Sporadic and von Hippel–Lindau Related Hemangioblastomas of Brain and Spinal Cord: Multimodal Imaging for Intraoperative Strategy
Abstract
:Simple Summary
Abstract
1. Introduction
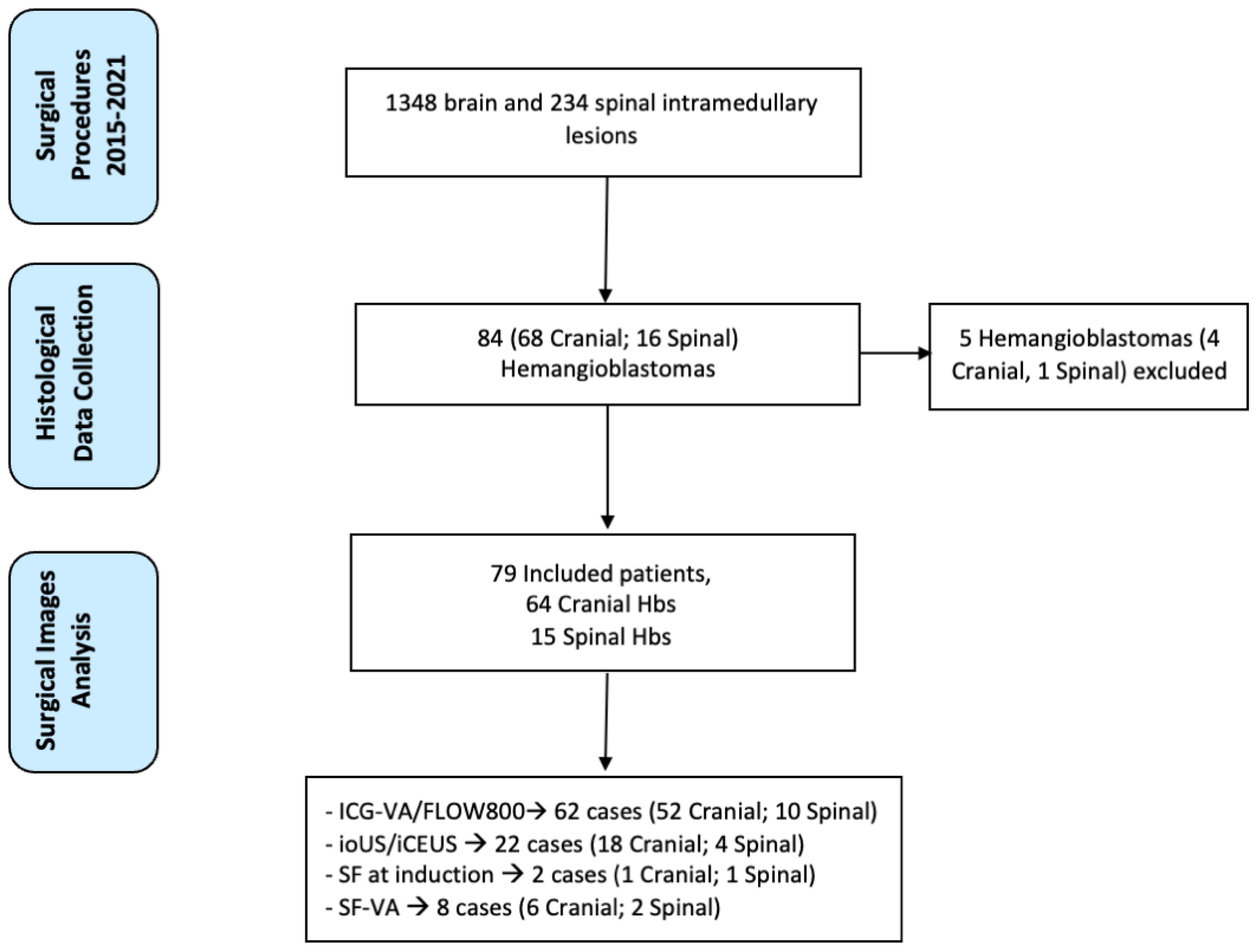
2. Materials and Methods
2.1. Surgical Protocol
2.2. Intraoperative Imaging Modalities
2.2.1. IOUS and iCEUS
2.2.2. ICG Videoangiography with FLOW 800 Analysis
2.2.3. Sodium Fluorescein Protocol
3. Results
3.1. Clinical Series
3.2. Use of Intraoperative Imaging Modalities during HB Resection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Kuharic, M.; Jankovic, D.; Splavski, B.; Boop, F.A.; Arnautovic, K.I. Hemangioblastomas of the Posterior Cranial Fossa in Adults: Demographics, Clinical, Morphologic, Pathologic, Surgical Features, and Outcomes. A Systematic Review. World Neurosurg. 2018, 110, e1049–e1062. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Rutkowski, M.J.; Yalcin, C.E.; Eren, O.C.; Caliskan, I.; Tihan, T. Sporadic and Von-Hippel Lindau Disease-Associated Spinal Hemangioblastomas: Institutional Experience on Their Similarities and Differences. J. Neurooncol. 2019, 143, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Lohle, P.N.M.; Van Mameren, H.; Zwinderman, K.H.; Teepen, H.L.J.M.; Go, K.G.; Wilmink, J.T. On the Pathogenesis of Brain Tumour Cysts: A Volumetric Study of Tumour, Oedema and Cyst. Neuroradiology 2000, 42, 639–642. [Google Scholar] [CrossRef]
- Nguyen, H.S.; Doan, N.B.; Gelsomino, M.; Shabani, S.; Awad, A.J.; Kaushal, M.; Mortazavi, M.M. Intracranial Hemangioblastoma—A SEER-Based Analysis 2004–2013. Oncotarget 2018, 9, 28009–28015. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Dornbos, D.; Kim, H.J.; Butman, J.A.; Lonser, R.R. Review of the Neurological Implications of von Hippel-Lindau Disease. JAMA Neurol. 2018, 75, 620–627. [Google Scholar] [CrossRef]
- Lonser, R.R.; Glenn, G.M.; Walther, M.; Chew, E.Y.; Libutti, S.K.; Linehan, W.M.; Oldfield, E.H. Von Hippel-Lindau Disease. Lancet 2003, 361, 2059–2067. [Google Scholar] [CrossRef]
- Melmon, K.L.; Rosen, S.W. Lindau’s Disease. Review of the Literature and Study of a Large Kindred. Am. J. Med. 1964, 36, 595–617. [Google Scholar] [CrossRef]
- Wanebo, J.E.; Lonser, R.R.; Glenn, G.M.; Oldfield, E.H. The Natural History of Hemangioblastomas of the Central Nervous System in Patients with von Hippel-Lindau Disease. J. Neurosurg. 2003, 98, 82–94. [Google Scholar] [CrossRef]
- Lonser, R.R.; Butman, J.A.; Kiringoda, R.; Song, D.; Oldfield, E.H. Pituitary Stalk Hemangioblastomas in von Hippel–Lindau Disease: Clinical Article. J. Neurosurg. 2009, 110, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Reste, P.J.; Henaux, P.L.; Morandi, X.; Carsin-Nicol, B.; Brassier, G.; Riffaud, L. Sporadic Intracranial Haemangioblastomas: Surgical Outcome in a Single Institution Series. Acta Neurochir. 2013, 155, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-Q.; Cui, H.; Wang, Y. Surgical Management of Large Solid Hemangioblastomas of the Posterior Fossa. J. Clin. Neurosci. 2011, 18, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Del Bene, M.; Mattei, L.; Lodigiani, L.; Debeni, S.; Kolev, V.; Vetrano, I.; Solbiati, L.; Sakas, G.; Dimeco, F. Preoperative Magnetic Resonance and Intraoperative Ultrasound Fusion Imaging for Real-Time Neuronavigation in Brain Tumor Surgery. Ultraschall Med. 2014, 9, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prada, F.; Del Bene, M.; Moiraghi, A.; Casali, C.; Legnani, F.G.; Saladino, A.; Perin, A.; Vetrano, I.G.; Mattei, L.; Richetta, C.; et al. From Grey Scale B-Mode to Elastosonography: Multimodal Ultrasound Imaging in Meningioma Surgery—Pictorial Essay and Literature Review. Biomed Res. Int. 2015, 2015, 925729. [Google Scholar] [CrossRef]
- Vetrano, I.G.; Prada, F.; Nataloni, I.F.; Del Bene, M.; DiMeco, F.; Valentini, L.G. Discrete or Diffuse Intramedullary Tumor? Contrast-Enhanced Intraoperative Ultrasound in a Case of Intramedullary Cervicothoracic Hemangioblastomas Mimicking a Diffuse Infiltrative Glioma: Technical Note and Case Report. Neurosurg. Focus 2015, 39, E17. [Google Scholar] [CrossRef] [Green Version]
- Prada, F.; Perin, A.; Martegani, A.; Aiani, L.; Solbiati, L.; Lamperti, M.; Casali, C.; Legnani, F.; Mattei, L.; Saladino, A.; et al. Intraoperative Contrast-Enhanced Ultrasound for Brain Tumor Surgery. Neurosurgery 2014, 74, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Prada, F.; Mattei, L.; Del Bene, M.; Aiani, L.; Saini, M.; Casali, C.; Filippini, A.; Legnani, F.G.; Perin, A.; Saladino, A.; et al. Intraoperative Cerebral Glioma Characterization with Contrast Enhanced Ultrasound. Biomed Res. Int. 2014, 2014, 484261. [Google Scholar] [CrossRef] [Green Version]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated With a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Saladino, A.; Lamperti, M.; Mangraviti, A.; Legnani, F.G.; Prada, F.U.; Casali, C.; Caputi, L.; Borrelli, P.; DiMeco, F. The Semisitting Position: Analysis of the Risks and Surgical Outcomes in a Contemporary Series of 425 Adult Patients Undergoing Cranial Surgery. J. Neurosurg. 2016, 127, 867–876. [Google Scholar] [CrossRef]
- Sandalcioglu, I.E.; Gasser, T.; Asgari, S.; Lazorisak, A.; Engelhorn, T.; Egelhof, T.; Stolke, D.; Wiedemayer, H. Functional Outcome after Surgical Treatment of Intramedullary Spinal Cord Tumors: Experience with 78 Patients. Spinal Cord 2005, 43, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Jiang, X.; Wang, S.; Zhang, M.; Zhao, J.; Liu, H.; Ma, J.; Xiang, D.; Wang, L. Intraoperative Contrast-Enhanced Ultrasound for Brain Tumors. Clin. Imaging 2008, 32, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Hansen, C.; Eyding, J.; Wilkening, W.; Brenke, C.; Krogias, C.; Scholz, M.; Harders, A.; Ermert, H.; Schmieder, K. Feasibility of Contrast-Enhanced Sonography During Resection of Cerebral Tumours: Initial Results of a Prospective Study. Ultrasound Med. Biol. 2007, 33, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Kearns, K.N.; Sokolowski, J.D.; Chadwell, K.; Chandler, M.; Kiernan, T.; Prada, F.; Kalani, M.Y.S.; Park, M.S. The Role of Contrast-Enhanced Ultrasound in Neurosurgical Disease. Neurosurg. Focus 2019, 47, E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acerbi, F.; Prada, F.; Vetrano, I.G.; Falco, J.; Faragò, G.; Ferroli, P.; DiMeco, F. Indocyanine Green and Contrast-Enhanced Ultrasound Videoangiography: A Synergistic Approach for Real-Time Verification of Distal Revascularization and Aneurysm Occlusion in a Complex Distal Middle Cerebral Artery Aneurysm. World Neurosurg. 2019, 125, 277–284. [Google Scholar] [CrossRef]
- Prada, F.; Del Bene, M.; Faragò, G.; DiMeco, F. Spinal Dural Arteriovenous Fistula: Is There a Role for Intraoperative Contrast-Enhanced Ultrasound? World Neurosurg. 2017, 100, 712.e15–712.e18. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the Biodistribution of Fluorescein in Glioma-Infiltrated Mouse Brain and Histopathological Correlation of Intraoperative Findings in High-Grade Gliomas Resected under Fluorescein Fluorescence Guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Lane, B.C.; Cohen-Gadol, A.A. A Prospective Study of Microscope-Integrated Intraoperative Fluorescein Videoangiography during Arteriovenous Malformation Surgery: Preliminary Results. Neurosurg. Focus 2014, 36, E15. [Google Scholar] [CrossRef] [Green Version]
- Rey-Dios, R.; Cohen-Gadol, A.A. Technical Principles and Neurosurgical Applications of Fluorescein Fluorescence Using a Microscope-Integrated Fluorescence Module. Acta Neurochir. 2013, 155, 701–706. [Google Scholar] [CrossRef]
- Rey-Dios, R.; Cohen-Gadol, A.A. Intraoperative Fluorescence for Resection of Hemangioblastomas. Acta Neurochir. 2013, 155, 1287–1292. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; de Laurentis, C.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; Ferroli, P. The Role of Indocyanine Green Videoangiography with FLOW 800 Analysis for the Surgical Management of Central Nervous System Tumors: An Update. Neurosurg. Focus 2018, 44, E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feletti, A.; Marrone, F.; Barresi, V.; Sala, F. Hemangioblastoma with Contrast-Enhanced Cystic Wall: When the Surgical Rule Must Not Be Respected. World Neurosurg. 2021, 149, 190–194. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Novotny, A.; Heimann, A.; Sauer, O.; Kempski, O.; Plesnila, N.; Wietzorrek, J.; Reulen, H.J. In Vitro and in Vivo Porphyrin Accumulation by C6 Glioma Cells after Exposure to 5-Aminolevulinic Acid. J. Photochem. Photobiol. B Biol. 1998, 45, 160–169. [Google Scholar] [CrossRef]
- Ji, S.Y.; Kim, J.W.; Park, C.-K. Experience Profiling of Fluorescence-Guided Surgery II: Non-Glioma Pathologies. Brain Tumor Res. Treat. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, Y.; Tanaka, Y.; Hironaka, Y.; Shida, Y.; Nakase, H. Visualization of Vascular Structure of Spinal Hemangioblastoma Using Intraoperative Indocyanine Green Videoangiography and Temporary Feeder Occlusion. Eur. Spine J. 2015, 24, 585–589. [Google Scholar] [CrossRef]
- Hojo, M.; Arakawa, Y.; Funaki, T.; Yoshida, K.; Kikuchi, T.; Takagi, Y.; Araki, Y.; Ishii, A.; Kunieda, T.; Takahashi, J.C.; et al. Usefulness of Tumor Blood Flow Imaging by Intraoperative Indocyanine Green Videoangiography in Hemangioblastoma Surgery. World Neurosurg. 2014, 82, E495–E501. [Google Scholar] [CrossRef]
- Hao, S.; Li, D.; Ma, G.; Yang, J.; Wang, G. Application of Intraoperative Indocyanine Green Videoangiography for Resection of Spinal Cord Hemangioblastoma: Advantages and Limitations. J. Clin. Neurosci. 2013, 20, 1269–1275. [Google Scholar] [CrossRef]
- Acerbi, F.; Cavallo, C.; Schebesch, K.M.; Akçakaya, M.O.; de Laurentis, C.; Hamamcioglu, M.K.; Broggi, M.; Brawanski, A.; Falco, J.; Cordella, R.; et al. Fluorescein-Guided Resection of Intramedullary Spinal Cord Tumors: Results from a Preliminary, Multicentric, Retrospective Study. World Neurosurg. 2017, 108, 603–609. [Google Scholar] [CrossRef]
- Prada, F.; Ciocca, R.; Corradino, N.; Gionso, M.; Raspagliesi, L.; Vetrano, I.G.; Doniselli, F.; Del Bene, M.; DiMeco, F. Multiparametric Intraoperative Ultrasound in Oncological Neurosurgery: A Pictorial Essay. Front. Neurosci. 2022, 16, 881661. [Google Scholar] [CrossRef]
- Della Pappa, G.M.; Marchese, E.; Pedicelli, A.; Olivi, A.; Ricciardi, L.; Rapisarda, A.; Skrap, B.; Sabatino, G.; La Rocca, G. Contrast-Enhanced Ultrasonography and Color Doppler: Guided Intraoperative Embolization of Intracranial Highly Vascularized Tumors. World Neurosurg. 2019, 128, 547–555. [Google Scholar] [CrossRef]



| Patients’ Characteristics | Overall |
|---|---|
| N. Patients | 79 |
| Age, M ± SD | 53 ± 12 years |
| Gender | |
| Male | 32 (40.5%) |
| Female | 47 (59.5%) |
| Hydrocephalus or Syringomyelia, n (%) | 27 (34.1%) |
| VHL mutation | 12 (15.1%) |
| Locations | |
| Cerebellum | 42 (53.1%) |
| Brainstem | 14 (17.7%) |
| Supratentorial | 8 (10.1%) |
| Spinal cord | 15 (18.9%) |
| Tumor Size (mm), M ± SD | 24 ± 8 |
| Technique | Dosage | Advantages | Disadvantages |
|---|---|---|---|
| iCEUS | 2.4 mL | Completes and integrates standard B-mode and color Doppler imaging, providing dynamic and continuous real-time imaging and lesion characterization. | After tumor removal, bleedings or hemostatic agents could determine artifacts; need a learning curve mainly for anatomical orientation. |
| ICG-VA | 12.5 mg | Noninvasive modality provides rapid information on the vascular flow dynamics allowing identification of tumor nodules. | No good visualization of deep lesions; necessary to wait at least 20 min before a new administration. |
| SF-VA | 75 mg | More detailed identification of feeding and draining vessels. Good intraoperative visualization, no need to switch between fluorescent and white light mode during surgery. | Possible to do only a single administration per procedure. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzapicchi, E.; Restelli, F.; Falco, J.; Broggi, M.; Gatti, L.; Alongi, P.; Valentini, L.; Ferroli, P.; Vetrano, I.G.; DiMeco, F.; et al. Sporadic and von Hippel–Lindau Related Hemangioblastomas of Brain and Spinal Cord: Multimodal Imaging for Intraoperative Strategy. Cancers 2022, 14, 5492. https://doi.org/10.3390/cancers14225492
Mazzapicchi E, Restelli F, Falco J, Broggi M, Gatti L, Alongi P, Valentini L, Ferroli P, Vetrano IG, DiMeco F, et al. Sporadic and von Hippel–Lindau Related Hemangioblastomas of Brain and Spinal Cord: Multimodal Imaging for Intraoperative Strategy. Cancers. 2022; 14(22):5492. https://doi.org/10.3390/cancers14225492
Chicago/Turabian StyleMazzapicchi, Elio, Francesco Restelli, Jacopo Falco, Morgan Broggi, Laura Gatti, Pierpaolo Alongi, Laura Valentini, Paolo Ferroli, Ignazio G. Vetrano, Francesco DiMeco, and et al. 2022. "Sporadic and von Hippel–Lindau Related Hemangioblastomas of Brain and Spinal Cord: Multimodal Imaging for Intraoperative Strategy" Cancers 14, no. 22: 5492. https://doi.org/10.3390/cancers14225492
APA StyleMazzapicchi, E., Restelli, F., Falco, J., Broggi, M., Gatti, L., Alongi, P., Valentini, L., Ferroli, P., Vetrano, I. G., DiMeco, F., & Acerbi, F. (2022). Sporadic and von Hippel–Lindau Related Hemangioblastomas of Brain and Spinal Cord: Multimodal Imaging for Intraoperative Strategy. Cancers, 14(22), 5492. https://doi.org/10.3390/cancers14225492









