Anti-Hormonal Therapy in Breast Cancer and Its Effect on the Blood-Brain Barrier
Abstract
:Simple Summary
Abstract
1. Introduction
2. Breast Cancer
2.1. Molecular Characteristics
2.2. Current Therapies of Primary Hormone-Positive Breast Cancer
2.3. Current Therapies of Hormone Positive Metastatic Breast Cancer
3. Brain Metastases in Breast Cancer (BMBC)
3.1. Epidemiology
3.2. Overcoming the Blood-Brain-Barrier
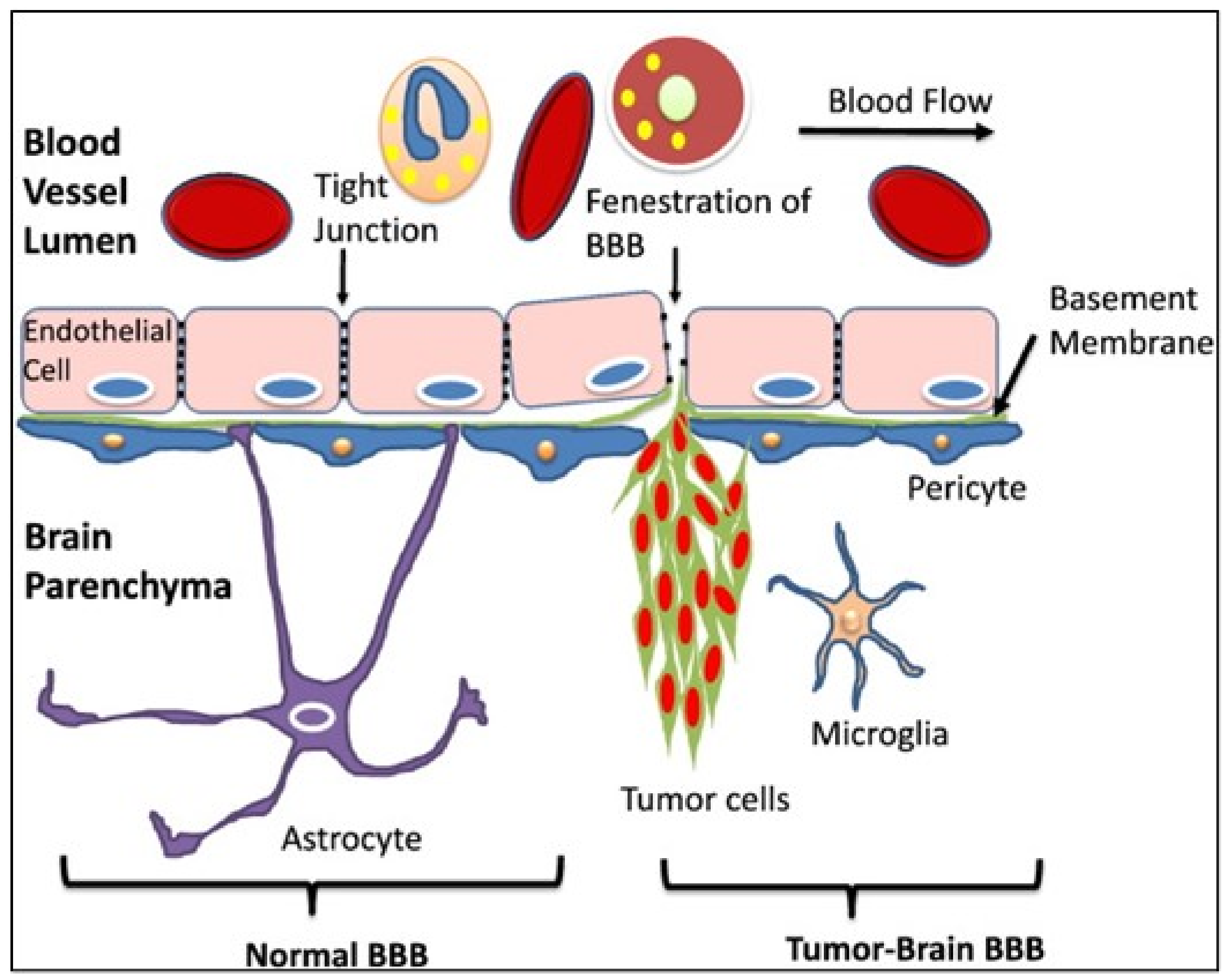
The CNS is respected as an immune-privileged site. The reason for this is that, compared to other tissues, very few neutrophils infiltrate the brain and this also represents a tightly regulated interaction between immune cells and BBB [86]. But inflammatory and other pathological conditions e.g., tumor metastases, can disrupt the TJs between endothelial cells. Cytokines and other pro-inflammatory agents may play a role in BBB specific permeability loss [12]
3.3. Therapy Options of Brain Metastases in Breast Cancer
4. Clinical Trials and Drug Approval for Bmbc
4.1. HER2+ BMBC
4.2. TNBC BMBC
4.3. Luminal BMBC
4.4. Triple-Positive BMBC
5. Anti-Hormonal Therapy Regimensand Their Impact on the BBB
5.1. Tamoxifen
5.2. Aromatase Inhibitor
5.3. Fulvestrant
5.4. GnRH-Analogue
5.5. Everolimus
5.6. CDK 4/6 Inhibitors- Palbociclib/Ribocilib/Abemacilib
5.6.1. Palbociclib
5.6.2. Ribociclib
5.6.3. Abemaciclib
5.7. Alpelisib
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Antibody-drug conjugate |
| AMT | adsorptive-mediated transcytosis |
| BBB | Blood-brain barrier |
| BM | Brain metastases |
| BMBC | Brain metastases in breast cancer |
| CDK 4/6i | CDK 4/6 inhibitors |
| CNS | Central nervous system |
| CMT | carrier-mediated transport |
| ESMO | European Society for Medical Oncology |
| ER | Estrogen receptor |
| MBC | metastatic breast cancer |
| NAC | neoadjuvant chemotherapy |
| OS | overall survival |
| PR | progesterone receptor |
| PFS | progression free survival |
| RMT | receptor-mediated transport |
| SG | sacituzumab-govitecan |
| SERM | selective estrogen receptor modulators |
| TPC | therapy of physician’s choice |
| TNBC | triple negative breast cancer |
References
- Robert Koch-Institut. Krebs in Deutschland 2015/2016; Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V.; Robert Koch-Institut: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hübner, J.; Katalinic, A.; Waldmann, A.; Kraywinkel, K. Long-term Incidence and Mortality Trends for Breast Cancer in Germany. Geburtshilfe und Frauenheilkunde 2020, 80, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Gonçalves, A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012, 32, 4655–4662. [Google Scholar]
- Quigley, M.R.; Fukui, O.; Chew, B.; Bhatia, S.; Karlovits, S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg. Rev. 2012, 36, 377–382. [Google Scholar] [CrossRef]
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Müller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Meattini, I.; Andratschke, N.; Kirby, A.M.; Sviri, G.; Offersen, B.V.; Poortmans, P.; Person, O.K. Challenges in the treatment of breast cancer brain metastases: Evidence, unresolved questions, and a practical algorithm. Clin. Transl. Oncol. 2020, 22, 1698–1709. [Google Scholar] [CrossRef]
- Thill, M.; Jackisch, C.; Janni, W.; Müller, V.; Albert, U.-S.; Bauerfeind, I.; Blohmer, J.; Budach, W.; Dall, P.; Diel, I.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2019. Breast Care 2019, 14, 247–255. [Google Scholar] [CrossRef]
- Krizbai, I.; Nyúl-Tóth, A.; Bauer, H.-C.; Farkas, E.A.; Traweger, A.; Haskó, J.; Bauer, H.; Wilhelm, I. Pharmaceutical Targeting of the Brain. Curr. Pharm. Des. 2016, 22, 5442–5462. [Google Scholar] [CrossRef]
- Curtaz, C.J.; Schmitt, C.; Blecharz-Lang, K.G.; Roewer, N.; Wöckel, A.; Burek, M. Circulating MicroRNAs and Blood-Brain-Barrier Function in Breast Cancer Metastasis. Curr. Pharm. Des. 2020, 26, 1417–1427. [Google Scholar] [CrossRef]
- Curtaz, C.J.; Schmitt, C.; Herbert, S.-L.; Feldheim, J.; Schlegel, N.; Gosselet, F.; Hagemann, C.; Roewer, N.; Meybohm, P.; Wöckel, A.; et al. Serum-derived factors of breast cancer patients with brain metastases alter permeability of a human blood-brain barrier model. Fluids Barriers CNS 2020, 17, 31. [Google Scholar] [CrossRef] [Green Version]
- Curtaz, C.J.; Reifschläger, L.; Strähle, L.; Feldheim, J.; Feldheim, J.J.; Schmitt, C.; Kiesel, M.; Herbert, S.-L.; Wöckel, A.; Meybohm, P.; et al. Analysis of microRNAs in Exosomes of Breast Cancer Patients in Search of Molecular Prognostic Factors in Brain Metastases. Int. J. Mol. Sci. 2022, 23, 3683. [Google Scholar] [CrossRef]
- Johansson, A.L.; Trewin, C.B.; Hjerkind, K.V.; Ellingjord-Dale, M.; Johannesen, T.B.; Ursin, G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int. J. Cancer 2018, 144, 1251–1261. [Google Scholar] [CrossRef]
- Cheng, S.H.-C.; Yu, B.-L.; Horng, C.-F.; Tsai, S.Y.; Chen, C.-M.; Chu, N.-M.; Tsou, M.-H.; Lin, C.K.; Shih, L.-S.; Liu, M.-C. Long-term survival and stage I breast cancer subtypes. J. Cancer Res. Pract. 2016, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Oehrlich, N.E.; Spineli, L.M.; Papendorf, F.; Park-Simon, T.-W. Clinical outcome of brain metastases differs significantly among breast cancer subtypes. Oncol. Lett. 2017, 14, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Witzel, I.; Laakmann, E.; Weide, R.; Neunhöffer, T.; Park-Simon, T.-J.; Schmidt, M.; Fasching, P.; Hesse, T.; Polasik, A.; Mohrmann, S.; et al. Treatment and outcomes of patients in the Brain Metastases in Breast Cancer Network Registry. Eur. J. Cancer 2018, 102, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Nunes, M.R.; Stearns, V. CDK4/6 Inhibitors: Game Changers in the Management of Hormone Receptor–Positive Advanced Breast Cancer? Oncology 2018, 32, 216–222. [Google Scholar]
- Spring, L.M.; Wander, S.A.; Zangardi, M.; Bardia, A. CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr. Oncol. Rep. 2019, 21, 25. [Google Scholar] [CrossRef]
- Batalini, F.; Moulder, S.L.; Winer, E.P.; Rugo, H.S.; Lin, N.U.; Wulf, G.M. Response of Brain Metastases From PIK3CA-Mutant Breast Cancer to Alpelisib. JCO Precis. Oncol. 2020, 4, 572–578. [Google Scholar] [CrossRef]
- Vasconcelos, I.; Hussainzada, A.; Berger, S.; Fietze, E.; Linke, J.; Siedentopf, F.; Schoenegg, W. The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival. Breast 2016, 29, 181–185. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Ingle, J.N.; Gelber, R.D.; Coates, A.S.; Thürlimann, B.; Senn, H.-J. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann. Oncol. 2009, 20, 1319–1329. [Google Scholar] [CrossRef]
- Vici, P.; Pizzuti, L.; Natoli, C.; Gamucci, T.; Di Lauro, L.; Barba, M.; Sergi, D.; Botti, C.; Michelotti, A.; Moscetti, L.; et al. Triple positive breast cancer: A distinct subtype? Cancer Treat. Rev. 2015, 41, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Thomssen, C.; Balic, M.; Harbeck, N.; Gnant, M. St. Gallen/Vienna 2021: A Brief Summary of the Consensus Discussion on Customizing Therapies for Women with Early Breast Cancer. Breast Care 2021, 16, 135–143. [Google Scholar] [CrossRef]
- Karrison, T.G.; Ferguson, D.J.; Meier, P. Dormancy of Mammary Carcinoma After Mastectomy. J. Natl. Cancer Inst. 1999, 91, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, R.N.; Esen, B.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The Incidence of Breast Cancer Recurrence 10-32 Years After Primary Diagnosis. JNCI J. Natl. Cancer Inst. 2021, 114, 391–399. [Google Scholar] [CrossRef]
- Van Maaren, M.C.; De Munck, L.; Strobbe, L.J.; Sonke, G.; Westenend, P.; Smidt, M.L.; Poortmans, P.M.; Siesling, S. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: A large population-based study. Int. J. Cancer 2018, 144, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thürlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin. Med. Insights Oncol. 2014, 8, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [Green Version]
- Pagani, O.; Gelber, S.; Colleoni, M.; Price, K.N.; Simoncini, E. Impact of SERM adherence on treatment effect: International Breast Cancer Study Group Trials 13-93 and 14-93. Breast Cancer Res. Treat. 2013, 142, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Francis, P.A.; Regan, M.M.; Fleming, G.F.; Láng, I.; Ciruelos, E.; Bellet, M.; Bonnefoi, H.R.; Climent, M.A.; Da Prada, G.A.; Burstein, H.J.; et al. Adjuvant Ovarian Suppression in Premenopausal Breast Cancer. N. Engl. J. Med. 2015, 372, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.N.; Stickeler, E.; Fasching, P.A.; Janni, W.; Kolberg-Liedtke, C.; Kolberg, H.-C.; Lüftner, D.; Müller, V.; Schütz, F.; Thomssen, C.; et al. Update Mammakarzinom 2021 Teil 3—Aktuelle Entwicklungen bei der Behandlung von Brustkrebspatientinnen mit frühen Krankheitsstadien: Übersicht und Beurteilung von speziellen Therapiesituationen durch ein internationales Expertenpanel. Senol.-Z. Mammadiagnostik-Ther. 2021, 19, 75–87. [Google Scholar] [CrossRef]
- Ditsch, N.; Kolberg-Liedtke, C.; Friedrich, M.; Jackisch, C.; Albert, U.-S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.-U.; Budach, W.; Dall, P.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2021. Breast Care 2021, 16, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Ditsch, N.; Wöckel, A.; Untch, M.; Jackisch, C.; Albert, U.-S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.-U.; Budach, W.; Dall, P.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer (EBC): Update 2022. Breast Care 2022, 17, 403–420. [Google Scholar] [CrossRef]
- Barbieri, E.; Gentile, D.; Bottini, A.; Sagona, A.; Gatzemeier, W.; Losurdo, A.; Fernandes, B.; Tinterri, C. Neo-Adjuvant Chemotherapy in Luminal, Node Positive Breast Cancer: Characteristics, Treatment and Oncological Outcomes: A Single Center’s Experience. Eur. J. Breast Health 2021, 17, 356–362. [Google Scholar] [CrossRef]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.; Shao, Z.; Fasching, P.; Huang, C.; Jaliffe, G.; Tryakin, A.; Goetz, M.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021, 32, 1571–1581. [Google Scholar] [CrossRef]
- Ibrar, M.; Peddie, N.; Agnew, S.; Diserholt, A.; Fleming, L. Breast Cancer Survivors’ Lived Experience of Adjuvant Hormone Therapy: A Thematic Analysis of Medication Side Effects and Their Impact on Adherence. Front. Psychol. 2022, 13, 861198. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Maya-Núñez, G.; Pérez-Solis, M.A.; López-Muñoz, E.; Guillén, N.; Olivo-Marin, J.-C.; Aguilar-Rojas, A. Treatment of Breast Cancer With Gonadotropin-Releasing Hormone Analogs. Front. Oncol. 2019, 9, 943. [Google Scholar] [CrossRef] [Green Version]
- Wöckel, A.; Kreienberg, R. Interdisziplinäre S3-Leitlinie “Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms”. Gynäkologe 2018, 51, 510–513. [Google Scholar] [CrossRef] [Green Version]
- Dowsett, M.; Forbes, J.F.; Bradley, R.; Ingle, J.N.; Aihara, T.; Bliss, J.M.; Boccardo, F.; Coates, A.S.; Coombes, R.C.; Cuzick, J.; et al. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Legha, S.S.; Davis, H.L.; Muggia, F.M. Hormonal Therapy of Breast Cancer: New Approaches and Concepts. Ann. Intern. Med. 1978, 88, 69–77. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil Gil, M.J.; Borrego, M.R.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. PARSIFAL: A randomized, multicenter, open-label, phase II trial to evaluate palbociclib in combination with fulvestrant or letrozole in endocrine-sensitive patients with estrogen receptor (ER)[+]/HER2[-] metastatic breast cancer. J. Clin. Oncol. 2020, 38, 1007. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Hess, K.R.; Esteva, F. Effect of HER2 status on distant recurrence in early stage breast cancer. Breast Cancer Res. Treat. 2013, 137, 449–455. [Google Scholar] [CrossRef]
- Ishihara, M.; Mukai, H.; Nagai, S.; Onozawa, M.; Nihei, K.; Shimada, T.; Wada, N. Retrospective Analysis of Risk Factors for Central Nervous System Metastases in Operable Breast Cancer: Effects of Biologic Subtype and Ki67 Overexpression on Survival. Oncology 2013, 84, 135–140. [Google Scholar] [CrossRef]
- Nie, F.; Yang, J.; Wen, S.; An, Y.-L.; Ding, J.; Ju, S.-H.; Zhao, Z.; Chen, H.-J.; Peng, X.-G.; Wong, S.T.C.; et al. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer 2012, 118, 5198–5209. [Google Scholar] [CrossRef] [Green Version]
- Soni, D.A.; Ren, Z.; Hameed, O.; Chanda, D.; Morgan, C.J.; Siegal, G.P.; Wei, S. Breast Cancer Subtypes Predispose the Site of Distant Metastases. Am. J. Clin. Pathol. 2015, 143, 471–478. [Google Scholar] [CrossRef]
- Lin, N.U.; Gaspar, L.E.; Soffietti, R. Breast Cancer in the Central Nervous System: Multidisciplinary Considerations and Management. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 45–56. [Google Scholar] [CrossRef]
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; et al. The incidence of brain metastases among patients with metastatic breast cancer: A systematic review and meta-analysis. Neuro-Oncology 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Arvold, N.D.; Oh, K.S.; Niemierko, A.; Taghian, A.G.; Lin, N.U.; Abi-Raad, R.F.; Sreedhara, M.; Harris, J.R.; Alexander, B.M. Brain metastases after breast-conserving therapy and systemic therapy: Incidence and characteristics by biologic subtype. Breast Cancer Res. Treat. 2012, 136, 153–160. [Google Scholar] [CrossRef]
- Bachmann, C.; Grischke, E.M.; Staebler, A.; Schittenhelm, J.; Wallwiener, D. Receptor change-clinicopathologic analysis of matched pairs of primary and cerebral metastatic breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1909–1916. [Google Scholar] [CrossRef]
- Bachmann, C.; Grischke, E.M.; Fehm, T.; Staebler, A.; Schittenhelm, J.; Wallwiener, D. CNS metastases of breast cancer show discordant immunohistochemical phenotype compared to primary. J. Cancer Res. Clin. Oncol. 2013, 139, 551–556. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, J.-S.; Kim, I.A. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J. Cancer Res. Clin. Oncol. 2018, 144, 1803–1816. [Google Scholar] [CrossRef]
- Riecke, K.; Müller, V.; Weide, R.; Schmidt, M.; Park-Simon, T.-W.; Möbus, V.; Mundhenke, C.; Polasik, A.; Lübbe, K.; Hesse, T.; et al. Predicting Prognosis of Breast Cancer Patients with Brain Metastases in the BMBC Registry—Comparison of Three Different GPA Prognostic Scores. Cancers 2021, 13, 844. [Google Scholar] [CrossRef]
- Laakmann, E.; Riecke, K.; Goy, Y.; Kersten, J.F.; Krüll, A.; Müller, V.; Petersen, C.; Witzel, I. Comparison of nine prognostic scores in patients with brain metastases of breast cancer receiving radiotherapy of the brain. J. Cancer Res. Clin. Oncol. 2016, 142, 325–332. [Google Scholar] [CrossRef]
- Darlix, A.; Louvel, G.; Fraisse, J.; Jacot, W.; Brain, E.; Debled, M.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; Delaloge, S.; et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br. J. Cancer 2019, 121, 991–1000. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Lorger, M.; Felding-Habermann, B. Capturing Changes in the Brain Microenvironment during Initial Steps of Breast Cancer Brain Metastasis. Am. J. Pathol. 2010, 176, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Lan, H.; Cai, X.; Zhang, Y.; Liang, A.; Li, J. Blood–Brain Barrier, Cell Junctions, and Tumor Microenvironment in Brain Metastases, the Biological Prospects and Dilemma in Therapies. Front. Cell Dev. Biol. 2021, 9, 722917. [Google Scholar] [CrossRef] [PubMed]
- Paku, S.; Döme, B.; Tóth, R.; Timár, J. Organ-specificity of the extravasation process: An ultrastructural study. Clin. Exp. Metastasis 2000, 18, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnár, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Tiwary, S.; Morales, J.E.; Kwiatkowski, S.C.; Lang, F.F.; Rao, G.; Mccarty, J.H. Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci. Rep. 2018, 8, 8267. [Google Scholar] [CrossRef] [Green Version]
- Fidler, I.J. The role of the organ microenvironment in brain metastasis. Semin. Cancer Biol. 2011, 21, 107–112. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [Green Version]
- Ljubimova, J.Y.; Sun, T.; Mashouf, L.; Ljubimov, A.V.; Israel, L.L.; Ljubimov, V.A.; Falahatian, V.; Holler, E. Covalent nano delivery systems for selective imaging and treatment of brain tumors. Adv. Drug Deliv. Rev. 2017, 113, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Lipid Solubility and Drug Penetration of the Blood Brain Barrier. Exp. Biol. Med. 1974, 147, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Brossi, A.; Pei, X.F.; Ingram, D.K.; Soncrant, T.T. Designing Drugs for Optimal Nervous System Activity. In New Concepts of a Blood—Brain Barrier; Greenwood, J., Begley, D.J., Segal, M.B., Eds.; Springer: Boston, MA, USA, 1995; pp. 251–264. [Google Scholar]
- Miller, D.S. Regulation of ABC transporters blood-brain barrier: The good, the bad, and the ugly. Adv. Cancer Res. 2015, 125, 43–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordon-Cardo, C.; O’Brien, J.P.; Casals, D.; Rittman-Grauer, L.; Biedler, J.L.; Melamed, M.R.; Bertino, J.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA 1989, 86, 695–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, B.; Doran, A.C.; Di, L.; West, M.A.; Osgood, S.M.; Mancuso, J.Y.; Shaffer, C.L.; Tremaine, L.; Liras, J. Prediction of Human Brain Penetration of P-glycoprotein and Breast Cancer Resistance Protein Substrates Using In Vitro Transporter Studies and Animal Models. J. Pharm. Sci. 2018, 107, 2225–2235. [Google Scholar] [CrossRef]
- Gao, B.; Hagenbuch, B.; A Kullak-Ublick, G.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar]
- Westholm, D.E.; Rumbley, J.N.; Salo, D.R.; Rich, T.P.; Anderson, G.W. Organic Anion-Transporting Polypeptides at the Blood–Brain and Blood–Cerebrospinal Fluid Barriers. Curr. Top. Dev. Biol. 2007, 80, 135–170. [Google Scholar] [CrossRef]
- Iorio, A.L.; Da Ros, M.; Fantappiè, O.; Lucchesi, M.; Facchini, L.; Stival, A.; Becciani, S.; Guidi, M.; Favre, C.; De Martino, M.; et al. Blood-Brain Barrier and Breast Cancer Resistance Protein: A Limit to the Therapy of CNS Tumors and Neurodegenerative Diseases. Anti-Cancer Agents Med. Chem. 2016, 16, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Hartz, A.M.; Elmquist, W.F.; Bauer, B. Breast Cancer Resistance Protein and P-Glycoprotein in Brain Cancer: Two Gatekeepers Team Up. Curr. Pharm. Des. 2011, 17, 2793–2802. [Google Scholar] [CrossRef] [Green Version]
- Delange, E. Potential role of ABC transporters as a detoxification system at the blood–CSF barrier. Adv. Drug Deliv. Rev. 2004, 56, 1793–1809. [Google Scholar] [CrossRef]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as therapeutic targets: Emerging opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef] [Green Version]
- Zlokovic, B.V.; Begley, D.J.; Chain-Eliash, D.G. Blood-brain barrier permeability to leucine-enkephalin,d-Alanine2-d-leucine5-enkephalin and their N-terminal amino acid (tyrosine). Brain Res. 1985, 336, 125–132. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS Delivery Via Adsorptive Transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Sauer, I.; Dunay, I.R.; Weisgraber, K.; Bienert, M.; Dathe, M. An Apolipoprotein E-Derived Peptide Mediates Uptake of Sterically Stabilized Liposomes into Brain Capillary Endothelial Cells. Biochemistry 2005, 44, 2021–2029. [Google Scholar] [CrossRef]
- Harris, M.G.; Hulseberg, P.D.; Ling, C.; Karman, J.; Clarkson, B.D.; Harding, J.S.; Zhang, M.; Sandor, A.; Christensen, K.B.; Nagy, A.; et al. Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci. Rep. 2014, 4, 4422. [Google Scholar] [CrossRef] [Green Version]
- Le Rhun, E.; Guckenberger, M.; Smits, M.; Dummer, R.; Bachelot, T.; Sahm, F.; Galldiks, N.; de Azambuja, E.; Berghoff, A.; Metellus, P.; et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann. Oncol. 2021, 32, 1332–1347. [Google Scholar] [CrossRef]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.-P.; et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- Tsao, M.N.; Rades, D.; Wirth, A.; Lo, S.S.; Danielson, B.L.; Gaspar, L.E.; Sperduto, P.W.; Vogelbaum, M.A.; Radawski, J.D.; Wang, J.Z.; et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract. Radiat. Oncol. 2012, 2, 210–225. [Google Scholar] [CrossRef] [Green Version]
- Soffietti, R.; Abacioglu, U.; Baumert, B.; Combs, S.E.; Kinhult, S.; Kros, J.M.; Marosi, C.; Metellus, P.; Radbruch, A.; Freixa, S.S.V.; et al. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of Neuro-Oncology (EANO). Neuro-Oncology 2017, 19, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs Stereotactic Radiosurgery Alone for Treatment of Brain Metastases. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Ling, D.C.; Vargo, J.A.; Wegner, R.E.; Flickinger, J.C.; Burton, S.A.; Engh, J.; Amankulor, N.; Quinn, A.E.; Ozhasoglu, C.; Heron, D.E. Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases: Clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neurosurgery 2015, 76, 150–156; discussion 156–157; quiz 157. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; I Laack, N.N.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Mix, M.; Elmarzouky, R.; O’Connor, T.; Plunkett, R.; Prasad, D. Clinical outcomes in patients with brain metastases from breast cancer treated with single-session radiosurgery or whole brain radiotherapy. J. Neurosurg. 2016, 125, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Halasz, L.M.; Uno, H.; Hughes, M.; D’Amico, T.; Dexter, E.U.; Edge, S.B.; Hayman, J.A.; Niland, J.C.; Otterson, G.A.; Pisters, K.M.W.; et al. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non-small cell lung cancer. Cancer 2016, 122, 2091–2100. [Google Scholar] [CrossRef] [Green Version]
- Tsao, M.; Xu, W.; Sahgal, A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 2012, 118, 2486–2493. [Google Scholar] [CrossRef]
- Hartgerink, D.; Bruynzeel, A.; Eekers, D.; Swinnen, A.; Hurkmans, C.; Wiggenraad, R.; Swaak-Kragten, A.; Dieleman, E.; van der Toorn, P.-P.; Oei, B.; et al. A Dutch phase III randomized multicenter trial: Whole brain radiotherapy versus stereotactic radiotherapy for 4–10 brain metastases. Neuro-Oncol. Adv. 2021, 3, vdab021. [Google Scholar] [CrossRef]
- Lobos-Urbina, D.; Kittsteiner-Manubens, L.; Peña, J. ¿Es efectiva la prevención primaria con anticonvulsivantes en tumores o metástasis cerebrales? (Is primary prevention with antiepileptic drugs effective in brain tumors or brain metastases?). Medwave 2017, 17, e6871. [Google Scholar] [CrossRef]
- Ryken, T.C.; McDermott, M.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; Kondziolka, D.; et al. The role of steroids in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neuro-Oncol. 2009, 96, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.M.; Messersmith, H.; Ahluwalia, M.; Andrews, D.; Brastianos, P.K.; E Gaspar, L.; Gatson, N.T.; Jordan, J.T.; Khasraw, M.; Lassman, A.B.; et al. Anticonvulsant prophylaxis and steroid use in adults with metastatic brain tumors: Summary of SNO and ASCO endorsement of the Congress of Neurological Surgeons guidelines*. Neuro-Oncology 2019, 21, 424–427. [Google Scholar] [CrossRef]
- Nahed, B.V.; Alvarez-Breckenridge, C.; Brastianos, P.K.; Shih, H.; Sloan, A.; Ammirati, M.; Kuo, J.S.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Surgery in the Management of Adults with Metastatic Brain Tumors. Neurosurgery 2019, 84, E152–E155. [Google Scholar] [CrossRef] [Green Version]
- Hulsbergen, A.F.C.; Claes, A.; Kavouridis, V.K.; Ansaripour, A.; Nogarede, C.; Hughes, M.E.; Smith, T.R.; Brastianos, P.K.; Verhoeff, J.J.C.; Lin, N.U.; et al. Subtype switching in breast cancer brain metastases: A multicenter analysis. Neuro Oncol. 2020, 22, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Estrogen/progesterone receptor and HER2 discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival. Neuro-Oncology 2020, 22, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Tonse, R.; Rubens, M.; McDermott, M.; Odia, Y.; Appel, H.; Mehta, M. 33. Systematic review and meta-analysis of breast cancer brain metastasis and primary tumor receptor expression discordance. Neuro-Oncol. Adv. 2020, 2, ii6. [Google Scholar] [CrossRef]
- Willman, M.; Willman, J.; Lucke-Wold, B. Endocrine resistant breast cancer: Brain metastasis. Explor. Target. Anti-Tumor Ther. 2022, 3, 240–251. [Google Scholar] [CrossRef]
- Bachelot, T.; Romieu, G.; Campone, M.; Diéras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.-Y.; Gonçalves, A.; et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2−positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef]
- Pivot, X.; Manikhas, A.; Żurawski, B.; Chmielowska, E.; Karaszewska, B.; Allerton, R.; Chan, S.; Fabi, A.; Bidoli, P.; Gori, S.; et al. CEREBEL (EGF111438): A Phase III, Randomized, Open-Label Study of Lapatinib Plus Capecitabine Versus Trastuzumab Plus Capecitabine in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer. J. Clin. Oncol. 2015, 33, 1564–1573. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Miles, D.; Im, Y.-H.; Quah, C.; Lee, L.F.; Cortes, J. Incidence of central nervous system metastases in patients with HER2−positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann. Oncol. 2014, 25, 1116–1121. [Google Scholar] [CrossRef]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Diéras, V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2−positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann. Oncol. 2015, 26, 113–119. [Google Scholar] [CrossRef]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2−Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619. [Google Scholar] [CrossRef]
- Jerusalem, G.H.M.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Modi, S.; Andre, F.; Krop, I.E.; Gonzalez, X.; Hall, P.S.; You, B.; et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: A subgroup analysis of the DESTINY-Breast01 trial. J. Clin. Oncol. 2021, 39, 526. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab deruxtecan in HER2−positive breast cancer with brain metastases: A single-arm, phase 2 trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2−Positive Metastatic Breast Cancer Previously Treated with ≥ 2 HER2−Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Diéras, V.; Weaver, R.; Tolaney, S.M.; Bardia, A.; Punie, K.; Brufsky, A.; Rugo, H.S.; Kalinsky, K.; Traina, T.; Klein, L.; et al. Abstract PD13-07: Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res. 2021, 81, PD13-07. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef]
- Schlam, I.; Tolaney, S.M. Is there a role for CDK 4/6 inhibitors in breast cancer brain metastases? Oncotarget 2021, 12, 873–875. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Chao, S.T.; Shanley, R.; Luo, X.; Sneed, P.K.; Suh, J.; Weil, R.J.; Jensen, A.W.; et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J. Neuro-Oncol. 2013, 112, 467–472. [Google Scholar] [CrossRef]
- Melisko, M.E.; Moore, D.H.; Sneed, P.K.; De Franco, J.; Rugo, H.S. Brain metastases in breast cancer: Clinical and pathologic characteristics associated with improvements in survival. J. Neuro-Oncol. 2008, 88, 359–365. [Google Scholar] [CrossRef]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment strategies for breast cancer brain metastases. Br. J. Cancer 2021, 124, 142–155. [Google Scholar] [CrossRef]
- Jensen, E.V.; Jacobson, H.I. Fate of Steroid Estrogens in Target Tissues. In Biological Activities of Steroids in Relation to Cancer; Elsevier: Amsterdam, The Netherlands, 1960; pp. 161–178. [Google Scholar]
- Harper, M.J.K.; Walpole, A.L. A new derivative of triphenylethylene: Effect on implantation and mode of action in rats. Reproduction 1967, 13, 101–119. [Google Scholar] [CrossRef]
- Cole, M.P.; Jones, C.T.; Todd, I.D.H. A New Anti-oestrogenic Agent in Late Breast Cancer: An Early Clinical Appraisal of ICI46474. Br. J. Cancer 1971, 25, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Bedford, G.R.; Richardson, D.N. Preparation and Identification of cis and trans Isomers of a Substituted Triarylethylene. Nature 1966, 212, 733–734. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Ward, H.W.C. Anti-oestrogen Therapy for Breast Cancer: A Trial of Tamoxifen at Two Dose Levels. BMJ 1973, 1, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, S.R.; Siddhanti, S.; Ciaccia, A.V.; Plouffe, L. A pharmacological review of selective oestrogen receptor modulators. Hum. Reprod. Updat. 2000, 6, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Mourits, M.J.; De Vries, E.G.; Willemse, P.H.; Hoor, K.A.T.; Hollema, H.; Van Der Zee, A.G. Tamoxifen treatment and gynecologic side effects: A review. Obstet. Gynecol. 2001, 97, 855–866. [Google Scholar] [CrossRef]
- Novick, A.M.; Scott, A.T.; Epperson, C.N.; Schneck, C.D. Neuropsychiatric effects of tamoxifen: Challenges and opportunities. Front. Neuroendocr. 2020, 59, 100869. [Google Scholar] [CrossRef]
- Demissie, S.; Silliman, R.; Lash, T.L. Adjuvant Tamoxifen: Predictors of Use, Side Effects, and Discontinuation in Older Women. J. Clin. Oncol. 2001, 19, 322–328. [Google Scholar] [CrossRef]
- Pluss, J.L.; DiBella, N.J. Reversible Central Nervous System Dysfunction Due to Tamoxifen in a Patient with Breast Cancer. Ann. Intern. Med. 1984, 101, 652. [Google Scholar] [CrossRef]
- Noureddin, B.; Seoud, M.; Bashshur, Z.F.; Salem, Z.; Shamseddin, A.; Khalil, A. Ocular toxicity in low-dose tamoxifen: A prospective study. Eye 1999, 13 Pt 6, 729–733. [Google Scholar] [CrossRef]
- Tang, R.; Shields, J.; Schiffman, J.; Li, H.; Locher, D.; Hampton, J.; Prager, T.; Pardo, G. Retinal changes associated with tamoxifen treatment for breast cancer. Eye 1997, 11 Pt 3, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.A.; Solheim, E.; Ueland, P.M. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991, 51, 4837–4844. [Google Scholar] [PubMed]
- Pareto, D.; Alvarado, M.; Hanrahan, S.; Biegon, A. In vivo occupancy of female rat brain estrogen receptors by 17β-estradiol and tamoxifen. NeuroImage 2004, 23, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Pors, H.; Von Eyben, F.E.; Sørensen, O.S.; Larsen, M. Longterm remission of multiple brain metastases with tamoxifen. J. Neuro-Oncol. 1991, 10, 173–177. [Google Scholar] [CrossRef]
- Salvati, M.; Cervoni, L.; Innocenzi, G.; Bardella, L. Prolonged Stabilization of Multiple and Single Brain Metastases from Breast Cancer with Tamoxifen. Report of Three Cases. Tumori J. 1993, 79, 359–362. [Google Scholar] [CrossRef]
- Colomer, R.; Cosos, D.; Del Campo, J.M.; Boada, M.; Rubio, D.; Salvador, L.; Casas, D. Brain metastases from breast cancer may respond to endocrine therapy. Breast Cancer Res. Treat. 1988, 12, 83–86. [Google Scholar] [CrossRef]
- Furr, B.J. (Ed.) Aromatase Inhibitors. In Milestones in Drug Therapy MDT; Birkhäuser Verlag: Basel, Switzerland, 2006. [Google Scholar]
- Thompson, E.A.; Siiteri, P.K. The Involvement of Human Placental Microsomal Cytochrome P-450 in Aromatization. J. Biol. Chem. 1974, 249, 5373–5378. [Google Scholar] [CrossRef]
- Brodie, A.M.; Santen, R.J.; Henderson, I.C. Aromatase in breast cancer and the role of aminoglutethimide and other aromatase inhibitors. Crit. Rev. Oncol. Hematol. 1986, 5, 361–396. [Google Scholar] [CrossRef]
- Barone, R.M.; Shamonki, I.M.; Siiteri, P.K.; Judd, H.L. Inhibition of peripheral aromatization of androstenedione to estrone in postmenopausal women with breast cancer using delta 1-testololactone. J. Clin. Endocrinol. Metab. 1979, 49, 672–676. [Google Scholar] [CrossRef]
- Howell, A.; Cuzick, J.; Baum, M.; Buzdar, A.; Dowsett, M.; Forbes, J.F.; Hoctin-Boes, G.; Houghton, J.; Locker, G.Y.; Tobias, J.S.; et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005, 365, 60–62. [Google Scholar] [CrossRef]
- Baum, M.; Budzar, A.U.; Cuzick, J.; Forbes, J.; Houghton, J.H.; Klijn, J.G.M.; Sahmoud, T.; ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet 2002, 359, 2131–2139. [Google Scholar] [CrossRef]
- Dave, N.; Gudelsky, G.A.; Desai, P.B. The pharmacokinetics of letrozole in brain and brain tumor in rats with orthotopically implanted C6 glioma, assessed using intracerebral microdialysis. Cancer Chemother. Pharmacol. 2013, 72, 349–357. [Google Scholar] [CrossRef]
- Miyajima, M.; Kusuhara, H.; Takahashi, K.; Takashima, T.; Hosoya, T.; Watanabe, Y.; Sugiyama, Y. Investigation of the effect of active efflux at the blood–brain barrier on the distribution of nonsteroidal aromatase inhibitors in the central nervous system. J. Pharm. Sci. 2013, 102, 3309–3319. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, B.; Liu, C.; Shi, S.; Ding, L.; Liu, J.; Wu, S. Brain metastases from breast cancer may respond to endocrine therapy: Report of two cases. OncoTargets Ther. 2019, 12, 1389–1393. [Google Scholar] [CrossRef] [Green Version]
- Bergen, E.S.; Berghoff, A.S.; Medjedovic, M.; Rudas, M.; Fitzal, F.; Bago-Horvath, Z.; Dieckmann, K.; Mader, R.M.; Exner, R.; Gnant, M.; et al. Continued Endocrine Therapy Is Associated with Improved Survival in Patients with Breast Cancer Brain Metastases. Clin. Cancer Res. 2019, 25, 2737–2744. [Google Scholar] [CrossRef]
- Martín, L.M.N.; Fernández, A.O.; Sánchez, C.A.R.; Martín, I.R.; Hernández, J.J.C. Durable clinical benefit with exemestane in leptomeningeal metastasis of breast cancer. Clin. Transl. Oncol. 2005, 7, 358–360. [Google Scholar] [CrossRef]
- Goyal, S.; Puri, T.; Julka, P.K.; Rath, G.K. Excellent response to letrozole in brain metastases from breast cancer. Acta Neurochir. 2008, 150, 613–614; discussion 614–615. [Google Scholar] [CrossRef]
- Carlson, R.W. The History and Mechanism of Action of Fulvestrant. Clin. Breast Cancer 2005, 6 (Suppl. S1), S5–S8. [Google Scholar] [CrossRef]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Final Overall Survival: Fulvestrant 500 mg vs. 250 mg in the Randomized CONFIRM Trial. JNCI J. Natl. Cancer Inst. 2014, 106, djt337. [Google Scholar] [CrossRef] [Green Version]
- Howell, A.; Abram, P. Clinical development of fulvestrant (‘Faslodex’). Cancer Treat. Rev. 2005, 31 (Suppl. 2), S3–S9. [Google Scholar] [CrossRef]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.I.; Rolski, J.; Papai, Z.; Mauriac, L.; Cardoso, F.; Chang, J.; Panasci, L.; Ianuli, C.; Kahan, Z.; Fukase, K.; et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2). Breast Cancer Res. Treat. 2010, 123, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Osborne, C.K.; Morris, C.; Wakeling, A.E. ICI 182,780 (Faslodex?). Cancer 2000, 89, 817–825. [Google Scholar] [CrossRef]
- Karten, M.J. An Overview of GnRH Antagonist Development: Two Decades of Progress. In Modes of Action of GnRH and GnRH Analogs; Crowley, W.F., Conn, P.M., Eds.; Springer: New York, NY, USA, 1992; pp. 277–297. [Google Scholar]
- Robertson, J.; Blamey, R. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur. J. Cancer 2003, 39, 861–869. [Google Scholar] [CrossRef]
- Jonat, W. Goserelin (Zoladex)—Its role in early breast cancer in pre- and perimenopausal women. Br. J. Cancer 2001, 85 (Suppl 2), 1–5. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.J.; Juneau-Norcross, M.; Rein, M.S. Adverse effects of leuprolide acetate depot treatment. Fertil. Steril. 1993, 59, 448–450. [Google Scholar] [CrossRef]
- Kaufmann, M.; Jonat, W.; Blamey, R.; Cuzick, J.; Namer, M.; Fogelman, I.; de Haes, J.; Schumacher, M.; Sauerbrei, W. Survival analyses from the ZEBRA study: Goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer. Eur. J. Cancer 2003, 39, 1711–1717. [Google Scholar] [CrossRef]
- Ermisch, A.; Rühle, H.-J.; Klauschenz, E.; Kretzschmar, R. On the Blood-Brain Barrier to Peptides: [3H]Gonadotropin-Releasing Hormone Accumulation by Eighteen Regions of the Rat Brain and by Anterior Pituitary. Exp. Clin. Endocrinol. Diabetes 1984, 84, 112–116. [Google Scholar] [CrossRef]
- Wilson, A.C.; Clemente, L.; Liu, T.; Bowen, R.L.; Meethal, S.V.; Atwood, C.S. Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2008, 1782, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., III; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Yardley, D.A.; Noguchi, S.; Pritchard, K.I.; Burris, H.A., III; Baselga, J.; Gnant, M.; Hortobagyi, G.N.; Campone, M.; Pistilli, B.; Piccart, M.; et al. Everolimus Plus Exemestane in Postmenopausal Patients with HR+ Breast Cancer: BOLERO-2 Final Progression-Free Survival Analysis. Adv. Ther. 2013, 30, 870–884. [Google Scholar] [CrossRef] [Green Version]
- Royce, M.; Bachelot, T.; Villanueva, C.; Özgüroglu, M.; Azevedo, S.J.; Cruz, F.M.; Debled, M.; Hegg, R.; Toyama, T.; Falkson, C.; et al. Everolimus Plus Endocrine Therapy for Postmenopausal Women With Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer. JAMA Oncol. 2018, 4, 977–984. [Google Scholar] [CrossRef]
- Bachelot, T.; Bourgier, C.; Cropet, C.; Ray-Coquard, I.; Ferrero, J.-M.; Freyer, G.; Abadie-Lacourtoisie, S.; Eymard, J.-C.; Debled, M.; Spaëth, D.; et al. Randomized Phase II Trial of Everolimus in Combination With Tamoxifen in Patients With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer With Prior Exposure to Aromatase Inhibitors: A GINECO Study. J. Clin. Oncol. 2012, 30, 2718–2724. [Google Scholar] [CrossRef]
- Kornblum, N.; Zhao, F.; Manola, J.; Klein, P.; Ramaswamy, B.; Brufsky, A.; Stella, P.J.; Burnette, B.; Telli, M.; Makower, D.F.; et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. 2018, 36, 1556–1563. [Google Scholar] [CrossRef]
- Fox, J.H.; Connor, T.; Chopra, V.; Dorsey, K.; A Kama, J.; Bleckmann, D.; Betschart, C.; Hoyer, D.; Frentzel, S.; DiFiglia, M.; et al. The mTOR kinase inhibitor Everolimus decreases S6 kinase phosphorylation but fails to reduce mutant huntingtin levels in brain and is not neuroprotective in the R6/2 mouse model of Huntington’s disease. Mol. Neurodegener. 2010, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, T.; McSheehy, P.M.J.; Kawai, R.; Kretz, O.; McMahon, L.; Brueggen, J.; Bruelisauer, A.; Gschwind, H.-P.; Allegrini, P.R.; Lane, H.A. Comparative pharmacokinetics of RAD001 (everolimus) in normal and tumor-bearing rodents. Cancer Chemother. Pharmacol. 2010, 65, 625–639. [Google Scholar] [CrossRef]
- Galanis, E.; Buckner, J.C.; Maurer, M.J.; Kreisberg, J.I.; Ballman, K.; Boni, J.; Peralba, J.M.; Jenkins, R.B.; Dakhil, S.R.; Morton, R.F.; et al. Phase II Trial of Temsirolimus (CCI-779) in Recurrent Glioblastoma Multiforme: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2005, 23, 5294–5304. [Google Scholar] [CrossRef]
- Mason, W.P.; MacNeil, M.; Kavan, P.; Easaw, J.; Macdonald, D.; Thiessen, B.; Urva, S.; Lwin, Z.; McIntosh, L.; Eisenhauer, E. A phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: An NCIC CTG study. Investig. New Drugs 2012, 30, 2344–2351. [Google Scholar] [CrossRef]
- Van Swearingen, A.E.D.; Siegel, M.B.; Deal, A.M.; Sambade, M.J.; Hoyle, A.; Hayes, D.N.; Jo, H.; Little, P.; Dees, E.C.; Muss, H.; et al. LCCC 1025: A phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2−positive breast cancer brain metastases. Breast Cancer Res. Treat. 2018, 171, 637–648. [Google Scholar] [CrossRef]
- Hurvitz, S.; Singh, R.; Adams, B.; Taguchi, J.A.; Chan, D.; Dichmann, R.A.; Castrellon, A.; Hu, E.; Berkowitz, J.; Mani, A.; et al. Phase Ib/II single-arm trial evaluating the combination of everolimus, lapatinib and capecitabine for the treatment of HER2−positive breast cancer with brain metastases (TRIO-US B-09). Ther. Adv. Med Oncol. 2018, 10, 1758835918807339. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.-B.; Li, G.-L.; Zheng, Y.-B.; Chen, Z.-H.; Cao, W.-M.; Wang, X.-J.; Shao, X.-Y. Combined everolimus and endocrine therapy in advanced HR-positive, HER2−negative Chinese breast cancer patients: A retrospective study. Ann. Transl. Med. 2021, 9, 1334. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Agamennone, M.; De Filippis, B.; Fantacuzzi, M. Development of CDK4/6 Inhibitors: A Five Years Update. Molecules 2021, 26, 1488. [Google Scholar] [CrossRef]
- Cersosimo, R.J. Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women. Am. J. Health Pharm. 2019, 76, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2−negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Pedersini, R.; Cabiddu, M.; Borgonovo, K.; Parati, M.C.; Ghilardi, M.; Amoroso, V.; Berruti, A.; Barni, S. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: An adjusted indirect analysis of randomized controlled trials. Breast Cancer Res. Treat. 2019, 174, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Berchialla, P.; Giannarelli, D.; Nisticò, C.; Ferretti, G.; Gasparro, S.; Russillo, M.; Catania, G.; Vigna, L.; Mancusi, R.L.; et al. Should All Patients With HR-Positive HER2−Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers 2019, 11, 1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Gao, S.; Li, D.; Ran, X.; Sheng, Z.; Wu, W.; Yang, X. CDK4/6 inhibitors plus endocrine therapy improve overall survival in advanced HR+/HER2− breast cancer: A meta-analysis of randomized controlled trials. Breast J. 2019, 26, 1439–1443. [Google Scholar] [CrossRef]
- Finn, R.S.; Boer, K.; Bondarenko, I.; Patel, R.; Pinter, T.; Schmidt, M.; Shparyk, Y.V.; Thummala, A.; Voitko, N.; Bananis, E.; et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 2020, 183, 419–428. [Google Scholar] [CrossRef]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil-Gil, M.; Ruíz-Borrego, M.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Fulvestrant-Palbociclib vs. Letrozole-Palbociclib as Initial Therapy for Endocrine-Sensitive, Hormone Receptor–Positive, ERBB2-Negative Advanced Breast Cancer. JAMA Oncol. 2021, 7, 1791–1799. [Google Scholar] [CrossRef]
- Yardley, D.A.; Hart, L.; Favret, A.; Blau, S.; Diab, S.; Richards, D.; Sparano, J.; Beck, J.T.; Richards, P.; Ward, P.; et al. Efficacy and Safety of Ribociclib With Letrozole in US Patients Enrolled in the MONALEESA-2 Study. Clin. Breast Cancer 2019, 19, 268–277.e1. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef]
- Johnston, S.; O’Shaughnessy, J.; Martin, M.; Huober, J.; Toi, M.; Sohn, J.; André, V.A.M.; Martin, H.R.; Hardebeck, M.C.; Goetz, M.P. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. npj Breast Cancer 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Im, S.-A.; Mukai, H.; Park, I.H.; Masuda, N.; Shimizu, C.; Kim, S.-B.; Im, Y.-H.; Ohtani, S.; Bartlett, C.H.; Lu, D.R.; et al. Palbociclib Plus Letrozole as First-Line Therapy in Postmenopausal Asian Women With Metastatic Breast Cancer: Results From the Phase III, Randomized PALOMA-2 Study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Thill, M.; Schmidt, M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758835918793326. [Google Scholar] [CrossRef] [Green Version]
- Michaud, K.; Solomon, D.A.; Oermann, E.; Kim, J.-S.; Zhong, W.-Z.; Prados, M.D.; Ozawa, T.; James, C.D.; Waldman, T. Pharmacologic Inhibition of Cyclin-Dependent Kinases 4 and 6 Arrests the Growth of Glioblastoma Multiforme Intracranial Xenografts. Cancer Res. 2010, 70, 3228–3238. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Hong, Y.; Mao, Y.; Chen, N.; Wang, Q.; Wang, Z.; Zhang, L.; Wang, L.; Shi, C.; Shi, W.; et al. SPH3643: A novel cyclin-dependent kinase 4/6 inhibitor with good anticancer efficacy and strong blood-brain barrier permeability. Cancer Sci. 2020, 111, 1761–1773. [Google Scholar] [CrossRef]
- Parrish, K.E.; Pokorny, J.L.; Mittapalli, R.K.; Bakken, K.; Sarkaria, J.N.; Elmquist, W.F. Efflux Transporters at the Blood-Brain Barrier Limit Delivery and Efficacy of Cyclin-Dependent Kinase 4/6 Inhibitor Palbociclib (PD-0332991) in an Orthotopic Brain Tumor Model. J. Pharmacol. Exp. Ther. 2015, 355, 264–271. [Google Scholar] [CrossRef]
- De Gooijer, M.C.; Zhang, P.; Thota, N.; Mayayo-Peralta, I.; Buil, L.C.M.; Beijnen, J.H.; van Tellingen, O. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Investig. New Drugs 2015, 33, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, C.A.; Kumthekar, P.; Rademaker, A.; Gross, L.; Jain, S.; Flaum, L.E.; Gradishar, W.J.; Cristofanilli, M. A pilot study of palbociclib in patients with HER2−positive breast cancer with brain metastasis. J. Clin. Oncol. 2017, 35, TPS1110. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, G.; Li, W.; Wang, T.; Zhao, Y.; Wu, Q. CDK4/6 Inhibitors in Combination With Hormone Therapy for HR+/HER2− Advanced Breast Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Breast Cancer 2018, 18, e943–e953. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.T.; Davis, A.; Baker, S.J.; Campagne, O.; Stewart, C.F. CNS penetration of the CDK4/6 inhibitor ribociclib in non-tumor bearing mice and mice bearing pediatric brain tumors. Cancer Chemother. Pharmacol. 2019, 84, 447–452. [Google Scholar] [CrossRef]
- Miller, T.W.; Traphagen, N.A.; Li, J.; Lewis, L.D.; Lopes, B.; Asthagiri, A.; Loomba, J.; De Jong, J.; Schiff, D.; Patel, S.H.; et al. Tumor pharmacokinetics and pharmacodynamics of the CDK4/6 inhibitor ribociclib in patients with recurrent glioblastoma. J. Neuro-Oncol. 2019, 144, 563–572. [Google Scholar] [CrossRef]
- Martínez-Chávez, A.; van Hoppe, S.; Rosing, H.; Lebre, M.C.; Tibben, M.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein Limits Ribociclib Brain Exposure and CYP3A4 Restricts Its Oral Bioavailability. Mol. Pharm. 2019, 16, 3842–3852. [Google Scholar] [CrossRef]
- Radke, I.; von Wahlde, M.-K.; Schülke, C.; Tio, J. Ribociclib in Breast Cancer Brain Metastases: A Case Report. Breast Care 2019, 15, 543–547. [Google Scholar] [CrossRef]
- Cottu, P.; Ring, A.; Abdel-Razeq, H.; Marchetti, P.; Cardoso, F.; Bofill, J.S.; Martín, M.; Menon-Singh, L.; Wu, J.; De Laurentiis, M. Ribociclib plus letrozole in subgroups of special clinical interest with hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: Subgroup analysis of the phase IIIb CompLEEment-1 trial. Breast 2022, 62, 75–83. [Google Scholar] [CrossRef]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Lin, N.U.; Thornton, D.; Klise, S.; Costigan, T.M.; Turner, P.K.; Anders, C.K. Abemaciclib for the treatment of brain metastases (BM) secondary to hormone receptor positive (HR+), HER2 negative breast cancer. J. Clin. Oncol. 2017, 35, 1019. [Google Scholar] [CrossRef]
- Raub, T.J.; Wishart, G.N.; Kulanthaivel, P.; Staton, B.A.; Ajamie, R.T.; Sawada, G.A.; Gelbert, L.M.; Shannon, H.E.; Sanchez-Martinez, C.; De Dios, A. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab. Dispos. 2015, 43, 1360–1371. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas (TCGA) Research Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Christgen, M.; Noskowicz, M.; Schipper, E.; Christgen, H.; Heil, C.; Krech, T.; Länger, F.; Kreipe, H.; Lehmann, U. OncogenicPIK3CAmutations in lobular breast cancer progression. Genes Chromosom. Cancer 2013, 52, 69–80. [Google Scholar] [CrossRef]
- Berlin, 19.04.2021—Marktrücknahme von Alpelisib zulasten von BrustkrebspatientInnen. Geburtshilfe Frauenheilkd 2021, 81, 604–605. [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Fitzgerald, D.; Muzikansky, A.; Pinto, C.; Henderson, L.; Walmsley, C.; Allen, R.; Ferraro, G.; Isakoff, S.; Moy, B.; Oh, K.; et al. Association between PIK3CA mutation status and development of brain metastases in HR+/HER2− metastatic breast cancer. Ann. Oncol. 2019, 30, v110. [Google Scholar] [CrossRef]
- Morin, G.; Degrugillier-Chopinet, C.; Vincent, M.; Fraissenon, A.; Aubert, H.; Chapelle, C.; Hoguin, C.; Dubos, F.; Catteau, B.; Petit, F.; et al. Treatment of two infants with PIK3CA-related overgrowth spectrum by alpelisib. J. Exp. Med. 2022, 219, e20212148. [Google Scholar] [CrossRef]
- Hedges, C.; Boix, J.; Jaiswal, J.; Shetty, B.; Shepherd, P.; Merry, T. Efficacy of Providing the PI3K p110α Inhibitor BYL719 (Alpelisib) to Middle-Aged Mice in Their Diet. Biomolecules 2021, 11, 150. [Google Scholar] [CrossRef]
| Antihormonal Therapy | Blood-Brain Barrier Permeability |
|---|---|
| Tamoxifen | positive |
| AI | positive |
| Fulvestrant | negative |
| GnRH-analogue | positive |
| Everolimus | positive |
| CDK 4/6 inhibitors | |
| Palbocolib | positive |
| Ribociclib | positive |
| Abemaciclib | positive |
| Alpelisib | negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtaz, C.J.; Kiesel, L.; Meybohm, P.; Wöckel, A.; Burek, M. Anti-Hormonal Therapy in Breast Cancer and Its Effect on the Blood-Brain Barrier. Cancers 2022, 14, 5132. https://doi.org/10.3390/cancers14205132
Curtaz CJ, Kiesel L, Meybohm P, Wöckel A, Burek M. Anti-Hormonal Therapy in Breast Cancer and Its Effect on the Blood-Brain Barrier. Cancers. 2022; 14(20):5132. https://doi.org/10.3390/cancers14205132
Chicago/Turabian StyleCurtaz, Carolin J., Ludwig Kiesel, Patrick Meybohm, Achim Wöckel, and Malgorzata Burek. 2022. "Anti-Hormonal Therapy in Breast Cancer and Its Effect on the Blood-Brain Barrier" Cancers 14, no. 20: 5132. https://doi.org/10.3390/cancers14205132
APA StyleCurtaz, C. J., Kiesel, L., Meybohm, P., Wöckel, A., & Burek, M. (2022). Anti-Hormonal Therapy in Breast Cancer and Its Effect on the Blood-Brain Barrier. Cancers, 14(20), 5132. https://doi.org/10.3390/cancers14205132







