Imaging Hallmarks of Sarcoma Progression Via X-ray Computed Tomography: Beholding the Flower of Evil
Abstract
:Simple Summary
Abstract
1. Introduction
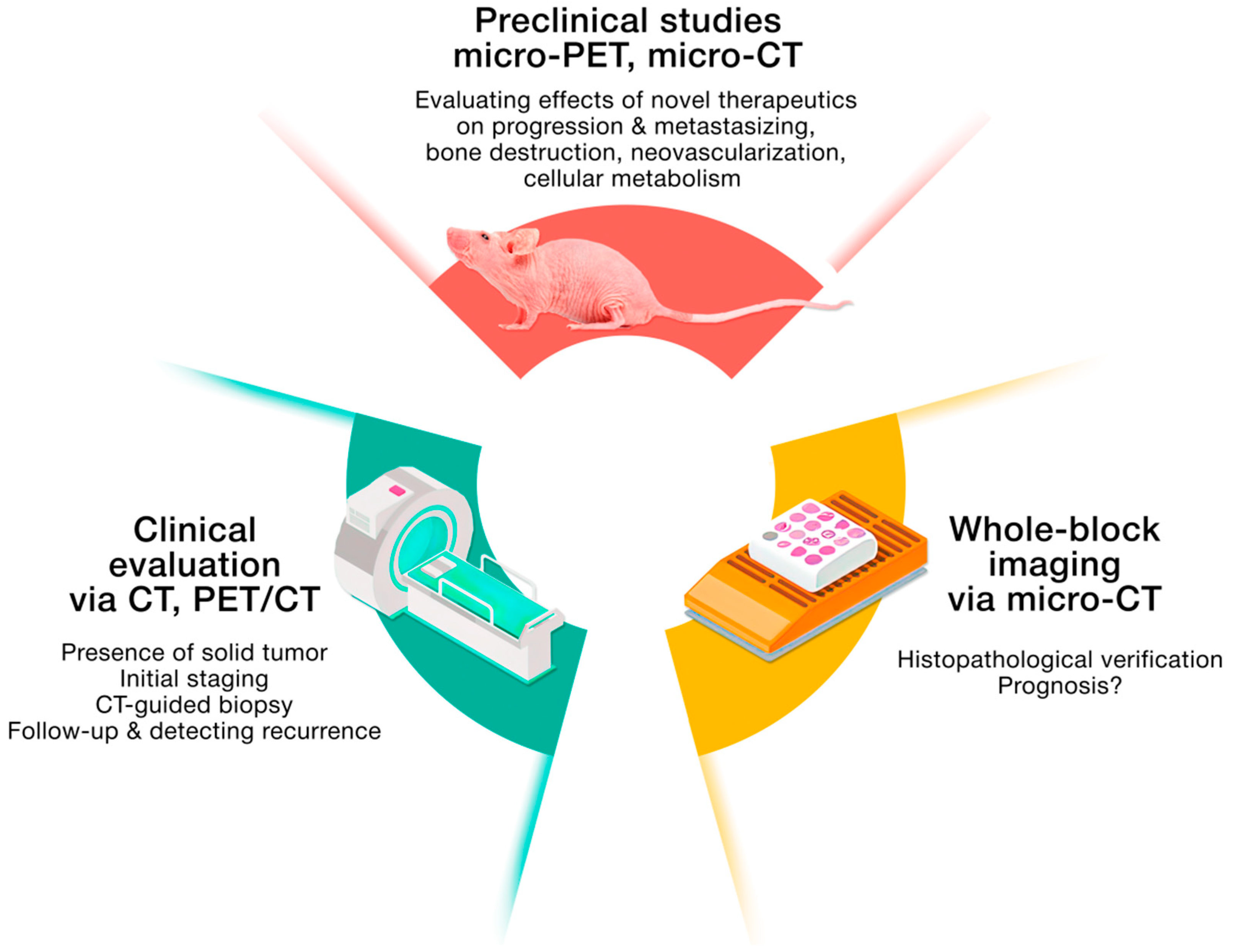
2. Clinical Imaging, or Seeing Many Things at Once
3. PET Imaging: A Tool for Revealing and Deregulating Cellular Metabolism and Overcoming the Avoidance of Immune Destruction
4. Bone Destruction as a Result of Sarcomas’ Progression and Metastasis
5. Untrodden Path: Vasculature Access in Sarcomas
6. Conclusions
Funding
Authors Contributions
Acknowledgments
Conflicts of Interest
References
- Mohseny, B.A.; Hogendoorn, P.C.W. Concise Review: Mesenchymal Tumors: When Stem Cells Go Mad. Stem Cells 2011, 29, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Lye, K.L.; Nordin, N.; Vidyadaran, S.; Thilakavathy, K. Mesenchymal stem cells: From stem cells to sarcomas. Cell Biol. Int. 2016, 40, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Baker, L.H.; Beech, D.; Benjamin, R.; Casper, E.S.; Conrad, E.U.; DeLaney, T.F.; Ettinger, D.S.; Heslin, M.J.; Hutchinson, R.J.; et al. Soft tissue sarcoma clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2005, 3, 158–194. [Google Scholar] [PubMed]
- Fletcher, C.D.M.; Bridge, J.A.; Hogendoorn, P.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; van der Zwan, J.M.; Casali, P.G.; Siesling, S.; Tos, A.P.D.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G.; RARECARE Working Group. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef]
- Ferrari, A.; Sultan, I.; Huang, T.T.; Rodriguez-Galindo, C.; Shehadeh, A.; Meazza, C.; Ness, K.K.; Casanova, M.; Spunt, S.L. Soft tissue sarcoma across the age spectrum: A population-based study from the surveillance epidemiology and end results database. Pediatr. Blood Cancer 2011, 57, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Baili, P.; Di Salvo, F.; Marcos-Gragera, R.; Siesling, S.; Mallone, S.; Santaquilani, M.; Micheli, A.; Lillini, R.; Francisci, S.; Hackl, M.; et al. Age and case mix-standardised survival for all cancer patients in Europe 1999–2007: Results of EUROCARE-5, a population-based study. Eur. J. Cancer 2015, 51, 2120–2129. [Google Scholar] [CrossRef]
- Andritsch, E.; Beishon, M.; Bielack, S.; Bonvalot, S.; Casali, P.; Crul, M.; Delgado-Bolton, R.; Donati, D.M.; Douis, H.; Haas, R.; et al. ECCO Essential Requirements for Quality Cancer Care: Soft Tissue Sarcoma in Adults and Bone Sarcoma. A critical review. Crit. Rev. Oncol./Hematol. 2017, 110, 94–105. [Google Scholar] [CrossRef]
- Weitz, J.; Antonescu, C.R.; Brennan, M.F. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J. Clin. Oncol. 2003, 21, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Bleloch, J.S.; Ballim, R.D.; Kimani, S.; Parkes, J.; Panieri, E.; Willmer, T.; Prince, S. Managing sarcoma: Where have we come from and where are we going? Ther. Adv. Med. Oncol. 2017, 9, 637–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie-Large, M.; James, S.; Tiessen, L.; Davies, A.; Grimer, R. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. Eur. J. Cancer 2008, 44, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Mariani, L.; Miceli, R.; Kattan, M.W.; Brennan, M.F.; Colecchia, M.; Fiore, M.; Casali, P.G.; Gronchi, A. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005, 103, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Amer, K.M.; Thomson, J.E.; Congiusta, D.; Dobitsch, A.; Chaudhry, A.; Li, M.; Chaudhry, A.; Bozzo, A.; Siracuse, B.; Aytekin, M.N.; et al. Epidemiology, Incidence, and Survival of Rhabdomyosarcoma Subtypes: SEER and ICES Database Analysis. J. Orthop. Res. 2019, 37, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- de Pinieux, G.; Karanian, M.; Le Loarer, F.; Le Guellec, S.; Chabaud, S.; Terrier, P.; Bouvier, C.; Batistella, M.; Neuville, A.; Robin, Y.M.; et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS ONE 2021, 16, e0246958. [Google Scholar] [CrossRef]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef]
- Bone Cancer (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf (accessed on 1 July 2022).
- Soft Tissue Sarcoma (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on 1 July 2022).
- Ray-Coquard, I.; Thiesse, P.; Ranchère-Vince, D.; Chauvin, F.; Bobin, J.-Y.; Sunyach, M.-P.; Carret, J.-P.; Mongodin, B.; Marec-Bérard, P.; Philip, T.; et al. Conformity to clinical practice guidelines, multidisciplinary management and outcome of treatment for soft tissue sarcomas. Ann. Oncol. 2004, 15, 307–315. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Montesco, M.; Coindre, J.M.; Tos, A.D.; Lurkin, A.; Ranchère-Vince, D.; Vecchiato, A.; Decouvelaere, A.V.; Mathoulin-Pélissier, S.; Albert, S.; et al. Sarcoma: Concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann. Oncol. 2012, 23, 2442–2449. [Google Scholar] [CrossRef]
- Soomers, V.L.M.N.; Husson, O.; Desar, I.M.E.; Sande, M.A.J.V.D.; De Haan, J.J.; Verhoef, C.; Vriens, I.J.H.; Van Houdt, W.J.; Van De Poll-Franse, L.; A Van Der Graaf, W.T. Patient and diagnostic intervals of survivors of sarcoma: Results from the SURVSARC study. Cancer 2020, 126, 5283–5292. [Google Scholar] [CrossRef]
- Lawrence, W., Jr.; Donegan, W.L.; Natarajan, N.; Mettlin, C.; Beart, R.; Winchester, D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann. Surg. 1987, 205, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Skubitz, K.M.; D’Adamo, D.R. Sarcoma. Mayo Clin. Proc. 2007, 82, 1409–1432. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Thomas, J.M. Delay in referral to a specialist soft-tissue sarcoma unit. Eur. J. Surg. Oncol. 2005, 31, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.; O’Connor, M.; Smith, R.C.; Halkett, G.K. The complexity of diagnosing sarcoma in a timely manner: Perspectives of health professionals, patients, and carers in Australia. BMC Health Serv. Res. 2020, 20, 711. [Google Scholar] [CrossRef]
- Kallen, M.E.; Hornick, J.L. From the ashes of “Ewing-like” sarcoma: A contemporary update of the classification, immunohistochemistry, and molecular genetics of round cell sarcomas. Semin. Diagn. Pathol. 2022, 39, 29–37. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA A Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [Green Version]
- Noebauer-Huhmann, I.M.; Weber, M.-A.; Lalam, R.K.; Trattnig, S.; Bohndorf, K.; Vanhoenacker, F.; Tagliafico, A.; Van Rijswijk, C.; Vilanova, J.C.; Afonso, P.D.; et al. Soft Tissue Tumors in Adults: ESSR-Approved Guidelines for Diagnostic Imaging. Semin. Musculoskelet. Radiol. 2015, 19, 475–482. [Google Scholar]
- Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of Histopathologic and Radiologic Grading of Cartilaginous Neoplasms in Long Bones. JBJS 2007, 89, 2113–2123. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Robert, A. Weinberg, Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hwang, S.; Hameed, M.; Kransdorf, M. The 2020 World Health Organization classification of bone tumors: What radiologists should know. Skelet. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Tos, A.P.D. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Kransdorf, M.J.; Murphey, M.D. Imaging of Soft-Tissue Musculoskeletal Masses: Fundamental Concepts. RadioGraphics 2016, 36, 1931–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoenacker, F.M.; Parizel, P.M.; Gielen, J.L. Imaging of Soft Tissue Tumors; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Zając, A.; Kopeć, S.; Szostakowski, B.; Spałek, M.; Fiedorowicz, M.; Bylina, E.; Filipowicz, P.; Szumera-Ciećkiewicz, A.; Tysarowski, A.; Czarnecka, A.; et al. Chondrosarcoma-from Molecular Pathology to Novel Therapies. Cancers 2021, 13, 2390. [Google Scholar] [CrossRef]
- Jelinek, J.S.; Kransdorf, M.J.; Shmookler, B.M.; Aboulafia, A.J.; Malawer, M.M. Liposarcoma of the extremities: MR and CT findings in the histologic subtypes. Radiology 1993, 186, 455–459. [Google Scholar] [CrossRef]
- Dubois, J.; Alison, M. Vascular anomalies: What a radiologist needs to know. Pediatr. Radiol. 2010, 40, 895–905. [Google Scholar] [CrossRef]
- Toti, L.; Manzia, T.M.; Roma, S.; Meucci, R.; Blasi, F.; Ferlosio, A.; Tisone, G.; Orlacchio, A. Rare malignant glomus tumor of the stomach with liver metastases. Radiol. Case Rep. 2019, 14, 463–467. [Google Scholar] [CrossRef]
- Braham, E.; Zairi, S.; Mlika, M.; Ayadi-Kaddour, A.; Ismail, O.; El Mezni, F. Malignant glomus tumor of trachea: A case report with literature review. Asian Cardiovasc. Thorac. Ann. 2015, 24, 104–106. [Google Scholar] [CrossRef]
- Goyal, N.; Kalra, M.; Soni, A.; Baweja, P.; Ghonghe, N.P. Multi-modality imaging approach to bone tumors-State-of-the art. J. Clin. Orthop. Trauma 2019, 10, 687–701. [Google Scholar] [CrossRef]
- Gaume, M.; Chevret, S.; Campagna, R.; Larousserie, F.; Biau, D. The appropriate and sequential value of standard radiograph, computed tomography and magnetic resonance imaging to characterize a bone tumor. Sci. Rep. 2022, 12, 6196. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J. Use of Imaging Prior to Referral to a Musculoskeletal Oncologist. J. Am. Acad. Orthop. Surg. 2019, 27, e1001–e1008. [Google Scholar] [CrossRef] [PubMed]
- Vanhoenacker, F.; Parizel, P.M.; Gielen, J. Imaging of Soft Tissue Tumors, 4th ed.; Springer: Cham, Switzerland, 2017; p. 666. [Google Scholar]
- Mori, T.; Fujii, M.; Akisue, T.; Yamamoto, T.; Kurosaka, M.; Sugimura, K. Three-dimensional images of contrast-enhanced MDCT for preoperative assessment of musculoskeletal masses: Comparison with MRI and plain radiographs. Radiat. Med. 2005, 23, 398–406. [Google Scholar] [PubMed]
- Zhang, X.; Zhang, Y.; Zhang, G.; Qiu, X.; Tan, W.; Yin, X.; Liao, L. Deep Learning with Radiomics for Disease Diagnosis and Treatment: Challenges and Potential. Front. Oncol. 2022, 12, 773840. [Google Scholar] [CrossRef]
- Arthur, A.; Johnston, E.W.; Winfield, J.M.; Blackledge, M.D.; Jones, R.L.; Huang, P.H.; Messiou, C. Virtual Biopsy in Soft Tissue Sarcoma. How Close Are We? Front. Oncol. 2022, 12, 892620. [Google Scholar] [CrossRef]
- Thrussell, I.; Winfield, J.M.; Orton, M.R.; Miah, A.B.; Zaidi, S.H.; Arthur, A.; Thway, K.; Strauss, D.C.; Collins, D.J.; Koh, D.-M.; et al. Radiomic Features from Diffusion-Weighted MRI of Retroperitoneal Soft-Tissue Sarcomas Are Repeatable and Exhibit Change After Radiotherapy. Front. Oncol. 2022, 12, 899180. [Google Scholar] [CrossRef] [PubMed]
- Hayano, K.; Tian, F.; Kambadakone, A.R.; Yoon, S.S.; Duda, D.G.; Ganeshan, B.; Sahani, D.V. Texture Analysis of Non-Contrast-Enhanced Computed Tomography for Assessing Angiogenesis and Survival of Soft Tissue Sarcoma. J. Comput. Assist. Tomogr. 2015, 39, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. RadioGraphics 2017, 37, 1483–1503. [Google Scholar] [CrossRef]
- Tian, F.; Hayano, K.; Kambadakone, A.R.; Sahani, D.V. Response assessment to neoadjuvant therapy in soft tissue sarcomas: Using CT texture analysis in comparison to tumor size, density, and perfusion. Abdom Imaging 2015, 40, 1705–1712. [Google Scholar] [CrossRef]
- Peng, Y.; Bi, L.; Guo, Y.; Feng, D.; Fulham, M.; Kim, J. Deep multi-modality collaborative learning for distant metastases predication in PET-CT soft-tissue sarcoma studies. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Gitto, S.; Cuocolo, R.; Albano, D.; Morelli, F.; Pescatori, L.C.; Messina, C.; Imbriaco, M.; Sconfienza, L.M. CT and MRI radiomics of bone and soft-tissue sarcomas: A systematic review of reproducibility and validation strategies. Insights Imaging 2021, 12, 68. [Google Scholar] [CrossRef]
- Esperança-Martins, M.; Fernandes, I.; Brito, J.S.D.; Macedo, D.; Vasques, H.; Serafim, T.; Costa, L.; Dias, S. Sarcoma Metabolomics: Current Horizons and Future Perspectives. Cells 2021, 10, 1432. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Hack, R.I.; Becker, A.S.; Bode-Lesniewska, B.; Exner, G.U.; Müller, D.A.; Ferraro, D.A.; Warnock, G.I.; Burger, I.A.; Britschgi, C. When SUV Matters: FDG PET/CT at Baseline Correlates with Survival in Soft Tissue and Ewing Sarcoma. Life 2021, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Benz, M.R.; Dry, S.M.; Eilber, F.C.; Allen-Auerbach, M.S.; Tap, W.D.; Elashoff, D.; Phelps, M.E.; Czernin, J. Correlation Between Glycolytic Phenotype and Tumor Grade in Soft-Tissue Sarcomas by 18F-FDG PET. J. Nucl. Med. 2010, 51, 1174–1181. [Google Scholar] [CrossRef] [Green Version]
- Benz, M.R.; Crompton, J.G.; Harder, D. PET/CT Variants and Pitfalls in Bone and Soft Tissue Sarcoma. Semin. Nucl. Med. 2021, 51, 584–592. [Google Scholar] [CrossRef]
- Younis, M.H.; Abu-Hijleh, H.A.; Aldahamsheh, O.O.; Abualruz, A.; Thalib, L. Meta-Analysis of the Diagnostic Accuracy of Primary Bone and Soft Tissue Sarcomas by 18F-FDG-PET. Med. Princ. Pract. 2020, 29, 465–472. [Google Scholar] [CrossRef]
- Seth, N.; Seth, I.; Bulloch, G.; Siu, A.H.Y.; Guo, A.; Chatterjee, R.; MacManus, M.; Donnan, L. 18F-FDG PET and PET/CT as a diagnostic method for Ewing sarcoma: A systematic review and meta-analysis. Pediatr. Blood Cancer 2022, 69, e29415. [Google Scholar] [CrossRef]
- Saranovic, D.P.S.; Nikitovic, M.; Saponjski, J.; Milojevic, I.G.; Paripovic, L.; Saranovic, D.; Beatovic, S.; Artiko, V.M. Post-treatment FDG PET/CT predicts progression-free survival in young patients with small round blue cell tumors: Ewing sarcoma and PNET. Eur. J. Radiol. 2020, 129, 109076. [Google Scholar] [CrossRef]
- Kessler, L.; Ferdinandus, J.; Hirmas, N.; Bauer, S.; Dirksen, U.; Zarrad, F.; Nader, M.; Chodyla, M.; Milosevic, A.; Umutlu, L.; et al. (68)Ga-FAPI as a Diagnostic Tool in Sarcoma: Data from the (68)Ga-FAPI PET Prospective Observational Trial. J. Nucl. Med. 2022, 63, 89–95. [Google Scholar] [CrossRef]
- Kessler, L.; Ferdinandus, J.; Hirmas, N.; Zarrad, F.; Nader, M.; Kersting, D.; Weber, M.; Kazek, S.; Sraieb, M.; Hamacher, R.; et al. Pitfalls and common findings in 68Ga-FAPI-PET—A pictorial analysis. J. Nucl. Med. 2022, 63, 890–896. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Kim, M.; Choi, B.K.; Kim, D.H.; Choi, I.; You, A.H.J. TJP1 Contributes to Tumor Progression through Supporting Cell-Cell Aggregation and Communicating with Tumor Microenvironment in Leiomyosarcoma. Mol. Cells 2021, 44, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, S.; Mizutani, T.; Ishikane, S.; Martinez, M.E.; Kiyono, Y.; Miura, K.; Hosoda, H.; Imamichi, Y.; Kangawa, K.; Miyamoto, K.; et al. Establishment and characterization of a novel orthotopic mouse model for human uterine sarcoma with different metastatic potentials. Cancer Lett. 2015, 366, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.-C.; Wu, C.-Y.; Chang, W.-T.; Lin, C.-Y.; Tseng, Y.-L.; Liu, R.-S.; Alauddin, M.M.; Lin, W.-J.; Wang, H.-E. Monitoring tumor response with [18F]FMAU in a sarcoma-bearing mouse model after liposomal vinorelbine treatment. Nucl. Med. Biol. 2013, 40, 1035–1042. [Google Scholar] [CrossRef]
- Liu, R.-S.; Chou, T.-K.; Chang, C.-H.; Wu, C.-Y.; Chang, C.-W.; Chang, T.-J.; Wang, S.-J.; Lin, W.-J.; Wang, H.-E. Biodistribution, pharmacokinetics and PET Imaging of [18F]FMISO, [18F]FDG and [18F]FAc in a sarcoma- and inflammation-bearing mouse model. Nucl. Med. Biol. 2009, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, T.; Probst, P.; Giovannoni, L.; Green, A.J.; Meyer, T.; Neri, D. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br. J. Cancer 2013, 109, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.C.; Tang, T.; Dasgupta, A.; Kurenbekova, L.; Shuck, R.; Gaber, M.; Yustein, J.T. In Vitro and In Vivo Characterization of a Preclinical Irradiation-Adapted Model for Ewing Sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Mach, R.H.; Zeng, C.; Hawkins, W.G. The σ2 receptor: A novel protein for the imaging and treatment of cancer. J. Med. Chem. 2013, 56, 7137–7160. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.K.; Kwon, J.; Na, K.S.; Jeong, H.S.; Hwang, H.; Oh, P.S.; Kim, D.H.; Jang, K.Y.; Lim, S.T.; Sohn, M.H.; et al. Evaluation of Selective Arterial Embolization Effect by Chitosan Micro-Hydrogels in Hindlimb Sarcoma Rodent Models Using Various Imaging Modalities. Nucl. Med. Mol. Imaging 2015, 49, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.F.; Zhu, H.; Li, G.H.; Xie, Q.; Yang, X.T.; Xu, X.X.; Tian, X.B.; Wan, Y.K.; Yang, Z. Construction of Anti-hPD-L1 HCAb Nb6 and in Situ (124)I Labeling for Noninvasive Detection of PD-L1 Expression in Human Bone Sarcoma. Bioconjug. Chem. 2019, 30, 2614–2623. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, H.; Xie, Q.; Tian, X.; Yang, X.; Feng, F.; Jiang, Q.; Sheng, X.; Yang, Z. Evaluation of 124I-JS001 for hPD1 immuno-PET imaging using sarcoma cell homografts in humanized mice. Acta Pharm. Sin. B 2020, 10, 1321–1330. [Google Scholar] [CrossRef]
- Karkare, S.; Allen, K.J.; Jiao, R.; Malo, M.E.; Dawicki, W.; Helal, M.; Godson, D.L.; Dickinson, R.; MacDonald-Dickinson, V.; Yang, R.; et al. Detection and targeting insulin growth factor receptor type 2 (IGF2R) in osteosarcoma PDX in mouse models and in canine osteosarcoma tumors. Sci. Rep. 2019, 9, 11476. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lu, Y.; Zhu, X.; Liu, L.; Chen, J.; Ma, Q.; Zhang, Y.; Wen, Y.; Yang, L.; Liu, T.; et al. CXCR4-targeted near-infrared imaging allows detection of orthotopic and metastatic human osteosarcoma in a mouse model. Sci. Rep. 2015, 5, 15244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, A.F.; Dearling, J.L.; Wang, Y.; Tupper, T.; Sun, Y.; Aster, J.C.; Calicchio, M.L.; Perez-Atayde, A.R.; Packard, A.B.; Kung, A.L. Targeted imaging of ewing sarcoma in preclinical models using a 64Cu-labeled anti-CD99 antibody. Clin. Cancer Res. 2014, 20, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, T.; Igarashi, K.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Bouvet, M.; Tsuchiya, H.; Hoffman, R.M. Osteosarcoma patient-derived orthotopic xenograft (PDOX) models used to identify novel and effective therapeutics: A review. Anticancer Res. 2021, 41, 5865–5871. [Google Scholar] [CrossRef]
- Jacques, C.; Renema, N.; Ory, B.; Walkley, C.R.; Grigoriadis, A.E.; Heymann, D. Murine models of bone sarcomas. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; pp. 331–342. [Google Scholar]
- Bone Research Protocols. Methods in Molecular Biology; Humana: Totowa, NJ, USA, 2017. [Google Scholar]
- Kersten, K.; E De Visser, K.; Van Miltenburg, M.H.; Jonkers, J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017, 9, 137–153. [Google Scholar] [CrossRef]
- Talbot, J.; Brion, R.; Picarda, G.; Amiaud, J.; Chesneau, J.; Bougras, G.; Stresing, V.; Tirode, F.; Heymann, D.; Redini, F.; et al. Loss of connexin43 expression in Ewing’s sarcoma cells favors the development of the primary tumor and the associated bone osteolysis. Biochim. Biophys. Acta 2013, 1832, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Georges, S.; Chesneau, J.; Hervouet, S.; Taurelle, J.; Gouin, F.; Redini, F.; Padrines, M.; Heymann, D.; Fortun, Y.; Verrecchia, F. A Disintegrin and Metalloproteinase 12 produced by tumour cells accelerates osteosarcoma tumour progression and associated osteolysis. Eur. J. Cancer 2013, 49, 2253–2263. [Google Scholar] [CrossRef]
- Moding, E.J.; Clark, D.P.; Qi, Y.; Li, Y.; Ma, Y.; Ghaghada, K.; Johnson, G.A.; Kirsch, D.G.; Badea, C.T. Dual-energy micro-computed tomography imaging of radiation-induced vascular changes in primary mouse sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1353–1359. [Google Scholar] [CrossRef] [Green Version]
- Heymann, D.; Ory, B.; Blanchard, F.; Heymann, M.-F.; Coipeau, P.; Charrier, C.; Couillaud, S.; Thiery, J.; Gouin, F.; Redini, F. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone 2005, 37, 74–86. [Google Scholar] [CrossRef]
- Molina, E.R.; Chim, L.K.; Salazar, M.C.; Koons, G.L.; Menegaz, B.A.; Ruiz-Velasco, A.; Lamhamedi-Cherradi, S.E.; Vetter, A.M.; Satish, T.; Cuglievan, B.; et al. 3D Tissue-Engineered Tumor Model for Ewing’s Sarcoma That Incorporates Bone-like ECM and Mineralization. ACS Biomater. Sci. Eng. 2020, 6, 539–552. [Google Scholar] [CrossRef]
- Oshiro, H.; Kiyuna, T.; Tome, Y.; Miyake, K.; Kawaguchi, K.; Higuchi, T.; Miyake, M.; Zhang, Z.; Razmjooei, S.; Barangi, M.; et al. Detection of Metastasis in a Patient-derived Orthotopic Xenograft (PDOX) Model of Undifferentiated Pleomorphic Sarcoma with Red Fluorescent Protein. Anticancer Res. 2019, 39, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kiyuna, T.; Murakami, T.; Tome, Y.; Igarashi, K.; Kawaguchi, K.; Russell, T.; Eckardt, M.A.; Crompton, J.; Singh, A.; Bernthal, N.; et al. Labeling the Stroma of a Patient-Derived Orthotopic Xenograft (PDOX) Mouse Model of Undifferentiated Pleomorphic Soft-Tissue Sarcoma with Red Fluorescent Protein for Rapid Non-Invasive Imaging for Drug Screening. J. Cell Biochem. 2017, 118, 361–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshiro, H.; Tome, Y.; Miyake, K.; Higuchi, T.; Sugisawa, N.; Kanaya, F.; Nishida, K.; Hoffman, R.M. An mTOR and VEGFR inhibitor combination arrests a doxorubicin resistant lung metastatic osteosarcoma in a PDOX mouse model. Sci. Rep. 2021, 11, 8583. [Google Scholar] [CrossRef]
- Kiyuna, T.; Murakami, T.; Tome, Y.; Kawaguchi, K.; Igarashi, K.; Miyake, K.; Kanaya, F.; Singh, A.; Eilber, F.C.; Hoffman, R.M. Analysis of Stroma Labeling During Multiple Passage of a Sarcoma Imageable Patient-Derived Orthotopic Xenograft (iPDOX) in Red Fluorescent Protein Transgenic Nude Mice. J. Cell. Biochem. 2017, 118, 3367–3371. [Google Scholar] [CrossRef]
- Blattmann, C.; Thiemann, M.; Stenzinger, A.; Roth, E.K.; Dittmar, A.; Witt, H.; Lehner, B.; Renker, E.; Jugold, M.; Eichwald, V.; et al. Establishment of a patient-derived orthotopic osteosarcoma mouse model. J. Transl. Med. 2015, 13, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, K. Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Albers, J.; Pacilé, S.; Markus, M.A.; Wiart, M.; Velde, G.V.; Tromba, G.; Dullin, C. X-ray-Based 3D Virtual Histology-Adding the Next Dimension to Histological Analysis. Mol. Imaging Biol. 2018, 20, 732–741. [Google Scholar] [CrossRef]
- Bertin, H.; Guilho, R.; Brion, R.; Amiaud, J.; Battaglia, S.; Moreau, A.; Brouchet-Gomez, A.; Longis, J.; Piot, B.; Heymann, D.; et al. Jaw osteosarcoma models in mice: First description. J. Transl. Med. 2019, 17, 56. [Google Scholar] [CrossRef]
- Cheng, J.N.; Frye, J.B.; Whitman, S.A.; Funk, J.L. Skeletal impact of 17β-estradiol in T cell-deficient mice: Age-dependent bone effects and osteosarcoma formation. Clin. Exp. Metastasis 2020, 37, 269–281. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Yang, S. Verteporfin Inhibits the Progression of Spontaneous Osteosarcoma Caused by Trp53 and Rb1 Deficiency in Ctsk-Expressing Cells via Impeding Hippo Pathway. Cells 2022, 11, 1361. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Vottis, C.T.; Megaloikonomos, P.D.; Agrogiannis, G.D.; Theocharis, S. Neovascularization in Ewing’s sarcoma. Neoplasma 2018, 65, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Hernández de la Cruz, O.N.; López-González, J.S.; García-Vázquez, R.; Salinas-Vera, Y.M.; Muñiz-Lino, M.A.; Aguilar-Cazares, D.; López-Camarillo, C.; Carlos-Reyes, Á. Regulation Networks Driving Vasculogenic Mimicry in Solid Tumors. Front. Oncol. 2019, 9, 1419. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Zhang, S.; Zhao, X.; Zhang, W.; Hao, X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int. J. Oncol. 2004, 25, 1609–1614. [Google Scholar] [CrossRef]
- Giner, F.; A López-Guerrero, J.; Fernández-Serra, A.; Machado, I.; Mayordomo-Aranda, E.; Peydró-Olaya, A.; Llombart-Bosch, A. Chemokine Expression Is Involved in the Vascular Neogenesis of Ewing Sarcoma: A Preliminary Analysis of the Early Stages of Angiogenesis in a Xenograft Model. Pediatr. Dev. Pathol. 2019, 22, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kalt, I.; Borodianskiy-Shteinberg, T.; Schachor, A.; Sarid, R. GLTSCR2/PICT-1, a putative tumor suppressor gene product, induces the nucleolar targeting of the Kaposi’s sarcoma-associated herpesvirus KS-Bcl-2 protein. J. Virol. 2010, 84, 2935–2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, K.; Sharma, V.; Sharma, M. Optimization, in vitro cytotoxicity and penetration capability of deformable nanovesicles of paclitaxel for dermal chemotherapy in Kaposi sarcoma. Artif. Cells Nanomed Biotechnol. 2016, 44, 1671–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagorchev, L.; Oses, P.; Zhuang, Z.W.; Moodie, K.; Mulligan-Kehoe, M.J.; Simons, M.; Couffinhal, T. Micro computed tomography for vascular exploration. J. Angiogenes Res. 2010, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Li, C.; Li, M.; Yin, X.; Wu, T.; Duan, C.; Cao, Y.; Lu, H.; Hu, J. Simultaneous 3D Visualization of the Microvascular and Neural Network in Mouse Spinal Cord Using Synchrotron Radiation Micro-Computed Tomography. Neurosci. Bull 2021, 37, 1469–1480. [Google Scholar] [CrossRef]
- Marxen, M.; Thornton, M.M.; Chiarot, C.B.; Klement, G.; Koprivnikar, J.; Sled, J.G.; Henkelman, R.M. MicroCT scanner performance and considerations for vascular specimen imaging. Med. Phys. 2004, 31, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xue, J.; Xi, Y.; Tang, R.; Jin, W.; Chen, J.-J.; Zhang, X.; Shao, Z.-M.; Wu, J. Evaluating the effect of Avastin on breast cancer angiogenesis using synchrotron radiation. Quant. Imaging Med. Surg. 2019, 9, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Tunset, H.M.; Cebulla, J.; Vettukattil, R.; Helgesen, H.; Feuerherm, A.J.; Engebråten, O.; Mælandsmo, G.M.; Johansen, B.; Moestue, S.A. Anti-vascular effects of the cytosolic phospholipase A2 inhibitor AVX235 in a patient-derived basal-like breast cancer model. BMC Cancer 2016, 16, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leszczyński, B.; Śniegocka, M.; Wróbel, A.; Pędrys, R.; Szczygieł, M.; Romanowska-Dixon, B.; Urbańska, K.; Elas, M. Visualization and Quantitative 3D Analysis of Intraocular Melanoma and Its Vascularization in a Hamster Eye. Int. J. Mol. Sci. 2018, 19, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downey, C.M.; Aghaei, M.; Schwendener, R.A.; Jirik, F.R. DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2’3’-cGAMP, induces M2 macrophage repolarization. PLoS ONE 2014, 9, e99988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byers, R.A.; Fisher, M.; Brown, N.J.; Tozer, G.M.; Matcher, S.J. Vascular patterning of subcutaneous mouse fibrosarcomas expressing individual VEGF isoforms can be differentiated using angiographic optical coherence tomography. Biomed Opt. Express 2017, 8, 4551–4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, H.Y.; Lee, Y.H.; Juhng, S.K.; Lee, M.S.; Oh, J.; Yoon, K.-H. Micro-CT measurements of tumoral vessels supplied by portal circulation in hepatic colorectal metastasis mouse model. Microsc. Res. Tech. 2014, 77, 415–421. [Google Scholar] [CrossRef]
- Deng, L.; Tang, H.; Qiang, J.; Wang, J.; Xiao, S. Blood Supply of Early Lung Adenocarcinomas in Mice and the Tumor-supplying Vessel Relationship: A Micro-CT Angiography Study. Cancer Prev. Res. 2020, 13, 989–996. [Google Scholar] [CrossRef]
- Farahani, N.; Braun, A.; Jutt, D.; Huffman, T.; Reder, N.; Liu, Z.; Yagi, Y.; Pantanowitz, L. Three-dimensional Imaging and Scanning: Current and Future Applications for Pathology. J. Pathol. Inf. 2017, 8, 36. [Google Scholar] [CrossRef]
- Xu, B.; Teplov, A.; Ibrahim, K.; Inoue, T.; Stueben, B.; Katabi, N.; Hameed, M.; Yagi, Y.; Ghossein, R. Detection and assessment of capsular invasion, vascular invasion and lymph node metastasis volume in thyroid carcinoma using microCT scanning of paraffin tissue blocks (3D whole block imaging): A proof of concept. Mod. Pathol. 2020, 33, 2449–2457. [Google Scholar] [CrossRef]
- Kayı Cangır, A.; Dizbay Sak, S.; Güneş, G.; Orhan, K. Differentiation of benign and malignant regions in paraffin embedded tissue blocks of pulmonary adenocarcinoma using micro CT scanning of paraffin tissue blocks: A pilot study for method validation. Surg. Today 2021, 51, 1594–1601. [Google Scholar] [CrossRef]
- Ohnishi, T.; Teplov, A.; Kawata, N.; Ibrahim, K.; Ntiamoah, P.; Firat, C.; Haneishi, H.; Hameed, M.; Shia, J.; Yagi, Y. Three-Dimensional Vessel Segmentation in Whole-Tissue and Whole-Block Imaging Using a Deep Neural Network: Proof-of-Concept Study. Am. J. Pathol. 2021, 191, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Downey, C.M.; Singla, A.K.; Villemaire, M.L.; Buie, H.R.; Boyd, S.K.; Jirik, F.R. Quantitative Ex-Vivo Micro-Computed Tomographic Imaging of Blood Vessels and Necrotic Regions within Tumors. PLoS ONE 2012, 7, e41685. [Google Scholar] [CrossRef]
- Sakamoto, H.; Nishimura, M.; Teplov, A.; Leung, G.; Ntiamoah, P.; Cesmecioglu, E.; Kawata, N.; Ohnishi, T.; Kareem, I.; Shia, J.; et al. A pilot study of micro-CT-based whole tissue imaging (WTI) on endoscopic submucosal dissection (ESD) specimens. Sci. Rep. 2022, 12, 9889. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Cebulla, J.; Ward, B.D.; Rhie, K.; Zhang, J.; Pathak, A.P. Assessing breast cancer angiogenesis in vivo: Which susceptibility contrast MRI biomarkers are relevant? Magn. Reson. Med. 2013, 70, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Darpolor, M.M.; Molthen, R.C.; Schmainda, K.M. Multimodality Imaging of Abnormal Vascular Perfusion and Morphology in Preclinical 9L Gliosarcoma Model. PLoS ONE 2011, 6, e16621. [Google Scholar] [CrossRef]


| CT Features | References | |
|---|---|---|
| Chondro-osseous malignant tumors Chondrosarcoma Intramedullary Clear cell | A lesion with calcifications (“ring and arc” or “popcorn” pattern) and aggressive growth features; lytic lesions are also common mixed and sclerotic lesions with visible calcifications (mineralized chondroid matrix present in most cases). Calcifications would be present only in 30% of cases. Heterogeneous pattern that would depend on the proportion of low- and high-grade areas in the lesion. | [39] |
| Malignant adipocytic tumors Well-differentiated liposarcoma: lipoma-like, sclerosing, inflammatory Dedifferentiated liposarcoma Myxoid liposarcoma Pleomorphic liposarcoma Myxoid pleomorphic liposarcoma | The fatty nature of the mass can be proved by the measurement at the field of view (FOV) in Hounsfield units (HU). Fat will show the lowest attenuation of any tissue, and a benign lipoma can be distinguished from a malignant tumor on CT by the uniformly low attenuation (−70 to −130 HU), but it is not possible to reliably differentiate a lipoma from a well-differentiated liposarcoma on CT. However, the presence of a combination of fat and solid components is suggestive of a low-grade liposarcoma. Nonfatty components within an adipocytic tumor should always suggest the possibility of a high-grade liposarcoma. However, it is not always possible to distinguish between the dedifferentiated type and other high-grade liposarcomas. A well-marginated mass of fat attenuation resembling a benign adipocytic tumor, clearly delineating the bony excrescences and adjacent bony cortex; some thickened (more than 2 mm wide) linear or nodular soft-tissue septa during contrast-enhanced CT. Should be suspected if a non-adipocytic component appears in a previously known well-differentiated liposarcoma; retains some of the features of the well-differentiated liposarcoma, while some mass-like areas develop a nonspecific appearance. These areas display tissue attenuation greater than fat on CT scans; calcification or even ossification may be present. Homogeneous or slightly heterogeneous mass that is less attenuating than the surrounding muscle. May occasionally resemble a cyst, due to the lack of fat content, with sharply demarcated margins. It displays attenuation values within the water range (+0 HU). Not distinguishable from other sarcomas because it contains little or no fat. | [38,40] |
| Fibroblastic/myofibroblastic malignant tumors Dermatofibrosarcoma protuberans, fibrosarcomatous Solitary fibrous tumor Inflammatory myofibroblastic tumor Low-grade myofibroblastic sarcoma Superficial CD34-positive fibroblastic tumor Myxoinflammatory fibroblastic sarcomaInfantile fibrosarcoma Solitary fibrous tumor, malignant Fibrosarcoma NOS Myxofibrosarcoma Low grade fibromyxoid sarcoma Sclerosing epithelioid fibrosarcoma) | The lesions have variable attenuation and enhancement on CT scans. Extra-abdominal desmoids are iso- or hypodense relative to the muscle and enhance to +100–110 HU after injection of iodinated contrast material. | [38] |
| Malignant tenosynovial giant cell tumor | A dense soft tissue mass (intra-articular or related to the tendon). CT is useful to detect underlying bone erosions or cysts, contrast-enhanced CT shows hypervascular nature. | [38] |
| Malignant vascular tumors Epithelioid haemangioendothelioma Angiosarcoma Malignant pericytic (perivascular) tumors Glomus tumor | Vascular malformations, such as phleboliths and dystrophic calcifications. The involvement of the adjacent joints or bones is possible, such as cortical erosion, periosteal reaction, regional osteopenia, and bony overgrowth. Nonspecific calcified intralesional septa, shown in the contrast enhancement. | [41,42,43] |
| Smooth muscle malignant tumors Inflammatory leiomyosarcoma Leiomyosarcoma | Well-defined, homogeneously enhancing tumors, often associated with fascial edema, with variable signal intensities, central necrosis, and marked peripheral and septal enhancement | [38] |
| Skeletal muscle malignant tumors Embryonal rhabdomyosarcoma Alveolar rhabdomyosarcoma Pleomorphic rhabdomyosarcoma Spindle cell/sclerosing rhabdomyosarcoma Ectomesenchymoma | The majority of STSs have an attenuation value slightly less than that of normal muscle. A nonspecific soft-tissue mass may show local bone invasion, which is seen in about 25% of cases. Bone metastases may occur and are usually lytic and rarely mixed. | [38] |
| Peripheral nerve sheath malignant tumors Malignant peripheral nerve sheath tumor Melanotic malignant nerve sheath tumor Granular cell tumor, malignant Perineurioma | Heterogeneous tumors with necrotic foci. PET/CT: SUVmax can assist to separate malignant from benign lesions (especially in case of neurofibromatosis type 1). | [38] |
| Malignant tumors of uncertain differentiation Synovial sarcoma Epithelioid sarcoma: proximal and classic variant Alveolar soft part sarcoma Clear cell sarcoma Desmoplastic small round cell tumor Intimal sarcoma | A soft-tissue mass, which may infiltrate adjacent structures, having a slightly higher density than muscle. Joint invasion and bony involvement, cortical bone erosion, or invasion. Intratumoral calcification or ossification is also more easily seen on CT.Extensive vascular supply led to marked enhancement after injection of contrast medium. A nonspecific soft-tissue mass which may occasionally show punctate calcifications. A nonspecific soft-tissue mass. A nonspecific soft-tissue mass. Multiple omental or serosal soft-tissue masses which have a low attenuation and only moderate homogeneous enhancement, foci of necrosis and calcification. Polypoid intraluminal soft-tissue masses. If not polypoid and no other signs of malignancy are present, the non-enhancing defect may be not distinguishable from thrombus or embolus material. | [38] |
| Undifferentiated small round cell sarcomas of bone and soft tissue Ewing sarcoma Primitive neuroectodermal tumor (PNET) | Tumor with low attenuation. The presence of only focal areas of hypodensity and moderate post-contrast enhancement reflects the differentvascularization pattern. A large, ill-defined mass with a heterogeneous appearance due to extensive cystic degeneration, may be the presence of calcifications. After the injection of iodinated contrast, the tumor has a heterogeneous appearance | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, E.; Tkachev, S.; Reshetov, I.; Timashev, P.; Ulasov, I. Imaging Hallmarks of Sarcoma Progression Via X-ray Computed Tomography: Beholding the Flower of Evil. Cancers 2022, 14, 5112. https://doi.org/10.3390/cancers14205112
Popova E, Tkachev S, Reshetov I, Timashev P, Ulasov I. Imaging Hallmarks of Sarcoma Progression Via X-ray Computed Tomography: Beholding the Flower of Evil. Cancers. 2022; 14(20):5112. https://doi.org/10.3390/cancers14205112
Chicago/Turabian StylePopova, Elena, Sergey Tkachev, Igor Reshetov, Peter Timashev, and Ilya Ulasov. 2022. "Imaging Hallmarks of Sarcoma Progression Via X-ray Computed Tomography: Beholding the Flower of Evil" Cancers 14, no. 20: 5112. https://doi.org/10.3390/cancers14205112
APA StylePopova, E., Tkachev, S., Reshetov, I., Timashev, P., & Ulasov, I. (2022). Imaging Hallmarks of Sarcoma Progression Via X-ray Computed Tomography: Beholding the Flower of Evil. Cancers, 14(20), 5112. https://doi.org/10.3390/cancers14205112






