Mannose and Hyaluronic Acid Dual-Modified Iron Oxide Enhances Neoantigen-Based Peptide Vaccine Therapy by Polarizing Tumor-Associated Macrophages
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hyaluronic Acid-Coated Fe3O4 Nanoparticles (HA@Fe3O4) and Oxidized Hyaluronic Acid-Coated Iron Oxide Nanoparticles (HA@Fe3O4-O2)
2.2. Synthesis of Hyaluronic Acid and Mannose Co-Modified Fe3O4 Nanoparticles (HA-man@Fe3O4)
2.3. Synthesis of Mannose-Modified Polyethyleneimine (PEI-man)
2.4. Synthesis of Nanovaccine Consisting of Neoantigen Peptides and HA-man@Fe3O4 (HA-man@Fe3O4@pep)
2.5. Characterization of Synthesized Nanoparticles
2.6. Cellular Uptake
2.7. Effect of RAW 264.7 Cells on Repolarization In Vitro
2.8. Reactive Oxygen Species (ROS) Assay
2.9. Quantitative RT-PCR (qRT-PCR) Analysis of the Repolarization of RAW 264.7 Cells
2.10. TC1 Inhibition by Repolarized RAW 264.7 In Vitro
2.11. Tumor Growth Inhibition
2.12. Flow Cytometry Analysis of Tumor Tissue and Sentinel Lymph Nodes
2.13. Quantitative RT-PCR (qRT-PCR) Analysis of Tumor Tissue
2.14. Statistical Analysis
3. Results
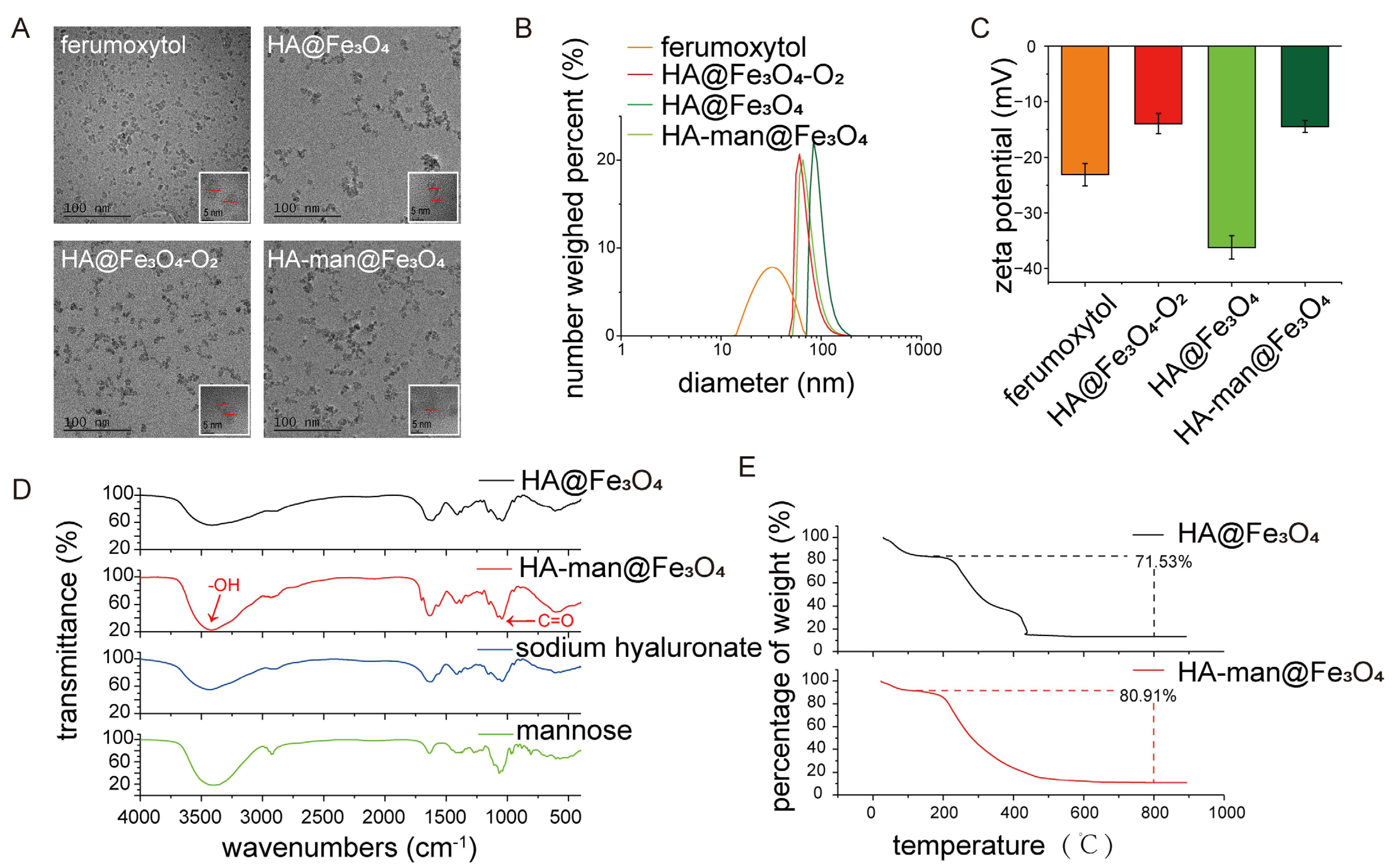
3.1. Synthesis and Characterization of HA@Fe3O4, HA@Fe3O4-O2 and HA-man@Fe3O4
| Molar Ratio (a) | Concentration of Filtrate Containing Mannose [mg] | AVE | STD | Amount of Remaining [mg] | Amount of Feeding [mg] | Load Efficiency (b) [%] | Modification Efficiency (c) [%] | ||
|---|---|---|---|---|---|---|---|---|---|
| 5:1 | 21.52 | 22.96 | 23.85 | 22.78 | 0.96 | 88.94 | 107.75 | 17.45 | 87.27 |
| 2:1 | 6.85 | 6.65 | 6.48 | 6.66 | 0.15 | 26.01 | 43.10 | 39.66 | 79.32 |
| 1:1 | 4.44 | 4.36 | 4.26 | 4.35 | 0.07 | 17.00 | 21.55 | 21.11 | 21.11 |
| 1:2 | 2.66 | 2.54 | 2.48 | 2.56 | 0.07 | 10.00 | 10.78 | 7.27 | 3.63 |
| 1:5 (d) | 2.41 | 2.38 | 2.36 | 2.38 | 0.02 | 9.31 | 4.31 | - | - |
| 1:10 (d) | 1.81 | 1.76 | 1.71 | 1.76 | 0.04 | 6.87 | 2.15 | - | - |
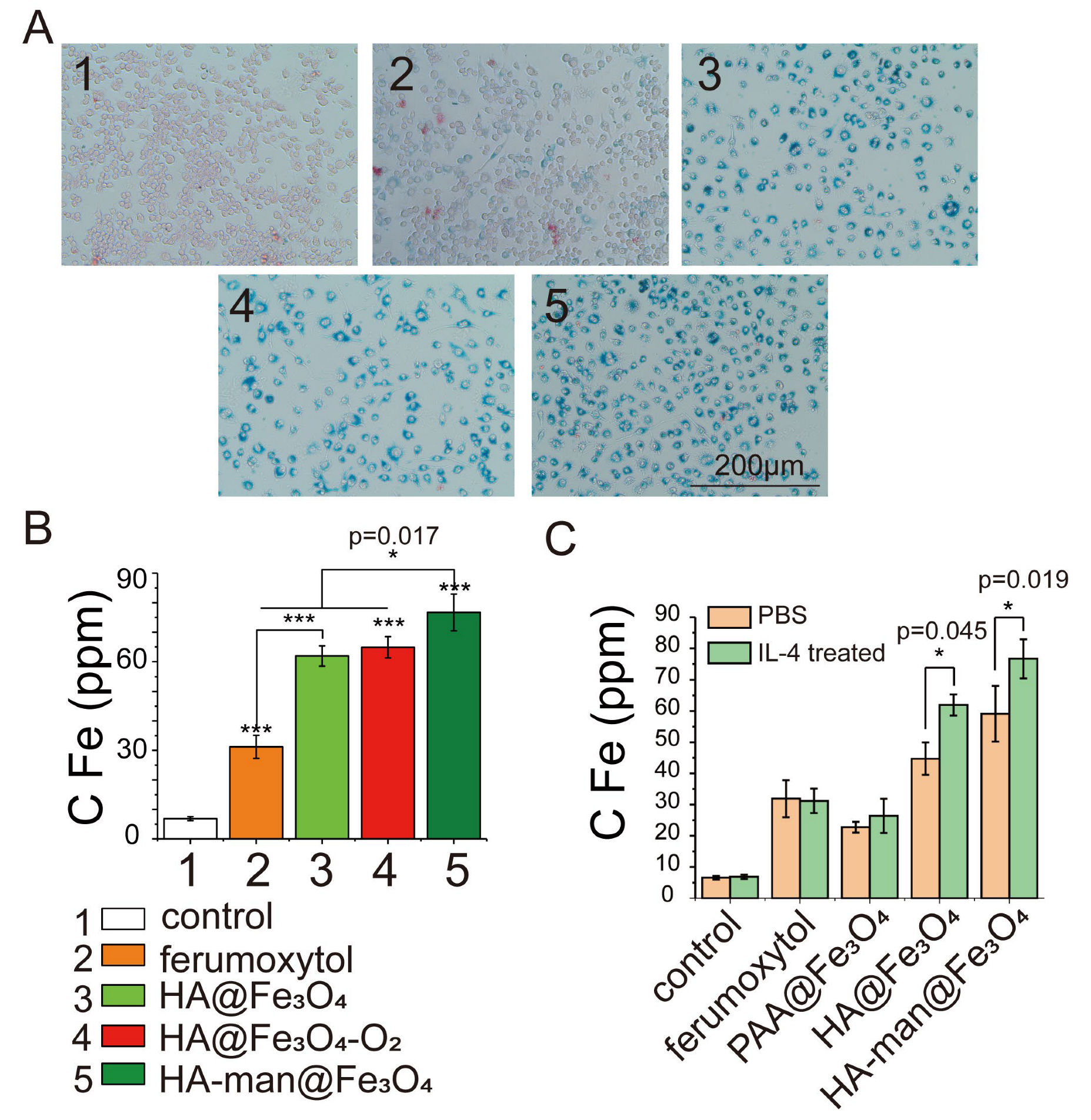
3.2. Enhanced Targeting towards M2-like Macrophages
3.3. Repolarization of M2-like Macrophages In Vitro
3.4. Effect of Macrophages on ROS Generation and Inflammatory Cell Pathways
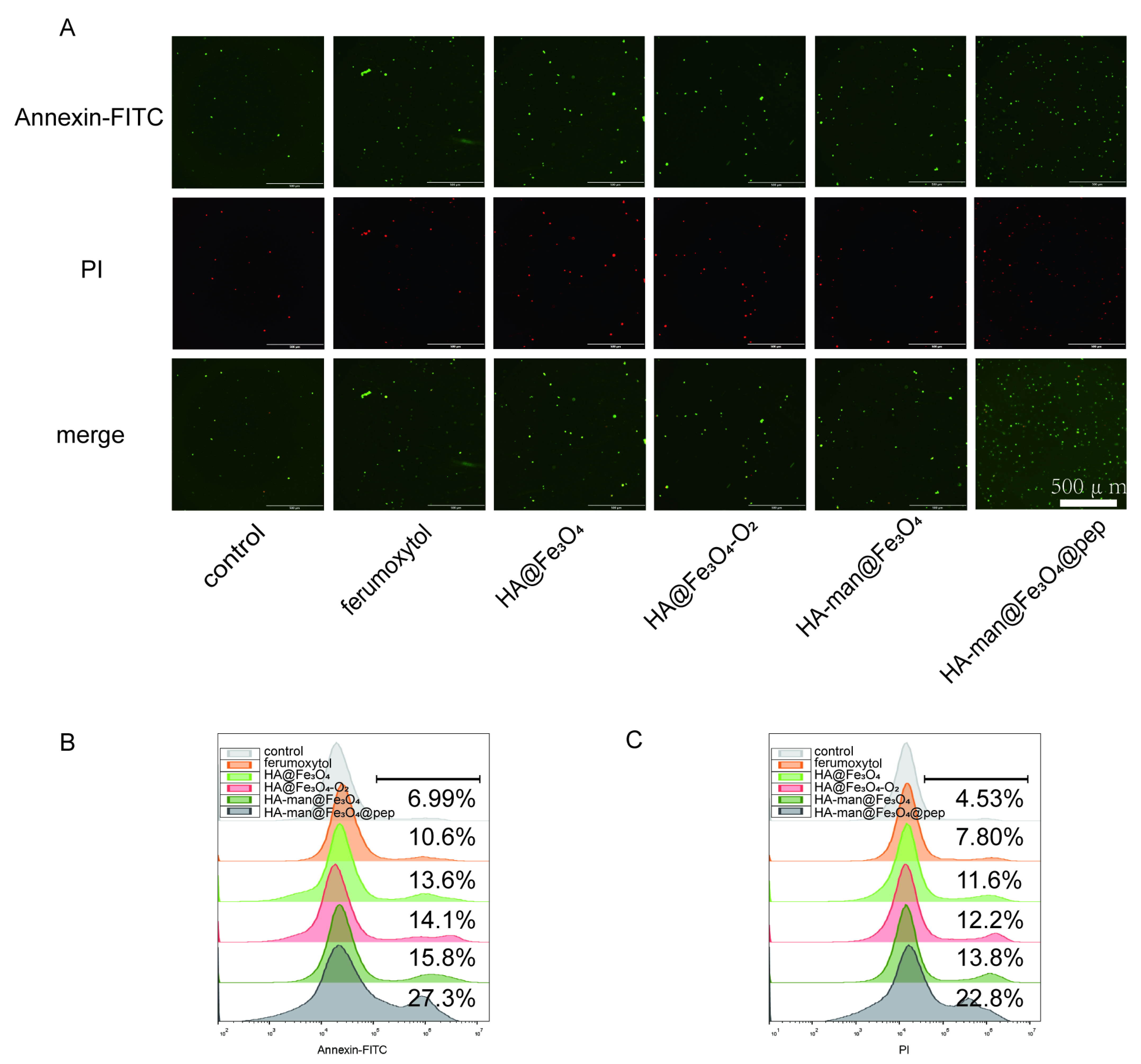
3.5. Construction and Characterization of HA-man@Fe3O4@pep
| ID | Name | Sequence |
|---|---|---|
| 1 | HPVp1 | ELQTTIHDI |
| 2 | HPVp2 | LLMGTLGIV |
| 3 | HPVp3 | YMLDLQPETT |
| 4 | HPVp4 | QAEPDRAHYNIVTFCCKCD |
| 5 | OVA | SIINFEKL |
3.6. Macrophages Mediated TC1 Inhibition In Vitro
3.7. Tumor Inhibition In Vivo
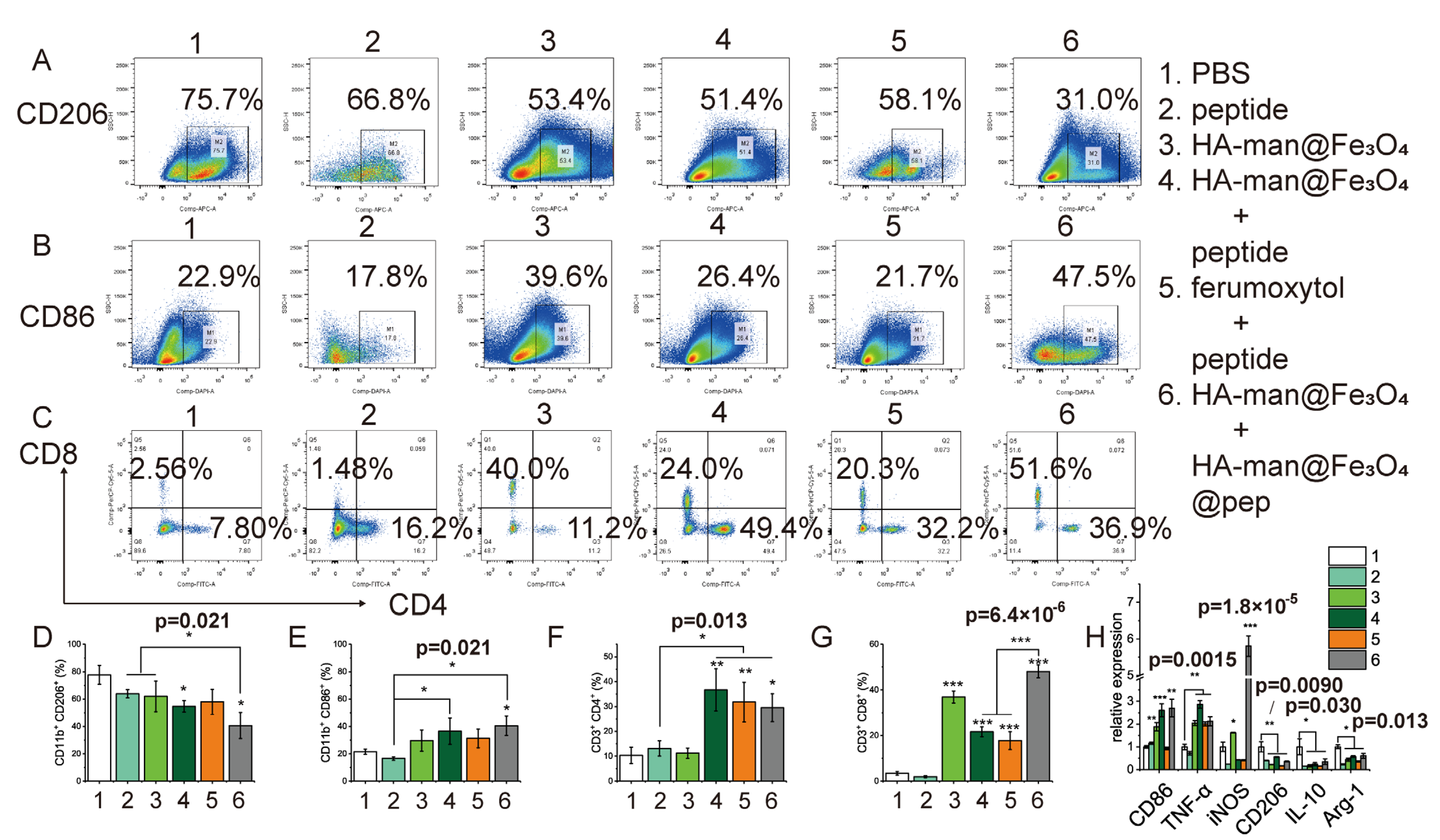
3.8. TAM Repolarization and Enhanced T Cell Infiltration into Tumor
3.9. Enhanced Antigen Presentation by DCs in Sentinel Lymph Nodes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Z.; Leet, D.E.; Allesoe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Lower, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrors, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer Neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef]
- Türeci, Ö.; Sahin, U. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef] [Green Version]
- Sylvestre, M.; Crane, C.A.; Pun, S.H. Progress on Modulating Tumor-Associated Macrophages with Biomaterials. Adv. Mater. 2020, 32, e1902007. [Google Scholar] [CrossRef] [PubMed]
- Sami, E.; Paul, B.T.; Koziol, J.A.; ElShamy, W.M. The Immunosuppressive Microenvironment in BRCA1-IRIS-Overexpressing TNBC Tumors Is Induced by Bidirectional Interaction with Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1102–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARgamma Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anfray, C.; Ummarino, A.; Andon, F.T.; Allavena, P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Guo, M.; Chen, C. Tailoring Nanomaterials for Targeting Tumor-Associated Macrophages. Adv. Mater. 2019, 31, e1808303. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Reviews. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Guerriero, J.L. Macrophages: Their Untold Story in T Cell Activation and Function. Int. Rev. Cell Mol. Biol. 2019, 342, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Muntjewerff, E.M.; Meesters, L.D.; van den Bogaart, G. Antigen Cross-Presentation by Macrophages. Front. Immunol. 2020, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Rojas, J.M.; Barber, D.F. The Use of Iron Oxide Nanoparticles to Reprogram Macrophage Responses and the Immunological Tumor Microenvironment. Front. Immunol. 2021, 12, 693709. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. A Biomimetic Polymer Magnetic Nanocarrier Polarizing Tumor-Associated Macrophages for Potentiating Immunotherapy. Small 2020, 16, e2003543. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, L.; Xue, C.; Liu, J.; He, Y.; Zhou, J.; Xia, Z.; Dai, L.; Luo, Z.; Mao, Y.; et al. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale 2020, 12, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, S.; Palazzo, M.; Zanobbio, L.; Selleri, S.; Sommariva, M.; Sfondrini, L.; Cavicchini, S.; Balsari, A.; Rumio, C. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J. Immunol. 2008, 181, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wei, P.; Seeberger, P.H.; Yin, J. Mannose-Functionalized Nanoscaffolds for Targeted Delivery in Biomedical Applications. Chem. Asian. J. 2018, 13, 3448–3459. [Google Scholar] [CrossRef]
- Patil, T.S.; Deshpande, A.S. Mannosylated nanocarriers mediated site-specific drug delivery for the treatment of cancer and other infectious diseases: A state of the art review. J. Control. Release 2020, 320, 239–252. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Soleymani, M.; Velashjerdi, M.; Shaterabadi, Z.; Barati, A. One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 2020, 237, 116130. [Google Scholar] [CrossRef]
- Gennari, A.; Pelliccia, M.; Donno, R.; Kimber, I.; Tirelli, N. Mannosylation Allows for Synergic (CD44/C-Type Lectin) Uptake of Hyaluronic Acid Nanoparticles in Dendritic Cells, but Only upon Correct Ligand Presentation. Adv. Healthc. Mater. 2016, 5, 966–976. [Google Scholar] [CrossRef]
- Zarif, J.C.; Valle, J.A.B.-D.; Hicks, J.L.; Heaphy, C.M.; Vidal, I.; Luo, J.; Lotan, T.L.; Hooper, J.E.; Isaacs, W.B.; Pienta, K.J.; et al. Abstract 4582: Mannose receptor positive macrophage infiltrate correlates with prostate cancer onset and metastatic castration-resistant disease. Cancer Res. 2019, 79, 4582. [Google Scholar] [CrossRef]
- Zhou, Y.; Do, D.C.; Ishmael, F.T.; Squadrito, M.L.; Tang, H.M.; Tang, H.L.; Hsu, M.H.; Qiu, L.; Li, C.; Zhang, Y.; et al. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. J. Allergy Clin. Immunol. 2018, 141, 350–364.e358. [Google Scholar] [CrossRef] [Green Version]
- Mahor, S.; Dash, B.C.; O’Connor, S.; Pandit, A. Mannosylated polyethyleneimine-hyaluronan nanohybrids for targeted gene delivery to macrophage-like cell lines. Bioconjugate Chem. 2012, 23, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios de la Rosa, J.M.; Tirella, A.; Gennari, A.; Stratford, I.J.; Tirelli, N. The CD44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv. Healthc. Mater. 2017, 6, 1601012. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, T.; Tang, J.; Yang, Y.; Song, H.; Tuong, Z.K.; Fu, J.; Yu, C. Mechanism of Iron Oxide-Induced Macrophage Activation: The Impact of Composition and the Underlying Signaling Pathway. J. Am. Chem. Soc. 2019, 141, 6122–6126. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhen, M.; Wang, H.; Sun, Z.; Jia, W.; Zhao, Z.; Zhou, C.; Liu, S.; Wang, C.; Bai, C. Functional Gadofullerene Nanoparticles Trigger Robust Cancer Immunotherapy Based on Rebuilding an Immunosuppressive Tumor Microenvironment. Nano Lett. 2020, 20, 4487–4496. [Google Scholar] [CrossRef] [PubMed]
- Formentini, L.; Santacatterina, F.; Nunez de Arenas, C.; Stamatakis, K.; Lopez-Martinez, D.; Logan, A.; Fresno, M.; Smits, R.; Murphy, M.P.; Cuezva, J.M. Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Rep. 2017, 19, 1202–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.W.; Sobral, M.C.; Badrinath, S.; Choi, Y.; Graveline, A.; Stafford, A.G.; Weaver, J.C.; Dellacherie, M.O.; Shih, T.Y.; Ali, O.A.; et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 2018, 17, 528–534. [Google Scholar] [CrossRef]
- Robles-Oteiza, C.; Wu, C.J. Editorial overview: Vaccines: Reinvigorating therapeutic cancer vaccines. Curr. Opin. Immunol. 2022, 76, 102176. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Reviews. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Shi, L.; Gu, H. Emerging Nanoparticle Strategies for Modulating Tumor-Associated Macrophage Polarization. Biomolecules 2021, 11, 1912. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Wu, X.; Zhang, Q.; Chen, S.; Ma, X.; Yu, M. Ironing Out the Details: How Iron Orchestrates Macrophage Polarization. Front. Immunol. 2021, 12, 669566. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; O'Connor, M.A.; Walls, A.C.; Hemann, E.A.; Murapa, P.; Archer, J.; Leventhal, S.; Fuller, J.T.; Lewis, T.B.; et al. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020, 12, eabc9396. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.; Huang, L. Tackling TAMs for Cancer Immunotherapy: It’s Nano Time. Trends Pharmacol. Sci. 2020, 41, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Alonso, V.; Pratt, E.C.; Zong, H.; Lara-Martinez, A.; Kaittanis, C.; Rabie, M.O.; Longo, V.; Becker, M.W.; Roboz, G.J.; Grimm, J.; et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019, 14, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.G.; Camps, M.G.M.; Li, T.; Chan, A.B.; Ossendorp, F.; Cruz, L.J. Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines. Biomaterials 2019, 220, 119417. [Google Scholar] [CrossRef]
- Li, C.X.; Zhang, Y.; Dong, X.; Zhang, L.; Liu, M.D.; Li, B.; Zhang, M.K.; Feng, J.; Zhang, X.Z. Artificially Reprogrammed Macrophages as Tumor-Tropic Immunosuppression-Resistant Biologics to Realize Therapeutics Production and Immune Activation. Adv. Mater. 2019, 31, e1807211. [Google Scholar] [CrossRef]
- Nascimento, C.S.; Alves, É.A.R.; de Melo, C.P.; Corrêa-Oliveira, R.; Calzavara-Silva, C.E. Immunotherapy for cancer: Effects of iron oxide nanoparticles on polarization of tumor-associated macrophages. Nanomed. Nanotechnol. Biol. Med. 2021, 29, 2633–2650. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhu, W.; Li, D.; Yang, L.; Duan, J.; Cai, Z.; Nie, Y.; Zhang, Y.; Gong, Q.; et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials 2019, 203, 23–30. [Google Scholar] [CrossRef]
- Irvine, D.J.; Swartz, M.A.; Szeto, G.L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Y.; Shi, L.; Zhang, Y.; Guo, Y.; Gu, H. Mannose and Hyaluronic Acid Dual-Modified Iron Oxide Enhances Neoantigen-Based Peptide Vaccine Therapy by Polarizing Tumor-Associated Macrophages. Cancers 2022, 14, 5107. https://doi.org/10.3390/cancers14205107
Nie Y, Shi L, Zhang Y, Guo Y, Gu H. Mannose and Hyaluronic Acid Dual-Modified Iron Oxide Enhances Neoantigen-Based Peptide Vaccine Therapy by Polarizing Tumor-Associated Macrophages. Cancers. 2022; 14(20):5107. https://doi.org/10.3390/cancers14205107
Chicago/Turabian StyleNie, Ying, Lu Shi, Yanan Zhang, Yunfei Guo, and Hongchen Gu. 2022. "Mannose and Hyaluronic Acid Dual-Modified Iron Oxide Enhances Neoantigen-Based Peptide Vaccine Therapy by Polarizing Tumor-Associated Macrophages" Cancers 14, no. 20: 5107. https://doi.org/10.3390/cancers14205107





