TRAIL in the Treatment of Cancer: From Soluble Cytokine to Nanosystems
Abstract
:Simple Summary
Abstract
1. Introduction
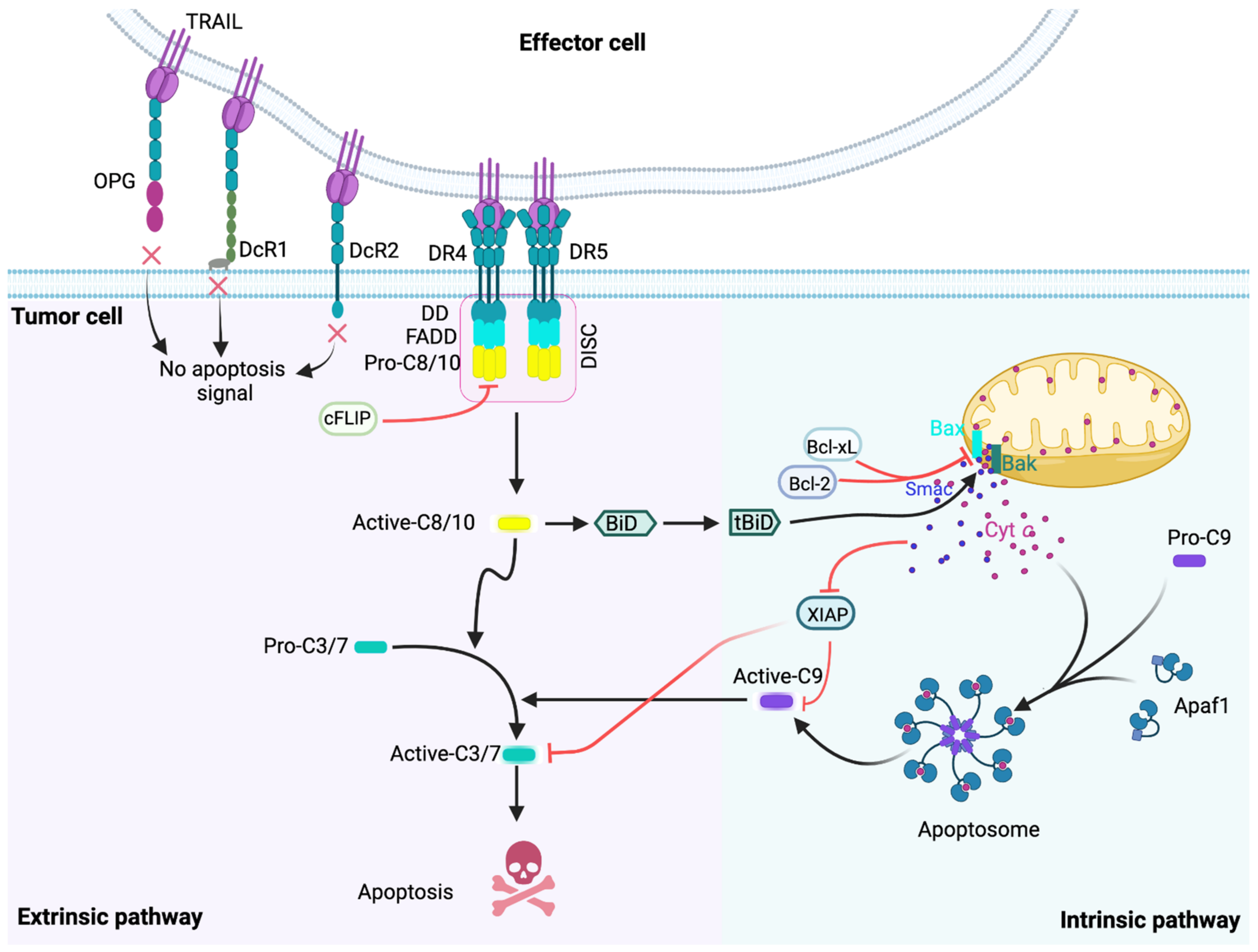
2. The TRAIL-Induced Apoptotic Pathway
3. TRAIL Formulations to Increase Its Serum Half-Life Time
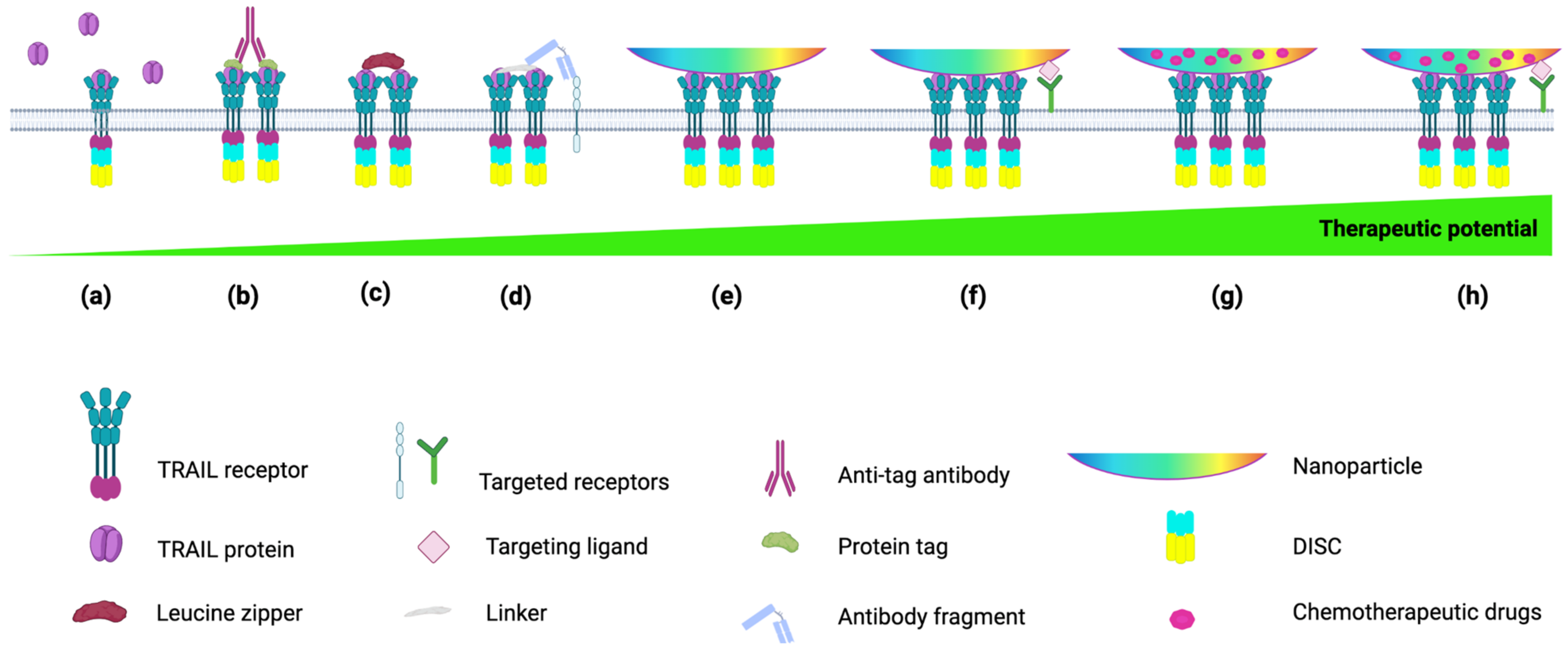
4. TRAIL Formulations to Mimic Membrane-Bound TRAIL
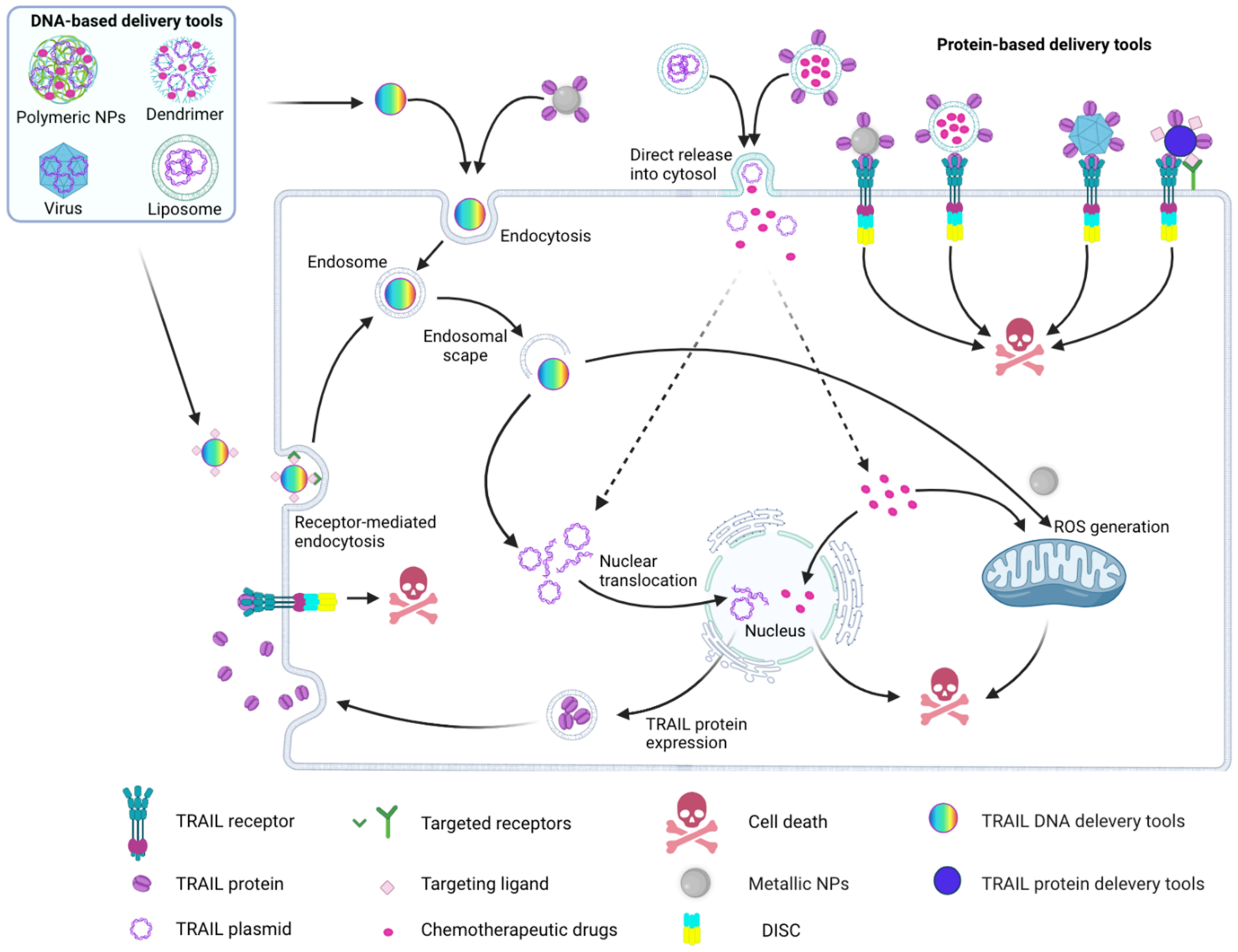
4.1. Cell- and Virus-Based Delivery Tools
4.2. Nanoparticle-Based TRAIL Delivery
4.2.1. Cell Component-Based Nanoparticles for TRAIL Delivery
4.2.2. Liposome-Based TRAIL Delivery
4.2.3. Active Targeting
| Formulation | TRAIL Form/Location | Targeting Strategy | Tumor Type | Ref. |
|---|---|---|---|---|
| Inhalable HSA NPs | rhTRAIL/Surface | HSA to target gp60 transcytosis pathway | Lung cancer, mouse model | [214] |
| HSA NPs | rhTRAIL/Surface | Transferrin on HSA NPs to target transferrin receptor on tumor cells and HSA to target gp60 transcytosis pathway | Colorectal cancer, mouse model | [215] |
| Liposome | pTRAIL/Surface | Angiopeptide-2 to target the low-density LRP on BBB and glioma cells | Glioblastoma, mouse model | [216] |
| Polymeric NPs coated with platelet membrane | rhTRAIL/Surface | Platelet membrane to target CD44 on tumor cells via p-selectin | Metastatic breast cancer, mouse model | [217] |
| Polymeric NPs | pTRAIL/Inside | Reconstituted HDL on polymeric NPs to target scavenger receptor class B type I on MSCs | Pulmonary melanoma, mouse metastasis model | [218] |
| Polymeric NPs | pTRAIL/Surface | EGFR-specific peptide to target EGFR on laryngeal cancer cells | Hep-2 laryngeal squamous cell carcinoma, mouse model | [219] |
| PEI-coated gold nanocomposite | pTRAIL/Surface | Dexamethasone to target nucleus | Hep3B cell, mouse model | [220] |
| Polymeric NPs | rhTRAIL conjugated to PEG/Inside | HA to target CD44 on tumor cells | Collagen-induced arthritis, mouse model | [221] |
| Liposome inside a hyaluronic acid crosslinked-gel shell | rhTRAIL/ B/w liposome and gel shell | HA to target CD44 on tumor cells | MDA-MB-231, breast cancer xenograft model | [222] |
| ZnFe2O4 magnetic NPs w/mesoporous silica shell with PEI | TRAIL DNA/Surface | Adipose tissue-derived MSCs treated with NPs to target tumor cells | Ovarian cancer, mouse model | [223] |
| Carbon dot coated polyethyleneimine | pTRAIL/ Surface | MSCs for targeting tumor cells | Lung cancer cell line (A549 cells) | [224] |
| PEG-crosslinked albumin hydrogel | rhTRAIL/Inside | HSA to target gp60 transcytosis pathway | Pancreatic cancer, mouse model | [225] |
| Magnetic ternary nanohybrids (iron oxide NPs coated with HA) | TRAIL DNA/Inside | MSCs for targeting tumor cells | Glioma, mouse model | [226] |
| Polymeric NPs | pTRAIL/Inside | Choline-derivate to target choline transporters on BBB and glioma cells | Glioma, mouse model | [227] |
4.2.4. Nanoparticles for TRAIL-Based Drug Combination Therapies
4.2.5. Combination of Chemotherapeutic Reagent Encapsulating-Nanoparticles with TRAIL
4.2.6. Combination of ROS Producing-Nanoparticles with TRAIL
4.2.7. Combination Phototherapy with TRAIL
5. Conclusions and Future Perspectives
- -
- Most TRAIL-NP formulations have only been tested in immunodeficient mouse models engrafted with cancer cell lines. These models cannot replicate crucial properties of the human body to assess targeting efficiency, the heterogeneity of primary tumors or the role of the immune system. Testing in patient-derived tumor xenografts or genetically engineered mouse models is crucial to progress TRAIL-NPs towards clinical development.
- -
- With the advance of personalized therapy, it is becoming possible to target the exact molecular mechanisms of TRAIL resistance in individual tumors. Cells become sensitive to TRAIL during the first stages of malignant transformation [288], but this sensitivity is lost as the tumor evolves by the up-regulation of one (but not multiple) inhibitory pathways (e.g., increased Bcl-2 expression, XIAP expression) [289]. On the contrary, healthy cells have redundant (multiple) mechanisms to maintain their TRAIL resistance [289]. Targeting the specific apoptosis inhibitor, instead of combining TRAIL with a broad-spectrum, and often toxic cell stressor (e.g., doxorubicin), is likely to achieve higher efficiency and/or lower systemic toxicity.
- -
- In most of the current formulations for co-delivery of TRAIL and TRAIL sensitizers, TRAIL is conjugated to the surface of the NPs, and its sensitizers are loaded inside the NPs. This means that first, TRAIL engages with DRs on the target cell, and after that, or in parallel, when the cell endocytoses the NPs, the sensitizing agent can exert its effect, when it might be too late. Formulations for time-dependent release nanoparticles, or development of sequential treatment regimes, might solve this issue.
- -
- Selective tumor-targeting with higher-specificity tumor markers have a great potential to maximize safety as well as potency of TRAIL-NPs and enable controlled activation and/or cargo-release. Thus, identifying and targeting more specific cancer biomarkers, such as CLL-1 (targeting leukemic stem cells) is required in order to minimize off-target toxicity [290].
Author Contributions
Funding
Conflicts of Interest
References
- Ashkenazi, A.; Shahrokh, Z.; Schwall, R.H.; Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; et al. Schwall, Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Voss, O.H.; Arango, D.; Tossey, J.C.; Calero, M.A.V.; Doseff, A.I. Splicing reprogramming of TRAIL/DISC-components sensitizes lung cancer cells to TRAIL-mediated apoptosis. Cell Death Dis. 2021, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Knudsen, S.; Mazin, W.; Dahlgaard, J.; Zhang, B. A 71-Gene Signature of TRAIL Sensitivity in Cancer CellsBiomarker of TRAIL Response. Mol. Cancer Ther. 2021, 11, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemke, J.; von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor–related apoptosis–inducing ligand in vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef]
- Yagita, H.; Takeda, K.; Hayakawa, Y.; Smyth, M.J.; Okumura, K. TRAIL and its receptors as targets for cancer therapy. Cancer Sci. 2004, 95, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Robertson, N.M.; Zangrilli, J.G.; Hastie, A.; Lindemeyer, R.G.; Planeta, A.; Smith, M.K.; Innocent, N.; Musani, A.; Pascual, R.; Peters, S.; et al. Differential Expression of TRAIL and TRAIL Receptors in Allergic Asthmatics Following Segmental Antigen Challenge: Evidence for a Role of TRAIL in Eosinophil Survival. J. Immunol. 2002, 169, 5986–5996. [Google Scholar] [CrossRef] [Green Version]
- Herold, S.; Steinmueller, M.; von Wulff, W.; Cakarova, L.; Pinto, R.; Pleschka, S.; Mack, M.; Kuziel, W.A.; Corazza, N.; Brunner, T.; et al. Lung epithelial apoptosis in infl uenza virus pneumonia: The role of macrophage- expressed TNF-related apoptosis- inducing ligand. J. Exp. Med. 2008, 205, 3065–3077. [Google Scholar] [CrossRef]
- Vik, R.; Espevik, T. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand. J. Immunol. 2000, 51, 244–250. [Google Scholar]
- Tecchio, C.; Huber, V.; Scapini, P.; Calzetti, F.; Margotto, D.; Todeschini, G.; Pilla, L.; Martinelli, G.; Pizzolo, G.; Rivoltini, L. IFNα-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood 2004, 103, 3837–3844. [Google Scholar] [CrossRef] [Green Version]
- Kamohara, H.; Matsuyama, W.; Shimozato, O.; Abe, K.; Galligan, C. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor expression in human neutrophils. Immunology 2004, 111, 186–194. [Google Scholar] [CrossRef]
- Zahn, S.; Rehkämper, C.; Ferring-Schmitt, S.; Bieber, T.; Tüting, T.; Wenzel, J. Interferon-α stimulates TRAIL expression in human keratinocytes and peripheral blood mononuclear cells: Implications for the pathogenesis of cutaneous lupus erythematosus. Br. J. Dermatol. 2011, 165, 1118–1123. [Google Scholar] [CrossRef]
- Fanger, N.A.; Maliszewski, C.R.; Schooley, K.; Griffith, T.S. Human Dendritic Cells Mediate Cellular Apoptosis via Tumor Necrosis Factor–related Apoptosis-inducing Ligand (TRAIL). J. Exp. Med. 1999, 190, 1155–1164. [Google Scholar] [CrossRef]
- Griffith, B.T.S.; Wiley, S.R.; Kubin, M.Z.; Sedger, L.M.; Maliszewski, C.R.; Fanger, N.A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor–related cytokine, TRAIL. J. Exp. Med. 1999, 189, 1343–1354. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Mariani, S.M.; Krammer, P.H. Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur. J. Immunol. 1998, 28, 1492–1498. [Google Scholar] [CrossRef]
- Mariani, S.M.; Krammer, P.H. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur. J. Immunol. 1998, 28, 973–982. [Google Scholar] [CrossRef]
- Wajant, H. CD95L/FasL and TRAIL in tumour surveillance and cancer therapy. In The Link Between Inflammation and Cancer; Part of the book series: Cancer Treatment and Research (CTAR, volume 130); Rosen, S.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 141–165. [Google Scholar]
- Kelley, S.K.; Ashkenazi, A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr. Opin. Pharmacol. 2004, 4, 333–339. [Google Scholar] [CrossRef]
- Smyth, M.J.; Takeda, K.; Hayakawa, Y.; Peschon, J.J.; Van Den Brink, M.R.; Yagita, H. Nature’s TRAIL—On a path to cancer immunotherapy. Immunity 2003, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Eckhardt, S.G.; Kurzrock, R.; Ebbinghaus, S.; O’Dwyer, P.J.; Gordon, M.S.; Novotny, W.; Goldwasser, M.A.; Tohnya, T.M.; Lum, B.L. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J. Clin. Oncol. 2010, 28, 2839–2846. [Google Scholar] [CrossRef]
- Singh, D.; Tewari, M.; Singh, S.; Narayan, G. Revisiting the role of TRAIL/TRAIL-R in cancer biology and therapy. Future Oncol. 2021, 17, 581–596. [Google Scholar] [CrossRef]
- Naka, T.; Sugamura, K.; Hylander, B.L.; Widmer, M.B.; Rustum, Y.M.; Repasky, E.A. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients’ colon tumors grown in SCID mice. Cancer Res. 2002, 62, 5800–5806. [Google Scholar]
- Ashkenazi, A.; Holland, P.; Eckhardt, S.G. Ligand-based targeting of apoptosis in cancer: The potential of recombinant human apoptosis ligand 2/tumor necrosis factor–related apoptosis-inducing ligand (rhApo2L/TRAIL). J. Clin. Oncol. 2008, 26, 3621–3630. [Google Scholar] [CrossRef]
- Stöhr, D.; Jeltsch, A.; Rehm, M. TRAIL receptor signaling: From the basics of canonical signal transduction toward its entanglement with ER stress and the unfolded protein response. Int. Rev. Cell Mol. Biol. 2020, 351, 57–99. [Google Scholar]
- Danish, L.; Stöhr, D.; Scheurich, P.; Pollak, N. TRAIL-R3/R4 and Inhibition of TRAIL Signalling in Cancer. In TRAIL, Fas Ligand, TNF and TLR3 in Cancer; Micheau, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 27–57. [Google Scholar]
- Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2002, 2, 420–430. [Google Scholar] [CrossRef]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Sun, S.Y.; Hail, N., Jr.; Lotan, R. Apoptosis as a novel target for cancer chemoprevention. J. Natl. Cancer Inst. 2004, 96, 662–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yang, Y.; Xing, D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011, 278, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.C.; Ní Chonghaile, T.; Gupta, S.; Samali, A. Bcl-2 family on guard at the ER. Am. J. Physiol. Cell Physiol. 2009, 296, C941–C953. [Google Scholar]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [Green Version]
- Cory, S.; Huang, D.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef] [Green Version]
- Denicourt, C.; Dowdy, S.F. Targeting apoptotic pathways in cancer cells. Science 2004, 305, 1411–1413. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.Y.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef] [Green Version]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [CrossRef]
- Strauss, G.; Westhoff, M.A.; Fischer-Posovszky, P.; Fulda, S.; Schanbacher, M.; Eckhoff, S.M.; Stahnke, K.; Vahsen, N.; Kroemer, G.; Debatin, K.-M. 4-hydroperoxy-cyclophosphamide mediates caspase-independent T-cell apoptosis involving oxidative stress-induced nuclear relocation of mitochondrial apoptogenic factors AIF and EndoG. Cell Death Differ. 2008, 15, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c–dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Elmallah, M.I.; Micheau, O. Marine drugs regulating apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Mar. Drugs 2015, 13, 6884–6909. [Google Scholar] [CrossRef] [Green Version]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–630. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1309. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.C. Apoptosis-targeted therapies for cancer. Cancer Cell 2003, 3, 17–22. [Google Scholar] [CrossRef]
- Hunter, A.M.; LaCasse, E.C.; Korneluk, R.G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 2007, 12, 1543–1568. [Google Scholar]
- Fu, T.M.; Li, Y.; Lu, A.; Li, Z.; Vajjhala, P.R.; Cruz, A.C.; Srivastava, D.B.; DiMaio, F.; Penczek, P.A.; Siegel, R.M.; et al. Cryo-EM structure of caspase-8 tandem DED filament reveals assembly and regulation mechanisms of the death-inducing signaling complex. Mol. Cell 2016, 64, 236–250. [Google Scholar] [CrossRef] [Green Version]
- Schleich, K.; Buchbinder, J.H.; Pietkiewicz, S.; Kähne, T.; Warnken, U.; Öztürk, S.; Schnölzer, M.; Naumann, M.; Krammer, P.H.; Lavrik, I.N. Molecular architecture of the DED chains at the DISC: Regulation of procaspase-8 activation by short DED proteins c-FLIP and procaspase-8 prodomain. Cell Death Differ. 2016, 23, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Dickens, L.S.; Boyd, R.S.; Jukes-Jones, R.; Hughes, M.A.; Robinson, G.L.; Fairall, L.; Schwabe, J.W.R.; Cain, K.; MacFarlane, M. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Mol. Cell 2012, 47, 291–305. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, L.M.; Fox, J.P.; Higgins, C.A.; Majkut, J.; Sessler, T.; McLaughlin, K.; McCann, C.; Roberts, J.Z.; Crawford, N.T.; McDade, S.S.; et al. A revised model of TRAIL-R2 DISC assembly explains how FLIP (L) can inhibit or promote apoptosis. EMBO Rep. 2020, 21, e49254. [Google Scholar] [CrossRef]
- Sessler, T.; Healy, S.; Samali, A.; Szegezdi, E. Structural determinants of DISC function: New insights into death receptor-mediated apoptosis signalling. Pharmacol. Ther. 2013, 140, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Eskes, R.; Desagher, S.; Antonsson, B.; Martinou, J.C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 2000, 20, 929–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özören, N.; El-Deiry, W.S. Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia 2002, 4, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnhart, B.C.; Alappat, E.C.; Peter, M.E. The CD95 type I/type II model. Semin. Immunol. 2003, 15, 185–193. [Google Scholar] [CrossRef]
- Algeciras-Schimnich, A.; Pietras, E.M.; Barnhart, B.C.; Legembre, P.; Vijayan, S.; Holbeck, S.L.; Peter, M.E. Two CD95 tumor classes with different sensitivities to antitumor drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 11445–11450. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.F.; Harris, T.J.; Tran, S.; Evangelista, M.; Arulananda, S.; John, T.; Ramnac, C.; Hobbs, C.; Zhu, H.; Gunasingh, G. BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell survival. Cell Death Dis. 2019, 10, 342. [Google Scholar] [CrossRef]
- Mukherjee, N.; Skees, J.; Todd, K.J.; West, D.A.; Lambert, K.A.; Robinson, W.A.; Amato, C.M.; Couts, K.L.; van Gulick, R.; MacBeth, M.; et al. MCL1 inhibitors S63845/MIK665 plus Navitoclax synergistically kill difficult-to-treat melanoma cells. Cell Death Dis. 2020, 11, 443. [Google Scholar] [CrossRef]
- Mukherjee, N.; Almeida, A.; Partyka, K.A.; Lu, Y.; Schwan, J.V.; Lambert, K.; Rogers, M.; Robinson, W.A.; Robinson, S.E.; Applegate, A.J.; et al. Combining a GSI and BCL-2 inhibitor to overcome melanoma’s resistance to current treatments. Oncotarget 2016, 7, 84594. [Google Scholar] [CrossRef] [Green Version]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer TherapeuticsBCL2 Family and Targeted Therapies. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, C.; Chen, C.; Zhuang, G. Correlation between expression of DcR3 on tumor cells and sensitivity to FasL. Cell. Mol. Immunol. 2007, 4, 455–460. [Google Scholar]
- Mocellin, S.; Rossi, C.R.; Pilati, P.; Nitti, D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005, 16, 35–53. [Google Scholar] [CrossRef]
- Eberle, J. Countering TRAIL resistance in melanoma. Cancers 2019, 11, 656. [Google Scholar] [CrossRef] [Green Version]
- Panaitiu, A.E.; Basiashvili, T.; Mierke, D.F.; Pellegrini, M. An engineered construct of cFLIP provides insight into DED1 structure and interactions. Structure 2022, 30, 229–239. [Google Scholar] [CrossRef]
- Hajra, K.M.; Liu, J.R. Apoptosome dysfunction in human cancer. Apoptosis 2004, 9, 691–704. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Riedl, S.J.; Renatus, M.; Schwarzenbacher, R.; Zhou, Q.; Sun, C.; Fesik, S.W.; Liddington, R.C.; Salvesen, G.S. Structural basis for the inhibition of caspase-3 by XIAP. Cell 2001, 104, 791–800. [Google Scholar] [CrossRef]
- Chai, J.; Shiozaki, E.; Srinivasula, S.M.; Wu, Q.; Dataa, P.; Alnemri, E.S.; Shi, Y. Structural basis of caspase-7 inhibition by XIAP. Cell 2001, 104, 769–780. [Google Scholar] [CrossRef]
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S. NF-κB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683. [Google Scholar] [CrossRef]
- Chen, C.; Edelstein, L.C.; Gélinas, C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol. Cell. Biol. 2000, 20, 2687–2695. [Google Scholar] [CrossRef]
- Zarnegar, B.J.; Wang, Y.; Mahoney, D.J.; Dempsey, P.W.; Cheung, H.H.; He, J.; Shiba, T.; Yang, X.; Yeh, W.; Mak, T.W.; et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 2008, 9, 1371–1378. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.L.; McKinsey, T.A.; Liu, L.; Gentry, J.J.; Malim, M.H.; Ballard, D.W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc. Natl. Acad. Sci. USA 1997, 94, 10057–10062. [Google Scholar] [CrossRef] [Green Version]
- Karin, M.; Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]
- Caliceti, P.; Veronese, F.M. Pharmacokinetic and biodistribution properties of poly (ethylene glycol)–protein conjugates. Adv. Drug Deliv. Rev. 2003, 55, 1261–1277. [Google Scholar] [CrossRef]
- Seifert, O.; Plappert, A.; Fellermeier, S.; Siegemund, M.; Pfizenmaier, K.; Kontermann, R.E. Tetravalent Antibody–scTRAIL Fusion Proteins with Improved PropertiesTetravalent Fusion Proteins. Mol. Cancer Ther. 2014, 13, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Rozga, P.; Kloska, D.; Pawlak, S.; Teska-Kaminska, M.; Galazka, M.; Bukato, K.; Pieczykolan, A.; Jaworski, A.; Molga-Kaczmarska, A.; Kopacz, A.; et al. Novel engineered TRAIL-based chimeric protein strongly inhibits tumor growth and bypasses TRAIL resistance. Int. J. Cancer 2020, 147, 1117–1130. [Google Scholar] [CrossRef]
- Yan, C.; Li, S.; Li, Z.; Peng, H.; Yuan, X.; Jiang, L.; Zhang, Y.; Fan, D.; Hu, X.; Yang, M.; et al. Human umbilical cord mesenchymal stem cells as vehicles of CD20-specific TRAIL fusion protein delivery: A double-target therapy against non-Hodgkin’s lymphoma. Mol. Pharm. 2013, 10, 142–151. [Google Scholar] [CrossRef]
- Trebing, J.; El-Mesery, M.; Schäfer, V.; Weisenberger, D.; Siegmund, D.; Silence, K.; Wajant, H. CD70-restricted specific activation of TRAILR1 or TRAILR2 using scFv-targeted TRAIL mutants. Cell Death Dis. 2014, 5, e1035. [Google Scholar] [CrossRef] [Green Version]
- Müller, N.; Schneider, B.; Pfizenmaier, K.; Wajant, H. Superior serum half life of albumin tagged TNF ligands. Biochem. Biophys. Res. Commun. 2010, 396, 793–799. [Google Scholar] [CrossRef]
- Hendriks, D.; Choi, G.; de Bruyn, M.; Wiersma, V.R.; Bremer, E. Antibody-based cancer therapy: Successful agents and novel approaches. Int. Rev. Cell Mol. Biol. 2017, 331, 289–383. [Google Scholar]
- Kholodenko, R.V.; Kalinovsky, D.V.; Doronin, I.I.; Ponomarev, E.D.; Kholodenko, I.V. Antibody fragments as potential biopharmaceuticals for cancer therapy: Success and limitations. Curr. Med. Chem. 2019, 26, 396–426. [Google Scholar] [CrossRef]
- Madhumathi, J.; Sridevi, S.; Verma, R.S. Novel TNF-related apoptotic-inducing ligand-based immunotoxin for therapeutic targeting of CD25 positive leukemia. Target. Oncol. 2016, 11, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Gerspach, J.; Pfizenmaier, K. Engineering death receptor ligands for cancer therapy. Cancer Lett. 2013, 332, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Assohou-Luty, C.; Gerspach, J.; Siegmund, D.; Müller, N.; Huard, B.; Tiegs, G.; Pfizenmaier, K.; Wajant, H. A CD40–CD95L fusion protein interferes with CD40L-induced prosurvival signaling and allows membrane CD40L-restricted activation of CD95. J. Mol. Med. 2006, 84, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Bremer, E.; van Dam, G.M.; de Bruyn, M.; van Riezen, M.; Dijkstra, M.; Kamps, G.; Helfrich, W.; Haisma, H. Potent systemic anticancer activity of adenovirally expressed EGFR-selective TRAIL fusion protein. Mol. Ther. 2008, 16, 1919–1926. [Google Scholar] [CrossRef] [Green Version]
- Stieglmaier, J.; Bremer, E.; Kellner, C.; Liebig, T.M.; Cate, B.T.; Peipp, M.; Schulze-Koops, H.; Pfeiffer, M.; Bühring, H.-J.; Greil, J.; et al. Selective induction of apoptosis in leukemic B-lymphoid cells by a CD19-specific TRAIL fusion protein. Cancer Immunol. Immunother. 2008, 57, 233–246. [Google Scholar] [CrossRef]
- Li, R.; Yang, H.; Jia, D.; Nie, Q.; Cai, H.; Fan, Q.; Wan, L.; Li, L.; Lu, X. Fusion to an albumin-binding domain with a high affinity for albumin extends the circulatory half-life and enhances the in vivo antitumor effects of human TRAIL. J. Control. Release 2016, 228, 96–106. [Google Scholar] [CrossRef]
- Byeon, H.J.; Min, S.Y.; Kim, I.; Lee, E.S.; Oh, K.T.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Human serum albumin-TRAIL conjugate for the treatment of rheumatoid arthritis. Bioconjug. Chem. 2014, 25, 2212–2221. [Google Scholar] [CrossRef]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Brin, E.; Wu, K.; Dagostino, E.; Kuo, M.M.C.; He, Y.; Shia, W.J.; Chen, L.C.; Stempniak, M.; Hickey, R.; Almassy, R.; et al. TRAIL stabilization and cancer cell sensitization to its pro-apoptotic activity achieved through genetic fusion with arginine deiminase. Oncotarget 2018, 9, 36914. [Google Scholar] [CrossRef]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Naval, J.; de Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Importance of TRAIL molecular anatomy in receptor oligomerization and signaling. Implications for cancer therapy. Cancers 2019, 11, 444. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yu, B.; Wang, B.; Yan, J.; Feng, X.; Wang, Z.; Wang, L.; Zhang, H.; Wu, H.; Wu, J.; et al. A novel capsid-modified oncolytic recombinant adenovirus type 5 for tumor-targeting gene therapy by intravenous route. Oncotarget 2016, 7, 47287. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, W.; Wang, L.; Gao, P.; Li, Z.; Wu, J.; Zhang, H.; Wu, H.; Kong, W.; Yu, B.; et al. Enhancing the antitumor activity of an engineered TRAIL-coated oncolytic adenovirus for treating acute myeloid leukemia. Signal Transduct. Target. Ther. 2020, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, H.; Dou, G.; Li, J.; Zhang, X.; Jiang, M.; Li, P.; Huang, X.; Chen, H.; Li, L.; et al. Ginsenoside 20 (S)-Rh2 induces apoptosis and differentiation of acute myeloid leukemia cells: Role of orphan nuclear receptor Nur77. J. Agric. Food Chem. 2017, 65, 7687–7697. [Google Scholar] [CrossRef]
- Le, D.H.; Commandeur, U.; Steinmetz, N.F. Presentation and delivery of tumor necrosis factor-related apoptosis-inducing ligand via elongated plant viral nanoparticle enhances antitumor efficacy. ACS Nano 2019, 13, 2501–2510. [Google Scholar] [CrossRef]
- Lee, K.L.; Murray, A.A.; Le, D.H.T.; Sheen, M.R.; Shukla, S.; Commandeur, U.; Fiering, S.; Steinmetz, N.F. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Lett. 2017, 17, 4019–4028. [Google Scholar] [CrossRef] [Green Version]
- Le, D.H.; Lee, K.L.; Shukla, S.; Commandeur, U.; Steinmetz, N.F. Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale 2017, 9, 2348–2357. [Google Scholar] [CrossRef]
- Le, D.H.; Hu, H.; Commandeur, U.; Steinmetz, N.F. Chemical addressability of potato virus X for its applications in bio/nanotechnology. J. Struct. Biol. 2017, 200, 360–368. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [Green Version]
- Menon, L.G.; Kelly, K.; Yang, H.W.; Kim, S.; Black, P.M.; Carroll, R.S. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells 2009, 27, 2320–2330. [Google Scholar] [CrossRef]
- Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Golinelli, G.; Grisendi, G.; Prapa, M.; Bestagno, M.; Spano, C.; Rossignoli, F.; Bambi, F.; Sardi, I.; Cellini, M.; Horwitz, E.M.; et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2020, 27, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sharkey, C.C.; Wun, B.; Liesveld, J.L.; King, M.R. Genetic engineering of platelets to neutralize circulating tumor cells. J. Control. Release 2016, 228, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Kauer, T.M.; Figueiredo, J.L.; Hingtgen, S.; Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat. Neurosci. 2012, 15, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Labusca, L.; Herea, D.D.; Mashayekhi, K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World J. Stem Cells 2018, 10, 43. [Google Scholar] [CrossRef]
- Kawada, H.; Fujita, J.; Kinjo, K.; Matsuzaki, Y.; Tsuma, M.; Miyatake, H.; Muguruma, Y.; Tsuboi, K.; Itabashi, Y.; Ikeda, Y.; et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004, 104, 3581–3587. [Google Scholar] [CrossRef]
- Choi, Y.U.; Yoon, Y.; Jung, P.Y.; Hwang, S.; Hong, J.E.; Kim, W.-S.; Sohn, J.H.; Rhee, K.-J.; Bae, K.S.; Eom, Y.W. TRAIL-Overexpressing Adipose Tissue-Derived Mesenchymal Stem Cells Efficiently Inhibit Tumor Growth in an H460 Xenograft Model. Cancer Genom. Proteom. 2021, 18, 569–578. [Google Scholar] [CrossRef]
- Yoon, N.; Park, M.S.; Peltier, G.C.; Lee, R.H. Pre-activated human mesenchymal stromal cells in combination with doxorubicin synergistically enhance tumor-suppressive activity in mice. Cytotherapy 2015, 17, 1332–1341. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.J.; Park, S.H.; Yang, H.G.; Kang, M.C.; Choi, Y.W.; Kim, S.M.; Jeun, S.-S.; Sung, Y.C. Complete Regression of Metastatic Renal Cell Carcinoma by Multiple Injections of Engineered Mesenchymal Stem Cells Expressing Dodecameric TRAIL and HSV-TKComplete Cure of Metastatic Tumor by Engineered MSCs. Clin. Cancer Res. 2013, 19, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Redjal, N.; Zhu, Y.; Shah, K. Combination of Systemic Chemotherapy with Local Stem Cell Delivered S-TRAIL in Resected Brain Tumors. Stem Cells 2015, 33, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Woo, J.S.; Jeong, C.H.; Ryu, C.H.; Jang, J.D.; Jeun, S.S. Potential application of temozolomide in mesenchymal stem cell-based TRAIL gene therapy against malignant glioma. Stem Cells Transl. Med. 2014, 3, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lim, J.Y.; Park, S.I.; Jeong, C.H.; Oh, J.H.; Jeong, M.; Oh, W.; Park, S.H.; Sung, Y.C.; Jeun, S.-S. Gene therapy using TRAIL-secreting human umbilical cord blood–derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008, 68, 9614–9623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Shan, H.; Li, D.; Li, Z.R.; Zhu, K.S.; Jiang, Z.B. The inhibitory effect of MSCs expressing TRAIL as a cellular delivery vehicle in combination with cisplatin on hepatocellular carcinoma. Cancer Biol. Ther. 2012, 13, 1175–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grisendi, G.; Bussolari, R.; Cafarelli, L.; Petak, I.; Rasini, V.; Veronesi, E.; de Santis, G.; Spano, C.; Tagliazzucchi, M.; Barti-Juhasz, H.; et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor–related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010, 70, 3718–3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, L.P.; Luetzkendorf, J.; Widder, M.; Nerger, K.; Caysa, H.; Mueller, T. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2011, 18, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Albarenque, S.M.; Deedigan, L.; Yu, R.; Reidy, M.; Fulda, S.; Zwacka, R.M. Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells 2010, 28, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.S. Usage of human mesenchymal stem cells in cell-based therapy: Advantages and disadvantages. Dev. Reprod. 2017, 21, 1. [Google Scholar] [CrossRef]
- Nishikawa, S.; Goldstein, R.A.; Nierras, C.R. The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 2008, 9, 725–729. [Google Scholar] [CrossRef]
- Kimbrel, E.A.; Lanza, R. Next-generation stem cells—Ushering in a new era of cell-based therapies. Nat. Rev. Drug Discov. 2020, 19, 463–479. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Cell-mediated delivery of nanoparticles: Taking advantage of circulatory cells to target nanoparticles. J. Control. Release 2014, 190, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Jain, R.K.; Langer, R. Engineering and physical sciences in oncology: Challenges and opportunities. Nat. Rev. Cancer 2017, 17, 659–675. [Google Scholar] [CrossRef]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popović, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [Green Version]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Kih, M.; Lee, E.J.; Lee, N.K.; Kim, Y.K.; Lee, K.E.; Jeong, C.; Yang, Y.; Kim, D.H.; Kim, I.S. Designed trimer-mimetic TNF superfamily ligands on self-assembling nanocages. Biomaterials 2018, 180, 67–77. [Google Scholar] [CrossRef]
- De Miguel, D.; Gallego-Lleyda, A.; Ayuso, J.M.; Pejenaute-Ochoa, D.; Jarauta, V.; Marzo, I.; Fernández, L.J.; Ochoa, I.; Conde, B.; Anel, A.; et al. High-order TRAIL oligomer formation in TRAIL-coated lipid nanoparticles enhances DR5 cross-linking and increases antitumour effect against colon cancer. Cancer Lett. 2016, 383, 250–260. [Google Scholar] [CrossRef]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. USA 2016, 113, 13857–13862. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, D.; Gallego-Lleyda, A.; Ayuso, J.M.; Erviti-Ardanaz, S.; Pazo-Cid, R.; del Agua, C.; Fernández, L.J.; Ochoa, I.; Anel, A.; Martinez-Lostao, L. TRAIL-coated lipid-nanoparticles overcome resistance to soluble recombinant TRAIL in non-small cell lung cancer cells. Nanotechnology 2016, 27, 185101. [Google Scholar] [CrossRef]
- De Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Rozanov, D.V.; Savinov, A.Y.; Golubkov, V.S.; Rozanova, O.L.; Postnova, T.I.; Sergienko, E.A.; Vasile, S.; Aleshin, A.E.; Rega, M.F.; Pellecchia, M.; et al. Engineering a leucine zipper-TRAIL homotrimer with improved cytotoxicity in tumor cells. Mol. Cancer Ther. 2009, 8, 1515–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.J.; Hsu, S. TRAIL-functionalized gold nanoparticles selectively trigger apoptosis in polarized macrophages. Nanotheranostics 2017, 1, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkahla, H.; Haque, A.; Revzin, A.; Gharbi, T.; Constantinescu, A.A.; Micheau, O.; Hémadi, M.; Ammar, S. Coupling tumor necrosis factor-related apoptosis-inducing ligand to iron oxide nanoparticles increases its apoptotic activity on HCT116 and HepG2 malignant cells: Effect of magnetic core size. J. Interdiscip. Nanomed. 2019, 4, 34–50. [Google Scholar] [CrossRef]
- Ke, S.; Zhou, T.; Yang, P.; Wang, Y.; Zhang, P.; Chen, K.; Ren, L.; Ye, S. Gold nanoparticles enhance TRAIL sensitivity through Drp1-mediated apoptotic and autophagic mitochondrial fission in NSCLC cells. Int. J. Nanomed. 2017, 12, 2531. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jeong, D.; Kang, H.E.; Lee, K.C.; Na, K. A sulfate polysaccharide/TNF-related apoptosis-inducing ligand (TRAIL) complex for the long-term delivery of TRAIL in poly (lactic-co-glycolic acid)(PLGA) microspheres. J. Pharm. Pharmacol. 2013, 65, 11–21. [Google Scholar] [CrossRef]
- Chen, Z.; Penet, M.F.; Krishnamachary, B.; Banerjee, S.R.; Pomper, M.G.; Bhujwalla, Z.M. PSMA-specific theranostic nanoplex for combination of TRAIL gene and 5-FC prodrug therapy of prostate cancer. Biomaterials 2016, 80, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Li, J.; Han, L.; Liu, S.; Ma, H.; Huang, R.; Jiang, C. Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials 2011, 32, 6832–6838. [Google Scholar] [CrossRef]
- Hu, C.; Gu, F.; Tai, Z.; Yao, C.; Gong, C.; Xia, Q.; Gao, Y.; Gao, S. Synergistic effect of reduced polypeptide micelle for co-delivery of doxorubicin and TRAIL against drug-resistance in breast cancer. Oncotarget 2016, 7, 61832. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.M.; Flores, H.; Gogineni, A.; Marsters, S.; Lawrence, D.A.; Kelley, R.F.; Ngu, H.; Sagolla, M.; Komuves, L.; Bourgon, R.; et al. Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display. Proc. Natl. Acad. Sci. USA 2015, 112, 5679–5684. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Lleyda, A.; de Miguel, D.; Anel, A.; Martinez-Lostao, L. Lipid nanoparticles decorated with TNF-related aptosis-inducing ligand (TRAIL) are more cytotoxic than soluble recombinant TRAIL in sarcoma. Int. J. Mol. Sci. 2018, 19, 1449. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, D.; Basáñez, G.; Sánchez, D.; Malo, P.G.; Marzo, I.; Larrad, L.; Naval, J.; Pardo, J.; Anel, A.; Martinez-Lostao, L. Liposomes decorated with Apo2L/TRAIL overcome chemoresistance of human hematologic tumor cells. Mol. Pharm. 2013, 10, 893–904. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.-H.; Youn, Y.S.; Park, C.W.; Lim, S.M.; Jin, C.-H.; Tak, K.K.; Lee, H.S.; Lee, K.C. Preparation and characterization of Apo2L/TNF-related apoptosis-inducing ligand–loaded human serum albumin nanoparticles with improved stability and tumor distribution. J. Pharm. Sci. 2011, 100, 482–491. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Wang, A.; Li, Q.; Cui, W. Supramolecular assembly of protein-based nanoparticles based on tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) for cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124486. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chen, G.Y.; Chang, C.H.; Su, Y.C.; Chen, Y.C.; Jiang, Y.; Jan, J.S. TRAIL encapsulated to polypeptide-crosslinked nanogel exhibits increased anti-inflammatory activities in Klebsiella pneumoniae-induced sepsis treatment. Mater. Sci. Eng. C 2019, 102, 85–95. [Google Scholar] [CrossRef]
- Arroyo, N.; Herlem, G.; Picaud, F. Ligand nanovectorization using graphene to target cellular death receptors of cancer cell. Proteins Struct. Funct. Bioinform. 2020, 88, 94–105. [Google Scholar] [CrossRef]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zhang, Y.; Feng, N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Mater. Sci. Eng. C 2020, 106, 110298. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Zeng, X.; Zhang, X.Z. Cell primitive-based biomimetic functional materials for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50, 945–985. [Google Scholar] [CrossRef]
- Dash, P.; Piras, A.M.; Dash, M. Cell membrane coated nanocarriers-an efficient biomimetic platform for targeted therapy. J. Control. Release 2020, 327, 546–570. [Google Scholar] [CrossRef]
- Liu, W.; Yan, Q.; Xia, C.; Wang, X.; Kumar, A.; Wang, Y.; Liu, Y.; Pan, Y.; Liu, J. Recent advances in cell membrane coated metal–organic frameworks (MOFs) for tumor therapy. J. Mater. Chem. B 2021, 9, 4459–4474. [Google Scholar] [CrossRef]
- Li, J.; Ai, Y.; Wang, L.; Bu, P.; Sharkey, C.C.; Wu, Q.; Wun, B.; Roy, S.; Shen, X.; King, M.R. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials 2016, 76, 52–65. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Chang, C.H.; Pauklin, S. Extracellular vesicles in pancreatic cancer progression and therapies. Cell Death Dis. 2021, 12, 973. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, J.N.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Q.; Huyan, T.; Wang, Y.; Huang, Q.; Shi, J. Bifacial effects of engineering tumour cell-derived exosomes on human natural killer cells. Exp. Cell Res. 2018, 363, 141–150. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A system of cytokines encapsulated in extracellular vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.; Steadman, R.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010, 70, 9621–9630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Poggio, M.; Hu, T.; Pai, C.-C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019, 177, 414–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef] [Green Version]
- Andreola, G.; Rivoltini, L.; Castelli, C.; Huber, V.; Perego, P.; Deho, P.; Squarcina, P.; Accornero, P.; Lozupone, F.; Lugini, L.; et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 2002, 195, 1303–1316. [Google Scholar] [CrossRef] [Green Version]
- Abusamra, A.J.; Zhong, Z.; Zheng, X.; Li, M.; Ichim, T.E.; Chin, J.L.; Min, W.P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005, 35, 169–173. [Google Scholar] [CrossRef]
- Rivoltini, L.; Chiodoni, C.; Squarcina, P.; Tortoreto, M.; Villa, A.; Vergani, B.; Bürdek, M.; Botti, L.; Arioli, I.; Cova, A.; et al. TNF-related apoptosis-inducing ligand (TRAIL)–armed exosomes deliver proapoptotic signals to tumor site. Clin. Cancer Res. 2016, 22, 3499–3512. [Google Scholar] [CrossRef] [Green Version]
- Shamili, F.H.; Bayegi, H.R.; Salmasi, Z.; Sadri, K.; Mahmoudi, M.; Kalantari, M.; Ramezani, M.; Abnous, K. Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int. J. Pharm. 2018, 549, 218. [Google Scholar] [CrossRef]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.C.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef] [Green Version]
- Setroikromo, R.; Zhang, B.; Reis, C.R.; Mistry, R.H.; Quax, W.J. Death Receptor 5 Displayed on Extracellular Vesicles Decreases TRAIL Sensitivity of Colon Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 318. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Ho, E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J. Control. Release 2020, 329, 894–906. [Google Scholar] [CrossRef]
- Gutierrez-Millan, C.; Díaz, C.C.; Lanao, J.M.; Colino, C.I. Advances in Exosomes-Based Drug Delivery Systems. Macromol. Biosci. 2020, 21, 2000269. [Google Scholar] [CrossRef]
- Leggio, L.; Arrabito, G.; Ferrara, V.; Vivarelli, S.; Paternò, G.; Marchetti, B.; Pignataro, B.; Iraci, N. Mastering the Tools: Natural versus Artificial Vesicles in Nanomedicine. Adv. Healthc. Mater. 2020, 9, 2000731. [Google Scholar] [CrossRef]
- Glantz, M.J.; Jaeckle, K.A.; Chamberlain, M.C.; Phuphanich, S.; Recht, L.; Swinnen, L.J.; Maria, B.; LaFollette, S.; Schumann, G.B.; Cole, B.F.; et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 1999, 5, 3394–3402. [Google Scholar]
- Judson, I.; Radford, J.A.; Harris, M.; Blay, J.Y.; van Hoesel, Q.; le Cesne, A.; van Oosterom, A.T.; Clemons, M.J.; Kamby, C.; Hermans, C.; et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL®/CAELYX®) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer 2001, 37, 870–877. [Google Scholar] [CrossRef]
- Van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238-IN27. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660-IN10. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, P.P.G.; Gaglione, S.; Sewastianik, T.; Carrasco, R.D.; Langer, R.; Mitchell, M.J. Nanoparticles for immune cytokine TRAIL-based cancer therapy. ACS Nano 2018, 12, 912–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Liposome-bound TRAIL induces superior DR5 clustering and enhanced DISC recruitment in histiocytic lymphoma U937 cells. Leuk. Res. 2015, 39, 657–666. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Shao, N.; Hu, Z.; Chen, H.; Xu, L.; Wang, C.; Cheng, Y.; Xiao, J. Triazine-modified dendrimer for efficient TRAIL gene therapy in osteosarcoma. Acta Biomater. 2015, 17, 115–124. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Park, C.W.; Youn, Y.S.; Lee, S.; Chen, X.; Lee, K.C. PEGylated TNF-related apoptosis-inducing ligand (TRAIL)-loaded sustained release PLGA microspheres for enhanced stability and antitumor activity. J. Control. Release 2011, 150, 63–69. [Google Scholar] [CrossRef]
- Zakaria, A.B.; Picaud, F.; Rattier, T.; Pudlo, M.; Saviot, L.; Chassagnon, R.; Lherminier, J.; Gharbi, T.; Micheau, O.; Herlem, G. Nanovectorization of TRAIL with single wall carbon nanotubes enhances tumor cell killing. Nano Lett. 2015, 15, 891–895. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [CrossRef]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef] [Green Version]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Platt, V.M.; Szoka, F.C., Jr. Anticancer therapeutics: Targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol. Pharm. 2008, 5, 474–486. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Wayne, E.; Rana, K.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. USA 2014, 111, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Nimrichter, L.; Burdick, M.M.; Aoki, K.; Laroy, W.; Fierro, M.A.; Hudson, S.A.; von Seggern, C.E.; Cotter, R.J.; Bochner, B.S.; Tiemeyer, M.; et al. E-selectin receptors on human leukocytes, Blood. J. Am. Soc. Hematol. 2008, 112, 3744–3752. [Google Scholar]
- Larsen, G.R.; Sako, D.; Ahern, T.J.; Shaffer, M.; Erban, J.; Sajer, S.A.; Gibson, R.M.; Wagner, D.D.; Furie, B.C.; Furie, B. P-selectin and E-selectin. Distinct but overlapping leukocyte ligand specificities. J. Biol. Chem. 1992, 267, 11104–11110. [Google Scholar] [CrossRef]
- Wayne, E.C.; Chandrasekaran, S.; Mitchell, M.J.; Chan, M.F.; Lee, R.E.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer. J. Control. Release 2016, 223, 215–223. [Google Scholar]
- Jyotsana, N.; Zhang, Z.; Himmel, L.E.; Yu, F.; King, M.R. Minimal dosing of leukocyte targeting TRAIL decreases triple-negative breast cancer metastasis following tumor resection. Sci. Adv. 2019, 5, eaaw4197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, S.; McGuire, M.J.; King, M.R. Sweeping lymph node micrometastases off their feet: An engineered model to evaluate natural killer cell mediated therapeutic intervention of circulating tumor cells that disseminate to the lymph nodes. Lab Chip 2014, 14, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Chan, M.F.; Li, J.; King, M.R. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 2016, 77, 66–76. [Google Scholar] [PubMed] [Green Version]
- O’Reilly, E.; Tirincsi, A.; Logue, S.E.; Szegezdi, E. The Janus face of death receptor signaling during tumor immunoediting. Front. Immunol. 2016, 7, 446. [Google Scholar]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar]
- Seifert, O.; Pollak, N.; Nusser, A.; Steiniger, F.; Rüger, R.; Pfizenmaier, K.; Kontermann, R.E. Immuno-LipoTRAIL: Targeted delivery of TRAIL-functionalized liposomal nanoparticles. Bioconjug. Chem. 2014, 25, 879–887. [Google Scholar]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Tebbutt, N.; Pedersen, M.W.; Johns, T.G. Targeting the ERBB family in cancer: Couples therapy. Nat. Rev. Cancer 2013, 13, 663–673. [Google Scholar] [CrossRef]
- Choi, S.H.; Byeon, H.J.; Choi, J.S.; Thao, L.; Kim, I.; Lee, E.S.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J. Control. Release 2015, 197, 199–207. [Google Scholar] [CrossRef]
- Bae, S.; Ma, K.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Park, E.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials 2012, 33, 1536–1546. [Google Scholar] [CrossRef]
- Sun, X.; Pang, Z.; Ye, H.; Qiu, B.; Guo, L.; Li, J.; Ren, J.; Qian, Y.; Zhang, Q.; Chen, J.; et al. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials 2012, 33, 916–924. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer platelet-mimicking nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef]
- Chen, K.; Cao, X.; Li, M.; Su, Y.; Li, H.; Xie, M.; Zhang, Z.; Gao, H.; Xu, X.; Han, Y.; et al. A TRAIL-delivered lipoprotein-bioinspired nanovector engineering stem cell-based platform for inhibition of lung metastasis of melanoma. Theranostics 2019, 9, 2984. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, L.; Liu, M.; Lu, W.; Gao, C. Peptide GE11–polyethylene glycol–polyethylenimine for targeted gene delivery in laryngeal cancer. Med. Oncol. 2015, 32, 185. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; He, Y.; Li, Y. Sandwich-type Au-PEI/DNA/PEI-Dexa nanocomplex for nucleus-targeted gene delivery in vitro and in vivo. ACS Appl. Mater. Interfaces 2014, 6, 14196–14206. [Google Scholar] [CrossRef]
- Kim, Y.J.; Chae, S.Y.; Jin, C.H.; Sivasubramanian, M.; Son, S.; Choi, K.Y.; Jo, D.G.; Kim, K.; Kwon, I.C.; Lee, K.C.; et al. Ionic complex systems based on hyaluronic acid and PEGylated TNF-related apoptosis-inducing ligand for treatment of rheumatoid arthritis. Biomaterials 2010, 31, 9057–9064. [Google Scholar] [CrossRef]
- Jiang, T.; Mo, R.; Bellotti, A.; Zhou, J.; Gu, Z. Gel–liposome-mediated co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv. Funct. Mater. 2014, 24, 2295–2304. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Pasquale, N.J.; Garbuzenko, O.B.; Minko, T.; Lee, K.B. Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials 2016, 81, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Na, K. Transfection of the TRAIL gene into human mesenchymal stem cells using biocompatible polyethyleneimine carbon dots for cancer gene therapy. J. Ind. Eng. Chem. 2019, 80, 722–728. [Google Scholar] [CrossRef]
- Kim, I.; Choi, J.S.; Lee, S.; Byeon, H.J.; Lee, E.S.; Shin, B.S.; Choi, H.-G.; Lee, K.C.; Youn, Y.S. In situ facile-forming PEG cross-linked albumin hydrogels loaded with an apoptotic TRAIL protein. J. Control. Release 2015, 214, 30–39. [Google Scholar] [CrossRef]
- Huang, R.Y.; Lin, Y.H.; Lin, S.Y.; Li, Y.N.; Chiang, C.S.; Chang, C.W. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics 2019, 9, 2411. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Kuang, Y.; An, S.; Ma, H.; Jiang, C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials 2013, 34, 9142–9148. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Xu, T.; Kong, Q.; Zhang, Y.; Shen, Y.; Wei, Y.; Wang, G.; Chang, K.J. Overcoming resistance to TRAIL-induced apoptosis in solid tumor cells by simultaneously targeting death receptors, c-FLIP and IAPs. Int. J. Oncol. 2016, 49, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Kretz, A.-L.; Trauzold, A.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; von Karstedt, S.; Lemke, J. TRAILblazing strategies for cancer treatment. Cancers 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, D.; Shah, K. TRAIL of hope meeting resistance in cancer. Trends Cancer 2020, 6, 989–1001. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Immune escape of tumors: Apoptosis resistance and tumor counterattack. J. Leukoc. Biol. 2002, 71, 907–920. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005, 12, 228–237. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Q.; Wang, L.; Chen, W.; Li, N.; Cao, X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol. Immunol. 2007, 44, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Song, G.; Liu, T.; He, B.; Zhang, H.; Wang, X.; Zhang, Q. Combination antitumor therapy with targeted dual-nanomedicines. Adv. Drug Deliv. Rev. 2017, 115, 23–45. [Google Scholar] [CrossRef]
- Guo, L.; Fan, L.; Ren, J.; Pang, Z.; Ren, Y.; Li, J.; Wen, Z.; Jiang, X. A novel combination of TRAIL and doxorubicin enhances antitumor effect based on passive tumor-targeting of liposomes. Nanotechnology 2011, 22, 265105. [Google Scholar] [CrossRef]
- Jiang, H.H.; Kim, T.H.; Lee, S.; Chen, X.; Youn, Y.S.; Lee, K.C. PEGylated TNF-related apoptosis-inducing ligand (TRAIL) for effective tumor combination therapy. Biomaterials 2011, 32, 8529–8537. [Google Scholar] [CrossRef]
- Kim, I.; Byeon, H.J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded porous PLGA microparticles with surface attached TRAIL for the inhalation treatment of metastatic lung cancer. Biomaterials 2013, 34, 6444–6453. [Google Scholar] [CrossRef]
- Alvizo-Baez, C.A.; Luna-Cruz, I.E.; Vilches-Cisneros, N.; Rodríguez-Padilla, C.; Alcocer-González, J.M. Systemic delivery and activation of the TRAIL gene in lungs, with magnetic nanoparticles of chitosan controlled by an external magnetic field. Int. J. Nanomed. 2016, 11, 6449. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Cui, Y.; Zhao, J.; Li, J. Fabrication of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)/ALG modified CaCO3 as drug carriers with the function of tumor selective recognition. J. Mater. Chem. B 2013, 1, 1326–1332. [Google Scholar] [CrossRef]
- Perlstein, B.; Finniss, S.A.; Miller, C.; Okhrimenko, H.; Kazimirsky, G.; Cazacu, S.; Lee, H.K.; Lemke, N.; Brodie, S.; Umansky, F.; et al. TRAIL conjugated to nanoparticles exhibits increased anti-tumor activities in glioma cells and glioma stem cells in vitro and in vivo. Neuro-Oncol. 2013, 15, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, R.W.; Frew, A.J.; Smyth, M.J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 2008, 8, 782–798. [Google Scholar] [CrossRef]
- Ediriwickrema, A.; Saltzman, W.M. Nanotherapy for cancer: Targeting and multifunctionality in the future of cancer therapies. ACS Biomater. Sci. Eng. 2015, 1, 64–78. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Feng, C.; Han, X.; Chi, L.; Sun, J.; Gong, F.; Shen, Y. Synthesis, characterization, and in vitro evaluation of TRAIL-modified, cabazitaxel-loaded polymeric micelles for achieving synergistic anticancer therapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1729–1744. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today 2017, 22, 314–326. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Y.; Huang, R.; Li, J.; Huang, S.; Kuang, Y.; Han, L.; Jiang, C. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials 2012, 33, 4907–4916. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Moos, T. Revisiting nanoparticle technology for blood–brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J. Control. Release 2016, 222, 32–46. [Google Scholar] [CrossRef]
- Pang, Z.; Gao, H.; Yu, Y.; Chen, J.; Guo, L.; Ren, J.; Wen, Z.; Su, J.; Jiang, X. Brain delivery and cellular internalization mechanisms for transferrin conjugated biodegradable polymersomes. Int. J. Pharm. 2011, 415, 284–292. [Google Scholar] [CrossRef]
- Pishavar, E.; Ramezani, M.; Hashemi, M. Co-delivery of doxorubicin and TRAIL plasmid by modified PAMAM dendrimer in colon cancer cells, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2019, 45, 1931–1939. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Chen, H.; Liu, H.; Zhang, Q.; Cheng, Y. Fluorinated dendrimer for TRAIL gene therapy in cancer treatment. J. Mater. Chem. B 2016, 4, 1354–1360. [Google Scholar] [CrossRef]
- Merten, M.; Thiagarajan, P. P-selectin in arterial thrombosis. Z. Kardiol. 2004, 93, 855–863. [Google Scholar] [CrossRef]
- Li, L.; Ding, Q.; Zhou, J.; Wu, Y.; Zhang, M.; Guo, X.; Long, M.; Lü, S. Distinct binding kinetics of E-, P-and L-selectins to CD44. FEBS J. 2021, 289, 2877–2894. [Google Scholar] [CrossRef]
- Suryaprakash, S.; Lao, Y.-H.; Cho, H.-Y.; Li, M.; Ji, H.Y.; Shao, D.; Hu, H.; Quek, C.H.; Huang, D.; Mintz, R.L.; et al. Engineered mesenchymal stem cell/nanomedicine spheroid as an active drug delivery platform for combinational glioblastoma therapy. Nano Lett. 2019, 19, 1701–1705. [Google Scholar]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Li, F.; Zhang, L.; Liu, W.; Wang, X.; Zhu, R.; Qiao, Z.-A.; Yu, B.; Yu, X. TRAIL-modified, doxorubicin-embedded periodic mesoporous organosilica nanoparticles for targeted drug delivery and efficient antitumor immunotherapy. Acta Biomater. 2022, 143, 392–405. [Google Scholar]
- De Miguel, D.; Gallego-Lleyda, A.; Martinez-Ara, M.; Plou, J.; Anel, A.; Martinez-Lostao, L. Double-edged lipid nanoparticles combining liposome-bound TRAIL and encapsulated doxorubicin showing an extraordinary synergistic pro-apoptotic potential. Cancers 2019, 11, 1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Patel, S.B.; King, M.R. Micelle-in-Liposomes for Sustained Delivery of Anticancer Agents That Promote Potent TRAIL-Induced Cancer Cell Apoptosis. Molecules 2021, 26, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Liang, X.; Zhang, J.; Tao, W.; Zhu, X.; Chang, D.; Zeng, X.; Liu, G.; Mei, L. Iron oxide nanoparticles induce autophagosome accumulation through multiple mechanisms: Lysosome impairment, mitochondrial damage, and ER stress. Mol. Pharm. 2016, 13, 2578–2587. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yang, L.X.; Shi, X.Y.; Li, I.C.; Biazik, J.M.; Ratinac, K.R.; Chen, D.H.; Thordarson, P.; Shieh, D.B.; Braet, F. The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 2011, 32, 4565–4573. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Dashtestani, F.; AlizadehZeinabad, H. Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB 231. PLoS ONE 2017, 12, e0188639. [Google Scholar]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Alizadehzeinabad, H. Albumin coated cadmium nanoparticles as chemotherapeutic agent against MDA-MB 231 human breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2018, 46, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Chen, Y.; Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar]
- Chen, Z.; Yin, J.-J.; Zhou, Y.-T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.A.H.; Chauhan, L.K.S.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar]
- Ranji-Burachaloo, H.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. Cancer treatment through nanoparticle-facilitated fenton reaction. ACS Nano 2018, 12, 11819–11837. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Zhang, J.; Gu, Z.; Chen, Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials 2019, 211, 1–13. [Google Scholar] [CrossRef]
- Gao, S.; Jin, Y.; Ge, K.; Li, Z.; Liu, H.; Dai, X.; Zhang, Y.; Chen, S.; Liang, X.; Zhang, J. Self-supply of O2 and H2O2 by a Nanocatalytic medicine to enhance combined chemo/Chemodynamic therapy. Adv. Sci. 2019, 6, 1902137. [Google Scholar] [CrossRef] [Green Version]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Dianat-Moghadam, H.; Heidarifard, M.; Mahari, A.; Shahgolzari, M.; Keshavarz, M.; Nouri, M.; Amoozgar, Z. TRAIL in oncology: From recombinant TRAIL to nano-and self-targeted TRAIL-based therapies. Pharmacol. Res. 2020, 155, 104716. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Liu, J.; Lin, G.; Xie, F.; Pang, X.; Pei, Y.; Cheng, Y.; Zhang, Y.; Lin, Z.; et al. Oxidative stress-driven DR5 upregulation restores TRAIL/Apo2L sensitivity induced by iron oxide nanoparticles in colorectal cancer. Biomaterials 2020, 233, 119753. [Google Scholar] [CrossRef]
- Sur-Erdem, I.; Muslu, K.; Pınarbası, N.; Altunbek, M.; Seker-Polat, F.; Cingöz, A.; Aydın, S.O.; Kahraman, M.; Culha, M.; Solaroglu, I.; et al. TRAIL-conjugated silver nanoparticles sensitize glioblastoma cells to TRAIL by regulating CHK1 in the DNA repair pathway. Neurol. Res. 2020, 42, 1061–1069. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Karimi, H.; Khazaei, M. Review on metal-based nanoparticles: Role of reactive oxygen species in renal toxicity. Chem. Res. Toxicol. 2020, 33, 2503–2514. [Google Scholar] [CrossRef]
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The toxicity phenomenon and the related occurrence in metal and metal oxide nanoparticles: A brief review from the biomedical perspective. Front. Bioeng. Biotechnol. 2020, 8, 822. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, A.; Attar, F.; Babadaei, M.M.N.; Zeinabad, H.A.; Salehi, M.; Alizadeh, M.; Hassan, M.; Derakhshankhah, H.; Hamblin, M.R. Diagnostic and drug release systems based on microneedle arrays in breast cancer therapy. J. Control. Release 2021, 338, 341–357. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; O’hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zhang, Y.; Zhu, C.; Chu, C.; Shi, Y.; Pang, X.; Ren, E.; Wu, Y.; Mi, P.; Xia, H.; et al. Photo-excitable hybrid nanocomposites for image-guided photo/TRAIL synergistic cancer therapy. Biomaterials 2018, 176, 60–70. [Google Scholar] [CrossRef]
- Peng, L.-H.; Wang, M.-Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.-T.; Yuan, T.-J.; Jiang, Z.-H.; Gao, J.-Q.; Ning, X.-H. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL–programmed therapy against melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef]
- Nesterov, A.; Nikrad, M.; Johnson, T.; Kraft, A.S. Oncogenic Ras sensitizes normal human cells to tumor necrosis factor-α-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2004, 64, 3922–3927. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, M.; Halpin-McCormick, A.; Sessler, T.; Samali, A.; Szegezdi, E. Resistance to TRAIL in non-transformed cells is due to multiple redundant pathways. Cell Death Dis. 2013, 4, e702. [Google Scholar] [CrossRef] [Green Version]
- Gurney, M.; O’Reilly, E.; Corcoran, S.; Brophy, S.; Hardwicke, D.; Krawczyk, J.; Hermanson, D.; Childs, R.W.; Szegezdi, E.; O’Dwyer, M.E. Tc Buster Transposon Engineered CLL-1 CAR-NK Cells Efficiently Target Acute Myeloid Leukemia. Blood 2021, 138, 1725. [Google Scholar] [CrossRef]
| Formulation | Size (nm) | TRAIL Form/Localization | Main Findings | Tumor Type | Ref. |
|---|---|---|---|---|---|
| Liposome | 100–140 | rhTRAIL/Surface | Mimicking the membrane properties of natural TRAIL increased receptor clustering and improved cytotoxic potential of TRAIL thus overcoming resistance to soluble TRAIL. | Colorectal cancer, mouse model | [141] |
| Liposome | 100 | rhTRAIL/Surface | Enhanced clustering of DR5 overcoming resistance to soluble TRAIL. | Colorectal cancer, mouse model | [129] |
| Triazine modified dendrimer | 200 | pTRAIL/Surface | Enhancement in transfection efficacy of TRAIL gene. Improved tumor growth inhibition. | Osteosarcoma mouse model | [189] |
| PEG-TRAIL microencapsulated into PLGA | 11,000–15,500 | TRAIL/Inside | Increased biological half-life time and sustained TRAIL delivery up to 18 days. | Colorectal cancer, mouse model | [190] |
| Single-walled carbon nanotubes | ND | rhTRAIL/Surface | 20-fold increase in TRAIL cytotoxicity against cancer cells without toxicity against normal cells. | Colorectal cancer, NSCLC, hepatocellular carcinoma | [191] |
| Formulation | TRAIL Form/Location | Strategy to Overcome TRAIL Resistance | Tumor Type | Ref. |
|---|---|---|---|---|
| Inhalable HSA NPs; loaded w/Dox | rhTRAIL/Surface | DNA damage caused by DOX. | Lung cancer, mouse model | [214] |
| HSA NPs; loaded w/DOX | rhTRAIL/Surface | DNA damage caused by DOX. | Colorectal cancer, mouse model | [215] |
| Liposome, loaded w/DOX | rhTRAIL/Surface | DNA damage caused by DOX. | NSCLC, mouse model | [238] |
| Liposome, loaded w/PTX | pTRAIL/Surface | PTX induced M-phase cell cycle arrest. | Glioblastoma, mouse model | [216] |
| Polymeric NPs coated with platelet membrane, loaded w/DOX | rhTRAIL/Surface | DNA damage caused by DOX. | Metastatic breast cancer, mouse model | [217] |
| Polymeric NPs, loaded w/DOX | rhTRAIL/Inside | DNA damage caused by DOX. | Prostate and colon cancer, mouse model | [239] |
| PEI-coated gold nanocomposite | pTRAIL/Surface | ROS generation by iron oxide NPs inducing DNA damage. | Hep3B cell xenograft mouse model | [220] |
| Inhalable highly porous PLGA microparticles, loaded w/DOX | rhTRAIL/Surface | DNA damage caused by DOX. | Mouse model of H226 cell metastasis | [240] |
| Liposome inside a hyaluronic acid crosslinked-gel shell, loaded w/DOX | rhTRAIL/Between liposome and the gel shell | DNA damage caused by DOX. | MDA-MB-231 breast cancer cell, xenograft mouse model | [222] |
| Chitosan modified magnetic nanoparticles | pTRAIL/Inside | Hyperthermia induced by a magnetic field. | Pulmonary metastatic mouse model | [241] |
| Alginate modified CaCO3 NPs, loaded w/DOX | rhTRAIL/Surface | DNA damage caused by DOX. | Cervical cancer cell line (HeLa cells) | [242] |
| Magnetic ferric oxide NP | rhTRAIL/Surface | ROS generation by iron oxide causing DNA damage. | Glioma mouse model | [243] |
| Polymeric NPs, DOX intercalated | pTRAIL/Inside | DNA damage caused by DOX. | Glioma mouse model | [227] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizadeh Zeinabad, H.; Szegezdi, E. TRAIL in the Treatment of Cancer: From Soluble Cytokine to Nanosystems. Cancers 2022, 14, 5125. https://doi.org/10.3390/cancers14205125
Alizadeh Zeinabad H, Szegezdi E. TRAIL in the Treatment of Cancer: From Soluble Cytokine to Nanosystems. Cancers. 2022; 14(20):5125. https://doi.org/10.3390/cancers14205125
Chicago/Turabian StyleAlizadeh Zeinabad, Hojjat, and Eva Szegezdi. 2022. "TRAIL in the Treatment of Cancer: From Soluble Cytokine to Nanosystems" Cancers 14, no. 20: 5125. https://doi.org/10.3390/cancers14205125
APA StyleAlizadeh Zeinabad, H., & Szegezdi, E. (2022). TRAIL in the Treatment of Cancer: From Soluble Cytokine to Nanosystems. Cancers, 14(20), 5125. https://doi.org/10.3390/cancers14205125





