Simple Summary
Cancer is a disease which affects approximately 40% of people in their lifetime. Chemotherapy, the primary choice for treatment of cancer, is often ineffective or/and presents itself with many debilitating side effects, including loss of appetite, nausea, insomnia, and anxiety. Components of cannabis extracts, including cannabinoids and terpenes, may present an alternative for controlling side effects and may be used for tumor shrinkage together with chemodrugs. Cannabinoids act on so called endocannabinoid system (ECS) that operates in our body to maintain homeostasis. ECS promotes healthy development of tissues and regulates many processes in our organism and when disbalanced may lead to disease, including cancer. In this review, we will discuss the role of the ECS in relation with carcinogenesis and use of cannabis extracts and their components for primary and secondary care of cancer. Knowledge about the use of cannabinoids for cancer therapy may prolong the life of many cancer patients.
Abstract
The endocannabinoid system (ECS) is an ancient homeostasis mechanism operating from embryonic stages to adulthood. It controls the growth and development of many cells and cell lineages. Dysregulation of the components of the ECS may result in uncontrolled proliferation, adhesion, invasion, inhibition of apoptosis and increased vascularization, leading to the development of various malignancies. Cancer is the disease of uncontrolled cell division. In this review, we will discuss whether the changes to the ECS are a cause or a consequence of malignization and whether different tissues react differently to changes in the ECS. We will discuss the potential use of cannabinoids for treatment of cancer, focusing on primary outcome/care—tumor shrinkage and eradication, as well as secondary outcome/palliative care—improvement of life quality, including pain, appetite, sleep, and many more factors. Finally, we will complete this review with the chapter on sex- and gender-specific differences in ECS and response to cannabinoids, and equality of the access to treatments with cannabinoids.
1. Introduction
Endocannabinoid system (ECS) is an ancient (over 600 mln years old), evolutionary stable animal homeostasis system [1]. It consists of three components—ligands, including 2-arachidonoylglycerol (2-AG) and arachidonoyl ethanolamide (AEA or anandamide), receptors, such as cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), and the metabolizing enzymes—fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). As a regulator of homeostasis, ECS regulates the activity of brain, endocrine, and immune systems, among others. One such regulatory mechanism is the regulation of energy metabolism. ECS increases the energy intake, facilitates its storage, and decreases the expenditure [2]. Central regulators of energy metabolism, such as hypothalamic orexigenic neuropeptide Y (NPY) and anorexigenic cocaine and amphetamine regulated transcript (CART) peptide and peripheral regulators, such as leptin (LEP), ghrelin (GHRL) adiponectin (ADIPOQ), and cholecystokinin (CCK), are known to be dysregulated in various cancers and to contribute to malignancy (reviewed in [3]).
Genes responsible for energy homeostasis play essential roles in the organism, and dysregulation of energetic metabolism not only results in the development of metabolic syndrome, obesity, and diabetes, but is also linked to many cancers [4]. Detailed description of the role of ECS in controlling energy homeostasis is beyond the scope of this review and is presented well elsewhere [5].
Cancer is a disease of dysregulated and uncontrolled cell division and cell proliferation. Successful malignization requires mutations in multiple genes [6]. Numerous theories of cancer development and progression exist. Currently, most cancers have no cure; even so, significant progress in the development of chemotherapy and immune therapy of cancers has been achieved. Cancer therapy consists of primary care, directed at tumor eradication and palliative care, which aims to reduce side effects and suffering of a patient.
Cannabinoids as endogenous regulators of homeostasis are the molecules that can potentially be used for cancer therapy. They may be particularly useful in palliative care.
In this review, we will discuss the role of the endocannabinoid system in the control of cell growth and proliferation, describe changes in various cells and tissues that occur in ECS in response to carcinogenesis, describe major mechanism of action of endo- and phytocannabinoids on various cancers, discuss data from cell, animal, and human studies, and discuss the use of various cannabinoids for primary and palliative care.
2. Role of Endocannabinoids in the Human Body
ECS is active in virtually all cells of our organism. It plays an important role in the reproduction, function, and proper development of gametes [7], fertilization event, embryo implantation, and proper placenta development [8]. It is also active at all stages of embryogenesis, regulating cell division, and tissue and organ development, specifically, regulating differentiation of neural progenitors, synaptogenesis, and axonal migration [9]. During human adult life, it regulates homeostasis of many tissues, playing critical role in proper brain function by regulating neuronal synaptic communications affecting critical organismal functions, including general metabolism, growth and development, reproduction, learning and memory formation, mood, and behavior, among others [10]. In the peripheral tissues, endocannabinoids are involved in endocrine regulation and energy balance [11], as well as regulating the function of innate and adaptive immune system and immune response [12], regulating cell migration and apoptosis. The activity and functionality of ECS depends on many factors, from cell- and tissue-specific differences in the synthesis of endocannabinoids, to the number and the activity of endocannabinoid and auxiliary receptors, to the expression and the activity of enzymes involved in the degradation of circulating endocannabinoids.
In the cells, endocannabinoids acting in CB-receptor-dependent and independent manner exhibit anti-oxidative properties, are involved in clearance of damaged molecules and regulate mitochondrial activity. Anti-oxidative properties are associated with the inhibition of production of reactive oxygen species (ROS), metal chelation and prevention/alleviation of ROS-induced cell damage [13]. It should be noted that the anti-oxidative effects of cannabinoids are cell specific—while in most cells of the body, they mitigate oxidative stress, in hepatic cells they may cause it, leading to cell death [14]. Similarly, in cancer cells, such as gliomas and leukemia, cannabinoids promote oxidative stress [13].
Cannabinoids contribute to recycling of damaged molecules and are likely involved in autophagy in health tissues [15]—the activity well documented in cancer cells (discussed below). In normal cells, they increase lysosomal stability and integrity [15] through CB1 receptors found on the surface of lysosomes.
CB1 receptors are also present on the surface of mitochondria. They regulate mitochondrial oxidative phosphorylation in a positive and a negative manner, acting through the CB1 receptor, but it is not clear what modulates this activity [13]. When cells are stressed, cannabinoids attenuate mitochondrial damage [16] and decrease calcium-induced cytochrome c release [17].
2.1. Mechanism of Action—Ligand/Receptor
Cannabinoid receptors are ubiquitous and expressed on the cell surface as well on cell organelles, including mitochondria and lysosomes. Classical cannabinoid receptors include CB1 and CB2. CB1 is expressed at a higher level in central and peripheral nervous systems, while CB2 is expressed in many different tissues, including the immune system, internal organs, skin, bone, muscle, and glia in the brain [18]. CB1 and CB2 are GPCR (Gi/o) protein-coupled receptors, and when activated, they modulate various cellular functions through receptor internalization; interaction with other G-protein-coupled receptors; inhibition of adenylyl cyclase activity, changing the activity of calcium and potassium channels; increasing phosphorylation of various mitogen-activated protein kinases (MAPK); and many more functions [12] (Figure 1).
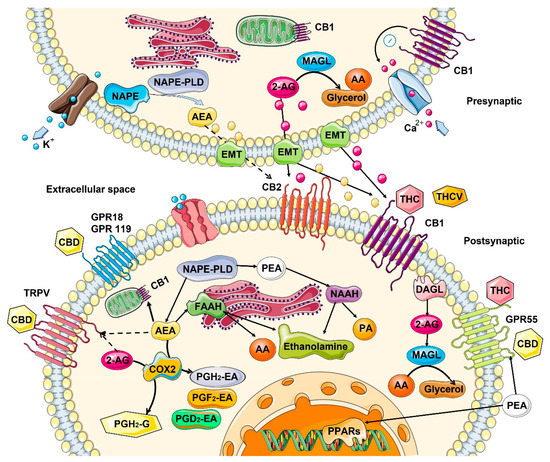
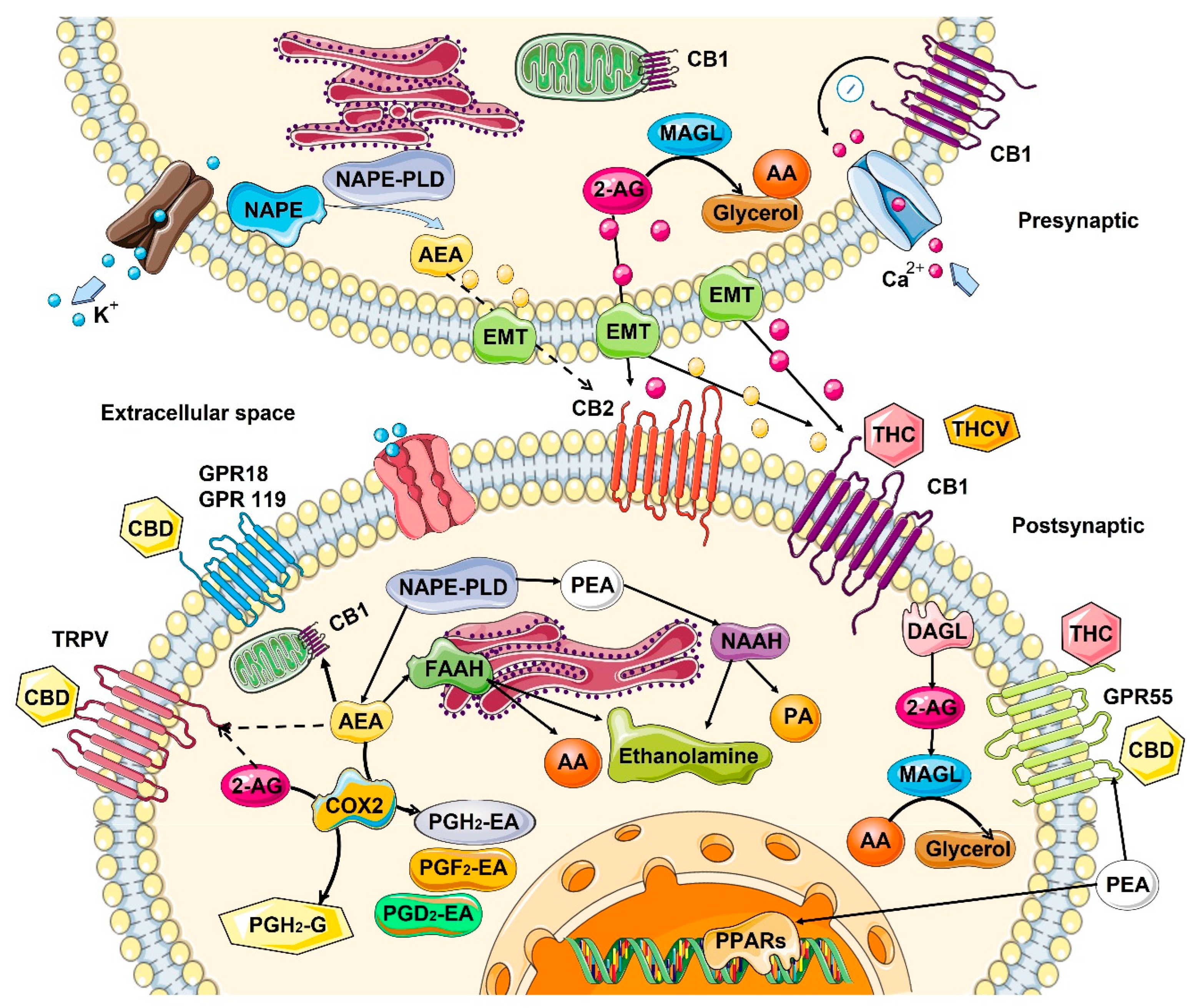
Figure 1.
Biosynthesis, degradation, and interaction of endocannabinoids with cannabinoid receptors. Biosynthesis and the inactivation of the two endogenous lipid messengers, such as endocannabinoids N-Arachidonoylethanolamine or anandamide (AEA), 2-arachidonoylglycerol (2-AG), and N-palmitoylethanolamide (PEA) act on cannabinoid receptors. AEA and 2-AG are typically released on demand from membrane lipids. AEA synthesized from N-arachidonoyl-phosphatidylethanolamines (NAPE) via the activity of N-acyl-phosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) and hydrolyzed by fatty acid amide hydrolase (FAAH) to ethanolamine and arachidonic acid (AA). 2-AG is 2-AG can also be produced from sn-2-arachidonate-containing diacylglycerols by sn-1-acyl-2-arachidonoylglycerol lipase (DAGL), and degraded by lipase (MAGL), releasing glycerol and AA. PEA is hydrolyzed by N-acylethanolamine-hydrolyzing acid amidase (NAAA) into ethanolamine and palmitic acid (PA). Cyclooxygenase-2 (COX-2) can also oxidize anandamide and 2-AG, followed by prostaglandin synthases to produce prostamides (from anandamide) and prostaglandin-ethanolamide, PG-EA (from 2-AG). Both AEA and 2-AG move across the plasma membrane via a purported endocannabinoid membrane transporter (EMT) and target CB1 and CB2, which show an extracellular binding site. 2-AG, AEA, and PEA directly activate orphan G-protein-coupled receptors (GPR55, GPR18, GPR119), the transient receptor potential of vanilloid (TRPV) channel, and peroxisome proliferator-activated nuclear receptors (PPARs). Dashed lines denote low-affinity bindings. Phytocannabinoids Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and Δ9-tetrahydrocannabivarin (THCV) showed to activate cannabinoid receptors. CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; ER, endoplasmic reticulum. This figure was created using images from Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com (accessed on 4 May 2022)).
Endo- and phytocannabinoids interact with other receptors throughout the body, including the ionotropic transient receptor potential (TRP) cation channels family, including TRPA1, TRPV2, TRPV3, and TRPV4; nuclear receptors/transcription factors called the peroxisome proliferator-activated receptor (PPAR) α and γ; along with the orphan GPCRs, including GPR18 and GPR55; serotonin 1A receptor (5-HT1A); and the adenosine A2A receptor [19,20,21]. The nature of interaction is not always apparent, but it was shown that phytocannabinoid delta-9-tetrahydrocannabinol (THC) functions as an agonist of GPR55, GPR18, PPARγ receptors, while acting as an antagonist on TRPM8 and 5-HT3A receptors. In contrast, cannabidiol (CBD) has a very weak affinity for CB2 or CB1, although it may work as a negative allosteric regulator of these receptors [22], modulating THC activity. TRPA1, TRPV1, TRPV2, TRPV3, PPARγ, 5-HT1A, A2 and A1 adenosine receptors, and CBD functions as an agonist, while on GPR55, GPR18, and 5-HT3A, it functions as an antagonist. In addition, CBD can have inverse agonist activity on the GPR3, GPR6, and GPR12 receptors [23]. THC and CBD also can affect the levels of anandamide in the brain. Moreover, THC can increase AEA and adenosine levels [24].
2.2. Role in the Control of Cell Division and Cell Proliferation
It appears that ECS controls the fate of many cells in the organism, regulating the cell division and proliferation, apoptosis, necrosis and autophagy in several organs and organ systems, including the brain, skin, and immune system.
In the central nervous system (CNS), the ECS system functions as a neuroprotective system that controls glutamate excitotoxicity, calcium influx, inflammation, and autophagy [25]. In the CNS, the interaction of endocannabinoids with CB1/CB2 and other receptors mediates synaptic plasticity or progenitor cell fate in the central nervous system, promoting self-repair of the brain [26]. It also appears that constitutive release of 2-arachidonoylglycero by late oligodendrocyte progenitors allows oligodendrocyte maturation by activating CB receptors and downstream ERK pathway [27].
In skin, ECS activity maintains the cutaneous homeostasis through the regulation of skin cell proliferation, survival, and differentiation [28]. Locally produced AEA inhibits the cellular growth and the differentiation of cultured NHEK and HaCaT keratinocytes, as well as inducing apoptosis of human HaCaT keratinocytes [28]. CB1 activity is higher in differentiated skin layers [29]. In human cultured hair follicles, AEA but not 2-AG inhibit elongation and proliferation of hair shaft and induce intraepithelial apoptosis in a CB1-dependent manner [30]. Both AEA and 2-AG induce apoptosis of human sebaceous-gland-derived SZ95 sebocytes in a CB2-dependent manner [31].
In the immune system, the central role is played by CB2 receptors that are mainly expressed by cells (T and B lymphocytes) and peripheral tissues of the immune system (spleen and thymus) where it regulates immune suppression, apoptosis, and cell migration [32]. In in vitro studies, it was demonstrated that anandamide inhibits mitogen-induced proliferation of T cells [33], while inhibiting the chemokine SDF-1-induced migration of CD8+ T cells [34]. In contrast, 2-AG, but not anandamide, induced CB2-dependent migration in natural killer cell line KHYG-1 cells [35]. In B cells, 2-AG chemo-attracts naïve B cells and marginal zone B cells and inhibits the function of activated B cells, while 2-AG and anandamide suppress the migration of neutrophils [36]. Additionally, anandamide induces the apoptosis of murine bone-marrow-derived DCs (BMDCs) in a CB1- and CB2-dependent manner [37].
2.3. Changes in the ECS with Age
Cancer can be considered an age-associated disease, due to the accumulation of cellular and DNA damage. From this perspective, it is interesting to understand what happens to ECS with age.
In general, information about age-related changes in the ECS is scarce. Most of the data are related to changes in the central nervous system, and even then, the data are very contradictory. In general, it is believed that the activity of ECS declines with age [13]. In rats, in one study, a general decrease in the expression of CB1 and a decrease in density of the receptors in various brain areas with age was observed [38], while in another study—in which only redistribution of the receptors was noted– they were reduced in the postrhinal, but elevated in the entorhinal and temporal cortices in old animals [39] (Table 1). In mice, no changes in the receptor density in most brain regions was found with age, but instead, a significantly reduced receptor/Gi protein coupling was observed [40]. In one study on humans, CB1 expression increased, predominantly in females, most drastically in the basal ganglia, the lateral temporal cortex, and in the hippocampus [41], while another study reported no change [39]. As for endocannabinoids, the picture is not clear either—some studies suggested a decrease, while others found no difference in different brain regions of young and old animals. [13]. However, animals lacking FAAH—the enzyme degrading anandamide showed less pronounced features of aging—decreased expression of pro-inflammatory genes and decreased decline in cardiac function [42] (Table 1).

Table 1.
Age-dependent changes to ECS components in different normal tissues.
Very little reliable data exist on changes in the ECS in skin. Concentration of AEA is 119-fold higher than 2-AG in human skin [43], although it is not known how it changes with age. Moderate CB1 activation in skin works as a suppressor of the differentiation, while high activation leads to anti-proliferative and pro-apoptotic events [19]. In mice, CB1 deficiency in skin may lead to premature aging [44]. CB1-deficient mice exhibit cognitive impairments and changes in the structure of skin, indicating that CB1 deficiency accelerates aging only in the brain and in the skin, but not other peripheral organs [44].
Expression of anandamide degrading enzyme FAAH decreases with age and in response to sunburn in skin [45], indicating that ECS may undergo similar changes upon skin aging and in response to UV damage. A decrease in FAAH with age may also mean that there is less anandamide produced with age.
There is even less information about ECS activity in other tissues. One report shows a 2-AG decrease in lungs and increase in blood, while AEA increases in lung and blood in mice [46].
3. ECS and Cancer
3.1. Changes in ECS in Cancer
The metabolic abnormality of lipids has been linked to cancer due to their crucial regulatory roles in signaling pathways involved in initiation and progression of malignancies. The ECS is a biological system comprised of lipid-derived endocannabinoids, cannabinoid receptors, and the enzymes responsible for endocannabinoid metabolism. The ECS is dysregulated in numerous diseases, including cancer. In this section, we will discuss changes in the ECS in human malignancies and the impact on cancer progression and patients’ prognosis. We will also discuss signaling pathways that mediate antitumorigenic or protumorigenic effects of the ECS activation. We will summarize the data for major cancers in Table 2.

Table 2.
Changes to ECS components in malignant tissues.
3.1.1. Changes in Expression Pattern of Cannabinoid Receptors in Cancer
As mentioned above, cannabinoids exert their biological actions primarily through the activation of various receptors, and many of them are likely to be altered in cancer. In this review, we will mainly focus on three well-characterized G-protein-coupled receptors: cannabinoid receptor 1 (CNR1, also known as CB1R and CB1), cannabinoid receptor 2 (CNR2, also known as CB2R and CB2), and orphan G-protein-coupled receptor 55 (GPR55) [80,81].
CB1R and CB2R
To date, many studies using immunohistochemical staining, Western blotting, qRT-PCR, or a combined method have demonstrated overexpression or expression of CB1R and/or CB2R in human cancers, including glioma [75,76,77,78], lymphoma [82,83], leukemia [84,85], breast [64,65,66,67], lung [60,61], ovarian [86,87], pancreatic [88], prostate [89,90,91], skin [52,92,93] and thyroid cancers [94], endometrial [95], esophageal [96], head and neck [97], hepatocellular [98,99,100], renal [101,102], and mobile tongue carcinomas [39,103]. In addition, CB1 and CB2 receptors were highly expressed in non-Hodgkin’s lymphoma, and CB1 in mantle cell lymphoma compared to reactive lymph nodes [83,104]. Correlation also exists between the CB2 receptors expression and estrogen and progesterone receptor, as well as ERBB2/HER-2 levels in breast cancer [105]. Another study showed higher expression of CB1 receptors in androgen-sensitive and androgen-independent prostate cancer cell lines compared to normal prostate epithelial cells [90].
A large body of evidence has indicated that the overexpression of CB1R or CB2R is correlated with reduced survival, increased risk of metastasis and recurrence, and poor prognosis and clinical outcomes [64,65,75,77,84,87,91,94,96,97,102]. Analysis of astrocytoma showed that expression of CB2 receptors correlates with tumor malignancy [75]. The ratio of CB2 to CB1 expression in gliomas correlates with the tumor grade [75]. Additionally, the over-expression of CB1 and TRPV1 correlated with increased grades of prostate tumors [89]. In pancreatic tumors, the over-expression of CB1 was associated with shorter survival rates [106]. Interestingly, several studies have also shown an association between the elevated expression of CB1R and/or CB2R and a better prognosis in hepatocellular carcinoma [99], ERα− and ERα+ breast cancer [66], and non-small-cell lung cancer [60]. Additionally, in hepatocellular carcinoma, the higher expression of CB1 and CB2 correlated with improved prognosis [99]. Importantly, numerous studies have also indicated a downregulation of CB1R and/or CB2R in a few human cancer types, including glioma [79], colorectal cancer [107,108], endometrial [95], hepatocellular [100], and renal cell carcinomas [109]. These data suggest cannabinoid receptors are potential prognostic indicators for cancer patients and should caution us that such indicators are very cancer specific.
In contrast with the extensive studies that have been conducted to determine the expression of cannabinoid receptors in cancers and the correlation with disease clinicopathological parameters and patients’ prognosis, the analysis of mechanisms underlying dysregulation of these receptors has drawn much less attention. Hypermethylation of CpG islands has been revealed in transcription factor binding sites in the CNR1 promoter of colorectal cancer cell lines and tissue samples examined [108]; it was further found that inhibition of DNA methyltransferase profoundly elevates the CNR1 transcription, suggesting a crucial contributing role of CNR1 promoter hypermethylation in downregulating CB1R expression in colorectal cancer. Another study has validated the involvement of DNA methylation in suppressing CNR1 transcription in the same cancer type [57]. In addition to DNA methylation, miRNAs (miRs)—well-characterized small non-coding RNA molecules (20–22 nt)—play pivotal roles in many biological and pathological processes, including cancer. Therefore, it is not surprising if these small RNA molecules also contribute to the dysregulation of cannabinoid receptors in cancer. The LoVo colorectal cancer cells overexpress miR-1273g-3p, which directly targets CNR1 [110], leading to activation of the Erb-B2 receptor tyrosine kinase 4 (ERBB4)/phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3)/mechanistic target of rapamycin (mTOR)/S6 kinase 2 (S6K2) pathway, eventually promoting the malignant behavior of LoVo cells. Another study has shown that miR-23b-3p and miR-130a-5p, which are downregulated in gastric cancer cells, directly silence CB1R, attenuating cell growth, migration, and invasion of the cancer cells [63].
GPR55
Growing evidence has demonstrated that GPR55 is overexpressed in numerous malignancies, including breast [68,69] and colorectal cancers [57,59], endometrial [111] and squamous cell carcinomas [112]. The elevated expression of GPR55 is significantly correlated with metastasis, reduced disease-free survival, and poor prognosis in breast cancer [68,69].
Mechanically, although GPR55 does not have a CpG island, its DNA methylation is globally reduced in colorectal cancer [57], which may contribute to the elevated transcription of GPR55 gene. GPR55 mRNA is a direct target of miR-675-5p [113]. Downregulation of miR-675-5p has been shown in non-small-cell lung cancer (NSCLC), which is correlated with TNM stage and lymph node metastasis of this disease [113], and may play a role in the upregulation of GPR55 expression in NSCLC. Overexpression of miR-675-5p inhibits tumor growth, proliferation, migration, and invasion of NSCLC cells in vivo and in vitro, by targeting GPR55 [113].
3.1.2. Changes in Cannabinoid Receptor Endogenous Ligands in Cancer
Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the two most bioactive endocannabinoids [114], which trigger activation of CB receptors and regulate downstream signaling pathways in a receptor-dependent or -independent manner. Growing evidence has indicated differential regulation of endocannabinoids AEA and 2-AG in numerous cancers.
The AEA and 2-AG were increased in many cancer cell lines and tissues, including glioblastoma, meningioma, pituitary adenoma, endometrial sarcoma, prostate, and colon carcinoma [70,71,72,73,74]. Additionally, the concentrations of 2-AG and AEA were higher in CRC cells than in healthy neighboring tissues [71,73,115,116]. The levels of plasma and/or tissue 2-AG are elevated in colorectal cancer [55], craniopharyngioma [117], diffuse large B-cell lymphoma [118], glioma [78], and hepatocellular carcinoma [100], whereas the levels of AEA are reduced in hepatocellular carcinoma [100] and glioma [78]. In consistence with downregulation of AEA in glioma and hepatocellular carcinoma [78,100], the functional studies support a tumor suppressive role of AEA in head and neck and laryngeal squamous cell carcinomas [119,120]. Although 2-AG was elevated in various human malignancies [55,78,100,117,118], the functional evidence does not support an oncogenic role of 2-AG in tumor progression. Indeed, 2-AG exhibits an anticancer property in several model systems. 2-AG suppresses pancreatic cancer cell proliferation and tumor growth in vitro and in vivo [121], which could be blocked by CB1R antagonist, but not CB2R antagonist, indicating that 2-AG-induced antiproliferative effect is CB1R dependent. 2-AG also inhibits proliferation of diffuse large B-cell lymphoma and laryngeal squamous cell carcinoma cells [118,120], although it has been shown to promote proliferation of a few diffuse large B-cell lymphoma cell lines [118].
Lysophosphatidylinositol (LPI) is an endogenous agonist of GPR55 receptor. The levels of LPI have been shown to be elevated in two cancer types: breast [63] and colorectal cancers. Functional studies have indicated that LPI profoundly promotes migration/invasion of breast and colorectal cancer cells, which could be blocked by GPR5 antagonist or GPR55 knockdown [56,63,122]. Furthermore, LPI produced by ovarian cancer cells promotes angiogenesis [123], which is prevented by GPR55 inhibition or GPR55 knockdown. Interestingly, N-docosahexaenoyl dopamine triggers apoptosis of cancer cells via activation of GPR55 [124], and LPI enhances the cytotoxic effect. These data suggest that the antiproliferative effect of endocannabinoids and the prometastatic effect of LPI are mainly mediated by cannabinoid receptors.
3.1.3. Changes in the Expression Pattern of Endocannabinoid Hydrolytic Enzymes in Cancer
AEA and 2-AG are synthesized from the substances N-arachidonoyl phosphatidylethanolamide (NAPE-PLD) and diacylglycerol (DAG) by phospholipase D and DAG lipase, respectively, and degraded by FAAH and MAGL, respectively. A large body of evidence has demonstrated that aberrant expression of enzymes responsible for endocannabinoid metabolism results in dysregulation of endocannabinoid metabolism that may drive progression of cancer [125,126,127]. Here, we will mainly discuss changes in FAAH and MAGL expression in cancer and their impact on cancer biology and prognosis.
FAAH is overexpressed in prostate cancer cell lines and tumor tissues; tumor FAAH immunoreactivity (FAAH-IR) is positively correlated with disease severity for cases with mid-range CB1 expression [128]. The high tumor FAAH-IR may serve as an indicator of poor prognosis [128,129]. Functional studies have shown that downregulation of FAAH in prostate cancer cells attenuates 2-AG hydrolysis and cell invasion [130], and the phenotypic effects can be reversed by FAAH overexpression. Furthermore, pharmacological inhibition of FAAH suppresses invasion and metastasis of lung cancer and colon adenocarcinoma cells in vivo and/or in vitro [62,131]. Moreover, pharmacological inactivation of CB2R attenuates FAAH-siRNA-induced TIMP-1 expression, suggesting a role of CB2R in mediating the anti-invasive and anti-metastatic signaling triggered by FAAH inhibition [62]. Data from The Human Protein Atlas (https://www.proteinatlas.org/ (accessed on 15 June 2022)) indicate that FAAH expression increases in most of the studied cancers, with the most drastic increase in prostate cancer [45]. These data suggest that FAAH may act as an oncogene. Interestingly, several lines of evidence have also indicated downregulation of FAAH in tumors, including endometrial carcinoma, glioma, and uterine leiomyoma [78,132,133].
Numerous studies have shown an overexpression of MAGL in various human malignancies, including prostate adenocarcinomas [130], malignant melanoma [53], osteosarcoma, hepatocellular carcinoma [134,135], cervical [136], colorectal [54], and endometrial cancers [137]. The overexpression of MAGL is correlated with larger tumor size, vascular invasion, poor differentiation, and clinicopathological stage of several cancers [53,134,135,137]. Therefore, the elevated MAGL may act as an indicator of poor prognosis in cancer patients. Functional studies have shown that inhibition or knockdown of MAGL significantly suppresses proliferation, migration, invasion, and xenograft tumor growth in vitro and/or in vivo [54,134,136,137,138]; knockdown of MAGL also induces apoptosis and cell cycle arrest via downregulation of cyclin D1 and Bcl-2 [54,137], or upregulation of Bax and cleaved caspase-3 [136]. Conversely, overexpression of MAGL promotes tumor growth, migration, invasion, and metastasis in nitro and/or in vivo [134,139,140,141], via a NF-κB-mediated EMT process. These data suggest an oncogenic role of MAGL in malignant progression.
3.2. Changes of ECS Signaling Pathways in Cancer—Potential Molecular Targets
Alterations in all components of endocannabinoid system contribute to cancer progression through multiple pathways of cellular signaling. Below, we will discuss ECS changes through receptor-dependent and -independent signaling.
3.2.1. Receptor-Dependent Signaling and Changes in Response to Cannabinoids
GPCRs are the biggest family of receptors targeted by approved drugs [142]. However, they are rarely targeted in cancer therapy, with the exception of endocrine tumors (pituitary, adrenal, testes, ovarian) and hormone-dependent tumors of breast and prostate. Although mutations in GPCRs are not often the “driver mutations”, they take a large part in regulation of cell signaling, which regulates cellular functions such as metabolism, growth, and proliferation [143]. Considering that CB receptors belong to the GPCR family of receptors with a wide variety of cell downstream signaling, they are likely the best therapeutic targets in cancer patients.
Pharmacological activation or upregulation of CB1R and/or CB2R inhibits tumor growth and metastasis in vivo, attenuated proliferation, migration/invasion, and angiogenesis, and induced apoptosis in vitro through inhibition of extracellular signal-regulated kinase (ERK), protein kinase B (PKB)/AKT, cAMP/protein kinase A (PKA), c-Jun N-terminal kinase (JNK), MAPK p38, nuclear factor kappa B (NF-κB), and/or vascular endothelial growth factor (VEGF) signaling pathways, in various human cancers, including glioma [144,145], leukemia [146,147,148], multiple myeloma [149], neuroblastoma [150], breast [151,152,153,154], cervical [105], colorectal [110,155,156,157], endometrial [158], hepatocellular [159], intestinal [108], non-small-cell lung [160,161], prostate [162,163], and thyroid cancers [164]. The antitumorigenic and/or proapoptotic effects can be blocked by inhibition and/or knockdown of CB1R and/or CB2R [144,145,146,149,152,153,155,160,162,164]. This is very interesting, since CB1 and CB2 are overexpressed is many cancers as well. The level of endocannabinoids is also often upregulated in cancers, as well as the level of FAAH and MAGL. So, it is counterintuitive that one can inhibit growth of cancer cells by overexpressing or/and activating the CB1/CB2 receptors or adding endocannabinoids.
It has been reported that the receptor-triggered antitumorigenic and/or proapoptotic effect can be mediated via activation of signaling pathways. Activation of CB1R and CB2R profoundly inhibits malignant glioma growth in rat and mouse models through activation of ERK pathway [165]. Pharmacological activation of CB1R and CB2R induces apoptosis of hepatocellular carcinoma cells through upregulation of proapoptotic factors Bax and Bcl-x(s) and downregulation of antiapoptotic factors Bcl-2 and survivin, through activation of JNK/p38 MAPK pathway [166]. Interestingly, stimulation of cannabinoid receptors may have a dual effect on signaling pathways. In addition to attenuating the Akt pathway, activation of CB1R and/or CB2R also causes activation of JNK [108,162] and ERK1/2 [163] pathways in prostate and non-small cell lung cancer cells.
Evidence has also demonstrated both protumorigenic and anti-apoptotic roles of the ECS. Pharmacological inhibition of CB1R attenuates proliferation and tumor growth, inducing apoptosis and cell cycle arrest in breast cancer through upregulation of p27KIP1 and downregulation of cyclin D and E [167]. The elevated expression of cannabinoid receptors induced by chronic intermittent hypoxia promotes tumor growth, migration, angiogenesis, and lung metastasis of breast cancer cells both in vitro and in vivo [168], through activation of insulin-like growth factor-1 receptor (IGF-1R)/AKT/glycogen synthase kinase-3β (GSK-3β) pathway, which can be blocked by knockdown of cannabinoid receptors. Furthermore, pharmacological activation of CB2R enhances proliferation and tumor growth of colon cancer cells in vitro and in vivo via activation of AKT/PKB pathway [169]. Knockdown of CNR1 diminishes proliferation and migration of progesterone-resistant endometrial cancer cells, and resensitizes these cells to progesterone, through inhibition of ERK and NF-κB pathways [158]. Moreover, activation of CB1R prevents astrocytoma cells from ceramide-induced apoptosis through activation of ERK pathway [170]. Inhibition or knockdown of cannabinoid receptors attenuates proliferation, migration, and induces apoptosis of HPV+ head and neck squamous cell carcinoma cells [171]. Conversely, pharmacological activation of CB1R and CB2R enhances cancer cell growth and migration, and attenuates apoptosis both in vitro and in vivo, via activation of p38 MAPK pathway [171].
GPR55 receptor also plays a pivotal role in tumor progression. Stimulation of GPR55 promotes cancer cell proliferation and tumor growth in vitro and in vivo via activation of ERK pathway [172]. Overexpression of miR-675-5p inhibits tumor growth, attenuates proliferation, migration, and invasion, and induces G1 cell cycle arrest of non-small-cell lung cancer cells both in vitro and in vivo via direct targeting of GPR55, through inhibition of ERK pathway [113]. Furthermore, the LPI-mediated activation of GPR55 promotes proliferation of ovarian and prostate cancer cells [80] and ovarian-cancer-cell-induced angiogenesis [123], via activation of Akt, ERK1/2, and p38 MAPK pathways. Moreover, pharmacological inhibition of GPR55 enhances doxorubicin cytotoxicity in cancer cells through inactivation of MEK/ERK and PI3K/AKT pathways [173]. These data indicate that GPR55 may function as an oncogene. Interestingly, pharmacological activation of GPR55 in cholangiocarcinoma cells, however, diminishes cancer cell proliferation through activation of JNK pathway [174], which can be blocked by GPR55 knockdown, suggesting that GPR55 may also act as a tumor suppressor.
3.2.2. Receptor-Independent Signaling and Changes in Response to Cannabinoids
The ECS is also able to exert its biological and pathological effects in a receptor-independent manner. Pharmacological activation of cannabinoid receptors enhances the radiation-mediated anti-proliferative effect on breast cancer cells via a receptor-independent sphingosine-1-phosphate (S1P)/ceramide pathway [175]. CBD triggers apoptosis of breast cancer cells by attenuating mitochondrial membrane potential and promoting the release of cytochrome c, eventually leading to activation of a receptor-independent intrinsic apoptotic pathway [176]. Interestingly, cannabinoids triggers apoptosis only in astrocytoma cells expressing low levels of cannabinoid receptors through activation of ERK1/2 pathway, not in the cells overexpressing the receptors [177]. Furthermore, AEA induces apoptosis of pheochromocytoma cells through activation of p38 MAPK/JNK pathway, which cannot be rescued by CB1R antagonist [178]. Moreover, the synthetic cannabinoid CP55940 induces apoptosis and cell cycle arrest of T-cell acute lymphoblastic leukemia cells by upregulation and activation of proapoptotic molecules via activation of c-Jun/JNK pathway [179]. However, the CP55940-induced apoptosis in leukemia cells cannot be rescued by cannabinoid receptor agonists, suggesting involvement of cannabinoid receptor-independent mechanism. These data indicate that cannabinoids can trigger programmed death of cancer cells via receptor-independent signaling.
3.2.3. Signaling When the Receptor Status Is Unknown
Numerous studies have also shown the antitumorigenic and/or proapoptotic effects of cannabinoids on human malignant cells, while not reporting the involvement of cannabinoid receptors in these processes. CBD profoundly attenuates proliferation and invasion of breast cancer cells in vitro through inactivation of EGFR, AKT, ERK, and NF-κB pathways [180]. Consistently, in a mouse model, CBD also suppresses tumor growth and metastasis of breast cancer cells via attenuation of macrophage recruitment to xenograft tumor sites [180]. Furthermore, CBD enhances radiation-induced glioblastoma cell death through inhibition of ERK1/2 and AKT pathways, and activation of JNK1/2 and p38 MAPK pathways [181].
Interestingly, endocannabinoids act differentially on tumor growth. AEA inhibits tumor growth of cholangiocarcinoma cells via upregulation and activation of Notch1 [182], whereas 2-AG promotes tumor growth through upregulation and activation of Notch2. The antitumorigenic effect of AEA on cholangiocarcinoma may also require activation of Wnt-JNK pathway [183], since that can be abolished by Wnt5a knockdown.
Recently, using two neuroblastoma cell lines as a model system, we have revealed a suppressive role of cannabinol (CBN) on neuroblastoma cell proliferation, invasion, and angiogenesis through miR-34a-mediated targeting via inhibition of AKT pathway [184]. We have found that a novel 31 nt tRNAiMet fragment tRiMetF31 generated from miR-34a-guided cleavage [185] can directly target 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), a key proangiogenic factor, and highlighted a crucial role of the miR-34a/tRiMetF31/PFKFB3 axis in CBN-mediated suppression in neuroblastoma biology. We have not studied the role of cannabinoid receptors in this process, however.
4. Effect of Cannabinoids on Various Hallmarks of Cancer
Various in vitro and in vivo experiments have shown that cannabinoids can target almost every hallmark of cancer (Figure 2) [186]. They inhibit proliferation, reduce inflammation, stimulate apoptosis, and inhibit tumor invasiveness, angiogenesis, and metastasis [187,188,189,190]. One of the most important effects of cannabinoids, besides their antitumor ability, is that they are less likely to affect non-transformed normal cells surrounding tumors, and they may even have protective effects. For instance, cannabinoids may induce cell death in glioma cells while protecting normal astroglial and oligodendroglial cells from apoptosis via CB1 receptors [187]. Studies on animals show the protective effects of cannabinoids against certain types of tumors. For example, a dose-dependent decrease in the incidence of hepatic adenomas and hepatocellular carcinomas in mice that were given THC over 2 years was noted. Additionally, lower incidence rates of benign tumors in mammary glands, uterus, testis, and pancreas were seen in tested rats [191].
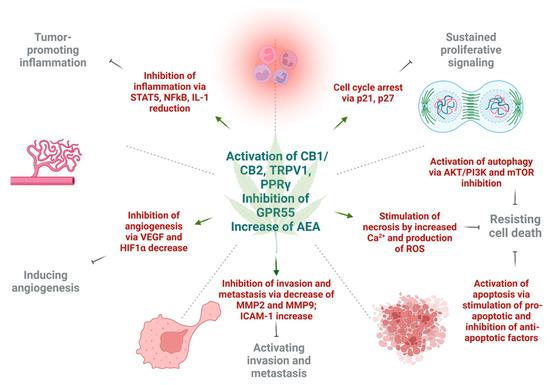
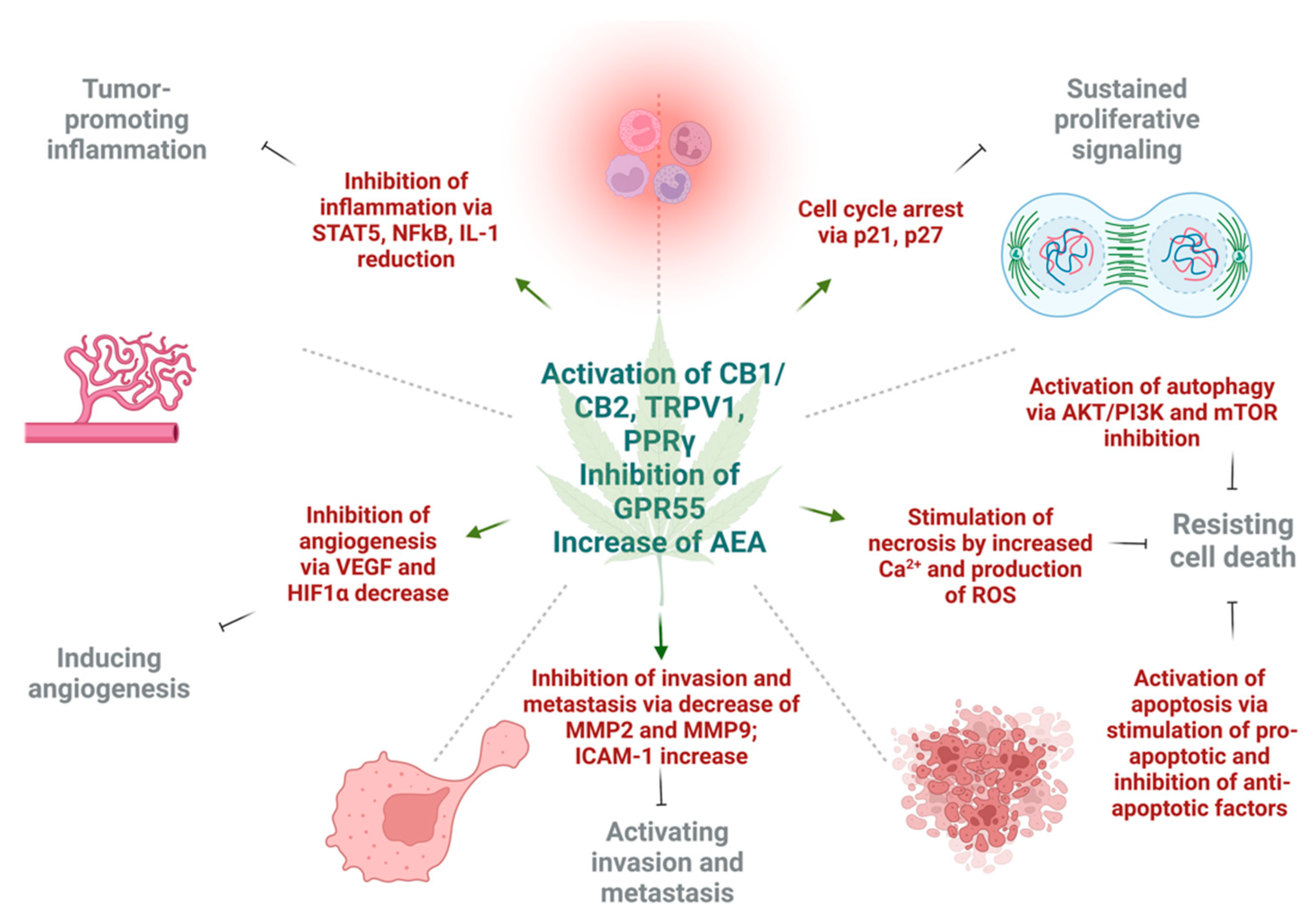
Figure 2.
The effects of cannabinoids on different hallmarks of cancer. The activation of cannabinoid 1 (CB1), cannabinoid 2 (CB2), and transient receptor potential cation channel 1 (TRPV1) increase levels of anandamide (AEA), as well as inhibition of G-protein coupled receptor 55 (GPR55), and exert different effects on tumor cells in respect to cancer hallmarks. Cannabinoids inhibit tumor-promoting inflammation via downregulation of nuclear factor κB (NFκB), signal transducer and activator of transcription 5 (STAT5), and interleukin 1 (IL1). The angiogenesis is inhibited by reduction in vascular endothelial growth factor (VEGF) and hypoxia inducible factor 1α (HIF1α). Next, invasion and metastasis are prevented by decrease of tissue degrading enzymes—matrix metalloproteinase 2 and 9 (MMP2, MMP9), as well as expression of intercellular adhesion molecule 1 (ICAM-1). Sustained proliferative signaling is opposed by the activation of p21 and p27 that leads to cell cycle arrest. Lastly, under the action of cannabinoids, cell death may be achieved by three mechanisms. Autophagy is triggered by inhibition of protein kinase B (AKT), phosphoinositide 3-kinase (PI3K) and mammalian inhibitor of rapamycin (mTOR). Apoptotic cell death is a result of upregulation of pro-apoptotic and downregulation of anti-apoptotic factors under the action of different cannabinoids. Necrosis can result due to high Ca2+ release and formation of ROS in cancer cells. This figure was created with BioRender.com.
4.1. Induction of Autophagy and Apoptosis
Autophagy and apoptosis are two essential mechanisms of regulation of uncontrolled growth. Autophagic activity of cannabinoids observed in several major cancers [192,193] is partially dependent on the CB1 or CB2 receptor. Mice deficient in CB1 receptor exhibit altered autophagosomal activity [13], while endocannabinoid palmitoylethanolamide increased the phagocytosis of murine microglial cells [194]. Additionally, the experimental study using delta-9-THC and a synthetic agonist decreased the cell viability of hepatocellular carcinoma xenografts in nude mice via the CB2 receptors. The anti-cancer effect was explained by activating the endoplasmic reticulum stress response, which leads to macro-autophagy and eventually apoptosis [195]. Studies on small-cell lung carcinoma [61] and breast cancer cells [67] supported the idea that CB1 and CB2 receptors may be potential targets to achieve apoptosis. The preclinical models of breast cancer showed evidence that CBD may induce apoptosis in estrogen-dependent and estrogen-independent breast cancer cells with little or no effect on normal mammary cells. Surprisingly, this was CB1-, CB2-, and vanilloid receptor-independent [176].
The well-established antineoplastic mechanisms of cannabinoids are alterations in ceramide de novo synthesis. In cancer cells, increased ceramide levels, a neutral lipid backbone of complex sphingolipids, can occur under chemotherapy, radiation, and stimulation of CB receptors [58,196]. As a result, ceramide activates endoplasmic reticulum stress response and causes inhibition of global translation of proteins. At the same time, there is an activation of C/EBP homology protein (CHOP) which can stimulate proapoptotic proteins BAD and BAX [197]. Moreover, cannabinoids can cause downregulation of AKT, which may have a variety of intracellular effects. Low AKT leads to activation of autophagy via the mTOR pathway, cell cycle arrest through p21, and activation of caspase 9 and 3, which eventually ends in apoptotic cell death [58,108,155,198,199,200].
Activation of CB1 and CB2 receptors by synthetic cannabinoid agonists could stimulate apoptosis via ceramide synthesis and TNF-receptor activation [58]. Another group showed that activation of CB1 receptors in different CRC cell lines causes inhibition of major cancer survival pathways such as RAS/MAPK, ERK1, and PI3K/AKT [155]. Additionally, CBD, a partial agonist of CB1/CB2 receptors and antagonist of GPR55, may suppress mTOR/AKT signaling and activate proapoptotic NOXA in CRC cells [201]. Moreover, CBD suppressed the production of inhibitors of apoptosis, such as survivin and c-FLIP in colon cancer cells [197].
4.2. Reduction of Inflammation and Inhibition of Proliferation
Inflammation is a large component of carcinogenesis. ECS plays a central in the regulation of function of immune system and control of inflammation. Similarly, many phytocannabinoids exert strong anti-inflammatory effects upon local [202] or systemic [203] application.
Cannabinoids inhibited proliferation by suppressing the AKT/PKB prosurvival pathway causing cell cycle arrest in G1/S phase. This was shown in multiple cancers, including melanoma, breast, gastric, lung, and liver carcinomas [93,151,159,160,204,205]. In a breast cancer model, cannabinoids were able to induce cell cycle arrest via inhibition of cyclin dependent kinase 1 (CDK1), induction of p21 and p27, a decrease in cyclin A and E levels, degradation of CDC25A, and finally, inactivation of CDK2 [206,207].
In the study on head and neck squamous cell carcinoma, cannabinoids were able to stimulate dual specificity phosphatase 1 (DUSP1), which is a negative regulator of MAPK [208]. DUSP1 is one of the central mediators in the resolution of inflammation in cells. Moreover, the levels of cyclin dependent kinase inhibitor, p21, as well as growth arrest and DNA damage-inducible protein α (GADD45A) were activated, resulting in cannabinoid’s antiproliferative effects. In human gastric cancer model, CBD upregulated ATM and p21, which caused a decrease in CDK2 and CCNE, resulting in cell arrest in G0/G1 stage [209]. In a xenograft model of human glioma, CBD was able to reduce the activity of 5-lipoxygenase, an enzyme that catalyzes synthesis of leukotrienes (LTs) and mediators of inflammation; a decrease in 5-lipoxygenase activity caused inhibition of LTB4 and had antiproliferative effect [210].
The eicosanoid system, which contains pro- and anti-inflammatory molecules, plays an important role in cannabinoid-induced tumor cell apoptosis. The addition of R(+)methanandamide to the glioma cells activated de novo ceramide synthesis, which eventually led to COX-2 expression with subsequent production of PGE2 that had proapoptotic effect [211,212]. It was shown that proapoptotic effects of eicosanoids was PPARγ receptor-dependent [213,214,215].
On the other hand, it was shown that the micromolar concentrations of THC, CB1 agonist—arachidonyl-2-chloroethylamine (ACEA), and CB2 agonist HU308 stimulated the proliferation of cancer cells, which can be explained by transactivation of EGFR [169,171,216,217].
The chemoprotective effect of CBD was also shown on colorectal cancer in mice. Adding CBD prevented premalignant and malignant lesions development in the azoxymethane model of colon cancer [218]. The effect was explained by DNA protection against oxidative damage, increased levels of endocannabinoids, and decreased cell proliferation [218]. The antiproliferative action was CB1 dependent [219].
4.3. Inhibition of Angiogenesis, Tumor Invasiveness, and Metastasis
There were multiple reports showing the inhibitory effects of cannabinoids on cancer cell migration, invasion, and metastasis [62,74,220]. CBD was shown to inhibit the invasiveness of lung cancer cell lines by inhibiting ICAM-1 [190]. As some experiments indicated, the induction of tissue inhibitor of metalloproteinase-1 (TIMP-1), and ICAM-1 by THC, Met-AEA and CBD had significant anti-invasive effects [105,190,221]. The action of TIMP-1 is achieved via reduction of collagen-degrading enzymes, MMP-2 and MMP-9, that promote cancer cell invasiveness [222].
Another way in which cannabinoids are diminishing tumor aggressiveness is inhibition of epithelial-to-mesenchymal transition. A study that involved 2-methyl-2′-F-anandamide (Met-F-AEA) showed a significant reduction in β-catenin, vimentin, N-cadherin, and fibronectin, which are considered mesenchymal markers in tumor invasion. Moreover, Met-F-AEA decreased the levels of EMT markers such as Snail1, Slug, and Twist [223]. Other studies showed that CBD may reverse an IL-1β-induced EMT in breast cancer cells [224], or TGF-β-induced reorganization of F-actin, which also corresponds to EMT in lung cancer cells [60]. Cannabinoids may inhibit the invasion and metastasis of cancer cells through downregulation of vascular endothelial growth factor (VEGF), matrix metalloproteinase 2, matrix metalloproteinase 9, E-cadherin, cyclooxygenase 2 (COX-2), and hypoxia-inducible factor α [225,226,227].
5. Effect of Terpenes and Flavonoids
Some preclinical studies have shown that Cannabis extracts may be more effective than cannabinoids alone for cancer treatment. For instance, high-CBD extract showed higher affinity for CB1 and CB2 receptors than CBD alone. As a result, a high-CBD extract was more potent in preventing intestinal polyps’ formation in animal models [219,228].
The cannabis plant is rich in terpenes and flavonoids, biologically active substances which can also be used in cancer treatment [229,230]. There are more than 20,000 terpenes in nature, with around 200 found in Cannabis plants [231]. The monoterpene myrcene, sesquiterpenes β-caryophyllene, and α-humulene are often present in Cannabis chemovars. However, the spectrum of terpenes can vary from plant to plant. We will describe only some of the common terpenes that have anti-neoplastic effects.
Myrcene is present in hop, bay, verbena, lemongrass, citrus, and even carrot. Surprisingly, in some animal studies, myrcene showed to be carcinogenic, causing kidney cancer in rats and liver cancer in mice [191]. Another study showed that myrcene protected human B lymphocytes from DNA damage caused by hydroperoxides [232]. However, it also had cytotoxic effects on breast, colon, cervical, lung cancer cell lines, and leukemia cells [231,233]. There is not much knowledge about the mechanisms of action of myrcene, and more studies should be undertaken considering its controversial effects on cancer cells.
β-caryophyllene is a sesquiterpenoid commonly present in black pepper, oregano, basil, and rosemary. This terpene can induce apoptosis and cause cell cycle arrest in lung and ovarian cancer cell lines [234,235]. It can also influence the production of free radicals and can have antiapoptotic and antiproliferative effects via activation of the JAK1/STAT3 pathway in osteosarcoma cells [236]. Importantly, β-caryophyllene may sensitize different cancer cell lines to conventional chemotherapy drug doxorubicin [237,238,239,240]. Additionally, it attenuated doxorubicin-induced chronic cardiotoxicity in rats via activation of CB2 receptors [241]. Moreover, the combination of β-caryophyllene with 5-fluorouracil (5-FU) or oxaliplatin on colorectal cancer cells sensitized those cells to chemotherapeutics [242]; similarly, combination of β-caryophyllene with sorafenib potentiated the effect on liver cancer cells [243]. Thus, combining different cannabinoids with β-caryophyllene may become advantageous in cancer therapy, which needs further investigation.
The monocyclic sesquiterpene, humulene, has cytotoxic activity on multiple cancer cell lines via increasing production of reactive oxygen species [244,245] and inhibition of AKT in hepatocellular carcinoma cells with activation of apoptosis [246]. In in vitro models, humulene enhanced 5-fluorouracil, oxaliplatin, and doxorubicin cytotoxic effects [239,242].
Another terpene, limonene, is a cyclic monoterpene mainly present in citrus plants and is also present in cannabis. In the bladder cancer cell line, limonene caused G2/M cell cycle arrest, decreased migration, and metastasis, and increased Bax and caspase 3, thus inducing apoptosis [247]. It inhibited PI3K/AKT, induced autophagy and enhanced sensitivity to docetaxel in in vitro cancer cell models [248,249,250,251]. In in vivo models, limonene decreased tumor growth, induced apoptosis, and reduced c-Jun and c-myc expression [251,252,253,254,255,256,257,258,259]. There was one small clinical trial in which breast cancer patients received limonene for a short period of time; limonene decreased cell cycle regulatory protein expression, including cyclin D1 in breast cancer patients [260].
Pinene is present in pine resins, rosemary, basil, and parsley. As multiple preclinical data show, pinene was able to reduce the cell viability, stimulate apoptosis, and induce cell cycle arrest in numerous cancer cell lines [261,262,263,264,265,266,267]. Moreover, it can act synergistically with paclitaxel in tested lung cancer [265]. In in vivo animal models, pinene showed reduced growth and number of tumors [268].
These data could also support the advantageous action of cannabis extracts rich in terpenes versus purified cannabinoids in fighting against different malignancies. Different modulatory (often referred to as “entourage”) effects of cannabinoids and other substances in the cannabis plant were extensively reviewed in the past [269], although exact mechanisms are still unclear.
6. Preclinical and Clinical Use of Cannabinoids
6.1. Cannabis and Cannabinoids for Primary Care—Tumor Shrinkage
6.1.1. Data on Humans Are Limited
Cannabis has been used for medicinal purposes for thousands of years, until the 1940s, when the authorities prohibited it. In the USA, cannabis is classified as a Schedule I agent with risk for abuse and no approved medical use [270]. Despite the solid preclinical evidence regarding the antitumor properties of cannabinoids, there were not many human trials that supported this effect of cannabinoids (see Table 3 and Table 4). This could partially be because of the multiple legislation problems with cannabis. Thus, it is still kept on “the shelf” as a backup medication, mainly for palliative care in cancer patients. The number of clinical studies related to the role of cannabis and cannabinoids in cancer is critically low (see Supplementary Table S1). Today, there are few human trials regarding the palliative effects of cannabinoids in cancer patients, and even fewer regarding their anti-cancer effects (based on the clinicaltrials.gov database, 22 June 2022).

Table 3.
Preclinical studies regarding the primary (tumor shrinkage) and palliative effects of cannabinoids on different types of cancer.

Table 4.
Clinical studies regarding the primary (anti-cancer), palliative, and adverse effects of cannabinoids and cannabis use in cancer patients.
There were few human studies regarding delta-9-tetrahydrocannabinol (THC) in brain tumors. One of the first human trials involving cannabis as a cancer treatment was a small study on recurrent glioblastoma with intra-tumoral injections of delta-9-THC. Unfortunately, this study showed no beneficial effect [288]. However, a case report in two children with pilocytic astrocytoma after subtotal resection showed spontaneous regression of the tumor 3 years after the surgery. The patients did not receive any conventional adjuvant therapy, but they had inhalations of cannabinoids [312]. Another trial showed that the combination of temozolomide and Sativex increased 1-year survival rates in glioblastoma multiforme patients (NCT01812603, NCT01812616). In a pilot I study in nine glioblastoma patients that failed conventional therapies and had signs of tumor progression, patients received intratumoral injections of THC. Results showed the reduction of Ki67 immunostaining and antiproliferative effect of THC on tumor cells [288]. Another study in 2 glioblastoma patients receiving intratumoral injections of THC showed THC effectiveness in reducing VEGF and VEGFR-2 activation [145]. In the case study of a 14-year-old patient with an aggressive form of acute lymphoblastic leukemia, remission was achieved following the consumption of hemp oil, after bone marrow transplant, aggressive chemotherapy, and radiotherapy were revoked. A double-blind study tested oromucosal spray containing nabiximols in conjunction with temozolomide. Overall, the 1-year survival rates were higher in patients using nabiximols (83%) compared to the placebo group (44%) [289].
One interesting study included CBD as an immunosuppressive and anti-inflammatory agent in the adjunct treatment of acute graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation in 48 patients with acute leukemia and myelodysplastic syndrome. The results of the study showed that the combination of CBD with conventional graft-versus-host disease prophylactic treatment was safe and showed a lower incidence of GVHD [290].
6.1.2. Combination of Cannabinoids with Other Drugs—There Is Potential Benefit, but Caution Is to Be Exercised
The combination of cannabinoids with conventional anti-cancer therapy is also under investigation. For instance, the combination of cannabis with gemcitabine reduced the cell viability of pancreatic cells in vitro [192].
Moreover, adding THC to temozolomide treatment increased the sensitivity of chemotherapy-resistant glioma cells to the treatment in mice models [313]. Another study showed that the combination of THC with CBD enhanced radiation’s effects on the murine glioma model [314]. CBD may also overcome the oxaliplatin resistance of cancer cells via inhibition of superoxide dismutase 2 and activation of autophagic response [315]. The combination of CBD with a conventional chemotherapy agent, carmustine, caused inhibition of proliferation in glioblastoma multiforme cell line and overcame the carmustine resistance via activation of TRPV2 [316]. Additionally, the combination of CBD with THC showed higher antiproliferative action on glioblastoma multiforme cell lines. Furthermore, CBD stimulated TRPV2 and increased uptake of cytotoxic drugs by glioma cancer cells without affecting normal astrocytic cells [144]. The combination of THC with CBD also enhanced the action of temozolomide in mouse models [200,313].
Before cannabinoids can be prescribed as an actual treatment in cancer patients, their pharmacokinetics should be considered. In vitro studies showed that CBD can inhibit cytochrome P450, which is responsible for the metabolism of many medications, including conventional chemotherapeutics. As a result, a high concentration of CBD may increase the toxicity and decrease the potency of standard anti-cancer therapy [317,318]. Thus, the interaction of cannabinoids with cytochrome P450 raised a valuable concern about combining it with conventional chemotherapeutics. A clinical study involving 24 patients receiving irinotecan or docetaxel that were using cannabis showed that the addition of cannabis tea did not significantly affect clearance and medication exposure [319]. However, there is an obvious need for more data regarding cannabinoid pharmacokinetics and interaction with other medications, as many cancer patients are using cannabis for different purposes.
A significant concern for using cannabis with anti-cancer treatment was raised in patients undergoing immunotherapy. A retrospective observational study evaluated the influence of cannabis during nivolumab therapy in 140 patients with advanced melanoma, non-small-cell lung cancer, and renal cell carcinoma [310]. In this study, 89 patients received nivolumab and 51 received nivolumab and cannabis. As a result, cannabis reduced the response rate to immunotherapy. It was shown that the response rate to nivolumab alone was 37.5%, and for nivolumab, with cannabis, it was only 15.9%. However, there was no difference in overall survival [310]. Another prospective observational study from the same group followed 102 patients with metastatic cancers that started immunotherapy. In this study, 68 patients received immunotherapy, and 34 were on immunotherapy while using cannabis. Participants using cannabis had 39% of clinical benefit, whereas patients receiving immunotherapy had 59%. Moreover, in a cannabis arm, the tumor progression took 3.4 months compared to nonusers, for whom it took 13.1 months. The overall survival in cannabis users was only 6.4 months, and for patients on immunotherapy, it was 28.5 months. These results may be related to the immunosuppressive effects of cannabinoids, and cannabis use should be carefully considered in patients on immune checkpoint inhibitors [311].
6.2. Cannabis for Palliative Care
Cancer patients are accessing cannabis to alleviate various symptoms and improve their quality of life. Cannabis medication may reduce the devastating symptoms experienced by cancer patients, such as pain, emesis, anxiety, loss of appetite, and poor sleep quality [320]. A cross-sectional survey of 936 cancer patients revealed that 24% considered themselves active cannabis users. The reasons for cannabis ingestion were to alleviate the physical symptoms such as pain, nausea, and loss of appetite (75%); neuropsychiatric symptoms (63%), recreational use (35%), and cancer treatment (26%) [321]. Thus, the addition of cannabinoids to cancer care seems inevitable regardless of the legislation procedures. However, what if the usage of cannabis as palliative care is affecting the conventional anti-cancer treatment? We already discussed that cannabinoids have anti-cancer properties. However, they can affect drug metabolism and have a negative impact on immunotherapy. Thus, there is a huge need for clinical trials regarding the specific anti-cancer therapy and cannabinoid use to uncover their antineoplastic benefits, as well as to ensure the safe conditions for their ingestion by cancer patients.
6.2.1. Cannabis for Pain
Pain is one of the most devastating symptoms in patients with advanced cancer. It is estimated that eight out of ten patients with advanced cancer experience moderate to severe pain, and around 55% of cancer patients have chronic cancer-related pain. The mechanism of cancer-related pain can be inflammation, invasion of organs, or nerve injury. Opioids are the essential treatment for cancer-related pain; however, they cause addiction, and the overdose can be lethal [322]. Thus, adjusting or lowering the dose of opioids and maintaining an analgesic effect is crucial for cancer patients.
Both cannabinoid and opioid receptors have similar neural transduction systems and are expressed in the parts of the brain responsible for nociception, such as periaqueductal gray, raphe nuclei, and central–medial thalamic nuclei [323]. Moreover, CB1 and μ-opioid receptors colocalize in peripheral pain afferent neurons [324]. The cannabinoid signaling in pain is related to the distribution of CB1 receptors in the spinal dorsal horn. CB1 receptors in presynaptic neurons are colocalized with transient receptor potential cation channel 1. Activation of the CB1 receptor decreases calcium influx, resulting in the lower release of neurotransmitters [325]. The CB1 receptors present in postsynaptic neurons cause an increase in potassium influx that results in hyperpolarization and reduction of neuron excitability [326]. It was also observed that CB2 receptors could indirectly stimulate opioid receptors in afferent pathways, thus enhancing the analgesic effects of opiates [327]. There is also evidence that anandamide, 2-AG, and exogenous cannabinoids can interact with opioid receptors [328]. All these data support the idea that cannabinoids may exert an antinociceptive effect alone and in combination with opiates.
A systematic review and meta-analysis published in 2015 included 79 trials with 6462 participants who assessed different cannabinoid indications, including chemotherapy-induced nausea and vomiting (CINV), appetite stimulation in HIV/AIDS, chronic pain, spasticity due to multiple sclerosis and paraplegia, depression, anxiety, sleep disorder, psychosis, glaucoma, or Tourette’s syndrome, showed the cannabinoid’s effectiveness in pain management [329]. In a systematic review that summarized 28 studies involving Cannabis, dronabinol, nabilone, and nabiximols, 12 included patients with neuropathic pain, 3 with cancer-associated pain, and 1 with chemotherapy-induced pain. The mean number of participants who indicated a reduction in pain of at least 30% was higher with cannabinoids than with placebo. The research showed a significant improvement in cancer-associated pain, with a 1.41 overall odds ratio in two trials [329]. Another review discussed eighteen randomized controlled trials involving 766 patients with chronic non-cancer-related pain and showed that fifteen reported a significant analgesic effect of cannabinoids [330].
Multiple clinical studies showed analgesic properties of cannabinoids in HIV neuropathy, neuropathic pain, spinal cord injury, and diabetic neuropathy [331,332,333,334]. In a meta-analysis of 19 preclinical studies that involved the administration of THC (14 studies), CB1 agonists (3 studies), and CB2 agonists (1 study), 90% demonstrated a statistically significant synergistic effect with opiates. Additionally, the median effective dose of morphine was 3.6, and codeine was 9.5 times lower in combination with delta-9-THC compared to opiates alone. Overall, cannabinoids, when co-administered with opioids, have the opioid-sparing effect, which means that opioid dosage may be reduced without losing its analgesic efficacy [335]. Another randomized controlled trial showed that adding dronabinol to patients on opioids reduced pain and increased patient satisfaction [336]. Moreover, nabiximols reduced pain and improved sleep in cancer patients with poorly controlled pain [337].
One multicenter, double-blind, randomized, placebo-controlled study tested the efficacy of THC/CBD combination and THC extract in patients with intractable cancer-related pain. The study revealed that patients who received THC/CBD extract had a more than 30% reduction in pain according to the Numerical Rating Scale for patients with advanced cancer. In contrast, THC extract showed no significant difference in pain management compared to placebo. The results imply that THC/CBD extract could be an effective adjuvant therapy for cancer-related pain in patients on opioids with inadequate analgetic effect [301].
One of the most disturbing symptoms in cancer patients is neuropathic pain [338]. However, the data involving patients with chemotherapy-induced peripheral neuropathy are very scarce. Preclinical research that modeled vincristine-induced peripheral neuropathy in rats showed that stimulation of CB1 and CB2 receptors prevented the development of neuropathy [339]. Another study involving cisplatin-induced neuropathy in mice presented that the addition of anandamide with the inhibitor of fatty acid amide hydrolase attenuated chemotherapy-induced peripheral neuropathy [340]. Moreover, pretreatment with CBD prevented paclitaxel-induced neuropathy in mice [333]. The only study that involved humans in chemotherapy-induced peripheral neuropathy was a crossover placebo-controlled trial with nabiximols [341]. However, that study reported no significant difference in pain scores between nabiximols and placebo [341].
Although, there were many trials regarding cannabinoids in pain management, their limitations are a small sample size, difficulties with dose adjustment, withdrawal due to adverse effects, and a short duration, which makes it difficult to objectively establish the analgesic durability of cannabis alone, and in combination with opioids. Another issue could be biphasic effect of cannabinoids. It was shown that low doses of THC reduced pain, whereas higher doses exacerbated nociception [320]. Thus, it is critical to gradually titrate the doses of cannabinoids from lower ones to higher and reach a therapeutic window for analgesia whilst avoiding the opposite effect. The presented data shows the necessity in more clinical studies that would be able to optimize cannabinoid ratios and adjust the doses to achieve the maximum analgesic effect.
6.2.2. Reducing Nausea and Vomiting
Nausea and vomiting are prevalent symptoms in cancer patients. Gastrointestinal obstruction, increased calcium levels, metastasis, intoxication, and even the medications prescribed to cancer patients can induce emesis [320]. Despite the wide availability of antiemetics, many cancer patients suffer from chemotherapy-induced nausea and vomiting (CINV), one of the major side effects of conventional anti-cancer therapeutics [342]. Studies suggest that cannabinoids can inhibit nausea physiologically via CB1 and CB2 receptors in the brainstem dorsal vagal complex, which regulates emesis [343]. The preclinical study showed that emesis is controlled by the endocannabinoid system, which is mediated by 5-hydroxytryptamine 3 (5-HT3) receptors. The 5-HT3 and CB1 receptors are both present on GABA-ergic neurons and have opposite effects on the release of neuromediators [344]. Cannabinoid agonists, including THC, can bind to 5-HT3 receptors and antagonize their signaling [328].
Both dronabinol and nabilone were FDA approved in 1985 for CINV in patients refractory to standard antiemetics [345]. However, after the introduction of highly effective 5-HT3 receptor antagonists in 1991, which are now standard therapy for acute and delayed CINV, cannabinoids became more of a last resort for CINV treatment [345].
There were multiple clinical trials regarding the antiemetic effects of cannabinoids. A systematic review looking at 30 randomized comparisons of nabilone, dronabinol, or levonantradol in 1366 patients found that cannabinoids are more effective antiemetics than standard prochlorperazine, metoclopramide, chlorpromazine, thietylperazine, haloperidol, domperidone, or alizapride. Across all trials, cannabinoids were more effective than their comparators and placebo when it came to completely controlling nausea and vomiting [346]. However, the side effects of cannabinoids, which included a feeling of “high”, drowsiness, somnolence, dysphoria, depression, paranoia, and hallucinations, caused some patients to withdraw from using cannabis as an antiemetic [346].
Another systematic review that included 28 studies (1772 participants) for CINV assessed the effectiveness of dronabinol (14 studies), nabilone (3 studies), nabiximols (1 study), levonandratol (4 studies), and THC (6 studies). Additionally, two studies included an ondansetron combination with other antiemetics such as prochlorperazine. Eight studies involved placebo control, with three of these involving an active comparator, and twenty studies included an active comparator. All trials showed a higher benefit of cannabinoids than other antiemetics and placebo; however, only a few had statistical significance. In three trials, cannabinoids compared to placebo showed a complete absence of nausea and vomiting (47% vs. 20%) response [329]. In patients on methotrexate, symptoms were significantly improved [347]. However, the same research group noted no effect of cannabis on patients receiving cyclophosphamide or doxorubicin after adding dronabinol [348]. Nabiximols, such as Sativex, were tested in 20 patients during a randomized crossover trial, in which 5 noted antiemetic effects [349].
The paucity of the existing clinical data, insufficient understanding of molecular mechanisms of ECS in CINV, and safety and efficacy of using cannabinoids in cancer patients justify the substantial need for more preclinical and clinical trials regarding cannabinoids.
6.2.3. Improving Appetite
Cachexia and anorexia are one of the most troubling cancer-related symptoms experienced by patients. Cachexia is a multifactorial syndrome with progressive loss of skeletal muscular mass that cannot be reversed with the standard nutrition. A randomized placebo-controlled clinical trial conducted on 243 patients with cancer cachexia–anorexia showed no changes in appetite or quality of life under cannabinoids ingestion [145]. Three trials studied the effects of THC on appetite, food appreciation, calorie intake, and weight loss in patients with advanced cancers. As a result, in each study, an administration of oral THC improved one or more of the tested symptoms [297,298,350,351]. Another study with 469 cancer patients receiving dronabinol, megestrol, or both showed no advantage of THC alone or in combination with megestrol [352]. The findings suggest that cannabinoids are not as effective in cancer patients as they are in healthy subjects in terms of their appetite-stimulating effects. However, the palliative options for patients with advanced cancers are very limited, and cannabinoids merit further study in this context.
6.2.4. Reducing Anxiety and Improving Sleep
A small parallel-group trial reported that CBD was associated with greater improvement in the anxiety factor compared with placebo during a simulated public speaking test in patients with a generalized anxiety disorder [329]. Four placebo-controlled studies in patients with chronic pain showed a greater benefit of dronabinol, nabilone, and nabiximols in reducing anxiety [329]. Another placebo-controlled study noted improved sleeping patterns in cancer patients with disordered chemosensory perception using dronabinol [297]. Additionally, a Canadian study assessing the quality of life in 74 patients with a newly diagnosed head and neck cancer reported that using marijuana significantly decreased anxiety/depression and pain/discomfort scores [304]. Currently, there is insufficient data regarding the effectiveness of cannabinoids for relieving the symptoms of anxiety in cancer patients. What is more, higher doses of cannabinoids may affect conventional anti-cancer treatment, which may limit the utility of cannabis as a palliative care.
7. Adverse, Unexpected, and Unintended Effects of Cannabinoids
There are also controversial data regarding the cannabinoid’s action on cancer cells. As stated in one published study, the administration of THC in a xenograft model of non-small cell lung carcinoma in immunodeficient mice showed antiangiogenic and antiproliferative effects [272]. However, some scientists reported that in immunocompetent animals, THC induced tumor growth, possibly due to its immunosuppressive effect [274,275]. On the other hand, the anti-inflammatory effects of endo- and phytocannabinoids can be used to prevent and treat colorectal cancer [353,354,355,356,357,358]. Such results are excellent proof that cannabinoids cannot be blindly taken as an anti-cancer agent in every case. Careful analysis of their various cellular effects, considering the molecular subtypes of cancer and possible drug interactions, must be done. Otherwise, they may cause more harm than benefit to struggling cancer patients.
One of the systematic reviews that evaluated 72 studies on cannabis showed that 62 studies reported adverse events (AEs) associated with cannabis use compared to controls. Cannabinoids were associated with a higher risk of AEs, serious AEs, and withdrawals due to AEs. The most common AEs included asthenia, balance problems, confusion, disorientation, diarrhea, euphoria, drowsiness, dry mouth, fatigue, hallucinations, nausea, somnolence, and vomiting [329]. However, there was no evidence as to whether the type of cannabinoid or mode of administration may affect the development of AEs [329]. These data suggest why cannabinoids are not the first line of treatment for various symptoms in cancer patients. If patients experience multiple AEs, they are likely to discontinue the medication and switch to something with the same or even lower potency, but with no AEs.
Overall, there is an extensive need for more well-designed, high-quality clinical trials regarding the anti-cancer and palliative properties of cannabinoids. However, as cannabis is classified as a Schedule I drug, it is difficult to conduct multicenter trials, regulatory hurdles delay such trials, and access to research-grade cannabis medications that match the products used by the cancer patients may be limited. All these factors affect cannabis research and data efficacy. Below, we summarize data from preclinical (Table 3) and clinical (Table 4) studies on the use of cannabinoids for tumor shrinkage and palliative care. As additional information, we compiled the list of clinical trials on cancer using cannabinoids sourced from clinicaltrials.gov as of 22 June 2022 in Supplementary Table S1.
8. Sex-Specific Differences in ECS, Ethical Considerations of Cannabis Use and Equal Access to Cannabis
Humans are diverse in numerous ways. We differ in sex, gender, sexuality, race, ethnicity, nationality, age, religious and cultural backgrounds, lifestyle, and more. Our different origins, life histories, preferences, and exposures shape who we are as individual human beings. As personalized medicine, also referred to as precision medicine, is developing and growing as a field, it becomes increasingly important to look at health and disease through the lens of diversity.
8.1. Sex-Specific Difference in Cancer and Use of Cannabis
Sex- and gender-based analysis is the first step toward proper implementation of precision medicine—a ground-breaking personalized approach aimed at tailoring disease diagnostics, treatment, and prevention to the needs of each patient based on genetics, epigenetics, environment, and the lifestyle of each individual. Sex encompasses biological attributes such as hormones, chromosomes, gene expression, anatomy, and physical features, while gender signifies the socially constructed behaviors, roles, expressions, and personalities of women, men, and gender-diverse people.
As cancer represents a leading cause of death globally, over the past few decades, a growing number of cancer epidemiology studies have reported the existence of sex disparities [359]. Significant sex disparities have been reported in cancer mortality, whereby lung, colorectal, esophageal, bladder, and stomach cancers, along with melanoma and leukemia, have higher mortality in males than in females [359,360,361]. Indeed, men overall have higher cancer incidence and mortality compared with women [359,362]. An exception is thyroid cancer, which occurs much more frequently in females than in males [363], while the incidence of colorectal, stomach, liver, and bladder cancers, as well as leukemia, is higher in males than in females [359,361,364]. An important and intriguing area of experimental and clinical oncology research is understanding the magnitude, nature, and mechanisms of sex differences in cancer predisposition, incidence, response to treatments, as well as mortality. Some of those may be associated with genetic and molecular changes, gene polymorphism in enzymes involved in drug metabolism, as well as functions of sex hormones that modulate gene expression in various cancers [359].
Treatment outcomes are also sex dependent, and genetic, molecular, and hormonal differences between males and females influence the effect of chemotherapy. While mounting evidence from preclinical models and clinical studies has reported sex disparities in chemotherapy outcomes, until now, chemotherapy has been administered without consideration of sex and gender differences, often leading to reduced efficacy, increased toxicity, and subpar outcomes [359].
Understanding sex and gender is critical in the field of medical cannabis. Indeed, sex differences exist in cannabis use, and research has begun to identify sex differences in the biological effects of cannabis. In recent years, the use of cannabis for pain relief has been consistently growing among women. Interestingly, sex differences have been reported in pain conditions and responses to pain medication, and new evidence is now suggesting that there may be sex differences in cannabinoid-mediated analgesia.
Emerging preclinical evidence, as well as data from human studies, strongly suggests sex differences in the endocannabinoid system (ECS) and cannabinoid pharmacology. Natural and synthetic cannabinoids led to sex differences in the antinociceptive response in animal models, which may correlate with those seen in the expression and function of ECS components. For example, female rodents were more sensitive to the effects of Δ9-tetrahydrocannabinol (Δ9-THC) due to the action of estradiol and progesterone, as well as differences in metabolism and cannabinoid receptor expression [365].
A recent rodent study reported the existence of sex differences in the ECS in two important regions of the central nervous system relevant to cortical spreading depression (V1M cortex) and descending modulatory networks in pain/anxiety (periaqueductal gray (PAG)). Analysis revealed significant differences in the concentrations of endocannabinoids 2-arachidonoylglycerol (2-AG) and anandamide between males and females, and these also varied between female estrous cycle stages. The 2-AG concentration was lower within the female PAG compared with the male PAG; this was confirmed using immunohistochemistry and proteomics. The observed sex differences in endogenous endocannabinoid mechanisms may in turn underlie the development of chronic pain conditions, as well as variations seen in therapy responses [366]. Sex differences in tolerance to THC were shown in mice with cisplatin-evoked chronic neuropathic pain [367]. Chronic adolescent exposure to cannabis in mice also resulted in sex-based changes in gene expression networks in the brain [368]. Interestingly, another rodent study reported that cannabinoids may be equally effective in both males and females in treating nausea [369].
Several other studies reported sex-driven modulation of endogenous cannabinoid signaling during the stress response [370], supporting the hypothesis that the ECS is engaged to a greater degree in males than in females during acute and severe stress and trauma, [371]. Indeed, the ECS plays a pivotal role in the activation and regulation of the stress response [372], albeit stress and anxiety disorders are more prevalent in women than in men [373].
The balanced function of the ECS is pivotal for maintaining mental health [374], and dysregulated endocannabinoid levels have been reported in humans with post-traumatic stress disorder [375]. Sex differences in the ECS function may underlie the sex differences regarding response to trauma and stress. Mechanistically, these differences may be due to hormonal regulation of endocannabinoids, where various sex differences are observed in production and function.
Furthermore, sex differences in the effects of cannabis may be attributed, at least in part, to the differences in fat-tissue distribution and muscle mass between males and females. Cannabinoids and other cannabis components are fat soluble and stored in fat cells, and women usually have higher percentages of body fat than men. Hence, women may experience different manifestations and magnitudes of cannabis effects.
Clinical trials that control for sex and stratify data by sex are pivotal for defining the extent to which medical cannabis and its components will be effective for both male and female patients. Moreover, researchers need to develop additional in-depth preclinical studies to uncover the impacts of sex in ECS functioning in health and disease [376].
While many studies included males and females to analyze palliative effects of cannabinoids on cancer [301,377,378,379], data were not analyzed as a function of sex. Two randomized, controlled trials enrolling healthy subjects analyzed sex differences in the acute effects of cannabis. The data showed that there were no variations in the acute effects of a moderate dose of vaporized cannabis between males and females [380]. A recent report by Meiri and coworkers compared medical-cannabis-related adverse effects between male and female patients with chronic non-cancer pain, and showed that sex differences exist, with adverse effects more frequently reported in females [381].
The only study focused on sex differences in the effects of cannabis on cancer was the large-scale analysis of the Quebec Cannabis Registry. Within the scope of this study, patients (171 males and 187 females) completed the revised Edmonton Symptom Assessment System (ESAS-r) questionnaire at baseline and three-month follow-up. The ESAS-r was used to assess pain, tiredness, anxiety, nausea, drowsiness, appetite, shortness of breath, and overall well-being. In addition, the interaction between sex and time on each ESAS-r symptom, as well as the interaction between time and THC:CBD ratios for each sex on total symptom burden, were analyzed [382]. While no sex differences were seen in the baseline ESAS-r scores, medical cannabis therapy led to significant improvements in pain, tiredness, anxiety, and well-being in both males and females. Improvements in drowsiness, nausea, appetite, and shortness of breath were seen only in females. Moreover, there were sex differences in the effects of THC-dominant cannabis, whereby it reduced pain only in males, and decreased nausea and led to overall improved well-being in females. This important pioneer study concluded that medical-cannabis-induced relief of cancer symptoms differs between sexes [382].
The majority of studies have analyzed cannabis as an adjunct modality used to control pain, anxiety, and treatment side effects in cancer patients. A recent Phase 1b study evaluated the safety and tolerability, as well as the pharmacokinetics and preliminary efficacy, of nabiximols and dose-intense temozolomide in 27 patients with recurrent glioblastoma following radiotherapy and temozolomide as first-line treatment [383]. The study enrolled males and females and laid the foundation for the future analysis of the potential efficacy of cannabinoids in recurrent glioblastoma. The large-scale study will help to establish the role of sex, if any, in the anti-cancer potential of cannabinoids.
8.2. Effect of Cannabis as a Function of Age
The effects of cannabis have to be analyzed across the age continuum. The use of cannabis is more common in adults, but it is expanding in pediatric populations. As yet, there are not enough data regarding the safety and efficacy of cannabis used as an anticancer agent or for symptom management in pediatric oncology [384]. Because the ECS system develops early in life, and in utero exposure data show negative outcomes, extreme caution is recommended in the use of cannabis in children and adolescents. Nevertheless, numerous studies and cases show the safety and efficacy of cannabis in the treatment of pediatric epilepsy and chemotherapy-induced nausea and vomiting (reviewed in [385]). A recent survey-based study of the use of cannabis in pediatric oncology showed that out of 14 participants who reported the use of a cannabis oil formulation for either cancer treatment or symptom management, all experienced symptom improvement [384]. Accumulating preclinical evidence suggests that cannabis may in turn have antitumor effects and may be a promising agent in pediatric oncology. Still, its use in pediatric practice remains controversial and requires more research [385].
More studies are needed to discern the safety and efficacy of cannabis for pain and cancer symptom management in older adults, as no studies have focused on this specific patient population or have stratified data by age, even though the majority of studies enrolled older adults. Cannabis has been shown to be effective in the palliative setting. A study of 2970 cancer patients (average age 59.5 ± 16.3 years) who received medical cannabis as palliative treatment for cancer showed that cannabis was a well-tolerated, effective, and safe option that helped patients cope with cancer-related symptoms such as pain, anxiety, and depression [291]. Analysis of cannabis effects in older adults is very important, as cancer is an aging-associated disease, and the prevalence of cannabis use among older adults (age 65 and older) for medicinal purposes is on the rise [386,387].
8.3. Equal Access to Cannabis for Everyone
Another important aspect of medical cannabis-based therapy is access, as cannabis-based therapies are still not covered by many major insurance plans and remain rather costly. Hence, cannabis-based formulations that may be used to manage cancer- and cancer treatment-related symptoms such as pain, mental health issues, loss of appetite, and cachexia may not be available to low-income and underprivileged groups of individuals, leading to even larger disparities in outcomes. Furthermore, there still is a huge stigma associated with cannabis use, which is often perceived as one of the bases of discrimination, alienation, and devaluation [388,389,390]. Up to 76% of patients who participated in the online cross-sectional survey study reported hiding their cannabis use from medical practitioners to avoid being judged [391]. Even in this day and age, a considerable number of approved medical cannabis patients in Canada report a lack of support and a need to conceal their medical cannabis use [391].
Stigmatization of medical cannabis users may also be more frequent in groups that already experience discrimination based on race and ethnic origins, sexual orientation, gender and gender identity, physical and mental disability, addiction, income, and age [392,393], causing even more trauma and distress that can lead to worsening health outcomes [394]. Regrettably, the medical establishment sometimes regards individuals experiencing poverty, mental health patients, and gender and sexual minorities as “problem patients,” and cannabis use may further contribute to their social devaluation and stigmatization [389,390].
Perceived stigmatization and discrimination and fear of judgment and rejection may sometimes lead to cancer patients refusing safe and effective cannabis-based adjuvant treatments, thus adding depression, anxiety, pain, suffering, stress, and trauma to an already grave and often deteriorating disease condition [390,395,396]. In an excellent review of stigma in medical cannabis use, Reid quoted a study by Rudski [396]: “Medicine can only be effective if it is taken, and stigma and lack of acceptability can interfere with compliance and safe access.” This is especially important in clinical oncology, where the benefits of adjuvant use of medical cannabis have been documented. Alienation, devaluation, and stigmatization of cannabis users may also depend upon their cultural, ethnic, and religious background, being more frequent in some communities and countries where cannabis use is still prohibited and criminalized [390]. As more studies are completed and results reported, the acceptance of medical cannabis will grow globally, leading to increased safe use and decreased stigma.
9. Conclusions
Cannabis and cannabinoids hold big promise for cancer therapy. First, however, we need to understand more about the role of ECS in normal human physiology and malignant transformations. How is it that all three components of ECS are typically upregulated in cancers, but addition of cannabinoids, overexpression, or sometimes downregulation of CB1/CB2 receptors actually inhibits the cancer growth? One hypothesis to be tested is that ECS is upregulated in cancer to a greater capacity to cope with the demands of continuous growth and that any changes in the balanced action of ECS—whether up- or downregulation—result in the growth inhibition or death of these cells.
Molecular mechanisms of ECS regulation and anti-cancer properties of cannabis also need to be clarified. What role do CB1 and CB2 receptors play? How other receptors contribute? How is it that some anti-cancer properties are independent of receptor activation?
Here, we showed substantial preclinical and clinical evidence of the potential of cannabinoids and cannabis extracts in primary and palliative care of cancer. However, we need more data on human consumption of cannabis, from case reports to clinical trials. As with any novel drug, we need to have good understanding about the interaction of individual active ingredients of the extracts with respect to their efficiency for primary and palliative care. Moreover, we need to know how cannabinoids interact with these drugs. We also actually need to find out what the active ingredients in cannabis are with respect to specific anti-cancer properties. Finally, we need to understand the sex-, gender- and age-specific differences in response to cannabis and cannabinoids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14205142/s1; Table S1: Clinical trials related to cannabinoid use in cancer (source clinicaltrials.gov as of 22 June 2022).
Author Contributions
All authors were involved in writing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MITACS grants IT11447, IT15089.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McPartland, J.M.; Matias, I.; Di Marzo, V.; Glass, M. Evolutionary origins of the endocannabinoid system. Gene 2006, 370, 64–74. [Google Scholar] [CrossRef]
- Cristino, L.; Becker, T.; Di Marzo, V. Endocannabinoids and energy homeostasis: An update. BioFactors 2014, 40, 389–397. [Google Scholar] [CrossRef]
- Rodríguez-Valentín, R.; Torres-Mejía, G.; Martínez-Matsushita, L.; Angeles-Llerenas, A.; Gómez-Flores-Ramos, L.; Wolff, R.K.; Baumgartner, K.B.; Hines, L.M.; Ziv, E.; Flores-Luna, L.; et al. Energy homeostasis genes modify the association between serum concentrations of IGF-1 and IGFBP-3 and breast cancer risk. Sci. Rep. 2022, 12, 1837. [Google Scholar] [CrossRef]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Kunos, G.; Osei-Hyiaman, D.; Liu, J.; Godlewski, G.; Bátkai, S. Endocannabinoids and the control of energy homeostasis. J. Biol. Chem. 2008, 283, 33021–33025. [Google Scholar] [CrossRef]
- Loeb, L.A.; Loeb, K.R.; Anderson, J.P. Multiple mutations and cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 776–781. [Google Scholar] [CrossRef]
- Battista, N.; Bari, M.; Maccarrone, M. Endocannabinoids and Reproductive Events in Health and Disease. Endocannabinoids 2015, 231, 341–365. [Google Scholar] [CrossRef]
- Kozakiewicz, M.L.; Grotegut, C.A.; Howlett, A.C. Endocannabinoid System in Pregnancy Maintenance and Labor: A Mini-Review. Front. Endocrinol. 2021, 12, 699951. [Google Scholar] [CrossRef]
- Nghdawagsb, E.F. The endocannabinoid system during development: Emphasis on perinatal events and delayed effects. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2009; pp. 139–158. [Google Scholar]
- Skaper, S.D.; di Marzo, V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3193–3200. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2005, 27, 73–100. [Google Scholar] [CrossRef]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A. The endocannabinoid system in normal and pathological brain ageing. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3326–3341. [Google Scholar] [CrossRef]
- Michela, G.; Giuseppe, C.; Ornella, P.; Patrizia, P.; Daniela, C.; Renza, V.; Romano, L.L. Anandamide-induced apoptosis in Chang liver cells involves ceramide and JNK/AP-1 pathway. Int. J. Mol. Med. 2006, 17, 811–819. [Google Scholar]
- Noonan, J.; Tanveer, R.; Klompas, A.; Gowran, A.; McKiernan, J.; Campbell, V.A. Endocannabinoids Prevent β-Amyloid-mediated Lysosomal Destabilization in Cultured Neurons. J. Biol. Chem. 2010, 285, 38543–38554. [Google Scholar] [CrossRef]
- Velez-Pardo, C.; Jimenez-Del-Rio, M.; Lores-Arnaiz, S.; Bustamante, J. Protective Effects of the Synthetic Cannabinoids CP55,940 and JWH-015 on Rat Brain Mitochondria upon Paraquat Exposure. Neurochem. Res. 2010, 35, 1323–1332. [Google Scholar] [CrossRef]
- Zaccagnino, P.; D’Oria, S.; Romano, L.L.; Di Venere, A.; Sardanelli, A.M.; Lorusso, M. The endocannabinoid 2-arachidonoylglicerol decreases calcium induced cytochrome c release from liver mitochondria. J. Bioenerg. Biomembr. 2012, 44, 273–280. [Google Scholar] [CrossRef]
- Mackie, K.; Mackie, K. Cannabinoid Receptors: Where They are and What They do. J. Neuroendocr. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid signaling in the skin: Therapeutic potential of the “c(ut)annabinoid” system. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache J. Head Face Pain 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- del, R.C.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. J. Cereb. Blood Flow Metab. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; del Castillo, M.D.; Abalo, R. Cannabidiol and other non-psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: Useful nutraceuticals? Int. J. Mol. Sci. 2020, 9, 3067. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. In Endocannabinoids; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 233–259. [Google Scholar]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef]
- Gomez, O.; Arevalo-Martin, A.; Garcia-Ovejero, D.; Ortega-Gutierrez, S.; Cisneros, J.A.; Almazan, G.; Sánchez-Rodriguez, M.A.; Molina-Holgado, F.; Molina-Holgado, E. The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia 2010, 58, 1913–1927. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Haskó, G.; Paus, R.; Pacher, P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef]
- Ständer, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005, 38, 177–188. [Google Scholar] [CrossRef]
- Telek, A.; Bíró, T.; Bodó, E.; Tóth, B.I.; Borbíró, I.; Kunos, G.; Sardanelli, A.M. Inhibition of human hair follicle growth by endo-and exocannabinoids. FASEB J. 2007, 21, 3534–3541. [Google Scholar] [CrossRef]
- Dobrosi, N.; Tóth, B.I.; Nagy, G.; Dózsa, A.; Géczy, T.; Nagy, L.; Harvey-White, J.; Loureiro, A.I. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008, 22, 3685–3695. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. NLM (Medlin.) 2017, 2, 48–60. [Google Scholar] [CrossRef]
- Schwarz, H.; Blanco, F.J.; Lotz, M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J. Neuroimmunol. 1994, 55, 107–115. [Google Scholar] [CrossRef]
- Joseph, J.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol. Immunother. 2004, 53, 723–728. [Google Scholar] [CrossRef]
- Kishimoto, S.; Muramatsu, M.; Gokoh, M.; Oka, S.; Waku, K.; Sugiura, T. Endogenous Cannabinoid Receptor Ligand Induces the Migration of Human Natural Killer Cells. J. Biochem. 2005, 137, 217–223. [Google Scholar] [CrossRef]
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through Cannabinoid Receptors 1 and 2 on Dendritic Cells Triggers NF-κB-Dependent Apoptosis: Novel Role for Endogenous and Exogenous Cannabinoids in Immunoregulation. J. Immunology. 2004, 173, 2373–2382. [Google Scholar] [CrossRef]
- Berrendero, F.; Romero, J.; García-Gil, L.; Suarez, I.; De la Cruz, P.; Ramos, J.; Fernández-Ruiz, J. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 1998, 1407, 205–214. [Google Scholar] [CrossRef]
- Liu, P.; Bilkey, D.K.; Darlington, C.L.; Smith, P.F. Cannabinoid CB1 receptor protein expression in the rat hippocampus and entorhinal, perirhinal, postrhinal and temporal cortices: Regional variations and age-related changes. Brain Res. 2003, 979, 235–239. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Harvey-White, J.; Zimmer, A.; Kunos, G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1393–1398. [Google Scholar] [CrossRef]
- Van Laere, K.; Goffin, K.; Casteels, C.; Dupont, P.; Mortelmans, L.; de Hoon, J.; Bormans, G. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F]MK-9470 PET. Neuroimage 2008, 39, 1533–1541. [Google Scholar] [CrossRef]
- Bátkai, S.; Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Cravatt, B.F.; Csiszár, A.; Ungvári, Z.; Pacher, P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase-1) expression and monocyte-endothelial adhesion in Downloaded from. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 909–918. [Google Scholar] [CrossRef]
- Correia-Sá, I.B.; Carvalho, C.M.; Serrão, P.V.; Loureiro, A.I.; Fernandes-Lopes, C.; Marques, M.; Vieira-Coelho, M.A. A new role for anandamide: Defective link between the systemic and skin endocannabinoid systems in hypertrophic human wound healing. Sci. Rep. 2020, 10, 3. [Google Scholar] [CrossRef]
- Leal, E.C.; Moura, L.I.; Pirzgalska, R.M.; Marques-da-Silva, D.; Ledent, C.; Köfalvi, A.; Carvalho, E. Diabetes and Cannabinoid CB1 receptor deficiency promotes similar early onset aging-like changes in the skin. Exp. Gerontol. 2021, 15, 154. [Google Scholar]
- Robben, M.; Nasr, M.S.; Das, A.; Huber, M.; Jaworski, J.; Weidanz, J.; Luber, J.M. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 28, D20–D26. [Google Scholar]
- Pati, S.; Krishna, S.; Lee, J.H.; Ross, M.K.; de La Serre, C.B.; Harn, D.A.; Wagner, J.; Filipov, N.M.; Cummings, B.S. Effects of high-fat diet and age on the blood lipidome and circulating endocannabinoids of female C57BL/6 mice. Biochim. et Biophys. Acta BBA-Mol. Cell Biol. Lipids 2017, 1863, 26–39. [Google Scholar] [CrossRef]
- Maccarrone, M.; Valverde, O.; Barbaccia, M.L.; Castañé, A.; Maldonado, R.; Ledent, C.; Parmentier, M.; Finazzi-Agrò, A. Age-related changes of anandamide metabolism in CB1cannabinoid receptor knockout mice: Correlation with behaviour. Eur. J. Neurosci. 2002, 15, 1178–1186. [Google Scholar] [CrossRef]
- Piyanova, A.; Lomazzo, E.; Bindila, L.; Lerner, R.; Albayram, O.; Ruhl, T.; Lutz, B.; Zimmer, A.; Bilkei-Gorzo, A. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech. Ageing Dev. 2015, 150, 55–64. [Google Scholar] [CrossRef]
- Romero, J.; Berrendero, F.; Garcia-Gil, L.; de la Cruz, P.; Ramos, J.; Fernandez-Ruiz, J. Loss of cannabinoid receptor binding and messenger RNA levels and cannabinoid agonist-stimulated [35s]guanylyl-5′-O-(thio)-triphosphate binding in the basal ganglia of aged rats. Neuroscience 1998, 84, 1075–1083. [Google Scholar] [CrossRef]
- Lee, T.T.-Y.; Hill, M.N.; Hillard, C.J.; Gorzalka, B.B. Temporal changes inN-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse 2012, 67, 4–10. [Google Scholar] [CrossRef]
- Sailler, S.; Schmitz, K.; Jäger, E.; Ferreiros, N.; Wicker, S.; Zschiebsch, K.; Pickert, G.; Geisslinger, G.; Walter, C.; Tegeder, I.; et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience 2014, 1, 272–282. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, J.; Zhao, H.; Fang, X.; Li, H. Cannabinoid receptor 2 is upregulated in melanoma. J. Cancer Res. Ther. 2012, 8, 549–554. [Google Scholar] [CrossRef]
- Baba, Y.; Funakoshi, T.; Emoto, K.; Masugi, Y.; Ekmekcioglu, S.; Amagai, M.; Mori, M.; Tanese, K. Expression of monoacylglycerol lipase as a marker of tumour invasion and progression in malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, B.; Seviour, E.G.; Tao, K.-X.; Liu, X.-H.; Ling, Y.; Chen, J.-Y.; Wang, G.-B. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011, 307, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Andersen, L.; Hasenöhrl, C.; Feuersinger, D.; Stančić, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. J. Cereb. Blood Flow Metab. 2015, 173, 142–154. [Google Scholar] [CrossRef]
- Hasenoehrl, C.; Feuersinger, D.; Sturm, E.M.; Bärnthaler, T.; Heitzer, E.; Graf, R.; Grill, M.; Pichler, M.; Beck, S.; Butcher, L.; et al. G protein-coupled receptor GPR55 promotes colorectal cancer and has opposing effects to cannabinoid receptor 1. Int. J. Cancer 2018, 142, 121–132. [Google Scholar] [CrossRef]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnelli, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P.; et al. Cannabinoid Receptor Activation Induces Apoptosis through Tumor Necrosis Factor α–Mediated Ceramide De novo Synthesis in Colon Cancer Cells. Clin. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef]
- Kitamura, C.; Sonoda, H.; Anzai, H.; Nagai, Y.; Abe, S.; Yokoyama, Y.; Ishii, H.; Kishikawa, J.; Emoto, S.; Murono, K.; et al. Expression of Lysophosphatidylinositol Signaling-relevant Molecules in Colorectal Cancer. Anticancer Res. 2021, 41, 2349–2355. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; De Llano, J.J.M.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Preet, A.; Qamri, Z.; Nasser, M.W.; Prasad, A.; Shilo, K.; Zou, X.; Groopman, J.E.; Ganju, R.K. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev. Res. 2011, 4, 65–75. [Google Scholar] [CrossRef]
- Winkler, K.; Ramer, R.; Dithmer, S.; Ivanov, I.; Merkord, J.; Hinz, B. Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget 2016, 7, 15047–15064. [Google Scholar] [CrossRef]
- Xian, X.; Tang, L.; Wu, C.; Huang, L. MiR-23b-3p and miR-130a-5p affect cell growth, migration and invasion by targeting CB1R via the Wnt/β-catenin signaling pathway in gastric carcinoma. Onco. Targets Ther. 2018, 11, 7503–7512. [Google Scholar] [CrossRef]
- Morin-Buote, J.; Ennour-Idrissi, K.; Poirier, É.; Lemieux, J.; Furrer, D.; Burguin, A.; Durocher, F.; Diorio, C. Association of Breast Tumour Expression of Cannabinoid Receptors CBR1 and CBR2 with Prognostic Factors and Survival in Breast Cancer Patients. J. Pers. Med. 2021, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gomez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; García-Taboada, E.; Villa-Morales, M.C.; Moreno, E.; Hamann, S.; Martin-Villar, E.; Flores, J.M.; et al. Role of Cannabinoid Receptor CB2 in HER2 Pro-oncogenic Signaling in Breast Cancer. J. Natl. Cancer Inst. 2015, 107, djv077. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.; Ahirwar, D.; Ravi, J.; Nasser, M.W.; Ganju, R.K. Novel role of cannabinoid receptor 2 in inhibiting EGF/EGFR and IGF-I/IGF-IR pathways in breast cancer. Oncotarget 2017, 8, 29668–29678. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.W.; Qamri, Z.; Deol, Y.S.; Smith, D.; Shilo, K.; Zou, X.; Ganju, R.K. Crosstalk between Chemokine Receptor CXCR4 and Cannabinoid Receptor CB2 in Modulating Breast Cancer Growth and Invasion. PLoS ONE 2011, 6, e23901. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Guo, X.; Song, Y.-P.; Zhu, C.-Y.; Zou, W. The LPI/GPR55 axis enhances human breast cancer cell migration via HBXIP and p-MLC signaling. Acta Pharmacol. Sin. 2017, 39, 459–471. [Google Scholar] [CrossRef]
- Andradas, C.; Benito, S.B.; Castillo-Lluva, S.; Pilla, P.D.; Alarcia, R.D.; Garcia, A.J.; García-Taboada, E.; Hernando-Llorente, R.; Soriano, J.; Hamann, S.; et al. Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple-negative breast cancer. Oncotarget 2016, 7, 47565–47575. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Pisanti, S.; Gazzerro, P. Cannabinoids and cancer: Pros and cons of an antitumour strategy. J. Cereb. Blood Flow Metab. 2006, 148, 123–135. [Google Scholar] [CrossRef]
- Petersen, G.; Moesgaard, B.; Schmid, P.C.; Schmid, H.H.O.; Broholm, H.; Kosteljanetz, M.; Hansen, H.S. Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non-tumour brain tissue. J. Neurochem. 2005, 93, 299–309. [Google Scholar] [CrossRef]
- Schmid, P.C.; Wold, L.E.; Krebsbach, R.J.; Berdyshev, E.V.; Schmid, H.H.O. Anandamide and other N-acylethanolamines in human tumors. Lipids 2002, 37, 907–912. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Fezza, F.; Theodoropoulou, M.; Grübler, Y.; Stalla, J.; Arzberger, T.; Milone, A.; Losa, M.; Di Marzo, V.; et al. Normal Human Pituitary Gland and Pituitary Adenomas Express Cannabinoid Receptor Type 1 and Synthesize Endogenous Cannabinoids: First Evidence for a Direct Role of Cannabinoids on Hormone Modulation at the Human Pituitary Level. J. Clin. Endocrinol. Metab. 2001, 86, 2687–2696. [Google Scholar] [PubMed]
- Nithipatikom, K.; Endsley, M.P.; Isbell, M.A.; Falck, J.R.; Iwamoto, Y.; Hillard, C.J. 2-Arachidonoylglycerol: A Novel Inhibitor of Androgen-Independent Prostate Cancer Cell Invasion. Cancer Res. 2004, 64, 8826–8830. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; de Ceballos, M.L.; Gomez del Pulgar, T.; Rueda, D.; Corbacho, C.; Velasco, G.; Galve-Roperh, I.; Huffman, J.W.; Ramón y Cajal, S.; Guzmán, M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001, 61, 5784–5789. [Google Scholar] [PubMed]
- Ellert-Miklaszewska, A.; Grajkowska, W.; Gabrusiewicz, K.; Kaminska, B.; Konarska, L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007, 1137, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Bashi, S.; Zali, A. The expression level of cannabinoid receptors type 1 and 2 in the different types of astrocytomas. Mol. Biol. Rep. 2020, 47, 5461–5467. [Google Scholar] [CrossRef]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of endocannabinoid system in human gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef]
- Choucair, N.; Saker, Z.; Eddine, H.K.; Bahmad, H.F.; Fares, Y.; Zaarour, M.; Harati, H.; Nabha, S. Immunohistochemical assessment of cannabinoid type-1 receptor (CB1R) and its correlation with clinicopathological parameters in glioma. Pathologica 2022, 114, 128–137. [Google Scholar] [CrossRef]
- Piñeiro, R.; Maffucci, T.; Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 2010, 30, 142–152. [Google Scholar] [CrossRef]
- Hu, G.; Ren, G.; Shi, Y. The putative cannabinoid receptor GPR55 promotes cancer cell proliferation. Oncogene 2010, 30, 139–141. [Google Scholar] [CrossRef]
- Benz, A.H.; Renné, C.; Maronde, E.; Koch, M.; Grabiec, U.; Kallendrusch, S.; Rengstl, B.; Newrzela, S.; Hartmann, S.; Hansmann, M.-L.; et al. Expression and Functional Relevance of Cannabinoid Receptor 1 in Hodgkin Lymphoma. PLoS ONE 2013, 8, e81675. [Google Scholar] [CrossRef]
- Islam, T.C.; Asplund, A.C.; Lindvall, J.M.; Nygren, L.; Liden, J.; Kimby, E.; Christensson, B.; Smith, C.I.; Sander, B. High level cannabinoid receptor 1, resistance of regulator G protein signaling 13 and differential expression of Cyclin D1 in mantle cell lymphoma. Leukemia 2003, 17, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Freund, P.; Porpaczy, E.A.; Le, T.; Gruber, M.; Pausz, C.; Staber, P.; Jäger, U.; Vanura, K. Cannabinoid Receptors Are Overexpressed in CLL but of Limited Potential for Therapeutic Exploitation. PLoS ONE 2016, 11, e0156693. [Google Scholar] [CrossRef] [PubMed]
- Jordà, M.A.; Rayman, N.; Tas, M.; Verbakel, S.E.; Battista, N.; van Lom, K.; Löwenberg, B.; Maccarrone, M.; Delwel, R. The peripheral cannabinoid receptor Cb2, frequently expressed on AML blasts, either induces a neutrophilic differentiation block or confers abnormal migration properties in a ligand-dependent manner. Blood 2004, 104, 526–534. [Google Scholar] [CrossRef]
- Ronchi, A.; Grauso, F.; Zito Marino, F.; Quagliariello, V.; Maurea, N.; Facchini, G.; Montopoli, M.; Franco, R.; Berretta, M.; Messalli, E.M. Endocannabinoid system expression in ovarian epithelial tumors according to the dualistic model of ovarian carcinogenesis. Eutopean Rev. Med. Pharmacol. Sci. 2021, 25, 4678–4686. [Google Scholar]
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 211, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzmán, M.; Velasco, G.; Iovanna, J.L. Cannabinoids Induce Apoptosis of Pancreatic Tumor Cells via Endoplasmic Reticulum Stress–Related Genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [CrossRef]
- Czifra, G.; Varga, A.; Nyeste, K.; Marincsák, R.; Tóth, B.I.; Kovács, I.; Kovács, L.; Bíró, T. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 507–514. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid Receptor as a Novel Target for the Treatment of Prostate Cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef]
- Casanova, M.L.; Blázquez, C.; Martínez-Palacio, J.; Villanueva, C.; Fernández-Aceñero, M.J.; Huffman, J.W.; Jorcano, J.L.; Guzmán, M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J. Clin. Investig. 2003, 111, 43–50. [Google Scholar] [CrossRef]
- Blázquez, C.; Carracedo, A.; Barrado, L.; Real, P.J.; Fernández-Luna, J.L.; Velasco, G.; Malumbres, M.; Guzmán, M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Lakiotaki, E.; Giaginis, C.; Tolia, M.; Alexandrou, P.; Delladetsima, I.; Giannopoulou, I.; Kyrgias, G.; Patsouris, E.; Theocharis, S. Clinical Significance of Cannabinoid Receptors CB1 and CB2 Expression in Human Malignant and Benign Thyroid Lesions. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef]
- Hijiya, N.; Shibata, T.; Daa, T.; Hamanaka, R.; Uchida, T.; Matsuura, K.; Tsukamoto, Y.; Nakada, C.; Iha, H.; Inomata, M.; et al. Overexpression of cannabinoid receptor 1 in esophageal squamous cell carcinoma is correlated with metastasis to lymph nodes and distant organs, and poor prognosis. Pathol. Int. 2016, 67, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nulent, T.J.; Van Diest, P.J.; Van Der Groep, P.; Leusink, F.K.; Kruitwagen, C.L.; Koole, R.; Van Cann, E.M. Cannabinoid receptor-2 immunoreactivity is associated with survival in squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 2013, 51, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.-T.; Mederacke, I.; Gwak, G.-Y.; Cho, S.W.; Adeyemi, A.; Friedman, R.; Schwabe, R.F. Opposite roles of cannabinoid receptors 1 and 2 in hepatocarcinogenesis. Gut 2016, 65, 1721–1732. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef]
- Yang, J.; Tian, Y.; Zheng, R.; Li, L.; Qiu, F. Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma. Oncol. Lett. 2019, 18, 1530–1538. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhu, L.; Zou, Y.; Kong, W.; Dong, B.; Huang, J.; Chen, Y.; Xue, W.; Huang, Y.; et al. Cannabinoid receptor 2 as a novel target for promotion of renal cell carcinoma prognosis and progression. J. Cancer Res. Clin. Oncol. 2017, 144, 39–52. [Google Scholar] [CrossRef]
- Larrinaga, G.; Sanz, B.; Blanco, L.; Perez, I.; Candenas, M.L.; Pinto, F.M.; Irazusta, A.; Gil, J.; López, J.I. Cannabinoid CB1 receptor is expressed in chromophobe renal cell carcinoma and renal oncocytoma. Clin. Biochem. 2013, 46, 638–641. [Google Scholar] [CrossRef]
- Theocharis, S.; Giaginis, C.; Alexandrou, P.; Rodríguez, J.; Tasoulas, J.; Danas, E.; Patsouris, E.; Klijanienko, J. Evaluation of cannabinoid CB1 and CB2 receptors expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patients’ survival. Tumor Biol. 2015, 37, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.B.; Lindgren, T.; Jonsson, M.; Jacobsson, S.O.P. Cannabinoid receptor-independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: Synergism with 5-fluorouracil. Cancer Chemother. Pharmacol. 2008, 63, 691–701. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Inhibition of Cancer Cell Invasion by Cannabinoids via Increased Expression of Tissue Inhibitor of Matrix Metalloproteinases-1. JNCI J. Natl. Cancer Inst. 2008, 100, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2007, 122, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; Caruso, M.G.; De Nunzio, V.; Lorusso, D.; Veronese, N.; Gigante, I.; Notarnicola, M.; Giannelli, G. Down-Regulation of Cannabinoid Type 1 (CB1) Receptor and its Downstream Signaling Pathways in Metastatic Colorectal Cancer. Cancers 2019, 11, 708. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; DuBois, R.N. Loss of Cannabinoid Receptor 1 Accelerates Intestinal Tumor Growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef]
- Larrinaga, G.; Begoña, S.; Itxaro, P.; Blanco, L.; Maria, L.C.; Pinto, F.M.; Gil, J.; José, I.L. Cannabinoid CB1 receptor is downregulated in clear cell renal cell carcinoma. J. Histochem. Cytochem. 2010, 58, 1129–1134. [Google Scholar] [CrossRef]
- Li, M.; Qian, X.; Zhu, M.; Li, A.; Fang, M.; Zhu, Y.; Zhang, J. MiR-1273g-3p promotes proliferation, migration and invasion of LoVo cells via cannabinoid receptor 1 through activation of ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Mol. Med. Rep. 2018, 17, 4619–4626. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Expression of the putative cannabinoid receptor GPR55 is increased in endometrial carcinoma. Histochem. Cell Biol. 2021, 156, 449–460. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Andradas, C.; Flores, J.M.; Quintanilla, M.; Paramio, J.M.; Guzmán, M.; Sánchez, C. The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 2012, 32, 2534–2542. [Google Scholar] [CrossRef]
- He, D.; Wang, J.; Zhang, C.; Shan, B.; Deng, X.; Li, B.; Zhou, Y.; Chen, W.; Hong, J.; Gao, Y.; et al. Down-regulation of miR-675-5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol. Cancer 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Cota, D. MECHANISMS IN ENDOCRINOLOGY: Endocannabinoids and metabolism: Past, present and future. Eur. J. Endocrinol. 2017, 176, R309–R324. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’Argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Li, Y.; Li, L.; Qiu, Y.; Ren, J. Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol. Rep. 2015, 34, 447–454. [Google Scholar] [CrossRef]
- Auer, M.K.; Gebert, D.; Biedermann, S.V.; Bindila, L.; Stalla, G.; Reisch, N.; Kopczak, A.; Fuss, J. Altered endocannabinoid-dynamics in craniopharyngioma patients and their association with HPA-axis disturbances. Eur. J. Endocrinol. 2021, 1, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Medina-Cleghorn, D.; Bernal-Mizrachi, L.; Bracci, P.M.; Hubbard, A.; Conde, L.; Riby, J.; Nomura, D.K.; Skibola, C.F. The potential relevance of the endocannabinoid, 2-arachidonoylglycerol, in diffuse large B-cell lymphoma. Oncoscience 2016, 3, 31–41. [Google Scholar] [CrossRef][Green Version]
- Park, S.-W.; Kim, J.-E.; Oh, S.-M.; Cha, W.-J.; Hah, J.-H.; Sung, M.-W. Anticancer effects of anandamide on head and neck squamous cell carcinoma cells via the production of receptor-independent reactive oxygen species. Head Neck 2014, 37, 1187–1192. [Google Scholar] [CrossRef]
- Önay, Ö.; Köse, S.; Süslü, N.; Korkusuz, P.; Nemutlu, E.; Aydın, C.; Hosal, S. Human laryngeal squamous cell carcinoma cell line release of endogenous anandamide and 2-arachidonoylglycerol, and their antiproliferative effect via exogenous supplementation: An in vitro study. Cell Tissue Bank 2022, 23, 93–100. [Google Scholar]
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108952. [Google Scholar] [CrossRef]
- Ford, L.A.; Roelofs, A.J.; Anavi-Goffer, S.; Mowat, L.; Simpson, D.G.; Irving, A.J.; Rogers, M.J.; Rajnicek, A.M.; Ross, R.A. A role for L-α-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 2010, 160, 762–771. [Google Scholar] [CrossRef]
- Hofmann, N.A.; Yang, J.; Trauger, S.A.; Nakayama, H.; Huang, L.; Strunk, D.; Moses, M.A.; Klagsbrun, M.; Bischoff, J.; Graier, W.F. The GPR 55 agonist, L-α-lysophosphatidylinositol, mediates ovarian carcinoma cell-induced angiogenesis. Br. J. Pharmacol. 2015, 172, 4107–4118. [Google Scholar] [CrossRef] [PubMed]
- Akimov, M.G.; Gamisonia, A.M.; Dudina, P.V.; Gretskaya, N.M.; Gaydaryova, A.A.; Kuznetsov, A.S.; Zinchenko, G.N.; Bezuglov, V.V. GPR55 Receptor Activation by the N-acyl Dopamine Family Lipids Induces Apoptosis in Cancer Cells via the Nitric Oxide Synthase (nNOS) Over-Stimulation. Int. J. Mol. Sci. 2021, 22, 622. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Soboci, A.A.; Czarnecka, A.; Król, M.; Botta, B.; Szczylik, C. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Ramer, R.; Hinz, B. Targeting the endocannabinoid system as a potential anticancer approach. Drug Metab. Rev. 2018, 50, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Hinz, B. Antitumorigenic targets of cannabinoids—Current status and implications. Expert Opin. Ther. Targets 2016, 20, 1219–1235. [Google Scholar] [CrossRef]
- Thors, L.; Bergh, A.; Persson, E.; Hammarsten, P.; Stattin, P.; Egevad, L.; Granfors, T.; Fowler, C.J. Fatty Acid Amide Hydrolase in Prostate Cancer: Association with Disease Severity and Outcome, CB1 Receptor Expression and Regulation by IL-4. PLoS ONE 2010, 5, e12275. [Google Scholar] [CrossRef]
- Fowler, C.J.; Josefsson, A.; Thors, L.; Chung, S.C.; Hammarsten, P.; Wikström, P.; Bergh, A. Tumour epithelial expression levels of endocannabinoid markers modulate the value of endoglin-positive vascular density as a prognostic marker in prostate cancer. Biochim. et Biophys. Acta BBA-Mol. Cell Biol. Lipids 2013, 1831, 1579–1587. [Google Scholar] [CrossRef]
- Endsley, M.P.; Thill, R.; Choudhry, I.; Williams, C.L.; Kajdacsy-Balla, A.; Campbell, W.B.; Nithipatikom, K. Expression and function of fatty acid amide hydrolase in prostate cancer. Int. J. Cancer 2008, 123, 1318–1326. [Google Scholar] [CrossRef]
- Wasilewski, A.; Krajewska, U.; Owczarek, K.; Lewandowska, U.; Fichna, J. Fatty acid amide hydrolase (FAAH) inhibitor PF-3845 reduces viability, migration and invasiveness of human colon adenocarcinoma Colo-205 cell line: An in vitro study. Acta Biochim. Pol. 2017, 64, 519–525. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Bari, M.; Mastrangelo, N.; Maccarrone, M.; Konje, J.C. Expression and Function of the Endocannabinoid Modulating Enzymes Fatty Acid Amide Hydrolase and N-Acylphosphatidylethanolamine-Specific Phospholipase D in Endometrial Carcinoma. Front. Oncol. 2019, 9, 1363. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Konje, J.C. New Insights of Uterine Leiomyoma Pathogenesis: Endocannabinoid System. Med. Sci. Monit. Basic Res. 2019, 25, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, W.; Zhao, Y.; Zhou, J.; Wang, X.; Pan, Q.; Zhang, N.; Wang, L.; Wang, M.; Zhan, Z.; et al. Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial-mesenchymal transition. J. Hematol. Oncol. 2016, 25, 9. [Google Scholar]
- Liu, Y.; Yang, X.L.; Gong, J.P.; Zhang, J.Y. Monoacylglycerol lipase high expression as an independent indicator of survival for patients with hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2019, 27, 760–765. [Google Scholar] [PubMed]
- Wang, C.; Li, Z.; Zhong, L.; Chen, Y. Inhibition of monoacylglycerol lipase restrains proliferation, migration, invasion, tumor growth and induces apoptosis in cervical cancer. J. Obstet. Gynaecol. Res. 2021, 15, 110. [Google Scholar] [CrossRef]
- Li, X.; Gao, S.; Li, W.; Liu, Z.; Shi, Z.; Qiu, C.; Jiang, J. Effect of monoacylglycerol lipase on the tumor growth in endometrial cancer. J. Obstet. Gynaecol. Res. 2019, 45, 2043–2054. [Google Scholar] [CrossRef]
- Gong, X.; Zheng, X.; Huang, Y.; Song, W.; Chen, G.; Chen, T. Monoacylglycerol Lipase (MAGL) Inhibition Impedes the Osteosarcoma Progression by Regulating Epithelial Mesenchymal Transition. Tohoku J. Exp. Med. 2022, 256, 19–26. [Google Scholar] [CrossRef]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.-W.; Cravatt, B.F. Monoacylglycerol Lipase Regulates a Fatty Acid Network that Promotes Cancer Pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef]
- Carbonetti, G.; Wilpshaar, T.; Kroonen, J.; Studholme, K.; Converso, C.; D’Oelsnitz, S.; Kaczocha, M. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep. 2019, 9, 18944. [Google Scholar] [CrossRef]
- Hu, W.R.; Lian, Y.F.; Peng, L.X.; Lei, J.J.; Deng, C.C.; Xu, M.; Feng, Q.S.; Chen, L.Z.; Bei, J.X.; Zeng, Y.X. Monoacylglycerol lipase promotes metastases in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 832. [Google Scholar]
- Sriram, K.; Insel, P.A. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? In: Molecular Pharmacology. Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Insel, P.A.; Sriram, K.; Wiley, S.Z.; Wilderman, A.; Katakia, T.; McCann, T.; Yokouchi, H.; Zhang, L.; Corriden, R.; Liu, D.; et al. GPCRomics: GPCR Expression in Cancer Cells and Tumors Identifies New, Potential Biomarkers and Therapeutic Targets. Front. Pharmacol. 2018, 9, 431. [Google Scholar] [CrossRef]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef]
- Blázquez, C.; González-Feria, L.; Lvarez, L.A.´.; Haro, A.; Llanos Casanova, M.; Guzmán, M. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Hegde, V.L.; Singh, N.P.; Sisco, D.; Grant, S.; Nagarkatti, M.; Nagarkatti, P.S. Δ9-Tetrahydrocannabinol-Induced Apoptosis in Jurkat Leukemia T Cells Is Regulated by Translocation of Bad to Mitochondria. Mol. Cancer Res. 2006, 4, 549–562. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of p22phox and Nox4 Expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, D.; Nigro, P.; Cotugno, R.; Gazzerro, P.; Bifulco, M.; Belisario, M.A. Rimonabant-induced apoptosis in leukemia cell lines: Activation of caspase-dependent and -independent pathways. Biochem. Pharmacol. 2010, 80, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Mannino, F.; Pallio, G.; Corsaro, R.; Minutoli, L.; Altavilla, D.; Vermiglio, G.; Allegra, A.; Eid, A.H.; Bitto, A.; Squadrito, F.; et al. Beta-Caryophyllene Exhibits Anti-Proliferative Effects through Apoptosis Induction and Cell Cycle Modulation in Multiple Myeloma Cells. Cancers 2021, 13, 5741. [Google Scholar] [CrossRef]
- Gado, F.; Ferrisi, R.; Di Somma, S.; Napolitano, F.; Mohamed, K.A.; Stevenson, L.A.; Rapposelli, S.; Saccomanni, G.; Portella, G.; Pertwee, R.G.; et al. Synthesis and In Vitro Characterization of Selective Cannabinoid CB2 Receptor Agonists: Biological Evaluation against Neuroblastoma Cancer Cells. Molecules 2022, 27, 3019. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S.; et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9, 196. [Google Scholar] [CrossRef]
- Melck, D.; Rueda, D.; Galve-Roperh, I.; de Petrocellis, L.; Guzmän, M.; Di Marzo, V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999, 463, 235–240. [Google Scholar] [CrossRef]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef] [PubMed]
- Khunluck, T.; Lertsuwan, K.; Chutoe, C.; Sooksawanwit, S.; Inson, I.; Teerapornpuntakit, J.; Tohtong, R.; Charoenphandhu, N. Activation of cannabinoid receptors in breast cancer cells improves osteoblast viability in cancer-bone interaction model while reducing breast cancer cell survival and migration. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Greenhough, A.; Patsos, H.A.; Williams, A.C.; Paraskeva, C. The cannabinoid Δ9-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int. J. Cancer 2007, 121, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Proto, M.C.; Gazzerro, P.; Di Croce, L.; Santoro, A.; Malfitano, A.M.; Pisanti, S.; Laezza, C.; Bifulco, M. Interaction of endocannabinoid system and steroid Hormones in the control of colon cancer cell growth. J. Cell. Physiol. 2011, 227, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; D’Alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti Proliferative and Pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J. Cell. Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef]
- Ding, F.; Qiu, C.; Li, W.; Liu, Z.; Kong, D.; Ma, X.; Jiang, J. CNR1 may reverse progesterone-resistance of endometrial cancer through the ERK pathway. Biochem. Biophys. Res. Commun. 2021, 548, 148–154. [Google Scholar] [CrossRef]
- Rao, M.; Chen, D.; Zhan, P.; Jiang, J. MDA19, a novel CB2 agonist, inhibits hepatocellular carcinoma partly through inactivation of AKT signaling pathway. Biol. Direct 2019, 14, 9. [Google Scholar] [CrossRef]
- Boyacıoğlu, C.; Bilgiç, E.; Varan, C.; Bilensoy, E.; Nemutlu, E.; Sevim, D.; Kocaefe, L.; Korkusuz, P. ACPA decreases non-small cell lung cancer line growth through Akt/PI3K and JNK pathways in vitro. Cell Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Xu, S.; Ma, H.; Bo, Y.; Shao, M. The oncogenic role of CB2 in the progression of non-small-cell lung cancer. Biomed. Pharmacother. 2019, 117, 109080. [Google Scholar] [CrossRef]
- Olea-Herrero, N.; Vara, D.; Malagarie-Cazenave, S.; Diazlaviada, I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: Involvement of CB2. Br. J. Cancer 2009, 101, 940–950. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G 1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef]
- Pisanti, S.; Borselli, C.; Oliviero, O.; Laezza, C.; Gazzerro, P.; Bifulco, M. Antiangiogenic activity of the endocannabinoid anandamide: Correlation to its tumor-suppressor efficacy. J. Cell. Physiol. 2006, 211, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, M.; Pellerito, O.; Portanova, P.; Calvaruso, G.; Santulli, A.; De Blasio, A.; Vento, R.; Tesoriere, G. Apoptosis induced in HepG2 cells by the synthetic cannabinoid WIN: Involvement of the transcription factor PPARγ. Biochimie 2009, 91, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Sarnataro, D.; Pisanti, S.; Santoro, A.; Gazzerro, P.; Malfitano, A.M.; Laezza, C.; Bifulco, M. The Cannabinoid CB1 Receptor Antagonist Rimonabant (SR141716) Inhibits Human Breast Cancer Cell Proliferation through a Lipid Raft-Mediated Mechanism. Mol. Pharmacol. 2006, 70, 1298–1306. [Google Scholar] [CrossRef]
- Li, L.-T.; Zhao, F.-F.; Jia, Z.-M.; Qi, L.-Q.; Zhang, X.-Z.; Zhang, L.; Li, Y.-Y.; Yang, J.-J.; Wang, S.-J.; Lin, H.; et al. Cannabinoid receptors promote chronic intermittent hypoxia-induced breast cancer metastasis via IGF-1R/AKT/GSK-3β. Mol. Ther.-Oncol. 2021, 23, 220–230. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Martín-Ruiz, A.; Martín, P.; Calvo, V.; Provencio, M.; García, J.M. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3β signaling pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Rueda, D.; del Pulgar, T.G.; Velasco, G.; Guzmán, M. Mechanism of Extracellular Signal-Regulated Kinase Activation by the CB1 Cannabinoid Receptor. Mol. Pharmacol. 2002, 62, 1385–1392. [Google Scholar] [CrossRef]
- Liu, C.; Sadat, S.H.; Ebisumoto, K.; Sakai, A.; Panuganti, B.A.; Ren, S.; Goto, Y.; Haft, S.; Fukusumi, T.; Ando, M.; et al. Cannabinoids Promote Progression of HPV-Positive Head and Neck Squamous Cell Carcinoma via p38 MAPK Activation. Clin. Cancer Res. 2020, 26, 2693–2703. [Google Scholar] [CrossRef]
- Andradas, C.; Caffarel, M.M.; Pérez-Gómez, E.; Salazar, M.; Lorente, M.; Velasco, G.; Guzmán, M.; Sánchez, C. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 2011, 30, 245–252. [Google Scholar] [CrossRef]
- Singh, N.S.; Bernier, M.; Wainer, I.W. Selective GPR55 antagonism reduces chemoresistance in cancer cells. Pharmacol. Res. 2016, 111, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ramirez, J.C.; A Frampton, G.; E Golden, L.; A Quinn, M.; Pae, H.Y.; Horvat, D.; Liang, L.-J.; DeMorrow, S. Anandamide exerts its antiproliferative actions on cholangiocarcinoma by activation of the GPR55 receptor. Lab. Investig. 2011, 91, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.M.; Alotaibi, M.R.; Tao, Q.; Selley, D.E.; Lichtman, A.H.; Gewirtz, D.A. Combined Antiproliferative Effects of the Aminoalkylindole WIN55,212-2 and Radiation in Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2013, 348, 293–302. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Cudaback, E.; Marrs, W.; Moeller, T.; Stella, N. The Expression Level of CB1 and CB2 Receptors Determines Their Efficacy at Inducing Apoptosis in Astrocytomas. PLoS ONE 2010, 5, e8702. [Google Scholar] [CrossRef]
- Sarker, K.P.; Biswas, K.K.; Yamakuchi, M.; Lee, K.-Y.; Hahiguchi, T.; Kracht, M.; Kitajima, I.; Maruyama, I. ASK1-p38 MAPK/JNK signaling cascade mediates anandamide-induced PC12 cell death. J. Neurochem. 2003, 85, 50–61. [Google Scholar] [CrossRef]
- Soto-Mercado, V.; Mendivil-Perez, M.; Jimenez-Del-Rio, M.; E Fox, J.; Velez-Pardo, C. Cannabinoid CP55940 selectively induces apoptosis in Jurkat cells and in ex vivo T-cell acute lymphoblastic leukemia through H2O2 signaling mechanism. Leuk. Res. 2020, 95, 106389. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E.; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Wu, J.; Hei, T.K. Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signaling pathways. Oncotarget 2017, 8, 74068–74095. [Google Scholar] [CrossRef]
- Frampton, G.; Coufal, M.; Li, H.; Ramirez, J.; DeMorrow, S. Opposing actions of endocannabinoids on cholangiocarcinoma growth is via the differential activation of Notch signaling. Exp. Cell Res. 2010, 316, 1465–1478. [Google Scholar] [CrossRef]
- DeMorrow, S.; Francis, H.; Gaudio, E.; Venter, J.; Franchitto, A.; Kopriva, S.; Onori, P.; Mancinelli, R.; Frampton, G.; Coufal, M.; et al. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am. J. Physiol. Liver Physiol. 2008, 295, G1150–G1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Cherkasova, V.; Gerasymchuk, M.; Narendran, A.; Kovalchuk, I.; Kovalchuk, O. Cannabinol Inhibits Cellular Proliferation, Invasion, and Angiogenesis of Neuroblastoma via Novel miR-34a/tRiMetF31/PFKFB3 Axis. Cancers 2022, 14, 1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Kovalchuk, I.; Apel, I.J.; Chinnaiyan, A.M.; Wóycicki, R.K.; Cantor, C.R.; Kovalchuk, O. miR-34a directly targets tRNAiMet precursors and affects cellular proliferation, cell cycle, and apoptosis. Proc. Natl. Acad. Sci. USA 2018, 115, 7392–7397. [Google Scholar] [CrossRef] [PubMed]
- Pyszniak, M.; Tabarkiewicz, J.; Łuszczki, J.J. Endocannabinoid system as a regulator of tumor cell malignancy–biological pathways and clinical significance. OncoTargets Ther. 2016, 9, 4323–4336. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nature Reviews Cancer. Eur. Assoc. Cardio-Thorac. Surg. 2003, 3, 745–755. [Google Scholar]
- Blázquez, C.; Casanova, M.L.; Planas, A.; del Pulgar, T.G.; Villanueva, C.; Fernández-Aceñero, M.J.; Aragones, J.; Huffman, J.W.; Jorcano, J.L.; Guzman, M. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biology. 2003, 17, 529–531. [Google Scholar] [CrossRef]
- Vaccani, A.; Massi, P.; Colombo, A.; Rubino, T.; Parolaro, D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. J. Cereb. Blood Flow Metab. 2005, 144, 1032–1036. [Google Scholar] [CrossRef]
- Ramer, R.; Bublitz, K.; Freimuth, N.; Merkord, J.; Rohde, H.; Haustein, M.; Borchert, P.; Schmuhl, E.; Linnebacher, M.; Hinz, B. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2011, 26, 1535–1548. [Google Scholar] [CrossRef]
- National Toxicology Program: NTP toxicology and carcinogenesis studies of 1-trans-delta(9)-tetrahydrocannabinol (CAS No. 1972-08-3) in F344 rats and B6C3F1 mice (gavage studies). NTP Tech. Rep. 1996, 446, 1–317.
- Donadelli, M.; Dando, I.; Zaniboni, T.; Costanzo, C.; Pozza, E.D.; Scupoli, M.; Scarpa, A.; Zappavigna, S.; Marra, M.; Abbruzzese, A.; et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011, 2, e152. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Redlich, S.; Ribes, S.; Schütze, S.; Czesnik, D.; Nau, R. Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 and Streptococcus pneumoniae R6 by microglial cells. J. Neuroimmunol. 2012, 244, 32–34. [Google Scholar] [CrossRef]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzmán, M.; Velasco, G.; Díaz-Laviada, I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Galve-Roperh, I.; Rueda, D.; Guzmán, M. Involvement of Sphingomyelin Hydrolysis and the Mitogen-Activated Protein Kinase Cascade in the Δ9-Tetrahydrocannabinol-Induced Stimulation of Glucose Metabolism in Primary Astrocytes. Mol. Pharmacol. 1998, 54, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, O.; Notaro, A.; Sabella, S.; De Blasio, A.; Vento, R.; Calvaruso, G.; Giuliano, M. WIN induces apoptotic cell death in human colon cancer cells through a block of autophagic flux dependent on PPARγ down-regulation. Apoptosis 2014, 19, 985. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Cascio, M.G. Known Pharmacological Actions of Delta-9-Tetrahydrocannabinol and of Four Other Chemical Constituents of Cannabis that Activate Cannabinoid Receptors. In Handbook of Cannabis; Oxford University Press: Oxford, UK, 2014; Volume 115–136, p. 6. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef]
- Tubaro, A.; Giangaspero, A.; Sosa, S.; Negri, R.; Grassi, G.; Casano, S.; Della Loggia, R.; Appendino, G.B. Comparative topical anti-inflammatory activity of cannabinoids and cannabivarins. Fitoterapia 2010, 81, 816–819. [Google Scholar] [CrossRef]
- Anil, S.M.; Peeri, H.; Koltai, H. Medical Cannabis Activity Against Inflammation: Active Compounds and Modes of Action. Front. Pharmacol. 2022, 13, 908198. [Google Scholar] [CrossRef]
- Xian, X.-S.; Park, H.; Cho, Y.K.; Lee, I.S.; Kim, S.W.; Choi, M.-G.; Chung, I.-S.; Han, K.-H.; Park, J.M. Effect of a synthetic cannabinoid agonist on the proliferation and invasion of gastric cancer cells. J. Cell. Biochem. 2010, 110, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Ramer, R. Cannabinoids as anticancer drugs: Current status of preclinical Res. Br. J. Cancer 2022, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Caffarel, M.M.; Sarrió, D.; Palacios, J.; Guzmán, M.; Sanchez, C. Δ9-Tetrahydrocannabinol Inhibits Cell Cycle Progression in Human Breast Cancer Cells through Cdc2 Regulation. Cancer Res. 2006, 66, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pisanti, S.; Crescenzi, E.; Bifulco, M. Anandamide inhibits Cdk2 and activates Chk1 leading to cell cycle arrest in human breast cancer cells. FEBS Lett. 2006, 580, 6076–6082. [Google Scholar] [CrossRef]
- Go, Y.Y.; Kim, S.R.; Kim, D.Y.; Chae, S.W.; Song, J.J. Cannabidiol enhances cytotoxicity of anti-cancer drugs in human head and neck squamous cell carcinoma. Sci. Rep. 2020, 1, 10. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef]
- Massi, P.; Valenti, M.; Vaccani, A.; Gasperi, V.; Perletti, G.; Marras, E.; Fezza, F.; Maccarrone, M.; Parolaro, D. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J. Neurochem. 2008, 104, 1091–1100. [Google Scholar] [CrossRef]
- Ramer, R.; Weinzierl, U.; Schwind, B.; Brune, K.; Hinz, B. Ceramide Is Involved in R()-Methanandamide-Induced Cyclooxygenase-2 Expression in Human Neuroglioma Cells. Mol. Pharmacol. 2003, 64, 1189–1198. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R.; Eichele, K.; Weinzierl, U.; Brune, K. Up-Regulation of Cyclooxygenase-2 Expression Is Involved in R(+)-Methanandamide-Induced Apoptotic Death of Human Neuroglioma Cells. Mol. Pharmacol. 2004, 66, 1643–1651. [Google Scholar] [CrossRef]
- Eichele, K.; Ramer, R.; Hinz, B. R(+)-Methanandamide-Induced Apoptosis of Human Cervical Carcinoma Cells Involves A Cyclooxygenase-2-Dependent Pathway. Pharm. Res. 2008, 26, 346–355. [Google Scholar] [CrossRef]
- Eichele, K.; Ramer, R.; Hinz, B. Decisive role of cyclooxygenase-2 and lipocalin-type prostaglandin D synthase in chemotherapeutics-induced apoptosis of human cervical carcinoma cells. Oncogene 2007, 27, 3032–3044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor-Converting Enzyme (TACE/ADAM17)-Mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 1943, 64, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Miyato, H.; Kitayama, J.; Yamashita, H.; Souma, D.; Asakage, M.; Yamada, J.; Nagawa, H. Pharmacological Synergism Between Cannabinoids and Paclitaxel in Gastric Cancer Cell Lines. J. Surg. Res. 2009, 155, 40–47. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. Klin. Wochenschr. 2012, 90, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Pagano, E.; Cascio, M.G.; Pertwee, R.G.; Izzo, A.A. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2013, 21, 631–639. [Google Scholar] [CrossRef]
- Ma, C.; Wu, T.-T.; Jiang, P.-C.; Li, Z.-Q.; Chen, X.-J.; Fu, K.; Wang, W.; Gong, R. Anti-carcinogenic activity of anandamide on human glioma in vitro and in vivo. Mol. Med. Rep. 2015, 13, 1558–1562. [Google Scholar] [CrossRef]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef]
- Cruz-Munoz, W.; Khokha, R. The Role of Tissue Inhibitors of Metalloproteinases in Tumorigenesis and Metastasis. Crit. Rev. Clin. Lab. Sci. 2008, 45, 291–338. [Google Scholar] [CrossRef]
- Laezza, C.; D’Alessandro, A.; Paladino, S.; Malfitano, A.M.; Proto, M.C.; Gazzerro, P.; Pisanti, S.; Santoro, A.; Ciaglia, E.; Bifulco, M. Anandamide inhibits the Wnt/β-catenin signalling pathway in human breast cancer MDA MB 231 cells. Eur. J. Cancer 2012, 48, 3112–3122. [Google Scholar] [CrossRef]
- García-Morales, L.; Castillo, A.M.; Ramírez, J.T.; Zamudio-Meza, H.; Domínguez-Robles, M.D.C.; Meza, I. CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1β. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Borrelli, F.; Orlando, P.; Romano, B.; Monti, M.; Morbidelli, L.; Aviello, G.; Imperatore, R.; Capasso, R.; Piscitelli, F.; et al. Pharmacological inhibition of MAGL attenuates experimental colon carcinogenesis. Pharmacol. Res. 2017, 119, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Kang, Y.; Park, P.-H.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Ku, S.K.; Jung, Y.; Kim, J.-A. Anti-tumor Activity of the Novel Hexahydrocannabinol Analog LYR-8 in Human Colorectal Tumor Xenograft Is Mediated through the Inhibition of Akt and Hypoxia-Inducible Factor-1α Activation. Biol. Pharm. Bull. 2012, 35, 924–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef]
- Nallathambi, R.; Mazuz, M.; Ion, A.; Selvaraj, G.; Weininger, S.; Fridlender, M.; Nasser, A.; Sagee, O.; Kumari, P.; Nemichenizer, D.; et al. Anti-Inflammatory Activity in Colon Models Is Derived from Δ9-Tetrahydrocannabinolic Acid That Interacts with Additional Compounds in Cannabis Extracts. Cannabis Cannabinoid Res. 2017, 2, 167–182. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Huang, M.-Q.; Bao, J.-L.; Chen, X.-P.; Wang, Y.-T. Terpenoids: Natural products for cancer therapy. Exp. Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Ćulafić, D.M.; Žegura, B.; Nikolić, B.; Vuković-Gačić, B.; Knežević-Vukčević, J.; Filipič, M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem. Toxicol. 2009, 47, 260–266. [Google Scholar] [CrossRef]
- Luis Da Silva, S.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amazon. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Chung, K.-S.; Hong, J.Y.; Lee, J.-H.; Lee, H.-J.; Park, J.Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Arul, S.; Rajagopalan, H.; Ravi, J.; Dayalan, H. Beta-Caryophyllene Suppresses Ovarian Cancer Proliferation by Inducing Cell Cycle Arrest and Apoptosis. Anticancer Agents Med. Chem. 2020, 20, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, V.; Kotakonda, M.; Periyannan, V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J. Biochem. Mol. Toxicol. 2020, 1, 34. [Google Scholar] [CrossRef]
- di Giacomo, S.; di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing properties of β-caryophyllene and β-caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar] [PubMed]
- di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of low-dose doxorubicin cytotoxicity by affecting p-glycoprotein through caryophyllane sesquiterpenes in hepg2 cells: An in vitro and in silico study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef]
- Ambrož, M.; Matoušková, P.; Skarka, A.; Zajdlová, M.; Žáková, K.; Skálová, L. The effects of selected sesquiterpenes from myrica rubra essential oil on the efficacy of doxorubicin in sensitive and resistant cancer cell lines. Molecules 2017, 22, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar] [CrossRef]
- Meeran, M.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid type-2 (CB2) receptors in rats. Chem. Interactions 2019, 304, 158–167. [Google Scholar] [CrossRef]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Hanusova Skarkova, V.; Kralova, V.; Skalova, L. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef]
- di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J.G. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.-C. Antitumor Activity of Balsam Fir Oil: Production of Reactive Oxygen Species Induced by α-Humulene as Possible Mechanism of Action. Planta Med. 2003, 69, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Dodaro, D.; Passalacqua, N.G.; Statti, G.; Menichini, F. In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat. Prod. Res. 2009, 23, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. Buon Off. J. Balk. Union Oncol. 2020, 25, 280–285. [Google Scholar]
- Jia, S.-S.; Xi, G.-P.; Zhang, M.; Chen, Y.-B.; Lei, B.; Dong, X.-S.; Yang, Y.-M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2012, 29, 349–354. [Google Scholar] [CrossRef]
- Berliocchi, L.; Chiappini, C.; Adornetto, A.; Gentile, D.; Cerri, S.; Russo, R.; Bagetta, G.; Corasaniti, M.T. Early LC3 lipidation induced by d -limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine 2018, 40, 98–105. [Google Scholar] [CrossRef]
- Russo, R.; Cassiano, M.G.V.; Ciociaro, A.; Adornetto, A.; Varano, G.P.; Chiappini, C.; Berliocchi, L.; Tassorelli, C.; Bagetta, G.; Corasaniti, M.T. Role of D-Limonene in Autophagy Induced by Bergamot Essential Oil in SH-SY5Y Neuroblastoma Cells. PLoS ONE 2014, 9, e113682. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Haag, J.D.; Lindstrom, M.J.; Gould, M.N. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992, 52, 4021–4027. [Google Scholar]
- Gould, M.N.; Moore, C.J.; Zhang, R.; Wang, B.; Kennan, W.S.; Haag, J.D. Limonene chemoprevention of mammary carcinoma induction following direct in situ transfer of v-Ha-ras. Cancer Res. 1994, 54, 3540–3543. [Google Scholar]
- Elegbede, J.A.; Elson, C.E.; Qureshi, A.; Tanner, M.A.; Gould, M.N. Regression of Rat Primary Mammary Tumors Following Dietary d-Limonene2. JNCI: J. Natl. Cancer Inst. 1986, 76, 323. [Google Scholar] [CrossRef]
- Nakaizumi, A.; Baba, M.; Uehara, H.; Iishi, H.; Tatsuta, M. d-Limonene inhibits N-nitrosobis(2-oxopropyl)amine induced hamster pancreatic carcinogenesis. Cancer Lett. 1997, 117, 99–103. [Google Scholar] [CrossRef]
- Manuele, M.G.; Arcos Barreiro, M.L.; Davicino, R.; Ferraro, G.; Cremaschi, G.; Anesini, C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2010, 28, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.G.; Zhan, L.B.; Feng, B.A.; Qu, M.Y.; Yu, L.H.; Xie, J.H. Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J. Gastroenterol. 2004, 10, 2140–2144. Available online: https://pubmed.ncbi.nlm.nih.gov/15237454 (accessed on 1 October 2022). [CrossRef] [PubMed]
- Uedo, N.; Tatsuta, M.; Iishi, H.; Baba, M.; Sakai, N.; Yano, H.; Otani, T. Inhibition by d-limonene of gastric carcinogenesis induced by N-methyl-N H-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999, 137, 131–136. [Google Scholar] [CrossRef]
- Kumar Giri, R.; Paru, T.; Das, B.R. d-limonene chemoprevention of hepatocarcinogenesis in AKR mice: Inhibition of c-jun and c-myc. Oncol. Rep. 1999, 6, 1123–1127. [Google Scholar]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.-H.S. Plasma Metabolomic Profiles of Breast Cancer Patients after Short-term Limonene Intervention. Cancer Prev. Res. 2015, 8, 86–93. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene Induces Apoptotic Cell Death via Caspase Activation in Human Ovarian Cancer Cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 11, 38. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. α-pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef]
- Li, Y.-L.; Yeung, C.-M.; Chiu, L.C.M.; Cen, Y.-Z.; Ooi, V.E.C. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytotherapy Res. 2008, 23, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic Antitumor Effect of α-pinene and β-pinene with Paclitaxel against Non-small-cell Lung Carcinoma (NSCLC). Drug Res. 2014, 65, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Q.; Xu, B.; Mao, J.-W.; Wei, F.-X.; Li, M.; Liu, T.; Jin, X.-B.; Zhang, L.-R. Inhibitory Effects of α-Pinene on Hepatoma Carcinoma Cell Proliferation. Asian Pac. J. Cancer Prev. 2014, 15, 3293–3297. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharm. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H.G. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef]
- McKallip, R.J.; Lombard, C.; Fisher, M.; Martin, B.R.; Ryu, S.; Grant, S.; Nagarkatti, P.S.; Nagarkatti, M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 2002, 100, 627–634. [Google Scholar] [CrossRef]
- Preet, A.; Ganju, R.K.; Groopman, J.E. Δ9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2008, 27, 339–346. [Google Scholar] [CrossRef]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Δ-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Δ-9-Tetrahydrocannabinol Enhances Breast Cancer Growth and Metastasis by Suppression of the Antitumor Immune Response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N. ?9-Tetrahydrocannabinol and Synthetic Cannabinoids Prevent Emesis Produced by the Cannabinoid CB1 Receptor Antagonist/Inverse Agonist SR 141716A. Neuropsychopharmacology 2001, 24, 198–203. [Google Scholar] [CrossRef]
- A Darmani, N. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol. Biochem. Behav. 2001, 69, 239–249. [Google Scholar] [CrossRef]
- Parker, L.A.; Kwiatkowska, M.; Burton, P.; Mechoulam, R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 2004, 171, 156–161. [Google Scholar] [CrossRef]
- Fride, E.; Bregman, T.; Kirkham, T.C. Endocannabinoids and Food Intake: Newborn Suckling and Appetite Regulation in Adulthood. Exp. Biol. Med. 2005, 230, 225–234. [Google Scholar] [CrossRef]
- Mechoulam, R.; Berry, E.M.; Avraham, Y.; di Marzo, V.; Fride, E. Endocannabinoids, feeding and suckling—From our perspective. Int. J. Obes. 2006, 30, S24–S28. [Google Scholar] [CrossRef]
- Walker, J.; Hohmann, A.G.; Martin, W.J.; Strangman, N.M.; Huang, S.M.; Tsou, K. The neurobiology of cannabinoid analgesia. Life Sci. 1999, 65, 665–673. [Google Scholar] [CrossRef]
- Meng Ian, D.; Manning Barton, H.; Martin William, J.; Fields Howard, L. An analgesia circuit activated by cannabinoids. Nature 1998, 395, 381–383. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Gielissen, J.; Chandiramani, A.; Harding-Rose, C.; Abu Odeh, D.; Simone, D.A.; Seybold, V.S. CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behav. Pharmacol. 2011, 22, 607–616. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; A Walker, E. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5- HT 1A receptors without diminishing nervous system function or chemotherapy efficacy. J. Cereb. Blood Flow Metab. 2014, 171, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Rahn, E.J.; Makriyannis, A.; Hohmann, A.G. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. J. Cereb. Blood Flow Metab. 2007, 152, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Guimarães, F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology 2008, 199, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, M.; Caynas-Rojas, S.; Santacruz, V.A.; Ruiz-Contreras, A.E.; Aguilar-Roblero, R.; Prospéro-García, O. Entopeduncular nucleus endocannabinoid system modulates sleep–waking cycle and mood in rats. Pharmacol. Biochem. Behav. 2013, 107, 29–35. [Google Scholar] [CrossRef]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sanchez, C.; Velasco, G.; González-Feria, L. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Twelves, C.; Sabel, M.; Checketts, D.; Miller, S.; Tayo, B.; Jove, M.; Brazil, L.; Short, S.C. A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br. J. Cancer 2021, 124, 1379–1387. [Google Scholar] [CrossRef]
- Yeshurun, M.; Shpilberg, O.; Herscovici, C.; Shargian, L.; Dreyer, J.; Peck, A.; Israeli, M.; Levy-Assaraf, M.; Gruenewald, T.; Mechoulam, R.; et al. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation: Results of a Phase II Study. Biol. Blood Marrow Transplant. 2015, 21, 1770–1775. [Google Scholar] [CrossRef]
- Bar-Lev Schleider, L.; Mechoulam, R.; Lederman, V.; Hilou, M.; Lencovsky, O.; Betzalel, O.; Shbiro, L.; Novack, V. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur. J. Intern. Med. 2018, 49, 37–43. [Google Scholar] [CrossRef]
- Weiss, M.C.; Hibbs, J.E.; Buckley, M.E.; Danese, S.R.; Leitenberger, A.; Bollmann-Jenkins, M.; Meske, S.W.; Aliano-Ruiz, K.E.; McHugh, T.W.; Larson, S.L.; et al. A Coala-T-Cannabis Survey Study of breast cancer patients’ use of cannabis before, during, and after treatment. Cancer 2022, 128, 160–168. [Google Scholar] [CrossRef]
- Ahmedzai, S.; Carlyle, D.L.; Calder, I.T.; Moran, F. Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. Br. J. Cancer 1983, 48, 657–663. [Google Scholar] [CrossRef]
- Chan, H.S.; Correia, J.A.; MacLeod, S.M. Nabilone versus prochlorperazine for control of cancer chemotherapy-induced emesis in children: A double-blind, crossover trial. Pediatrics 1987, 79, 946–952. [Google Scholar] [PubMed]
- Johansson, R.; Kilkku, P.; Groenroost, M. A double-bllnd, controlled trial of nabHone vs. procldorperazine for refractory emesis induced by cancer chemotherapy. Cancer Treat. Rev. 1982, 9, 25–33. [Google Scholar] [CrossRef]
- Aila, N.; Karin, M. A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy. Ann. J. Clin. Oncol. 1985, 8, 336–340. [Google Scholar]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Mirhosseini, M.; Lamoureux, D.C.; Chasen, M.; MacDonald, N.; Baracos, V.E.; Wismer, W.V. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double-blind, placebo-controlled pilot trial. Ann. Oncol. 2011, 22, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Turcott, J.G.; del Rocío Guillen Núñez, M.; Flores-Estrada, D.; Oñate-Ocaña, L.F.; Zatarain-Barrón, Z.L.; Barrón, F.; Arrieta, O. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Supportive Care Cancer 2018, 26, 3029–3038. [Google Scholar] [CrossRef]
- Noyes, R.; Brunk, S.F.; Baram, D.A.; Canter, A. Analgesic Effect of Delta-9-Tetrahydrocannabinol. J. Clin. Pharmacol. 1975, 15, 139–143. [Google Scholar] [CrossRef]
- Noyes, R.; Brunk, S.F.; Avery, D.H.; Canter, A. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin. Pharmacol. Ther. 1975, 18, 84–89. [Google Scholar] [CrossRef]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of the Efficacy, Safety, and Tolerability of THC:CBD Extract and THC Extract in Patients with Intractable Cancer-Related Pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef]
- Maida, V.; Ennis, M.; Irani, S.; Corbo, M.; Dolzhykov, M. Adjunctive nabilone in cancer pain and symptom management: A prospective observational study using propensity scoring. J. Support Oncol. 2008, 6, 119–124. [Google Scholar]
- Waissengrin, B.; Mirelman, D.; Pelles, S.; Bukstein, F.; Blumenthal, D.T.; Wolf, I.; Geva, R. Effect of cannabis on oxaliplatin-induced peripheral neuropathy among oncology patients: A retrospective analysis. Ther. Adv. Med. Oncol. 2021, 13, 203. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, M.; Archibald, S.D.; Jackson, B.S.; Gupta, M.K. Association of Marijuana Use with Psychosocial and Quality of Life Outcomes Among Patients With Head and Neck Cancer. JAMA Otolaryngol. Neck Surg. 2018, 144, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Allebeck, P.; Akre, O.; McGlynn, K.A.; Sidorchuk, A. Cannabis Use and Incidence of Testicular Cancer: A 42-Year Follow-up of Swedish Men between 1970 and 2011. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Wallner, L.; Quinn, V.; Slezak, J.; Chien, G.; Jacobsen, S. Association between cannabis use and the risk of bladder cancer: Results from the California Men’s Health Study. J. Urol. 2013, 189, 527. [Google Scholar] [CrossRef]
- Hashibe, M.; Morgenstern, H.; Cui, Y.; Tashkin, D.P.; Zhang, Z.-F.; Cozen, W.; Mack, T.M.; Greenland, S. Marijuana Use and the Risk of Lung and Upper Aerodigestive Tract Cancers: Results of a Population-Based Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1829–1834. [Google Scholar] [CrossRef]
- Marks, M.A.; Chaturvedi, A.K.; Kelsey, K.; Straif, K.; Berthiller, J.; Schwartz, S.M.; Smith, E.; Wyss, A.; Brennan, P.; Olshan, A.F.; et al. Association of Marijuana Smoking with Oropharyngeal and Oral Tongue Cancers: Pooled Analysis from the INHANCE Consortium. Cancer Epidemiol. Biomark. Prev. 2014, 23, 160–171. [Google Scholar] [CrossRef]
- Mehra, R.; Moore, B.A.; Crothers, K.; Tetrault, J.; Fiellin, D.A. The Association Between Marijuana Smoking and Lung Cancer a Systematic Review. Arch. Intern. Med. 2006, 166, 1359–1367. [Google Scholar] [CrossRef]
- Taha, T.; Meiri, D.; Talhamy, S.; Wollner, M.; Peer, A.; Bar-Sela, G. Cannabis Impacts Tumor Response Rate to Nivolumab in Patients with Advanced Malignancies. Oncologist 2019, 24, 549–554. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Cohen, I.; Campisi-Pinto, S.; Lewitus, G.M.; Oz-Ari, L.; Jehassi, A.; Peer, A.; Turgeman, I.; Vernicova, O.; Berman, P.; et al. Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers 2020, 12, 2447. [Google Scholar] [CrossRef]
- Foroughi, M.; Hendson, G.; Sargent, M.A.; Steinbok, P. Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas—Possible role of Cannabis inhalation. Child’s Nerv. Syst. 2011, 27, 671–679. [Google Scholar] [CrossRef]
- Torres, S.; Lorente, M.; Rodríguez-Fornés, F.; Hernández-Tiedra, S.; Salazar, M.; García-Taboada, E.; Barcia, J.; Guzmán, M.; Velasco, G. A Combined Preclinical Therapy of Cannabinoids and Temozolomide against Glioma. Mol. Cancer Ther. 2011, 10, 90–103. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The Combination of Cannabidiol and Δ9-Tetrahydrocannabinol Enhances the Anticancer Effects of Radiation in an Orthotopic Murine Glioma Model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2012, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol, a Major Phytocannabinoid, As a Potent Atypical Inhibitor for CYP2D6. Drug Metab. Dispos. 2011, 39, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol Is a Potent Inhibitor of the Catalytic Activity of Cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Engels, F.K.; de Jong, F.A.; Sparreboom, A.; Mathot, R.A.A.; Loos, W.J.; Kitzen, J.J.E.M.; de Bruijn, P.; Verweij, J.; Mathijssen, R.H.J. Medicinal Cannabis Does Not Influence the Clinical Pharmacokinetics of Irinotecan and Docetaxel. Oncologist 2007, 12, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sexton, M.; Garcia, J.M.; Jatoi, A.; Clark, C.S.; Wallace, M.S. The Management of Cancer Symptoms and Treatment-Induced Side Effects with Cannabis or Cannabinoids. J. Natl. Cancer Inst. Monogr. 2021, 2021, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Pergam, S.A.; Bs, M.C.W.; Lee, C.M.; Cheng, G.-S.; Ms, K.K.B.; Marquis, S.R.; Fann, J.R. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer 2017, 123, 4488–4497. [Google Scholar] [CrossRef]
- Page, R.; Blanchard, E. Opioids and Cancer Pain: Patients’ Needs and Access Challenges. J. Oncol. Pr. 2019, 15, 229–231. [Google Scholar] [CrossRef]
- Cichewicz, D.L. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2003, 74, 1317–1324. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Briley, E.M.; Herkenham, M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999, 822, 17–25. [Google Scholar] [CrossRef]
- Mackie, K.; Hillet, B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells (aminoalkylindole/cyclic AMP/guanine nucleotide-binding protein/w-conotoxln/pertussis toxin). Neurobiology 1992, 89, 3825–3829. [Google Scholar]
- Deadwyler, S.A.; Hampson, R.E.; Bennett, B.A.; Edwards, T.A.; Mu, J.; Pacheco, M.A.; Ward, S.J.; Childers, S.R. Cannabinoids modulate potassium current in cultured hippocampal neurons. Recept. Channels 1993, 1, 121–134. [Google Scholar] [PubMed]
- Ibrahim, M.M.; Rude, M.L.; Stagg, N.J.; Mata, H.P.; Lai, J.; Vanderah, T.W.; Porreca, F.; Buckley, N.E.; Makriyannis, A.; Malan, T.F., Jr.; et al. CB2 cannabinoid receptor mediation of antinociception. Pain 2006, 122, 36–42. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA-Journal of the American Medical Association. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Lynch, M.E.; Campbell, F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials Keywords cannabinoids, chronic non-cancer pain, neuropathic pain, systematic review. Br. J. Clin. Pharmacol. 2011, 72, 735–744. [Google Scholar] [CrossRef]
- Ellis, R.J.; Toperoff, W.; Vaida, F.; Brande, G.V.D.; Gonzales, J.; Gouaux, B.; Bentley, H.; Atkinson, J.H. Smoked Medicinal Cannabis for Neuropathic Pain in HIV: A Randomized, Crossover Clinical Trial. Neuropsychopharmacology 2008, 34, 672–680. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Gouaux, B.; Sakai, S.; Donaghe, H. Low-Dose Vaporized Cannabis Significantly Improves Neuropathic Pain. J. Pain 2012, 14, 136–148. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.D.; Deutsch, R.; Zhao, H.; Prasad, H.; Phan, A. An Exploratory Human Laboratory Experiment Evaluating Vaporized Cannabis in the Treatment of Neuropathic Pain from Spinal Cord Injury and Disease. J. Pain 2016, 17, 982–1000. [Google Scholar] [CrossRef]
- Wallace, M.S.; Marcotte, T.D.; Umlauf, A.; Gouaux, B.; Atkinson, J.H. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J. Pain 2015, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Sabioni, P.; Trigo, J.M.; A Ware, M.; Betz-Stablein, B.; Murnion, B.; Lintzeris, N.; Khor, K.E.; Farrell, M.; Smith, A.; et al. Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis. Neuropsychopharmacology 2017, 42, 1752–1765. [Google Scholar] [CrossRef] [PubMed]
- Narang, S.; Gibson, D.; Wasan, A.D.; Ross, E.L.; Michna, E.; Nedeljkovic, S.S.; Jamison, R.N. Efficacy of Dronabinol as an Adjuvant Treatment for Chronic Pain Patients on Opioid Therapy. J. Pain 2008, 9, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for Opioid-Treated Cancer Patients with Poorly-Controlled Chronic Pain: A Randomized, Placebo-Controlled, Graded-Dose Trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Price, R.S.; Feldman, E.L. Distal symmetric polyneuropathy a review. JAMA-J. Am. Med. Assoc. 2015, 314, 2172–2181. [Google Scholar] [CrossRef]
- Martín-Sánchez, E.; Furukawa, T.; Taylor, J.; Martin, J.L.R. Systematic Review and Meta-analysis of Cannabis Treatment for Chronic Pain. Pain Med. 2009, 10, 1353–1368. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Khasabov, S.; Paz, J.; Harding-Rose, C.; Simone, N.A.; Seybold, V.S. Cannabinoid Type-1 Receptor Reduces Pain and Neurotoxicity Produced by Chemotherapy. J. Neurosci. 2012, 32, 7091–7101. [Google Scholar] [CrossRef]
- Lynch, M.E.; Cesar-Rittenberg, P.; Hohmann, A.G. A Double-Blind, Placebo-Controlled, Crossover Pilot Trial with Extension Using an Oral Mucosal Cannabinoid Extract for Treatment of Chemotherapy-Induced Neuropathic Pain. J. Pain Symptom Manag. 2014, 47, 166–173. [Google Scholar] [CrossRef]
- Bloechl-Daum, B.; Deuson, R.R.; Mavros, P.; Hansen, M.; Herrstedt, J. Delayed Nausea and Vomiting Continue to Reduce Patients’ Quality of Life After Highly and Moderately Emetogenic Chemotherapy Despite Antiemetic Treatment. J. Clin. Oncol. 2006, 24, 4472–4478. [Google Scholar] [CrossRef]
- Sanger, G.J. Endocannabinoids and the gastrointestinal tract: What are the key questions? Br. J. Pharmacol. 2007, 152, 663–670. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Roswell, F.; Todaro, B.; York, N. Cannabinoids in the Treatment of Chemotherapy-Induced Nausea and Vomiting. J. Natl. Compr. Cancer Netw. 2012, 10, 487–492. [Google Scholar]
- Tramèr, M.R.; Carroll, D.; A Campbell, F.; Reynolds, D.J.M.; Moore, R.A.; McQuay, H.J. Cannabinoids for control of chemotherapy induced nausea and vomiting: Quantitative systematic. BMJ 2001, 323, 16. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. Delta-9-Tetrahydrocannabinol as an Antiemetic in Cancer Patients Receiving High-Dose Methotrexate a Prospective, Randomized Evaluation. Ann. Intern. Med. 1979, 91, 819–824. Available online: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19553/ (accessed on 1 October 2022). [CrossRef]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer 1981, 47, 1746–1751. [Google Scholar]
- ben Amar, M. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006, 105, 1–25. [Google Scholar] [CrossRef]
- Strasser, F.; Luftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of orally administered cannabis extract and delta-9- tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar]
- Nelson, K.; Walsh, D.; Deeter, P.; Sheehan, F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J. Palliat. Care 1994, 10, 14–18. [Google Scholar]
- Jatoi, A.; Windschitl, H.E.; Loprinzi, C.L.; Sloan, J.A.; Dakhil, S.R.; Mailliard, J.A.; Pundaleeka, S.; Kardinal, C.G.; Fitch, T.R.; Krook, J.E.; et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A North Central Cancer Treatment Group study. J. Clin. Oncol. 2002, 20, 567–573. [Google Scholar] [CrossRef]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.-L.; Sibaev, A.; Storr, M.; Lutz, B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef]
- Patsos, H.; Hicks, D.; Greenhough, A.; Williams, A.; Paraskeva, C. Cannabinoids and cancer: Potential for colorectal cancer therapy. Biochem. Soc. Trans. 2005, 33, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.W.; Dalgleish, A.G. Cannabis-Derived Substances in Cancer Therapy—An Emerging Anti-Inflammatory Role for the Cannabinoids. Curr. Clin. Pharmacol. 2010, 5, 281–287. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Ciaglia, E.; Gangemi, G.; Gazzerro, P.; Laezza, C.; Bifulco, M. Update on the endocannabinoid system as an anticancer target. Expert Opin. Ther. Targets 2011, 15, 297–308. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Afaq, F.; Mukhtar, H. Cannabinoids for Cancer Treatment: Progress and Promise. Cancer Res. 2008, 68, 339–342. [Google Scholar] [CrossRef]
- Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer. Cancers 2021, 13, 4353. [Google Scholar] [CrossRef]
- Kim, H.I.; Lim, H.; Moon, A. Sex differences in cancer: Epidemiology, genetics and therapy. Biomol. Therapeutics. Korean Soc. Appl. Pharmacol. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Tevfik Dorak, M.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 3, 1–11. [Google Scholar]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden of Disease Cancer Collaboration. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Blanton, H.L.; Barnes, R.C.; McHann, M.C.; Bilbrey, J.A.; Wilkerson, J.L.; Guindon, J. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 2021, 202, 173107. [Google Scholar] [CrossRef]
- Levine, A.; Liktor-Busa, E.; Lipinski, A.A.; Couture, S.; Balasubramanian, S.; Aicher, S.A.; Langlais, P.R.; Vanderah, T.W.; Largent-Milnes, T.M. Sex differences in the expression of the endocannabinoid system within V1M cortex and PAG of Sprague Dawley rats. Biol. Sex Differ. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Redmond, A.N.; Crawford, L.C.; Sepulveda, D.E.; Hale, D.E.; Lesperance, J.J.; Morgan, D.J. Sex Differences in Tolerance to Delta-9-Tetrahydrocannabinol in Mice with Cisplatin-Evoked Chronic Neuropathic Pain. Front. Mol. Biosci. 2021, 8, 4115. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Iemolo, A.; Montilla-Perez, P.; Li, H.-R.; Yang, X.; Telese, F. Chronic adolescent exposure to cannabis in mice leads to sex-biased changes in gene expression networks across brain regions. Neuropsychopharmacology 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Smoum, R.; Mechoulam, R.; Parker, L.A. Evaluation of Sex Differences in the Potential of Δ 9-Tetrahydrocannabinol, Cannabidiol, Cannabidiolic Acid, and Oleoyl Alanine to Reduce Nausea-Induced Conditioned Gaping Reactions in Sprague-Dawley Rats. Cannabis Cannabinoid Res. 2022, 2022, 158. [Google Scholar] [CrossRef]
- Wyrofsky, R.R.; Reyes, B.A.S.; Yu, D.; Kirby, L.G.; Van Bockstaele, E.J. Sex differences in the effect of cannabinoid type 1 receptor deletion on locus coeruleus-norepinephrine neurons and corticotropin releasing factor-mediated responses. Eur. J. Neurosci. 2018, 48, 2118–2138. [Google Scholar] [CrossRef]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2018, 94, 302–320. [Google Scholar] [CrossRef]
- Morena, M.; Patel, S.; Bains, J.; Hill, M.N. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2015, 41, 80–102. [Google Scholar] [CrossRef]
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Meron Ruscio, A.; Benjet, C.; Scott, K.; et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology 2017, 43, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Wilker, S.; Pfeiffer, A.; Elbert, T.; Ovuga, E.; Karabatsiakis, A.; Krumbholz, A.; Thieme, D.; Schelling, G.; Kolassa, I.-T. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology 2016, 67, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Commentary on “Sex differences in the effect of cannabinoid type 1 receptor deletion on locus coeruleus-norepinephrine neurons and corticotropin releasing factor-mediated responses”. Eur. J. Neurosci. 2019, 49, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Lossignol, D.; Burnell-Nugent, M.; Fallon, M.T. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J. Pain Symptom Manag. 2013, 46, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.T.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Lichtman, A.H.; Kornyeyeva, E. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: Two double-blind, randomized, placebo-controlled phase 3 studies. Br. J. Pain 2017, 11, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Kornyeyeva, E.; Fallon, M.T. Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. J. Pain Symptom Manag. 2018, 55, 179–188.e1. [Google Scholar] [CrossRef]
- Arkell, T.R.; Kevin, R.C.; Vinckenbosch, F.; Lintzeris, N.; Theunissen, E.; Ramaekers, J.G.; McGregor, I.S. Sex differences in acute cannabis effects revisited: Results from two randomized, controlled trials. Addict. Biol. 2021, 27, e13125. [Google Scholar] [CrossRef]
- Aviram, J.; Lewitus, G.M.; Vysotski, Y.; Berman, P.; Shapira, A.; Procaccia, S.; Meiri, D. Sex differences in medical cannabis-related adverse effects. Pain 2021, 163, 975–983. [Google Scholar] [CrossRef]
- Kasvis, P.; Canac-Marquis, M.; Aprikian, S.; Vigano, M.; Vigano, A. Sex differences exist in the perceived relief of cancer symptoms with medical cannabis: Results from the Quebec Cannabis Registry. Support. Care Cancer 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Doherty, G.J.; de Paula, B.H.R. Cannabinoids in glioblastoma multiforme—Hype or hope? Br. J. Cancer 2021, 124, 1341–1343. [Google Scholar] [CrossRef]
- Chapman, S.; Protudjer, J.; Bourne, C.; Kelly, L.E.; Oberoi, S.; Vanan, M.I. Medical cannabis in pediatric oncology: A survey of patients and caregivers. Support. Care Cancer 2021, 29, 6589–6594. [Google Scholar] [CrossRef] [PubMed]
- Malach, M.; Kovalchuk, I.; Kovalchuk, O. Medical Cannabis in Pediatric Oncology: Friend or Foe? Pharmaceuticals 2022, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Solomon, H.V.; Greenstein, A.P.; DeLisi, L.E. Cannabis Use in Older Adults: A Perspective. Harv. Rev. Psychiatry 2021, 29, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.; Bouchard, L. Medical Cannabis Use: Exploring the Perceptions and Experiences of Older Adults with Chronic Conditions. Clin. Gerontol. 2020, 44, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Skliamis, K.; Benschop, A.; Korf, D.J. Cannabis users and stigma: A comparison of users from European countries with different cannabis policies. Eur. J. Criminol. 2020, 23, 3560. [Google Scholar] [CrossRef]
- Bottorff, J.L.; Bissell, L.J.; Balneaves, L.G.; Oliffe, J.L.; Capler, N.R.; Buxton, J. Perceptions of cannabis as a stigmatized medicine: A qualitative descriptive study. Harm Reduct. J. 2013, 10, 2. [Google Scholar] [CrossRef]
- Reid, M. A qualitative review of cannabis stigmas at the twilight of prohibition. J. Cannabis Res. 2020, 2, 46. [Google Scholar] [CrossRef]
- Leos-Toro, C.; Shiplo, S.; Hammond, D. Perceived support for medical cannabis use among approved medical cannabis users in Canada. Drug Alcohol Rev. 2018, 37, 627–636. [Google Scholar] [CrossRef]
- Davis, B.A. Discrimination: A Social Determinant of Health Inequities. Health Aff. 2020. [Google Scholar] [CrossRef]
- Barry, C.L.; McGinty, E.E.; Pescosolido, B.A.; Goldman, H.H. Stigma, Discrimination, Treatment Effectiveness, and Policy: Public Views About Drug Addiction and Mental Illness. Psychiatr. Serv. 2014, 65, 1269–1272. [Google Scholar] [CrossRef]
- Atmanli, A.; İleri, E.; Yüksel, B. Experimental investigation of engine performance and exhaust emissions of a diesel engine fueled with diesel–n-butanol–vegetable oil blends. Energy Convers Manag. 2014, 81, 312–321. [Google Scholar] [CrossRef]
- Victorson, D.; McMahon, M.; Horowitz, B.; Glickson, S.; Parker, B.; Mendoza-Temple, L. Exploring cancer survivors’ attitudes, perceptions, and concerns about using medical cannabis for symptom and side effect management: A qualitative focus group study. Complement. Ther. Med. 2019, 47, 102204. [Google Scholar] [CrossRef] [PubMed]
- Rudski, J.M. Treatment Acceptability, Stigma, and Legal Concerns of Medical Marijuana Are Affected by Method of Administration. J. Drug Issues 2013, 44, 308–320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).