The Optimal Therapy after Progression on Immune Checkpoint Inhibitors in MSI Metastatic Gastrointestinal Cancer Patients: A Multicenter Retrospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
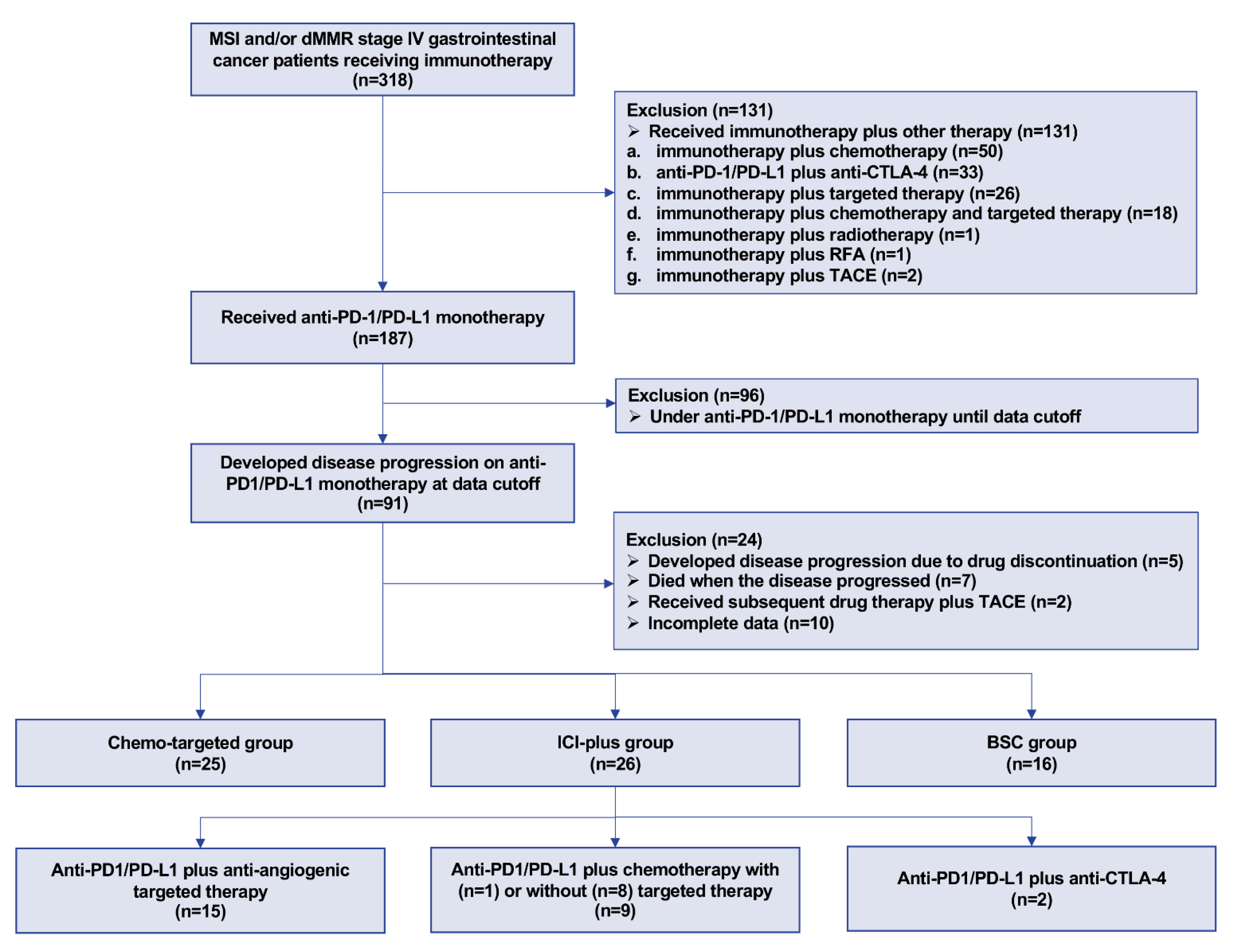
2.1. Patients
2.2. Outcome Evaluation
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
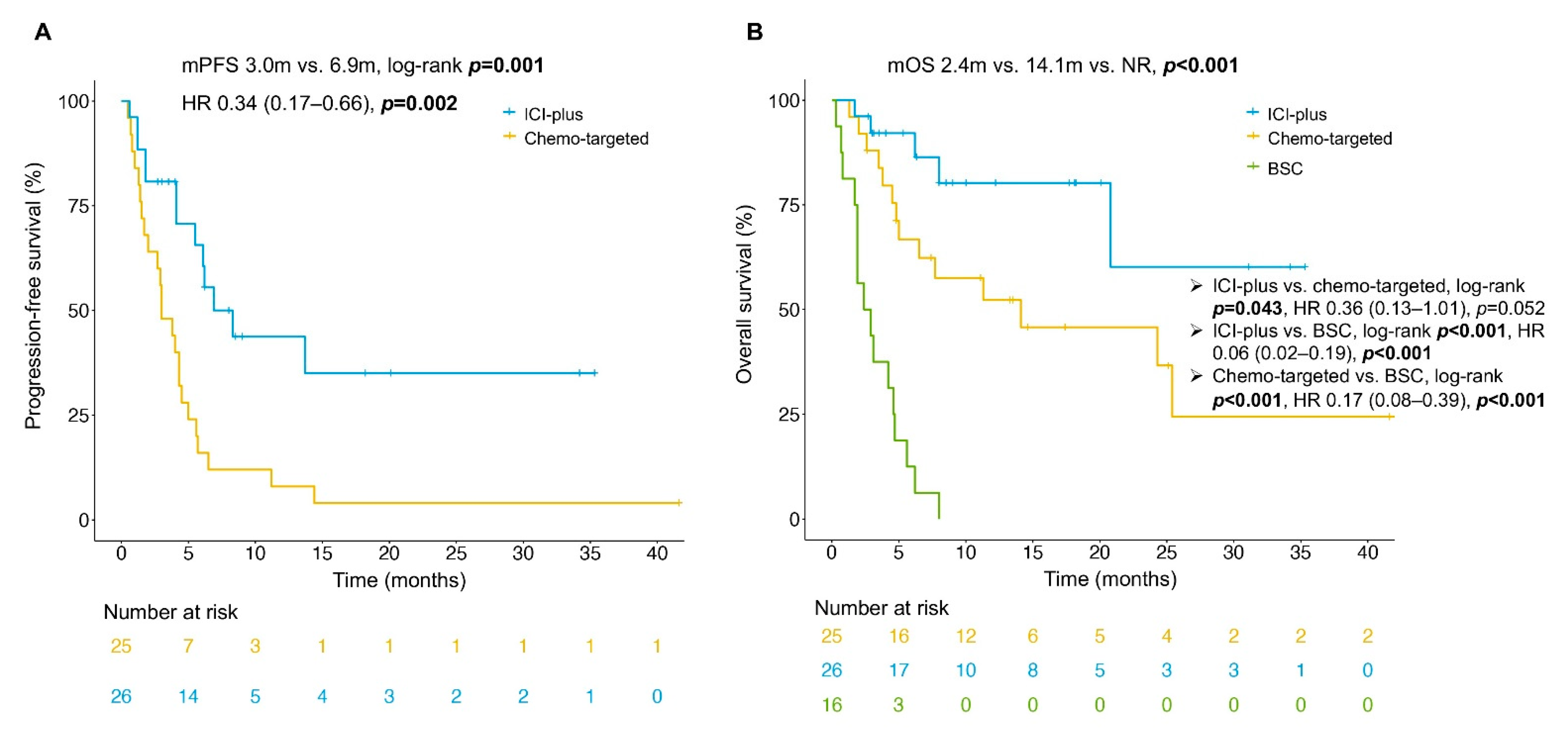
3.2. Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Akagi, K.; Oki, E.; Taniguchi, H.; Nakatani, K.; Aoki, D.; Kuwata, T.; Yoshino, T. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. 2021, 112, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.; Kortman, G.A.M.; Mekenkamp, L.; Ligtenberg, M.J.L.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.A.; van Krieken, J.H.J.M. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.-J.; De Vita, F.; Landers, G.; Yen, C.-J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; An, M.; Klempner, S.J.; Lee, H.; Kim, K.-M.; Sa, J.K.; Cho, H.J.; Hong, J.Y.; Lee, T.; Min, Y.W.; et al. Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability-High Gastric Cancer. Cancer Discov. 2021, 11, 2168–2185. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, Y.; Li, J.; Zhang, X.; Peng, Z.; Hu, Y.; Zhao, X.; Dong, K.; Zhang, B.; et al. Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med. 2022, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Kawazoe, A.; Kawazu, M.; Suzuki, T.; Nakamura, Y.; Nakatsura, T.; Kuwata, T.; Ueno, T.; Kuboki, Y.; Kotani, D.; et al. A Low Tumor Mutational Burden and Mutations Are Predictors of a Negative Response to PD-1 Blockade in MSI-H/dMMR Gastrointestinal Tumors. Clin. Cancer Res. 2021, 27, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Q.; Qi, C.; Bai, Y.; Zhao, F.; Chen, H.; Li, Z.; Wang, X.; Chen, M.; Gong, J.; et al. Combination of AKT1 and CDH1 mutations predicts primary resistance to immunotherapy in dMMR/MSI-H gastrointestinal cancer. J. Immunother. Cancer 2022, 10, e004703. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Liu, D.; Jiang, W.; Tang, J.; Kong, L.; Han, K.; Liao, L.; Li, Y.; Ou, Q.; Xiao, B.; et al. Dickkopf 1 impairs the tumor response to PD-1 blockade by inactivating CD8+ T cells in deficient mismatch repair colorectal cancer. J. Immunother. Cancer 2021, 9, e001498. [Google Scholar] [CrossRef] [PubMed]
- Bortolomeazzi, M.; Keddar, M.R.; Montorsi, L.; Acha-Sagredo, A.; Benedetti, L.; Temelkovski, D.; Choi, S.; Petrov, N.; Todd, K.; Wai, P.; et al. Immunogenomics of Colorectal Cancer Response to Checkpoint Blockade: Analysis of the KEYNOTE 177 Trial and Validation Cohorts. Gastroenterology 2021, 161, 1179–1193. [Google Scholar] [CrossRef]
- Morris, V.K.; Lam, M.; Wang, X.; Overman, M.J.; Johnson, B.; Kee, B.K.; Wolff, R.A.; Dasari, A.; Zorrilla, I.R.; Tam, A.; et al. Phase II trial of bintrafusp alfa in patients with metastatic MSI-H cancers following progression on immunotherapy. J. Clin. Oncol. 2021, 39 (Suppl. S3), 79. [Google Scholar] [CrossRef]
- Hollebecque, A.; Chung, H.C.; de Miguel, M.J.; Italiano, A.; Machiels, J.-P.; Lin, C.-C.; Dhani, N.C.; Peeters, M.; Moreno, V.; Su, W.-C.; et al. Safety and Antitumor Activity of α-PD-L1 Antibody as Monotherapy or in Combination with α-TIM-3 Antibody in Patients with Microsatellite Instability-High/Mismatch Repair-Deficient Tumors. Clin. Cancer Res. 2021, 27, 6393–6404. [Google Scholar] [CrossRef]
- Bui, Q.L.; Mas, L.; Hollebecque, A.; Tougeron, D.; de la Fouchardière, C.; Pudlarz, T.; Alouani, E.; Guimbaud, R.; Taieb, J.; André, T.; et al. Treatments after Immune Checkpoint Inhibitors in Patients with dMMR/MSI Metastatic Colorectal Cancer. Cancers 2022, 14, 406. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Watson, S.; Bonnet, C.; Menis, J.; Michot, J.M.; Paoletti, X. Evaluation of PFS ratio in patients with cancer enrolled in early-phase clinical trials: A single center, retrospective analysis. J. Clin. Oncol. 2017, 35 (Suppl. S15), e14025. [Google Scholar] [CrossRef]
- Guiard, E.; Clatot, F.; Even, C.; Perréard, M.; Abdeddaim, C.; Johnson, A.; Vauléon, E.; Rambeau, A. Impact of previous nivolumab treatment on the response to taxanes in patients with recurrent/metastatic head and neck squamous cell carcinoma. Eur. J. Cancer 2021, 159, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Tawbi, H.A.; Ascierto, M.L.; Bowden, M.; Callahan, M.K.; Cha, E.; Chen, H.X.; Drake, C.G.; Feltquate, D.M.; Ferris, R.L.; et al. Defining tumor resistance to PD-1 pathway blockade: Recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer 2020, 8, e000398. [Google Scholar] [CrossRef] [Green Version]
- Jeyakumar, G.; Kim, S.; Bumma, N.; Landry, C.; Silski, C.; Suisham, S.; Dickow, B.; Heath, E.; Fontana, J.; Vaishampayan, U. Neutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J. Immunother. Cancer 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, A.K.T.; Mimura, K.; Nakajima, S.; Okayama, H.; Saito, K.; Sakamoto, W.; Fujita, S.; Endo, H.; Saito, M.; Saze, Z.; et al. Therapeutic potential of anti-VEGF receptor 2 therapy targeting for M2-tumor-associated macrophages in colorectal cancer. Cancer Immunol. Immunother. 2021, 70, 289–298. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Zhang, J.; Luo, P. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front. Immunol. 2020, 11, 2039. [Google Scholar] [CrossRef]
- Hansen, T.F.; Jensen, L.H.; Spindler, K.L.G.; Lindebjerg, J.; Brandslund, I.; Jakobsen, A. The relationship between serum vascular endothelial growth factor A and microsatellite instability in colorectal cancer. Color. Dis. 2011, 13, 984–988. [Google Scholar] [CrossRef]
- Otto, W.; Macrae, F.; Sierdziński, J.; Smaga, J.; Król, M.; Wilińska, E.; Zieniewicz, K. Microsatellite instability and manifestations of angiogenesis in stage IV of sporadic colorectal carcinoma. Medicine 2019, 98, e13956. [Google Scholar] [CrossRef]
- Chida, K.; Kawazoe, A.; Suzuki, T.; Kawazu, M.; Ueno, T.; Takenouchi, K.; Nakamura, Y.; Kuboki, Y.; Kotani, D.; Kojima, T.; et al. Transcriptomic profiling of MSI-H/dMMR gastrointestinal tumors to identify determinants of responsiveness to anti-PD-1 therapy. Clin. Cancer Res. 2022, 28, 2110–2117. [Google Scholar] [CrossRef]
- Kasi, P.M.; Budde, G.; Krainock, M.; Aushev, V.N.; Koyen Malashevich, A.; Malhotra, M.; Olshan, P.; Billings, P.R.; Aleshin, A. Circulating tumor DNA (ctDNA) serial analysis during progression on PD-1 blockade and later CTLA-4 rescue in patients with mismatch repair deficient metastatic colorectal cancer. J. Immunother. Cancer 2022, 10, e003312. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Ghatalia, P.; Bubes, N.; Anari, F.; Varshavsky, A.; Kasireddy, V.; Liu, Y.; El-Deiry, W.S. Dual Checkpoint Inhibition with Ipilimumab plus Nivolumab After Progression on Sequential PD-1/PDL-1 Inhibitors Pembrolizumab and Atezolizumab in a Patient with Lynch Syndrome, Metastatic Colon, and Localized Urothelial Cancer. Oncol. 2019, 24, 1416–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Allen, A.; Berlin, J. Immunotherapy After Immunotherapy: Response Rescue in a Patient With Microsatellite Instability-high Colorectal Cancer Post-Pembrolizumab. Clin. Color. Cancer 2020, 19, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Jordan, F.; Trepel, M.; Claus, R. Restoring Immune Mediated Disease Control by Ipilimumab Re-exposition in a Heavily pretreated Patient With MSI-H mCRC. Clin. Color. Cancer 2022, 21, E148–E151. [Google Scholar] [CrossRef]
- Hamre, T.R.; Stougaard, J.K.; Havelund, B.M.; Jensen, L.H.; Hansen, T.F. Re-exposure to immunotherapy in metastatic colon cancer: A case report. Clin. Case Rep. 2021, 9, e04349. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Mazzoli, G.; Cohen, R.; Lonardi, S.; Corti, F.; Elez, E.; Fakih, M.; Jayachandran, P.; Colle, R.; Shah, A.T.; Salati, M.; et al. Prognostic impact of performance status on the outcomes of immune checkpoint inhibition strategies in patients with dMMR/MSI-H metastatic colorectal cancer. Eur. J. Cancer 2022, 172, 171–181. [Google Scholar] [CrossRef]
- Fucà, G.; Cohen, R.; Lonardi, S.; Shitara, K.; Elez, M.E.; Fakih, M.; Chao, J.; Klempner, S.J.; Emmett, M.; Jayachandran, P.; et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J. Immunother. Cancer 2022, 10, e004001. [Google Scholar] [CrossRef]

| Characteristics | All Patients with Post-ICI Therapy n = 51 | ICI-Plus Group n = 26 | Chemo-Targeted Group n = 25 | p Value | BSC Group n = 16 |
|---|---|---|---|---|---|
| Age | |||||
| <65 | 40 (78.4) | 21 (80.8) | 19 (76.0) | 0.941 | 8 (50.0) |
| ≥65 | 11 (21.6) | 5 (19.2) | 6 (24.0) | 8 (50.0) | |
| Sex | |||||
| Female | 21 (41.2) | 8 (30.8) | 13 (52.0) | 0.124 | 10 (62.5) |
| Male | 30 (58.8) | 18 (69.2) | 12 (48.0) | 6 (37.5) | |
| ECOG | |||||
| 0–1 | 44 (86.3) | 22 (84.6) | 22 (88.0) | 0.962 | 8 (50.0) |
| ≥2 | 5 (9.8) | 2 (7.7) | 3 (12.0) | 8 (50.0) | |
| NA | 2 (3.9) | 2 (7.7) | 0 (0.0) | 0 (0.0) | |
| Differentiation | |||||
| Moderate-high | 19 (37.3) | 11 (42.3) | 8 (32.0) | 0.376 | 5 (31.3) |
| Undifferentiation-low | 29 (56.9) | 13 (50.0) | 16 (64.0) | 10 (62.5) | |
| NA | 3 (5.9) | 2 (7.7) | 1 (4.0) | 1 (6.3) | |
| Site | |||||
| Gastric and GEJ | 10 (19.6) | 4 (15.4) | 6 (24.0) | 0.241 | 7 (43.8) |
| Colorectal | 35 (68.6) | 17 (65.4) | 18 (72.0) | 7 (43.8) | |
| Other sites | 6 (11.8) | 5 (19.2) | 1 (4.0) | 2 (12.5) | |
| No. of metastatic organs | |||||
| <3 | 41 (80.4) | 22 (84.6) | 19 (76.0) | 0.673 | 11 (68.8) |
| ≥3 | 10 (19.6) | 4 (15.4) | 6 (24.0) | 5 (31.3) | |
| Hepatic metastasis | |||||
| Absent | 34 (66.7) | 18 (69.2) | 16 (64.0) | 0.692 | 10 (62.5) |
| Present | 17 (33.3) | 8 (30.8) | 9 (36.0) | 6 (37.5) | |
| Pulmonary metastasis | |||||
| Absent | 43 (84.3) | 24 (92.3) | 19 (76.0) | 0.224 | 14 (87.5) |
| Present | 8 (15.7) | 2 (7.7) | 6 (24.0) | 2 (12.5) | |
| Distant lymph node metastasis | |||||
| Absent | 27 (52.9) | 14 (53.8) | 13 (52.0) | 0.895 | 5 (31.3) |
| Present | 24 (47.1) | 12 (46.2) | 12 (48.0) | 11 (68.8) | |
| Peritoneal metastasis | |||||
| Absent | 24 (47.1) | 11 (42.3) | 13 (52.0) | 0.488 | 7 (43.8) |
| Present | 27 (52.9) | 15 (57.7) | 12 (48.0) | 9 (56.3) | |
| No. of prior therapy lines | |||||
| <2 | 14 (27.5) | 12 (46.2) | 2 (8.0) | 0.006 | 5 (31.3) |
| ≥2 | 37 (72.5) | 14 (53.8) | 23 (92.0) | 11 (68.8) | |
| Time from metastasis to post-ICI therapy | |||||
| <12 months | 26 (51.0) | 12 (46.2) | 13 (52.0) | 0.886 | - |
| ≥12 months | 25 (49.0) | 14 (53.8) | 12 (48.0) | - | |
| Best response to prior anti-PD1/PD-L1 | |||||
| PR | 6 (11.8) | 2 (7.7) | 4 (16.0) | 0.590 | 1 (6.3) |
| SD + PD | 44 (86.3) | 24 (92.3) | 20 (80.0) | 15 (93.8) | |
| NA | 1 (2.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | |
| Type of resistance | |||||
| primary resistance | 35 (68.6) | 16 (61.5) | 19 (76.0) | 0.406 | 14 (87.5) |
| acquired resistance | 16 (31.4) | 10 (38.5) | 6 (24.0) | 2 (12.5) | |
| Anemia | |||||
| Absent | 18 (35.3) | 10 (38.5) | 8 (32.0) | 0.455 | - |
| Present | 25 (49.0) | 11 (42.3) | 14 (56.0) | ||
| NA | 8 (15.7) | 5 (19.2) | 3 (12.0) | ||
| NLR | |||||
| <3 | 19 (37.3) | 9 (34.6) | 10 (40.0) | 0.864 | - |
| ≥3 | 24 (47.1) | 12 (46.2) | 12 (48.0) | ||
| NA | 8 (15.7) | 5 (19.2) | 3 (12.0) |
| Variable | ICI-Plus Group n = 26 | Chemo-Targeted Group n = 25 | p Value |
|---|---|---|---|
| Best response, n (%) | |||
| Complete response | 0 (0.0) | 0 (0.0) | |
| Partial response | 6 (23.1) | 3 (12.0) | |
| Stable disease | 15 (57.7) | 8 (32.0) | |
| Progressive disease | 3 (11.5) | 10 (40.0) | |
| Unable to determine * | 2 (7.7) | 4 (16.0) | |
| Objective response, n (%) | 6 (23.1) | 3 (12.0) | 0.503 |
| Disease control, n (%) | 21 (80.8) | 11 (44.0) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wang, Z.; Liu, Z.; Liu, N.; Fang, W.; Zhang, H.; Jin, X.; Li, J.; Zhao, W.; Qu, H.; et al. The Optimal Therapy after Progression on Immune Checkpoint Inhibitors in MSI Metastatic Gastrointestinal Cancer Patients: A Multicenter Retrospective Cohort Study. Cancers 2022, 14, 5158. https://doi.org/10.3390/cancers14205158
Chen M, Wang Z, Liu Z, Liu N, Fang W, Zhang H, Jin X, Li J, Zhao W, Qu H, et al. The Optimal Therapy after Progression on Immune Checkpoint Inhibitors in MSI Metastatic Gastrointestinal Cancer Patients: A Multicenter Retrospective Cohort Study. Cancers. 2022; 14(20):5158. https://doi.org/10.3390/cancers14205158
Chicago/Turabian StyleChen, Mifen, Zhenghang Wang, Zimin Liu, Ning Liu, Weijia Fang, Hangyu Zhang, Xuan Jin, Jiayi Li, Weifeng Zhao, Huajun Qu, and et al. 2022. "The Optimal Therapy after Progression on Immune Checkpoint Inhibitors in MSI Metastatic Gastrointestinal Cancer Patients: A Multicenter Retrospective Cohort Study" Cancers 14, no. 20: 5158. https://doi.org/10.3390/cancers14205158
APA StyleChen, M., Wang, Z., Liu, Z., Liu, N., Fang, W., Zhang, H., Jin, X., Li, J., Zhao, W., Qu, H., Song, F., Chang, Z., Li, Y., Tang, Y., Xu, C., Zhang, X., Wang, X., Peng, Z., Cai, J., ... Shen, L. (2022). The Optimal Therapy after Progression on Immune Checkpoint Inhibitors in MSI Metastatic Gastrointestinal Cancer Patients: A Multicenter Retrospective Cohort Study. Cancers, 14(20), 5158. https://doi.org/10.3390/cancers14205158





