Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Prior Treatment
2.2. Histopathology
2.3. Tumor Molecular Profiling
2.4. Patient Evaluation
2.5. “MEMMAT-like” Antiangiogenic Treatment
2.6. Intraventricular Therapy
2.7. Treatment Response and Toxicity Evaluation
2.8. Statistical Methods
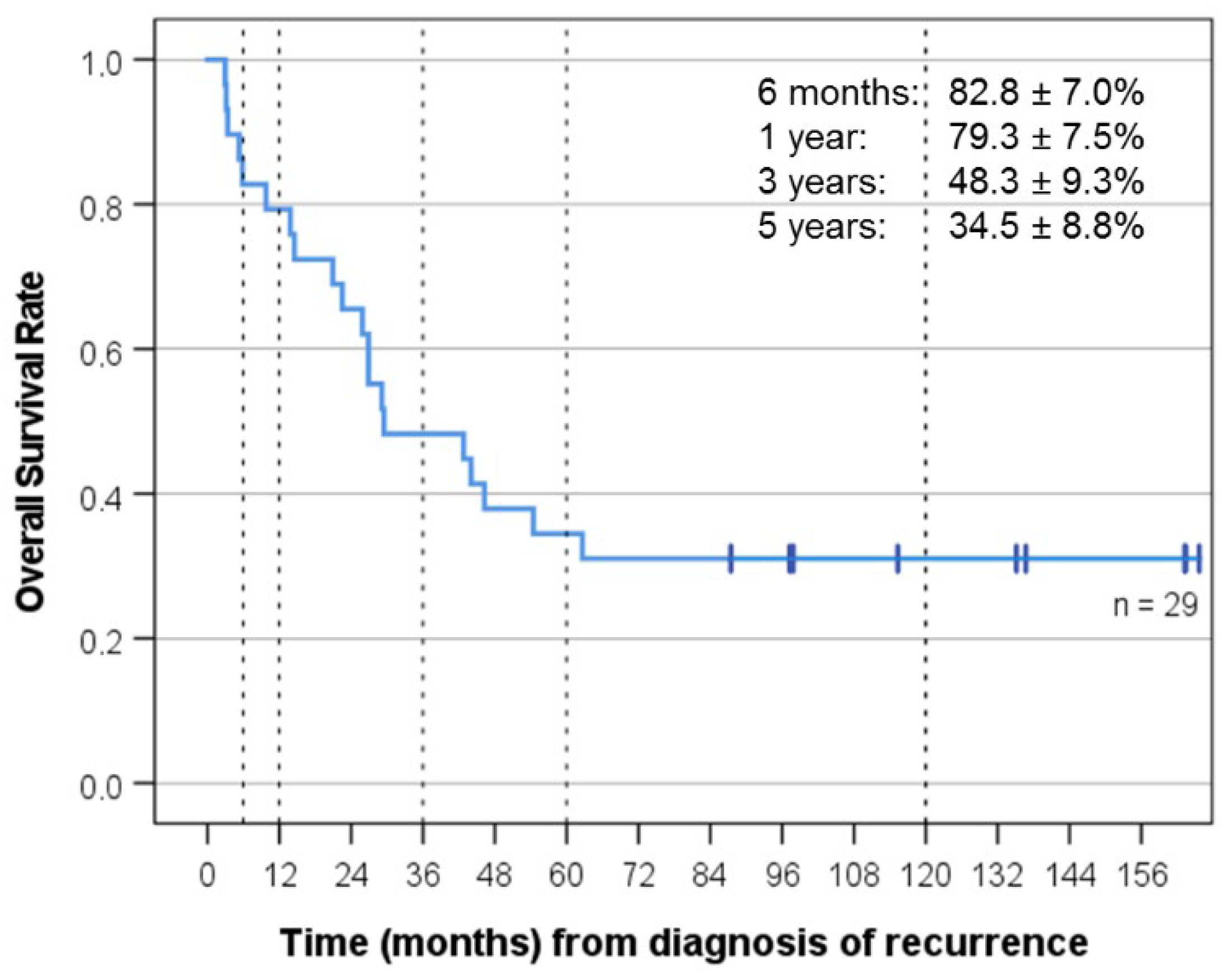
3. Results
3.1. Diagnosis of Recurrence
3.2. Patients with a Ventriculoperitoneal (VP) Shunt in Place
3.3. Histopathology
3.4. Response and Clinical Outcome after Relapse That Prompted “MEMMAT-like” Treatment
3.5. Molecular Profiling and Outcome Depending on Group Allocation
3.6. Treatment after “MEMMAT-like”
3.7. Feasibility and Tolerability of “MEMMAT-like” Treatment
3.8. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Ryan, K.; Edelson, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro-Oncol. 2022, 24, iii1–iii38. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.; Vezina, G.; Burger, P.; Gajjar, A.; Pollack, I.; Merchant, T.; Fitzgerald, T.; Booth, T.; Tarbell, N.; Shieh, I.; et al. Mb-109preliminary results of cog acns0331: A phase iii trial of involved field radiotherapy (ifrt) and low dose craniospinal irradiation (ld-csi) with chemotherapy in average risk medulloblastoma: A report from the children’s oncology group. Neuro-Oncol. 2016, 18, iii122. [Google Scholar] [CrossRef]
- Tarbell, N.J.; Friedman, H.; Polkinghorn, W.R.; Yock, T.; Zhou, T.; Chen, Z.; Burger, P.; Barnes, P.; Kun, L. High-Risk Medulloblastoma: A Pediatric Oncology Group Randomized Trial of Chemotherapy before or after Radiation Therapy (POG 9031). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2936–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoff, K.v.; Hinkes, B.; Gerber, N.U.; Deinlein, F.; Mittler, U.; Urban, C.; Benesch, M.; Warmuth-Metz, M.; Soerensen, N.; Zwiener, I.; et al. Long-Term Outcome and Clinical Prognostic Factors in Children with Medulloblastoma Treated in the Prospective Randomised Multicentre Trial HIT’91. Eur. J. Cancer Oxf. Engl. 1990 2009, 45, 1209–1217. [Google Scholar] [CrossRef]
- Johnston, D.L.; Keene, D.; Kostova, M.; Lafay-Cousin, L.; Fryer, C.; Scheinemann, K.; Carret, A.-S.; Fleming, A.; Percy, V.; Afzal, S.; et al. Survival of Children with Medulloblastoma in Canada Diagnosed between 1990 and 2009 Inclusive. J. Neurooncol. 2015, 124, 247–253. [Google Scholar] [CrossRef]
- Northcott, P.A.; Korshunov, A.; Witt, H.; Hielscher, T.; Eberhart, C.G.; Mack, S.; Bouffet, E.; Clifford, S.C.; Hawkins, C.E.; French, P.; et al. Medulloblastoma Comprises Four Distinct Molecular Variants. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Tsherniak, A.; Tamayo, P.; Santagata, S.; Ligon, A.; Greulich, H.; Berhoukim, R.; Amani, V.; Goumnerova, L.; Eberhart, C.G.; et al. Integrative Genomic Analysis of Medulloblastoma Identifies a Molecular Subgroup That Drives Poor Clinical Outcome. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1424–1430. [Google Scholar] [CrossRef]
- Thompson, M.C.; Fuller, C.; Hogg, T.L.; Dalton, J.; Finkelstein, D.; Lau, C.C.; Chintagumpala, M.; Adesina, A.; Ashley, D.M.; Kellie, S.J.; et al. Genomics Identifies Medulloblastoma Subgroups That Are Enriched for Specific Genetic Alterations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 1924–1931. [Google Scholar] [CrossRef]
- Kool, M.; Koster, J.; Bunt, J.; Hasselt, N.E.; Lakeman, A.; van Sluis, P.; Troost, D.; Meeteren, N.S.; Caron, H.N.; Cloos, J.; et al. Integrated Genomics Identifies Five Medulloblastoma Subtypes with Distinct Genetic Profiles, Pathway Signatures and Clinicopathological Features. PLoS ONE 2008, 3, e3088. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.-J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular Subgroups of Medulloblastoma: The Current Consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwalbe, E.C.; Lindsey, J.C.; Nakjang, S.; Crosier, S.; Smith, A.J.; Hicks, D.; Rafiee, G.; Hill, R.M.; Iliasova, A.; Stone, T.; et al. Novel Molecular Subgroups for Clinical Classification and Outcome Prediction in Childhood Medulloblastoma: A Cohort Study. Lancet Oncol. 2017, 18, 958–971. [Google Scholar] [CrossRef] [Green Version]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Gröbner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The Whole-Genome Landscape of Medulloblastoma Subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, G.W.; Rudneva, V.A.; Buchhalter, I.; Billups, C.A.; Waszak, S.M.; Smith, K.S.; Bowers, D.C.; Bendel, A.; Fisher, P.G.; Partap, S.; et al. Risk-Adapted Therapy for Young Children with Medulloblastoma (SJYC07): Therapeutic and Molecular Outcomes from a Multicentre, Phase 2 Trial. Lancet Oncol. 2018, 19, 768–784. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ellison, D.W.; Onilude, O.E.; Lindsey, J.C.; Lusher, M.E.; Weston, C.L.; Taylor, R.E.; Pearson, A.D.; Clifford, S.C. United Kingdom Children’s Cancer Study Group Brain Tumour Committee Beta-Catenin Status Predicts a Favorable Outcome in Childhood Medulloblastoma: The United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 7951–7957. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.C.; Lusher, M.E.; Lindsey, J.C.; Langdon, J.A.; Gilbertson, R.J.; Straughton, D.; Ellison, D.W. Wnt/Wingless Pathway Activation and Chromosome 6 Loss Characterize a Distinct Molecular Sub-Group of Medulloblastomas Associated with a Favorable Prognosis. Cell Cycle Georget. Tex 2006, 5, 2666–2670. [Google Scholar] [CrossRef] [Green Version]
- Gajjar, A.; Robinson, G.W.; Smith, K.S.; Lin, T.; Merchant, T.E.; Chintagumpala, M.; Mahajan, A.; Su, J.; Bouffet, E.; Bartels, U.; et al. Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 822–835. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk Stratification of Childhood Medulloblastoma in the Molecular Era: The Current Consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primer 2019, 5, 11. [Google Scholar] [CrossRef]
- Koschmann, C.; Bloom, K.; Upadhyaya, S.; Geyer, J.R.; Leary, S.E.S. Survival After Relapse of Medulloblastoma. J. Pediatr. Hematol. Oncol. 2016, 38, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Sabel, M.; Fleischhack, G.; Tippelt, S.; Gustafsson, G.; Doz, F.; Kortmann, R.; Massimino, M.; Navajas, A.; von Hoff, K.; Rutkowski, S.; et al. Relapse Patterns and Outcome after Relapse in Standard Risk Medulloblastoma: A Report from the HIT-SIOP-PNET4 Study. J. Neurooncol. 2016, 129, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Johnston, D.L.; Keene, D.; Strother, D.; Taneva, M.; Lafay-Cousin, L.; Fryer, C.; Scheinemann, K.; Carret, A.-S.; Fleming, A.; Afzal, S.; et al. Survival Following Tumor Recurrence in Children With Medulloblastoma. J. Pediatr. Hematol. Oncol. 2018, 40, e159–e163. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, A.; Pizer, B. Role of High-Dose Chemotherapy for Recurrent Medulloblastoma and Other CNS Primitive Neuroectodermal Tumors. Pediatr. Blood Cancer 2010, 54, 649–651. [Google Scholar] [CrossRef]
- Pizer, B.; Donachie, P.H.J.; Robinson, K.; Taylor, R.E.; Michalski, A.; Punt, J.; Ellison, D.W.; Picton, S. Treatment of Recurrent Central Nervous System Primitive Neuroectodermal Tumours in Children and Adolescents: Results of a Children’s Cancer and Leukaemia Group Study. Eur. J. Cancer Oxf. Engl. 1990 2011, 47, 1389–1397. [Google Scholar] [CrossRef]
- Bautista, F.; Fioravantti, V.; de Rojas, T.; Carceller, F.; Madero, L.; Lassaletta, A.; Moreno, L. Medulloblastoma in Children and Adolescents: A Systematic Review of Contemporary Phase I and II Clinical Trials and Biology Update. Cancer Med. 2017, 6, 2606–2624. [Google Scholar] [CrossRef]
- Kieran, M.W.; Chisholm, J.; Casanova, M.; Brandes, A.A.; Aerts, I.; Bouffet, E.; Bailey, S.; Leary, S.; MacDonald, T.J.; Mechinaud, F.; et al. Phase I Study of Oral Sonidegib (LDE225) in Pediatric Brain and Solid Tumors and a Phase II Study in Children and Adults with Relapsed Medulloblastoma. Neuro-Oncol. 2017, 19, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Q.; Day, B.W. Phase I and Phase II Sonidegib and Vismodegib Clinical Trials for the Treatment of Paediatric and Adult MB Patients: A Systemic Review and Meta-Analysis. Acta Neuropathol. Commun. 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frappaz, D.; Barritault, M.; Montané, L.; Laigle-Donadey, F.; Chinot, O.; Le Rhun, E.; Bonneville-Levard, A.; Hottinger, A.F.; Meyronnet, D.; Bidaux, A.-S.; et al. MEVITEM-a Phase I/II Trial of Vismodegib + Temozolomide vs Temozolomide in Patients with Recurrent/Refractory Medulloblastoma with Sonic Hedgehog Pathway Activation. Neuro-Oncol. 2021, 23, 1949–1960. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S.; Kamen, B.A. The Anti-Angiogenic Basis of Metronomic Chemotherapy. Nat. Rev. Cancer 2004, 4, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Kavallaris, M.; André, N. Metronomic Chemotherapy: New Rationale for New Directions. Nat. Rev. Clin. Oncol. 2010, 7, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Browder, T.; Butterfield, C.E.; Kräling, B.M.; Shi, B.; Marshall, B.; O’Reilly, M.S.; Folkman, J. Antiangiogenic Scheduling of Chemotherapy Improves Efficacy against Experimental Drug-Resistant Cancer. Cancer Res. 2000, 60, 1878–1886. [Google Scholar] [PubMed]
- Klement, G.; Baruchel, S.; Rak, J.; Man, S.; Clark, K.; Hicklin, D.J.; Bohlen, P.; Kerbel, R.S. Continuous Low-Dose Therapy with Vinblastine and VEGF Receptor-2 Antibody Induces Sustained Tumor Regression without Overt Toxicity. J. Clin. Invest. 2000, 105, R15–R24. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Bergers, G.; Bergsland, E. Less Is More, Regularly: Metronomic Dosing of Cytotoxic Drugs Can Target Tumor Angiogenesis in Mice. J. Clin. Invest. 2000, 105, 1045–1047. [Google Scholar] [CrossRef]
- Berthold, F.; Hömberg, M.; Proleskovskaya, I.; Mazanek, P.; Belogurova, M.; Ernst, A.; Sterba, J. Metronomic Therapy Has Low Toxicity and Is as Effective as Current Standard Treatment for Recurrent High-Risk Neuroblastoma. Pediatr. Hematol. Oncol. 2017, 34, 308–319. [Google Scholar] [CrossRef]
- El Kababri, M.; Benmiloud, S.; Cherkaoui, S.; El Houdzi, J.; Maani, K.; Ansari, N.; Khoubila, N.; Kili, A.; El Khorassani, M.; Madani, A.; et al. Metro-SMHOP 01: Metronomics Combination with Cyclophosphamide-Etoposide and Valproic Acid for Refractory and Relapsing Pediatric Malignancies. Pediatr. Blood Cancer 2020, 67, e28508. [Google Scholar] [CrossRef]
- Kerbel, R.S. Reappraising Antiangiogenic Therapy for Breast Cancer. Breast Edinb. Scotl. 2011, 20 (Suppl. 3), S56–S60. [Google Scholar] [CrossRef]
- Kieran, M.W.; Turner, C.D.; Rubin, J.B.; Chi, S.N.; Zimmerman, M.A.; Chordas, C.; Klement, G.; Laforme, A.; Gordon, A.; Thomas, A.; et al. A Feasibility Trial of Antiangiogenic (Metronomic) Chemotherapy in Pediatric Patients with Recurrent or Progressive Cancer. J. Pediatr. Hematol. Oncol. 2005, 27, 573–581. [Google Scholar] [CrossRef]
- Panigrahy, D.; Kaipainen, A.; Butterfield, C.E.; Chaponis, D.M.; Laforme, A.M.; Folkman, J.; Kieran, M.W. Inhibition of Tumor Angiogenesis by Oral Etoposide. Exp. Ther. Med. 2010, 1, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robison, N.J.; Campigotto, F.; Chi, S.N.; Manley, P.E.; Turner, C.D.; Zimmerman, M.A.; Chordas, C.A.; Werger, A.M.; Allen, J.C.; Goldman, S.; et al. A Phase II Trial of a Multi-Agent Oral Antiangiogenic (Metronomic) Regimen in Children with Recurrent or Progressive Cancer. Pediatr. Blood Cancer 2014, 61, 636–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrl, A.; Chocholous, M.; Kieran, M.W.; Azizi, A.A.; Prucker, C.; Czech, T.; Dieckmann, K.; Schmook, M.-T.; Haberler, C.; Leiss, U.; et al. Antiangiogenic Metronomic Therapy for Children with Recurrent Embryonal Brain Tumors. Pediatr. Blood Cancer 2012, 59, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lannering, B.; Rutkowski, S.; Doz, F.; Pizer, B.; Gustafsson, G.; Navajas, A.; Massimino, M.; Reddingius, R.; Benesch, M.; Carrie, C.; et al. Hyperfractionated versus Conventional Radiotherapy Followed by Chemotherapy in Standard-Risk Medulloblastoma: Results from the Randomized Multicenter HIT-SIOP PNET 4 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3187–3193. [Google Scholar] [CrossRef] [PubMed]
- Dietzsch, S.; Placzek, F.; Pietschmann, K.; von Bueren, A.O.; Matuschek, C.; Glück, A.; Guckenberger, M.; Budach, V.; Welzel, J.; Pöttgen, C.; et al. Evaluation of Prognostic Factors and Role of Participation in a Randomized Trial or a Prospective Registry in Pediatric and Adolescent Nonmetastatic Medulloblastoma—A Report From the HIT 2000 Trial. Adv. Radiat. Oncol. 2020, 5, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Kortmann, R.D.; Kühl, J.; Timmermann, B.; Mittler, U.; Urban, C.; Budach, V.; Richter, E.; Willich, N.; Flentje, M.; Berthold, F.; et al. Postoperative Neoadjuvant Chemotherapy before Radiotherapy as Compared to Immediate Radiotherapy Followed by Maintenance Chemotherapy in the Treatment of Medulloblastoma in Childhood: Results of the German Prospective Randomized Trial HIT ’91. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 269–279. [Google Scholar] [CrossRef]
- Dhall, G.; O’Neil, S.H.; Ji, L.; Haley, K.; Whitaker, A.M.; Nelson, M.D.; Gilles, F.; Gardner, S.L.; Allen, J.C.; Cornelius, A.S.; et al. Excellent Outcome of Young Children with Nodular Desmoplastic Medulloblastoma Treated on “Head Start” III: A Multi-Institutional, Prospective Clinical Trial. Neuro-Oncol. 2020, 22, 1862–1872. [Google Scholar] [CrossRef]
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; LaFond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III Study of Craniospinal Radiation Therapy Followed by Adjuvant Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 4202–4208. [Google Scholar] [CrossRef]
- Hovestadt, V.; Remke, M.; Kool, M.; Pietsch, T.; Northcott, P.A.; Fischer, R.; Cavalli, F.M.G.; Ramaswamy, V.; Zapatka, M.; Reifenberger, G.; et al. Robust Molecular Subgrouping and Copy-Number Profiling of Medulloblastoma from Small Amounts of Archival Tumour Material Using High-Density DNA Methylation Arrays. Acta Neuropathol. 2013, 125, 913–916. [Google Scholar] [CrossRef] [Green Version]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA Methylation-Based Classification of Central Nervous System Tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Fleischhack, G.; Reif, S.; Hasan, C.; Jaehde, U.; Hettmer, S.; Bode, U. Feasibility of Intraventricular Administration of Etoposide in Patients with Metastatic Brain Tumours. Br. J. Cancer 2001, 84, 1453–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavc, I.; Schuller, E.; Falger, J.; Günes, M.; Pillwein, K.; Czech, T.; Dietrich, W.; Rössler, K.; Dieckmann, K.; Prayer, D.; et al. Feasibility of Long-Term Intraventricular Therapy with Mafosfamide (n = 26) and Etoposide (n = 11): Experience in 26 Children with Disseminated Malignant Brain Tumors. J. Neurooncol. 2003, 64, 239–247. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Tippelt, S.; Siegler, N.; Reichling, S.; Zimmermann, M.; Mikasch, R.; Bode, U.; Gnekow, A.; Pietsch, T.; Benesch, M.; et al. Intraventricular Etoposide Safety and Toxicity Profile in Children and Young Adults with Refractory or Recurrent Malignant Brain Tumors. J. Neurooncol. 2016, 128, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Peyrl, A.; Sauermann, R.; Traunmueller, F.; Azizi, A.A.; Gruber-Olipitz, M.; Gupper, A.; Slavc, I. Pharmacokinetics and Safety of Intrathecal Liposomal Cytarabine in Children Aged <3 Years. Clin. Pharmacokinet. 2009, 48, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Peyrl, A.; Sauermann, R.; Chocholous, M.; Azizi, A.A.; Jäger, W.; Höferl, M.; Slavc, I. Pharmacokinetics and Toxicity of Intrathecal Liposomal Cytarabine in Children and Adolescents Following Age-Adapted Dosing. Clin. Pharmacokinet. 2014, 53, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.E.; Vezina, G.; Poussaint, T.Y.; Warmuth-Metz, M.; Chamberlain, M.C.; Packer, R.J.; Brandes, A.A.; Reiss, M.; Goldman, S.; Fisher, M.J.; et al. Response Assessment in Medulloblastoma and Leptomeningeal Seeding Tumors: Recommendations from the Response Assessment in Pediatric Neuro-Oncology Committee. Neuro-Oncol. 2018, 20, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czech, T.; Reinprecht, A.; Dietrich, W.; Hainfellner, J.A.; Slavc, I. Reversible Occlusion Shunt for Intraventricular Chemotherapy in Shunt-Dependent Brain Tumor Patients. Pediatr. Hematol. Oncol. 1997, 14, 375–380. [Google Scholar] [CrossRef]
- Grill, J.; Geoerger, B.; Gesner, L.; Perek, D.; Leblond, P.; Cañete, A.; Aerts, I.; Madero, L.; de Toledo Codina, J.S.; Verlooy, J.; et al. Phase II Study of Irinotecan in Combination with Temozolomide (TEMIRI) in Children with Recurrent or Refractory Medulloblastoma: A Joint ITCC and SIOPE Brain Tumor Study. Neuro-Oncol. 2013, 15, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and Antitumor Activities of Cyclooxygenase-2 Inhibitors. Cancer Res. 2000, 60, 1306–1311. [Google Scholar]
- Shaked, Y.; Emmenegger, U.; Man, S.; Cervi, D.; Bertolini, F.; Ben-David, Y.; Kerbel, R.S. Optimal Biologic Dose of Metronomic Chemotherapy Regimens Is Associated with Maximum Antiangiogenic Activity. Blood 2005, 106, 3058–3061. [Google Scholar] [CrossRef] [Green Version]
- Bocci, G.; Nicolaou, K.C.; Kerbel, R.S. Protracted Low-Dose Effects on Human Endothelial Cell Proliferation and Survival in Vitro Reveal a Selective Antiangiogenic Window for Various Chemotherapeutic Drugs. Cancer Res. 2002, 62, 6938–6943. [Google Scholar] [PubMed]
- Panigrahy, D.; Kaipainen, A.; Huang, S.; Butterfield, C.E.; Barnés, C.M.; Fannon, M.; Laforme, A.M.; Chaponis, D.M.; Folkman, J.; Kieran, M.W. PPARalpha Agonist Fenofibrate Suppresses Tumor Growth through Direct and Indirect Angiogenesis Inhibition. Proc. Natl. Acad. Sci. USA 2008, 105, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piha-Paul, S.A.; Shin, S.J.; Vats, T.; Guha-Thakurta, N.; Aaron, J.; Rytting, M.; Kleinerman, E.; Kurzrock, R. Pediatric Patients with Refractory Central Nervous System Tumors: Experiences of a Clinical Trial Combining Bevacizumab and Temsirolimus. Anticancer. Res. 2014, 34, 1939–1945. [Google Scholar] [PubMed]
- Bonney, P.A.; Santucci, J.A.; Maurer, A.J.; Sughrue, M.E.; McNall-Knapp, R.Y.; Battiste, J.D. Dramatic Response to Temozolomide, Irinotecan, and Bevacizumab for Recurrent Medulloblastoma with Widespread Osseous Metastases. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2016, 26, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Schiavetti, A.; Varrasso, G.; Mollace, M.G.; Dominici, C.; Ferrara, E.; Papoff, P.; Di Biasi, C. Bevacizumab-Containing Regimen in Relapsed/Progressed Brain Tumors: A Single-Institution Experience. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2019, 35, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.S.; Krailo, M.; Chi, S.; Villaluna, D.; Springer, L.; Williams-Hughes, C.; Fouladi, M.; Gajjar, A. Temozolomide with Irinotecan versus Temozolomide, Irinotecan plus Bevacizumab for Recurrent Medulloblastoma of Childhood: Report of a COG Randomized Phase II Screening Trial. Pediatr. Blood Cancer 2021, 68, e29031. [Google Scholar] [CrossRef]
- Fleischhack, G.; Jaehde, U.; Bode, U. Pharmacokinetics Following Intraventricular Administration of Chemotherapy in Patients with Neoplastic Meningitis. Clin. Pharmacokinet. 2005, 44, 1–31. [Google Scholar] [CrossRef]
- Rutkowski, S.; Bode, U.; Deinlein, F.; Ottensmeier, H.; Warmuth-Metz, M.; Soerensen, N.; Graf, N.; Emser, A.; Pietsch, T.; Wolff, J.E.A.; et al. Treatment of Early Childhood Medulloblastoma by Postoperative Chemotherapy Alone. N. Engl. J. Med. 2005, 352, 978–986. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Yang, S.; Zhao, X.; Xiao, J.; Ren, S.; Li, S.; Zhang, J.; Wang, Y.; Gong, X.; Li, M.; et al. Clinical Characteristics and Outcome of Children With Relapsed Medulloblastoma: A Retrospective Study at a Single Center in China. J. Pediatr. Hematol. Oncol. 2018, 40, 598–604. [Google Scholar] [CrossRef]
- Wasserstrom, W.R.; Glass, J.P.; Posner, J.B. Diagnosis and Treatment of Leptomeningeal Metastases from Solid Tumors: Experience with 90 Patients. Cancer 1982, 49, 759–772. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takahashi, S.; Sato, A.; Imaizumi, M.; Higano, S.; Sakamoto, K.; Asakawa, H.; Tada, K. Leukoencephalopathy in Childhood Hematopoietic Neoplasm Caused by Moderate-Dose Methotrexate and Prophylactic Cranial Radiotherapy--an MR Analysis. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 913–918. [Google Scholar] [CrossRef]
- Korshunov, A.; Sahm, F.; Zheludkova, O.; Golanov, A.; Stichel, D.; Schrimpf, D.; Ryzhova, M.; Potapov, A.; Habel, A.; Meyer, J.; et al. DNA Methylation Profiling Is a Method of Choice for Molecular Verification of Pediatric WNT-Activated Medulloblastomas. Neuro-Oncol. 2019, 21, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Bakst, R.L.; Dunkel, I.J.; Gilheeney, S.; Khakoo, Y.; Becher, O.; Souweidane, M.M.; Wolden, S.L. Reirradiation for Recurrent Medulloblastoma. Cancer 2011, 117, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, C.; Herington, D.; Lin, T.; Onar-Thomas, A.; Gajjar, A.; Merchant, T.E. Reirradiation of Recurrent Medulloblastoma: Does Clinical Benefit Outweigh Risk for Toxicity? Cancer 2014, 120, 3731–3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, T.; Maitre, M.; Sastri, G.J.; Krishnatry, R.; Shirsat, N.; Epari, S.; Sahay, A.; Chinnaswamy, G.; Patil, V.; Shetty, P.; et al. Outcomes of Salvage Re-Irradiation in Recurrent Medulloblastoma Correlate with Age at Initial Diagnosis, Primary Risk-Stratification, and Molecular Subgrouping. J. Neurooncol. 2019, 144, 283–291. [Google Scholar] [CrossRef]
- Tsang, D.S.; Sarhan, N.; Ramaswamy, V.; Nobre, L.; Yee, R.; Taylor, M.D.; Hawkins, C.; Bartels, U.; Huang, A.; Tabori, U.; et al. Re-Irradiation for Children with Recurrent Medulloblastoma in Toronto, Canada: A 20-Year Experience. J. Neurooncol. 2019, 145, 107–114. [Google Scholar] [CrossRef]
- Kumar, R.; Smith, K.S.; Deng, M.; Terhune, C.; Robinson, G.W.; Orr, B.A.; Liu, A.P.Y.; Lin, T.; Billups, C.A.; Chintagumpala, M.; et al. Clinical Outcomes and Patient-Matched Molecular Composition of Relapsed Medulloblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 807–821. [Google Scholar] [CrossRef]
- Dunkel, I.J.; Gardner, S.L.; Garvin, J.H.; Goldman, S.; Shi, W.; Finlay, J.L. High-Dose Carboplatin, Thiotepa, and Etoposide with Autologous Stem Cell Rescue for Patients with Previously Irradiated Recurrent Medulloblastoma. Neuro-Oncol. 2010, 12, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Wachsberger, P.; Burd, R.; Dicker, A.P. Tumor Response to Ionizing Radiation Combined with Antiangiogenesis or Vascular Targeting Agents: Exploring Mechanisms of Interaction. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 1957–1971. [Google Scholar]
- Timke, C.; Zieher, H.; Roth, A.; Hauser, K.; Lipson, K.E.; Weber, K.J.; Debus, J.; Abdollahi, A.; Huber, P.E. Combination of Vascular Endothelial Growth Factor Receptor/Platelet-Derived Growth Factor Receptor Inhibition Markedly Improves Radiation Tumor Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 2210–2219. [Google Scholar] [CrossRef]





| Case | Age at Primary Diagnosis (Years)/ Gender | Morphology | MB Group | Subgroup | Prior Therapy (RT/CT/ HDCT) | Stage at Primary Diagnosis | No. of Recurrences | Time to Relapse from Primary Diagnosis or Prior Relapse (Months) | Type of Recurrence | Age at MEMMAT Start (Years) | Duration of MEMMAT (Months) | i.th Therapy/ VP-Shunt | Best Response | RT during MEMMAT | Status/ Follow-Up in Months after First MEMMAT Start | Duration of Follow-Up after Discontinuation of Last MEMMAT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° | 12/M | classic | 4 | G34_VIII | +/+/− | M2/M3 | 1 | 132 | M2/M3 | 24 | 14 + 2 * | Yes/no | CR | focal | CCR, 164+ | 149+ |
| 2° | 4/F | classic | 3 | G34_IV | +/+/− | M1 | 1 | 34 | M3 | 7 | 12 + 16 * | Yes/yes | CR | focal | CCR, 160+ | 132+ |
| 3° | 10/M | classic | 4 | G34_VIII | + */+/− | M1 | 2 | 8 | M1 | 14 | 12 + 24 * | Yes/no | CR | no | CCR, 160+ | 124+ |
| 4° | 9/M | classic | NA | + */+/− | M0 | 3 | 9 | M2/M3 | 15 | 21 + 17 ** | Yes/no | PR | no | DOD, 63 | ||
| 5 | 7/M | classic | Non WNT/non SHH | +/+/− | M0 | 1 | 69 | Local/M3 | 13 | 19 + 12 | Yes/yes | CR | Focal # | AWD,131+ | 65+ | |
| 6° | 9/F | classic | WNT | WNT | +/+/− | M0 | 1 | 58 | Local | 14 | 25 | No/yes | NE | no | DOD, 27 | |
| 7° | 12/M | LCA | 3 | G34_II | +/+/− | M0 | 1 | 32 | Local | 15 | 10 + 13 * | Yes/IIIrd | CR | no | DOC, 23 | |
| 8 | 1/F | classic | 3 | G34_IV | −/+/+ | M2/M3 | 1 | 24 | M2 | 4 | 9 | Yes/no | CR | 18Gy CSI+focal | CCR, 96+ | 87+ |
| 9 | 12/M | classic | 3–4 *** | G34_V *** | +/+/− | M2 | 1 | 36 | M2 | 14 | 22 + 24 * | Yes/no | CR | focal | CR, 134+ | 57+ |
| 10 | 5/M | classic | 4 | G34_VIII | +/+/− | M0-1 | 2 | 3 | M2/M3 | 8 | 20 + 12 * | Yes/no | CR | focal | DOC, 54 | |
| 11 | 4/M | classic | 4 | G34_V | +/+/− | M0-1 | 1 | 26 | M2/M3 | 7 | 29 | Yes/no | PR | focal | DOD, 44 | |
| 12 | 7.5/M | classic | 4 | G34_VIII | +/+/− | NA | 1 | 22 | M2/M3 | 10 | 24 | Yes/yes | PR | no | DOD, 32 | |
| 13 | 7/M | classic | 4 | G34_VIII | +/+/− | M2/M3 | 1 | 25 | M1,M2 | 9 | 34 | Yes/IIIrd | PR | focal | CR, 86+ | 31+ |
| 14° | 6/M | LCA | NA | + §/+/− | M1 | 2 | 21 | Local | 10 | 6 | No/yes | PR | no | DOD, 10 | ||
| 15 | 8/M | desmoplastic | NA | +/+/− | NA | 2 | 21 | Local/M3 | 10 | 5 | Yes/yes | SD | no | DOD, 6 | ||
| 16 | 7/M | classic | 4 | G34_VIII | +/−/− | NA | 1 | 10 | M1–M3 | 8 | 10 | Yes/yes | PR | focal | DOC, 46 | |
| 17 | 9/F | classic | 4 | G34_VIII | +/+/− | M0 | 1 | 14 | M1–M3 | 11 | 12 | Yes/yes | SD | focal | DOD, 26 | |
| 18 | 0.4/F | desmoplastic | 2, SHH inf | SHH_Inf_1 | −/+/− | M0 | 1 | 7 | M2 | 1 | 3 | Yes/no | PD | no | DOD, 5 | |
| 19 | 1/M | LCA | 3, high MYC ampl | G34_II | −/+/− | M2/M3 | 1 | 4 | M2/M3 | 1 | 2 | Yes/yes SD | PD | no | DOD, 3 | |
| 20 | 10/M | classic | 4 | G34_VIII | + §/+/− | M0 | 3 | 12 | M2/M3 | 11 | 3 | Yes/no | SD | no | DOC, 3 | |
| 21 | 5/M | classic | Non WNT/non SHH | +/+/− | M0 | 2 | 16 | M2/M3 | Yes/IIIrd | SD | focal | DOD,30 | ||||
| 22 | 4.5/F | classic | 4 | + §/+/+ | M3 | 2 | 18 | M2/M3 | 10 | 36 | Yes/no | PR | focal& | DOD,42 | ||
| 23 | 5/M | classic | 4 | G34_V | +/+/− | M0 | 1 | 26 | Local/M2 | 7 | 14 + 14 * | Yes | CR | focal& | CCR, 111+ | 83+ |
| 24 | 7.5/M | classic | 4 | G34_V | +/+/− | M0 | 1 | 32 | M1–M3 | 10 | 15 | Yes | CR | focal& | DOD,26 | |
| 25 | 6.5/F | classic | 4 | G34_VI | +/+/− | M0 | 1 | 12 | M1–M3 | 7 | 3 | Yes | PD | no | DOD,3 | |
| 26 | 8/M | classic | NA | +/+/− | M0 | 1 | 109 | local | 17 | 10 | Yes | PD | focal | DOD,13 | ||
| 27 | 12/M | classic | 4 | G34_VIII | + §/+/− | NA | 3 | 31 | M2 | 17 | 19 | Yes | PR | no | DOC,20 | |
| 28 | 8/F | classic | 4 | +/+/− | M0 | 1 | 15 | M1-M3 | 9 | 8 | Yes | SD | no | DOD,13 | ||
| 29 | 12/F | classic | 3 | +/+/− | M0 | 1 | 18 | M3 | 14 | 11 | Yes | CR | no | AWD, 97+ | 88+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavc, I.; Mayr, L.; Stepien, N.; Gojo, J.; Aliotti Lippolis, M.; Azizi, A.A.; Chocholous, M.; Baumgartner, A.; Hedrich, C.S.; Holm, S.; et al. Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach. Cancers 2022, 14, 5128. https://doi.org/10.3390/cancers14205128
Slavc I, Mayr L, Stepien N, Gojo J, Aliotti Lippolis M, Azizi AA, Chocholous M, Baumgartner A, Hedrich CS, Holm S, et al. Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach. Cancers. 2022; 14(20):5128. https://doi.org/10.3390/cancers14205128
Chicago/Turabian StyleSlavc, Irene, Lisa Mayr, Natalia Stepien, Johannes Gojo, Maria Aliotti Lippolis, Amedeo A. Azizi, Monika Chocholous, Alicia Baumgartner, Cora S. Hedrich, Stefan Holm, and et al. 2022. "Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach" Cancers 14, no. 20: 5128. https://doi.org/10.3390/cancers14205128
APA StyleSlavc, I., Mayr, L., Stepien, N., Gojo, J., Aliotti Lippolis, M., Azizi, A. A., Chocholous, M., Baumgartner, A., Hedrich, C. S., Holm, S., Sehested, A., Leblond, P., Dieckmann, K., Haberler, C., Czech, T., Kool, M., & Peyrl, A. (2022). Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach. Cancers, 14(20), 5128. https://doi.org/10.3390/cancers14205128






