Exponential Slope from Absolute Lymphocyte Counts during Radio-Chemotherapy Can Predict an Aggressive Course of Cervical Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Samples
2.2. Patients
2.3. Variables
2.4. Exponential Functions from ALCs
2.5. Endpoints
2.6. Log2 Fold Change of RNAs
2.7. Selection of mRNAs
2.8. Survival Analysis
3. Results
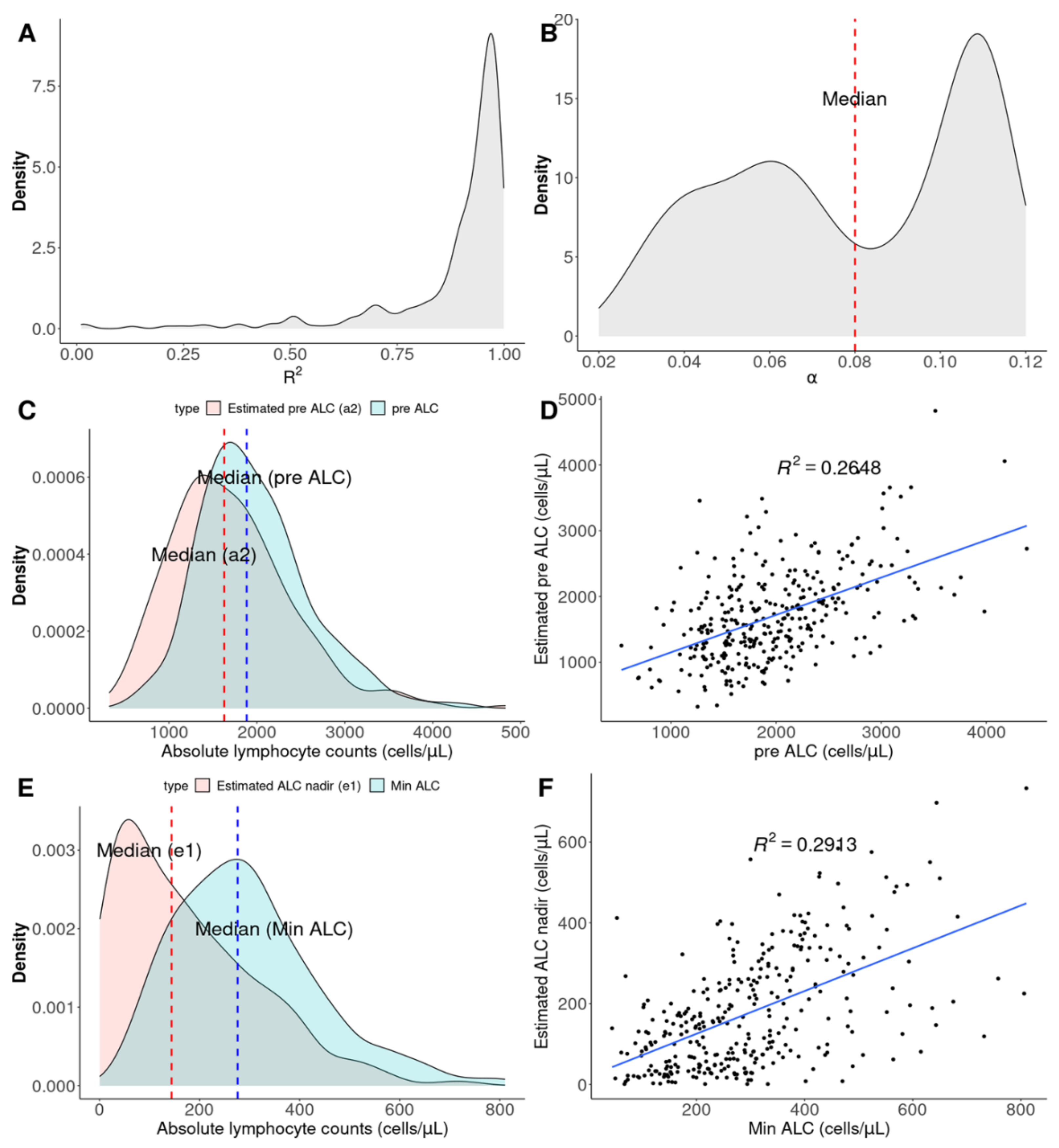
3.1. Concept of Exponential Functions from ALCs
3.2. Two Types of Disease Courses
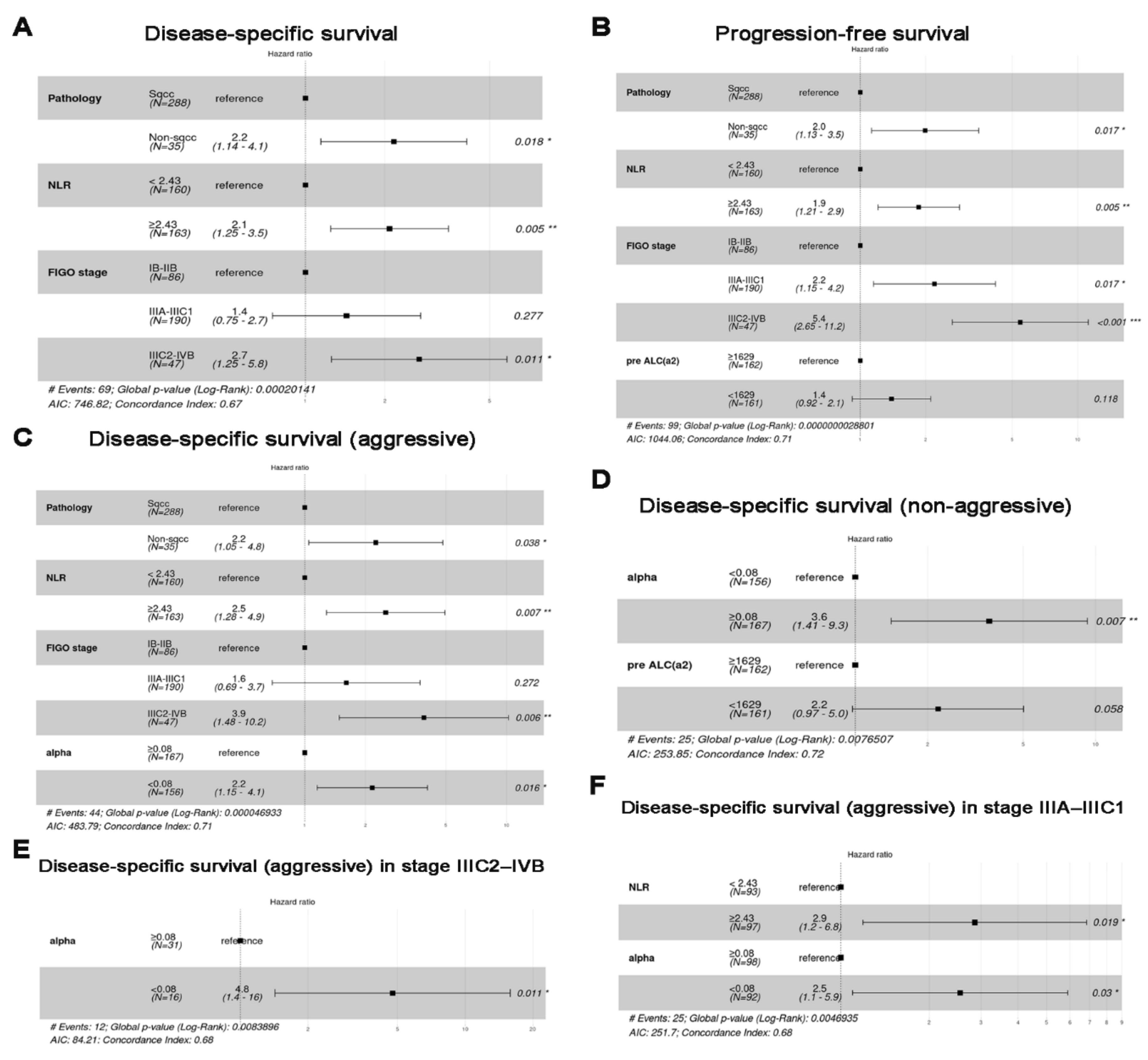
3.3. Survival Analysis (Cohort 1)
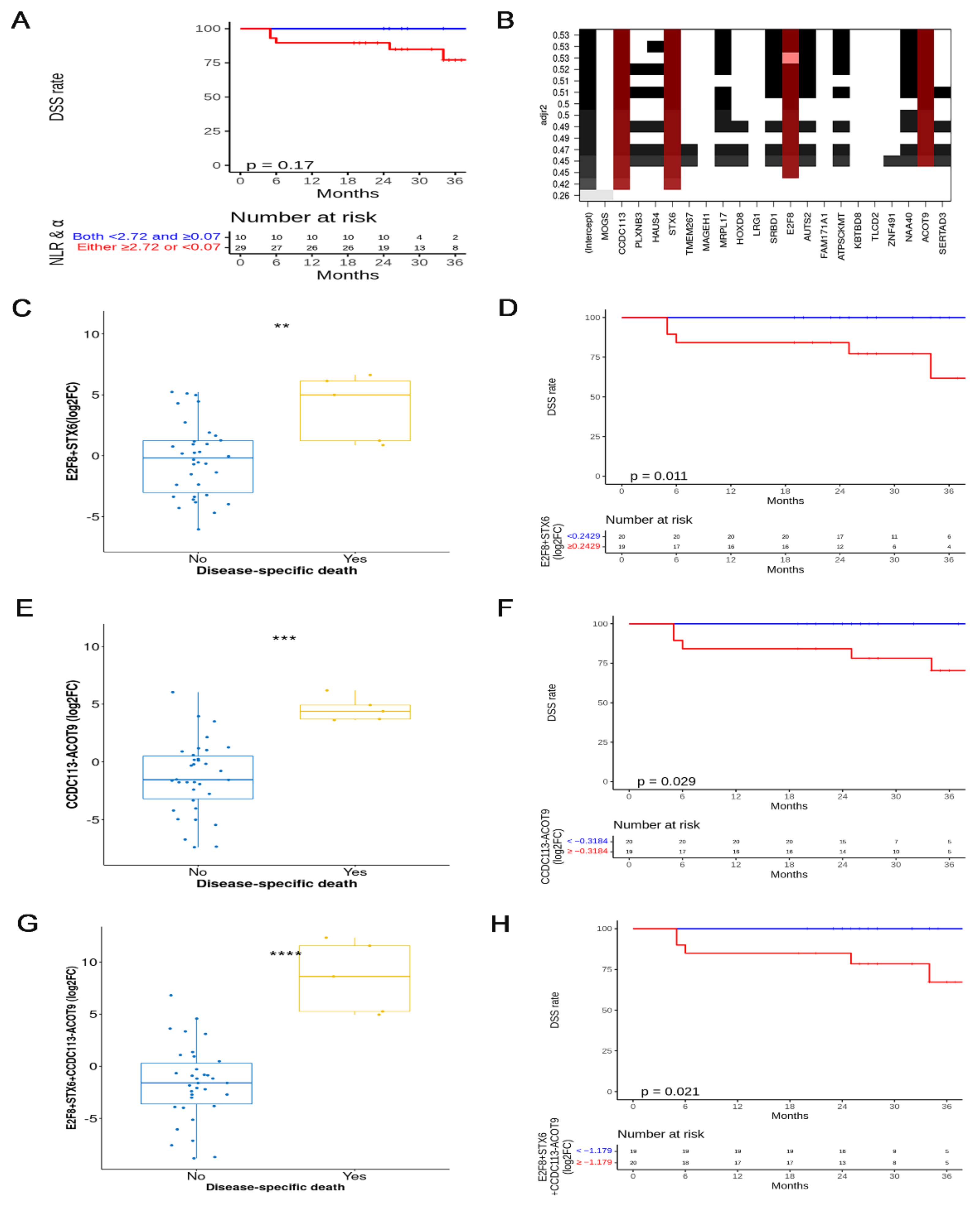
3.4. Selected mRNAs and Survival Analysis (Cohort 2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesulu, B.P.; Mallick, S.; Lin, S.H.; Krishnan, S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit. Rev. Oncol. Hematol. 2018, 123, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Menetrier-Caux, C.; Ray-Coquard, I.; Blay, J.Y.; Caux, C. Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: An opportunity for combination with Cytokines? J. Immunother. Cancer 2019, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Trapani, J.A. Tumor-mediated apoptosis of cancer-specific T lymphocytes—Reversing the “kiss of death”? Cancer Cell 2002, 2, 169–171. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [Green Version]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Surh, C.D.; Sprent, J. Homeostasis of naive and memory T cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef] [Green Version]
- Cabatingan, M.S.; Schmidt, M.R.; Sen, R.; Woodland, R.T. Naive B Lymphocytes Undergo Homeostatic Proliferation in Response to B Cell Deficit. J. Immunol. 2002, 169, 6795–6805. [Google Scholar] [CrossRef] [Green Version]
- Heylmann, D.; Ponath, V.; Kindler, T.; Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 2021, 11, 2478. [Google Scholar] [CrossRef]
- Heylmann, D.; Badura, J.; Becker, H.; Fahrer, J.; Kaina, B. Sensitivity of CD3/CD28-stimulated versus non-stimulated lymphocytes to ionizing radiation and genotoxic anticancer drugs: Key role of ATM in the differential radiation response. Cell Death Dis. 2018, 9, 1053. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Liu, A.; Li, W.; Liu, Y.; Zhang, G.; Ye, S.; Zhao, Z.; Shi, J.; Jia, Y.; Liu, X.; et al. Reference range of naive T and T memory lymphocyte subsets in peripheral blood of healthy adult. Clin. Exp. Immunol. 2022, 207, 208–217. [Google Scholar] [CrossRef]
- Franiak-Pietryga, I.; Miyauchi, S.; Kim, S.S.; Sanders, P.D.; Sumner, W.; Zhang, L.; Mundt, A.J.; Califano, J.A.; Sharabi, A.B. Activated B Cells and Plasma Cells Are Resistant to Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 514–528. [Google Scholar] [CrossRef]
- Cho, O.; Kim, D.W.; Cheong, J.Y. Plasma Exosomal miRNA Levels after Radiotherapy Are Associated with Early Progression and Metastasis of Cervical Cancer: A Pilot Study. J. Clin. Med. 2021, 10, 2110. [Google Scholar] [CrossRef]
- Cho, O.; Kim, D.W.; Cheong, J.Y. Screening Plasma Exosomal RNAs as Diagnostic Markers for Cervical Cancer: An Analysis of Patients Who Underwent Primary Chemoradiotherapy. Biomolecules 2021, 11, 1691. [Google Scholar] [CrossRef]
- Tsukishiro, T.; Donnenberg, A.D.; Whiteside, T.L. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol. Immunother. 2003, 52, 599–607. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Gao, Z.; Han, Y. Prognostic value of peripheral naive CD8+ T cells in oligometastatic non-small-cell lung cancer. Future Oncol. 2022, 18, 55–65. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, A.; Feng, J.; Xu, Z.; Wang, P.; Ruan, P.; Luo, R.; Weng, Y.; Peng, M. Prognostic Impact of Memory CD8(+) T Cells on Immunotherapy in Human Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 698076. [Google Scholar] [CrossRef]
- Das, R.K.; Vernau, L.; Grupp, S.A.; Barrett, D.M. Naive T-cell Deficits at Diagnosis and after Chemotherapy Impair Cell Therapy Potential in Pediatric Cancers. Cancer Discov. 2019, 9, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, C.; Stravokefalou, V.; Stellas, D.; Karaliota, S.; Felber, B.K.; Pavlakis, G.N. Heterodimeric IL-15 in Cancer Immunotherapy. Cancers 2021, 13, 837. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.T.; Kim, S.; Lee, B.; Lim, M.C.; Kim, J.W.; Won, Y.J. Prognosis of Cervical Cancer in the Era of Concurrent Chemoradiation from National Database in Korea: A Comparison between Squamous Cell Carcinoma and Adenocarcinoma. PLoS ONE 2015, 10, e0144887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelissier Vatter, F.A.; Cioffi, M.; Hanna, S.J.; Castarede, I.; Caielli, S.; Pascual, V.; Matei, I.; Lyden, D. Extracellular vesicle- and particle-mediated communication shapes innate and adaptive immune responses. J. Exp. Med. 2021, 218, e20202579. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Vecsler, M.; Liberzon, A.; Noach, M.; Zlotorynski, E.; Tzur, A. Unbiased transcriptome signature of in vivo cell proliferation reveals pro- and antiproliferative gene networks. Cell Cycle 2013, 12, 2992–3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharer, C.D.; Barwick, B.G.; Guo, M.; Bally, A.P.R.; Boss, J.M. Plasma cell differentiation is controlled by multiple cell division-coupled epigenetic programs. Nat. Commun. 2018, 9, 1698. [Google Scholar] [CrossRef] [Green Version]
- Stow, J.L.; Manderson, A.P.; Murray, R.Z. SNAREing immunity: The role of SNAREs in the immune system. Nat. Rev. Immunol. 2006, 6, 919–929. [Google Scholar] [CrossRef]
- Murray, R.Z.; Wylie, F.G.; Khromykh, T.; Hume, D.A.; Stow, J.L. Syntaxin 6 and Vti1b form a novel SNARE complex, which is up-regulated in activated macrophages to facilitate exocytosis of tumor necrosis Factor-alpha. J. Biol. Chem. 2005, 280, 10478–10483. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef]
- Marelli, G.; Morina, N.; Portale, F.; Pandini, M.; Iovino, M.; Di Conza, G.; Ho, P.C.; Di Mitri, D. Lipid-loaded macrophages as new therapeutic target in cancer. J. Immunother. Cancer 2022, 10, e004584. [Google Scholar] [CrossRef]



| Factors | Median [IQR] | ALL (n = 323) | p | |
|---|---|---|---|---|
| () | 0.08 [0.055–0.11] | ≥0.08 (n = 167) | <0.08 (n = 156) | |
| Age (years at diagnosis) | 57 [48–69] | 0.36 | ||
| ≥50 | 223 (69.0%) | 111 (66.5%) | 112 (71.8%) | |
| <50 | 100 (31.0%) | 56 (33.5%) | 44 (28.2%) | |
| Pathology | 0.011 | |||
| Adenocarcinoma | 20 (6.2%) | 8 (4.8%) | 12 (7.7%) | |
| ASC | 12 (3.7%) | 2 (1.2%) | 10 (6.4%) | |
| Carcinoma | 3 (0.9%) | 0 (0%) | 3 (1.9%) | |
| Squamous cell carcinoma | 288 (89.2%) | 157 (94.0%) | 131 (84.0%) | |
| FIGO stage | 0.056 | |||
| IB–IIB | 86 (26.6%) | 38 (22.8%) | 48 (30.8%) | |
| IIIA–IIIC1 | 190 (58.8%) | 98 (58.7%) | 92 (59.0%) | |
| IIC2–IVB | 47 (14.6%) | 31 (18.6%) | 16 (10.3%) | |
| Treatment time (days) | 53 [49–55] | 53.0 [49.0–58.0] | 53.0 [49.0–60.0] | 0.946 |
| Radiation therapy field | 0.026 | |||
| Pelvis and PALN | 63 (19.5%) | 41 (24.6%) | 22 (14.1%) | |
| Pelvis | 260 (80.5%) | 126 (75.5%) | 134 (85.9%) | |
| Total dose (EQD2) | 70.2 [68.1–73.2] | 69.8 [68.0–73.4] | 70.4 [68.1–72.9] | 0.976 |
| Pre-ALC (cells/μL) | 1884 [1543–2326] | 0.166 | ||
| ≥1884 | 160 (49.5%) | 76 (45.5%) | 84 (53.9%) | |
| <1884 | 163 (50.5%) | 91 (54.5%) | 72 (46.2%) | |
| Min-ALC (cells/μL) | 276 [193–377] | 0.288 | ||
| ≥276 | 163 (50.5%) | 79 (47.3%) | 84 (53.9%) | |
| <276 | 160 (49.5%) | 88 (52.7%) | 72 (46.2%) | |
| Neutrophil-to-lymphocyte ratio | 2.43 [1.41–3.35] | 1 | ||
| <2.43 | 160 (49.5%) | 83 (49.7%) | 77 (49.4%) | |
| ≥2.43 | 163 (50.5%) | 84 (50.3%) | 79 (50.6%) | |
| a2 = a1 + e1 (cells/μL) | 1629 [1237–2108] | 0 | ||
| ≥1629 | 162 (50.2%) | 105 (62.9%) | 57 (36.5%) | |
| <1629 | 161 (49.9%) | 62 (37.1%) | 99 (63.5%) | |
| e1 (cells/μL) | 144 [63–261] | 0 | ||
| ≥144 | 163 (50.5%) | 128 (76.7%) | 35 (22.4%) | |
| <144 | 160 (49.5%) | 39 (23.4%) | 121 (77.6%) | |
| Progression | 0.111 | |||
| No | 224 (69.4%) | 111 (66.5%) | 113 (72.4%) | |
| Locoregional progression (LP) | 23 (7.1%) | 14 (8.4%) | 9 (5.8%) | |
| Distant metastasis (DM) | 54 (16.7%) | 34 (20.4%) | 20 (12.8%) | |
| LP + DM | 22 (6.8%) | 8 (4.8%) | 14 (9.0%) | |
| DSD (aggressive) | 0.043 | |||
| No | 279 (86.4%) | 151 (90.4%) | 128 (82.1%) | |
| Yes | 44 (13.6%) | 16 (9.6%) | 28 (18.0%) | |
| DSD (non-aggressive) | 0.02 | |||
| No | 298 (92.3%) | 148 (88.6%) | 150 (96.2%) | |
| Yes | 25 (7.7%) | 19 (11.4%) | 6 (3.9%) | |
| DSD | 0.962 | |||
| No | 254 (78.6%) | 132 (79.0%) | 122 (78.2%) | |
| Yes | 69 (21.4%) | 35 (21.0%) | 34 (21.8%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, O.; Chun, M.; Chang, S.-J. Exponential Slope from Absolute Lymphocyte Counts during Radio-Chemotherapy Can Predict an Aggressive Course of Cervical Cancer. Cancers 2022, 14, 5109. https://doi.org/10.3390/cancers14205109
Cho O, Chun M, Chang S-J. Exponential Slope from Absolute Lymphocyte Counts during Radio-Chemotherapy Can Predict an Aggressive Course of Cervical Cancer. Cancers. 2022; 14(20):5109. https://doi.org/10.3390/cancers14205109
Chicago/Turabian StyleCho, Oyeon, Mison Chun, and Suk-Joon Chang. 2022. "Exponential Slope from Absolute Lymphocyte Counts during Radio-Chemotherapy Can Predict an Aggressive Course of Cervical Cancer" Cancers 14, no. 20: 5109. https://doi.org/10.3390/cancers14205109
APA StyleCho, O., Chun, M., & Chang, S.-J. (2022). Exponential Slope from Absolute Lymphocyte Counts during Radio-Chemotherapy Can Predict an Aggressive Course of Cervical Cancer. Cancers, 14(20), 5109. https://doi.org/10.3390/cancers14205109






