liu, T.; Li, J.; Song, D. Correlation of Metabolic Factors with Endometrial Atypical Hyperplasia and Endometrial Cancer: Development and Assessment of a New Predictive Nomogram. Cancer Manag. Res. 2021, 13, 7937–7949. [Google Scholar] [CrossRef]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stat facts: Uterine Cancer. Available online: http://seer.cancer.gov/statfacts/html/corp.html (accessed on 30 April 2022).
- Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, K.R.; Kim, Y.T.; Seong, S.J.; Kim, T.J.; Kim, J.W.; Kim, S.M.; et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur. J. Cancer 2013, 49, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xu, Y.; Zhu, Q.; Xie, L.; Shan, W.; Ning, C.; Xie, B.; Shi, Y.; Luo, X.; Zhang, H.; et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol. Oncol. 2019, 153, 55–62. [Google Scholar] [CrossRef]
- Gonthier, C.; Walker, F.; Luton, D.; Yazbeck, C.; Madelenat, P.; Koskas, M. Impact of obesity on the results of fertility-sparing management for atypical hyperplasia and grade 1 endometrial cancer. Gynecol. Oncol. 2014, 133, 33–37. [Google Scholar] [CrossRef]
- Chen, J.; Cao, D.; Yang, J.; Yu, M.; Zhou, H.; Cheng, N.; Wang, J.; Zhang, Y.; Peng, P.; Shen, K. Fertility-Sparing Treatment for Endometrial Cancer or Atypical Endometrial Hyperplasia Patients With Obesity. Front. Oncol. 2022, 12, 812346. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xie, L.; Zhang, H.; Zhu, Q.; Du, Y.; Luo, X.; Chen, X. Insulin resistance and overweight prolonged fertility-sparing treatment duration in endometrial atypical hyperplasia patients. J. Gynecol. Oncol. 2018, 29, e35. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, X.; Wang, Q.; Lv, Q.; Wu, P.; Liu, W.; Chen, X. Fertility-preserving treatment outcome in endometrial cancer or atypical hyperplasia patients with polycystic ovary syndrome. J. Gynecol. Oncol. 2021, 32, e70. [Google Scholar] [CrossRef]
- Li, X.; Fan, Y.; Wang, J.; Zhou, R.; Tian, L.; Wang, Y.; Wang, J. Insulin Resistance and Metabolic Syndrome Increase the Risk of Relapse For Fertility Preserving Treatment in Atypical Endometrial Hyperplasia and Early Endometrial Cancer Patients. Front. Oncol. 2021, 11, 744689. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Ning, C.; Luo, X.; Zhou, Q.; Gu, C.; Zhang, Z.; Chen, X. Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: A prospective cross-sectional study. Gynecol. Oncol. 2014, 132, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiang, H.; Zhang, B.; Wang, H.; Jia, X.; Huang, F.; Wang, L.; Wang, Z. Threshold-Effect Association of Dietary Cholesterol Intake with Dyslipidemia in Chinese Adults: Results from the China Health and Nutrition Survey in 2015. Nutrients 2019, 11, 2885. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian respondera plea for universal definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Farris, M.; McNeil, J.; Friedenreich, C. Obesity and Endometrial Cancer. Recent Results Cancer Res. 2016, 208, 107–136. [Google Scholar]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lu, J.; Wu, S.; Bi, Y. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. Am. J. Cancer Res. 2016, 6, 2334–2344. [Google Scholar] [PubMed]
- Piltonen, T.T.; Chen, J.C.; Khatun, M.; Kangasniemi, M.; Liakka, A.; Spitzer, T.; Tran, N.; Huddleston, H.; Irwin, J.C.; Giudice, L.C. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum. Reprod. 2015, 30, 1203–1215. [Google Scholar] [CrossRef]
- Ning, C.; Xie, B.; Zhang, L.; Li, C.; Shan, W.; Yang, B.; Luo, X.; Gu, C.; He, Q.; Jin, H.; et al. Infiltrating Macrophages Induce ERalpha Expression through an IL17A-mediated Epigenetic Mechanism to Sensitize Endometrial Cancer Cells to Estrogen. Cancer Res. 2016, 76, 1354–1366. [Google Scholar] [CrossRef]
- Hafiane, A.; Gasbarrino, K.; Daskalopoulou, S.S. The role of adiponectin in cholesterol efflux and HDL biogenesis and metabolism. Metabolism 2019, 100, 153953. [Google Scholar] [CrossRef] [PubMed]
- Hafiane, A.; Daskalopoulou, S.S. Adiponectin’s mechanisms in high-density lipoprotein biogenesis and cholesterol efflux. Metabolism 2020, 113, 154393. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose Tissue, Obesity and Adiponectin: Role in Endocrine Cancer Risk. Int. J. Mol. Sci. 2019, 20, 2863. [Google Scholar] [CrossRef]
- Yamauchi, N.; Takazawa, Y.; Maeda, D.; Hibiya, T. Expression levels of adiponectin receptors are decreased in human endometrial adenocarcinoma tissues. Int. J. Gynecol. Pathol. 2012, 31, 352–357. [Google Scholar] [CrossRef]
- Sato, M.; Tamura, Y.; Nakagata, T.; Someya, T. Prevalence and Features of Impaired Glucose Tolerance in Young Underweight Japanese Women. J. Clin. Endocrinol. Metab. 2021, 106, e2053–e2062. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, O.E.; Canbay, A.; Fuhrer, D.; Reger-Tan, S. Metabolic and androgen profile in underweight women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2017, 296, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, P.; Liu, K.; Lin, S.; Wang, M.; Tian, T.; Dai, C.; Deng, Y.; Li, N.; Hao, Q.; et al. Hyperuricemia and gout are associated with cancer incidence and mortality: A meta-analysis based on cohort studies. J. Cell Physiol. 2019, 234, 14364–14376. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Tang, L.J.; Luo, D.H.; Wang, Y.; Wu, N.; Chen, H.; Chen, M.X.; Jiang, L.; Jin, R. Metabolic Syndrome-Related Hyperuricemia is Associated with a Poorer Prognosis in Patients with Colorectal Cancer: A Multicenter Retrospective Study. Cancer Manag. Res. 2021, 13, 8809–8819. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yanagida, T.; Onda, A.; Tsukui, D.; Hosoyamada, M.; Kono, H. Soluble Uric Acid Promotes Atherosclerosis via AMPK (AMP-Activated Protein Kinase)-Mediated Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef]
- Hu, X.; Rong, S.; Wang, Q.; Sun, T.; Bao, W.; Chen, L.; Liu, L. Association between plasma uric acid and insulin resistance in type 2 diabetes: A Mendelian randomization analysis. Diabetes Res. Clin. Pract. 2021, 171, 108542. [Google Scholar] [CrossRef]
- D’Elia, L.; Giaquinto, A.; Cappuccio, F.P.; Iacone, R.; Russo, O.; Strazzullo, P.; Galletti, F. Circulating leptin is associated with serum uric acid level and its tubular reabsorption in a sample of adult middle-aged men. J. Endocrinol. Investig. 2020, 43, 587–593. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in cancer: More than a differentiation marker. Cancer Med. 2016, 5, 546–557. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Saccone, G.; D’Alessandro, P.; Arduino, B.; Mascolo, M.; De Placido, G.; Insabato, L.; Zullo, F. Diabetes Mellitus Is Associated with Occult Cancer in Endometrial Hyperplasia. Pathol. Oncol. Res. 2020, 26, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Gulinazi, Y.; Du, Y.; Ning, C.C.; Cheng, Y.L.; Shan, W.W.; Luo, X.Z.; Zhang, H.W.; Zhu, Q.; Ma, F.H.; et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: A randomised controlled trial. BJOG 2020, 127, 848–857. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total | Normal Weight (18.5 ≤ BMI < 25 kg/m2) | Overweight (25 ≤ BMI < 30 kg/m2) | Obese (BMI ≥ 30 kg/m2) | Underweight (BMI < 18.5 kg/m2) | p-Value |
|---|---|---|---|---|---|---|
| No. of patients | 286 | 135 (47.2%) | 85 (29.7%) | 52 (18.2%) | 14 (4.9%) | - |
| Age (years) | 32.0 (29.0–36.0) | 32.0 (29.0–36.5) | 32.0 (29.0–35.0) | 31.0 (28.0–35.0) | 33.0 (29.0–37.0) | 0.068 |
| Histology | 0.951 | |||||
| EAH | 209 (73.1%) | 98 (72.6%) | 64 (75.3%) | 37 (71.2%) | 10 (71.4%) | |
| EEC | 77 (26.9%) | 37 (27.4%) | 21 (24.7%) | 15 (28.8%) | 4 (28.6%) | |
| Diabetes | 32 (11.2%) | 5 (3.7%) | 15 (17.6%) | 12 (23.1%) | 0 (0.0%) | <0.001 |
| IR | 105 (36.7%) | 18 (13.3%) | 51 (60.0%) | 35 (67.3%) | 1 (7.1%) | <0.001 |
| Hyperlipidemia | 158 (55.2%) | 69 (51.1%) | 49 (57.6%) | 30 (57.7%) | 10 (71.4%) | 0.433 |
| Hyper-TC | 18 (6.3%) | 8 (6.0%) | 8 (9.4%) | 2 (3.8%) | 0 (0.0%) | 0.557 |
| Hyper-TG | 41 (14.3%) | 7 (5.2%) | 20 (23.5%) | 14 (26.9%) | 0 (0.0%) | <0.001 |
| Hypo-HDL | 116 (40.6%) | 58 (43.0%) | 29 (34.1%) | 19 (36.5%) | 10 (71.4%) | 0.053 |
| Hyper-LDL | 14 (4.9%) | 5 (3.7%) | 6 (7.1%) | 3 (5.8%) | 0 (0.0%) | 0.632 |
| MetS | 116 (40.6%) | 20 (14.8%) | 56 (65.9%) | 40 (76.9%) | 0 (0.0%) | <0.001 |
| Hypertension | 98 (34.3%) | 31 (23.0%) | 40 (47.1%) | 26 (50.0%) | 1 (7.1%) | <0.001 |
| DOR | 63 (22.0%) | 36 (26.7%) | 18 (22.8%) | 7 (14.0%) | 2 (14.3%) | 0.236 |
| PCOS | 104 (36.4%) | 37 (27.4%) | 35 (41.2%) | 28 (53.8%) | 4 (28.6%) | 0.005 |
| Hyperuricemia | 69 (24.1%) | 24 (17.8%) | 24 (28.2%) | 21 (40.4%) | 0 (0.0%) | 0.001 |
| Progestin therapy | 0.128 | |||||
| MA | 106 (37.1%) | 51 (37.8%) | 37 (43.5%) | 12 (23.1%) | 6 (42.9%) | |
| MA+LNG-IUS | 52 (18.2%) | 22 (16.3%) | 13 (15.3%) | 11 (21.2%) | 6 (42.9%) | |
| LNG-IUS | 55 (19.2%) | 29 (21.5%) | 14 (16.5%) | 12 (23.1%) | 0 (0.0%) | |
| MA+MET | 63 (22.0%) | 30 (22.2%) | 16 (18.8%) | 15 (28.8%) | 2 (14.3%) | |
| MA+LNG-IUS+MET | 10 (3.5%) | 3 (2.2%) | 5 (5.9%) | 2 (3.8%) | 0 (0.0%) | |
| Follow-up (months) | 19.1 (12.1–27.8) | 21.5 (13.1–28.9) | 17.8 (11.1–24.7) | 18.5 (11.4–24.8) | 20.7 (9.8–28.3) | 0.222 |
| Variables | Overweight vs. Normal Weight | Obese vs. Normal Weight | Underweight vs. Normal Weight | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Diabetes | 5.79 (2.01–16.74) | 0.001 | 8.00 (2.63–24.26) | <0.001 | - * | - * |
| IR | 9.94 (5.10–19.35) | <0.001 | 13.03 (6.06–28.00) | <0.001 | 0.52 (0.06–4.28) | 0.541 |
| Hyperlipidemia | 1.30 (0.75–2.26) | 0.346 | 1.37 (0.71–2.63) | 0.370 | 2.64 (0.77–9.04) | 0.122 |
| Hyper-TC | 1.79 (0.63–5.06) | 0.275 | 0.74 (0.15–3.67) | 0.708 | - * | - * |
| Hyper-TG | 6.37 (2.50–16.20) | <0.001 | 8.17 (2.95–22.60) | <0.001 | - * | - * |
| Hypo-HDL | 0.67 (0.38–1.19) | 0.174 | 0.77 (0.39–1.50) | 0.435 | 3.50 (1.02–11.97) | 0.047 |
| Hyper-LDL | 2.12 (0.64–7.69) | 0.212 | 1.94 (0.43–8.70) | 0.388 | - * | - * |
| MetS | 12.23 (6.19–24.15) | <0.001 | 24.06 (9.98–58.02) | <0.001 | - * | - * |
| Hypertension | 3.19 (1.75–5.79) | <0.001 | 3.90 (1.92–7.92) | <0.001 | 0.30 (0.37–2.46) | 0.263 |
| DOR | 0.83 (0.42–1.65) | 0.603 | 0.49 (0.20–1.22) | 0.124 | 0.65 (0.13–3.24) | 0.601 |
| PCOS | 1.71 (0.93–3.15) | 0.086 | 2.89 (1.44–5.82) | 0.003 | 0.67 (0.18–2.54) | 0.552 |
| Hyperuricemia | 1.90 (0.98–3.70) | 0.058 | 3.06 (1.50–6.25) | 0.002 | - * | - * |
| Variables | Total | Normal Weight (18.5 ≤ BMI < 25 kg/m2) | Overweight (25 ≤ BMI < 30 kg/m2) | Obese (BMI ≥ 30 kg/m2) | Underweight (BMI < 18.5 kg/m2) | p-Value |
|---|---|---|---|---|---|---|
| No. of patients | 286 | 135 (47.2%) | 85 (29.7%) | 52 (18.2%) | 14 (4.9%) | - |
| CR rate | ||||||
| Till 16-week | 25.7% | 34.1% | 23.7% | 11.6% | 7.1% | 0.004 |
| Till 32-week | 60.9% | 67.1% | 59.7% | 48.5% | 53.6% | 0.022 |
| Till 24-month | 98.2% | 97.1% | 100.0% | 100.0% | 100.0% | 0.098 |
| Time to CR (mo) | 6.2 (3.6–8.7) | 5.1 (3.3–7.9) | 5.9 (3.6–8.9) | 7.4 (5.7–11.9) | 6.8 (4.9–9.5) | 0.015 |
| Relapse rate a | 29.3% | 29.6% | 37.6% | 7.3% | 0.0% | 0.418 |
| Time to relapse (mo) | 13.8 (8.6–19.8) | 12.6 (9.6–22.2) | 14.3 (8.8–22.5) | 6.3 (6.1–6.5) | - * | 0.796 |
| Pregnant b | 36 (22.4%) | 17 (21.8%) | 10 (20.4%) | 6 (22.2%) | 3 (42.9%) | 0.610 |
| Miscarriage c | 8 (22.2%) | 5 (29.4%) | 1 (10.0%) | 2 (33.3%) | 0 (0.0%) | 0.565 |
| Live birth c | 18 (50.0%) | 8 (47.1%) | 6 (60.0%) | 2 (33.3%) | 2 (66.7%) | 0.737 |
| Variables | No. of Patients | 16-Week CR Rate | p-Value | 32-Week CR Rate | p-Value | Time to CR (mo) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Diabetes | No | 254 (88.8%) | 26.2% | 0.554 | 62.3% | 0.214 | 6.0 (3.6–8.5) | 0.045 |
| Yes | 32 (11.2%) | 21.9% | 50.7% | 7.1 (3.7–12.0) | ||||
| IR | No | 181 (63.3%) | 29.5% | 0.041 | 62.8% | 0.147 | 5.8 (3.4–8.4) | 0.073 |
| Yes | 105 (36.7%) | 19.0% | 57.8% | 6.7 (3.9–9.5) | ||||
| Hyperlipidemia | No | 128 (44.8%) | 27.0% | 0.594 | 63.4% | 0.391 | 5.8 (3.6–9.4) | 0.936 |
| Yes | 158 (55.2%) | 24.5% | 58.8% | 6.6 (3.6–8.5) | ||||
| Hyper-TC | No | 268 (93.7) | 26.6% | 0.158 | 61.9% | 0.261 | 6.2 (3.5–8.9) | 0.772 |
| Yes | 18 (6.3%) | 11.5% | 46.9% | 6.4 (4.0–8.0) | ||||
| Hyper-TG | No | 245 (85.7%) | 27.5% | 0.093 | 62.3% | 0.172 | 6.1 (3.5–8.9) | 0.492 |
| Yes | 41 (14.3%) | 14.7% | 53.0% | 7.1 (4.0–8.5) | ||||
| Hypo-HDL | No | 170 (59.4%) | 24.9% | 0.615 | 61.8% | 0.802 | 6.0 (3.7–8.6) | 0.847 |
| Yes | 116 (40.6%) | 26.8% | 59.8% | 6.6 (3.5–8.8) | ||||
| Hyper-LDL | No | 272 (95.1%) | 25.8% | 0.722 | 62.1% | 0.148 | 6.1 (3.6–8.8) | 0.507 |
| Yes | 14 (4.9%) | 22.1% | 37.7% | 7.5 (3.6–8.6) | ||||
| MetS | No | 170 (59.4%) | 29.6% | 0.055 | 63.9% | 0.037 | 5.9 (3.4–8.2) | 0.056 |
| Yes | 116 (40.6%) | 19.9% | 53.8% | 6.7 (3.9–9.6) | ||||
| Hypertension | No | 188 (65.7%) | 28.4% | 0.136 | 62.0% | 0.182 | 6.0 (3.5–8.9) | 0.560 |
| Yes | 98 (34.3%) | 20.5% | 53.5% | 6.8 (3.8–8.5) | ||||
| DOR | No | 223 (78.0%) | 23.4% | 0.147 | 58.7% | 0.199 | 6.6 (3.6–9.1) | 0.052 |
| Yes | 63 (22.0%) | 34.1% | 63.5% | 4.9 (3.5–7.8) | ||||
| PCOS | No | 182 (63.6%) | 31.6% | 0.003 | 61.0% | 0.203 | 5.4 (3.4–8.5) | 0.045 |
| Yes | 104 (36.4%) | 15.4% | 57.7% | 6.9 (4.1–9.4) | ||||
| Hyperuricemia | No | 217 (75.9%) | 28.3% | 0.071 | 64.9% | 0.009 | 5.8 (3.5–8.0) | 0.047 |
| Yes | 69 (24.1%) | 17.4% | 48.6% | 7.1 (4.0–10.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, L.; Wu, P.; Luo, S.; Shan, W.; Chen, X.; Luo, X. Effects of Weight Status and Related Metabolic Disorders on Fertility-Sparing Treatment Outcomes in Endometrial Atypical Hyperplasia and Endometrial Cancer: A Retrospective Study. Cancers 2022, 14, 5024. https://doi.org/10.3390/cancers14205024
Liu S, Wang L, Wu P, Luo S, Shan W, Chen X, Luo X. Effects of Weight Status and Related Metabolic Disorders on Fertility-Sparing Treatment Outcomes in Endometrial Atypical Hyperplasia and Endometrial Cancer: A Retrospective Study. Cancers. 2022; 14(20):5024. https://doi.org/10.3390/cancers14205024
Chicago/Turabian StyleLiu, Sijia, Lulu Wang, Pengfei Wu, Shuhan Luo, Weiwei Shan, Xiaojun Chen, and Xuezhen Luo. 2022. "Effects of Weight Status and Related Metabolic Disorders on Fertility-Sparing Treatment Outcomes in Endometrial Atypical Hyperplasia and Endometrial Cancer: A Retrospective Study" Cancers 14, no. 20: 5024. https://doi.org/10.3390/cancers14205024
APA StyleLiu, S., Wang, L., Wu, P., Luo, S., Shan, W., Chen, X., & Luo, X. (2022). Effects of Weight Status and Related Metabolic Disorders on Fertility-Sparing Treatment Outcomes in Endometrial Atypical Hyperplasia and Endometrial Cancer: A Retrospective Study. Cancers, 14(20), 5024. https://doi.org/10.3390/cancers14205024







