Should We Always Perform Preoperative Chest Computed Tomography in Patients with cT1a Renal Cell Carcinoma?
Abstract
Simple Summary
Abstract
1. Introduction
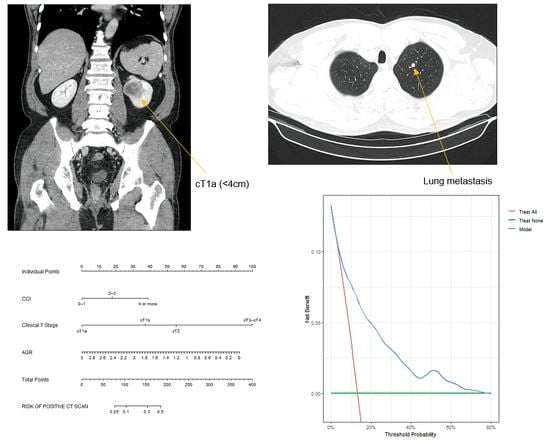
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martinek, A. Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2016, 160, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Hong, S.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2021. Cancer Res. Treat. 2021, 53, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res. Treat. 2019, 51, 417–430. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Dabestani, S.; Marconi, L.; Hofmann, F.; Stewart, F.; Lam, T.B.; Canfield, S.E.; Staehler, M.; Powles, T.; Ljungberg, B.; Bex, A. Local treatments for metastases of renal cell carcinoma: A systematic review. Lancet Oncol. 2014, 15, e549–e561. [Google Scholar] [CrossRef]
- Eggener, S.E.; Yossepowitch, O.; Pettus, J.A.; Snyder, M.E.; Motzer, R.J.; Russo, P. Renal cell carcinoma recurrence after nephrectomy for localized disease: Predicting survival from time of recurrence. J. Clin. Oncol. 2006, 24, 3101–3106. [Google Scholar] [CrossRef]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Beard, C.; Bhayani, S.; Bolger, G.B.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney cancer, version 3.2015. J. Natl. Compr. Canc. Netw. 2015, 13, 151–159. [Google Scholar] [CrossRef]
- Williamson, T.J.; Pearson, J.R.; Ischia, J.; Bolton, D.M.; Lawrentschuk, N. Guideline of guidelines: Follow-up after nephrectomy for renal cell carcinoma. BJU Int. 2016, 117, 555–562. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, W.J.; Cho, N.H.; Park, S.Y. Is Chest Computed Tomography Always Necessary Following Nephrectomy for Renal Cell Carcinoma? A Pilot Study in Single Tertiary Institution. J. Comput. Assist. Tomo. 2019, 43, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Larcher, A.; Dell’Oglio, P.; Fossati, N.; Nini, A.; Muttin, F.; Suardi, N.; De Cobelli, F.; Salonia, A.; Briganti, A.; Zhang, X.; et al. When to perform preoperative chest computed tomography for renal cancer staging. BJU Int. 2017, 120, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Fishman, M.; et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 804–834. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Carpenter, J.; Bithell, J. Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat. Med. 2000, 19, 1141–1164. [Google Scholar] [CrossRef]
- Rushing, C.; Bulusu, A.; Hurwitz, H.I.; Nixon, A.B.; Pang, H. A leave-one-out cross-validation SAS macro for the identification of markers associated with survival. Comput. Biol. Med. 2015, 57, 123–129. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Making 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Harding, G.; Cella, D.; Robinson, D.; Mahadevia, P.J.; Clark, J.; Revicki, D.A. Symptom burden among patients with Renal Cell Carcinoma (RCC): Content for a symptom index. Health Qual. Life Out. 2007, 5. [Google Scholar] [CrossRef]
- Lin, E.C. Radiation risk from medical imaging. Mayo Clin. Proc. 2010, 85, 1142–1146. [Google Scholar] [CrossRef]
- Huppmann, M.V.; Johnson, W.B.; Javitt, M.C. Radiation risks from exposure to chest computed tomography. Semin. Ultrasound CT MRI 2010, 31, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.; Heilbrun, M.E.; Conner, K.E.; Stevens, S.M.; Woller, S.C.; Elliott, C.G. Radiation and chest CT scan examinations: What do we know? Chest 2012, 142, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Belli, G.; Zappa, M.; Picozzi, G.; Falchini, M.; Della Nave, R.; Allescia, G.; Masi, A.; Pegna, A.L.; Villari, N.; et al. Risk-benefit analysis of X-ray exposure associated with lung cancer screening in the Italung-CT trial. AJR Am. J. Roentgenol. 2006, 187, 421–429. [Google Scholar] [CrossRef]
- Patel, U.; Sokhi, H. Imaging in the follow-up of renal cell carcinoma. AJR Am. J. Roentgenol. 2012, 198, 1266–1276. [Google Scholar] [CrossRef]
- Brufau, B.P.; Cerqueda, C.S.; Villalba, L.B.; Izquierdo, R.S.; Gonzalez, B.M.; Molina, C.N. Metastatic Renal Cell Carcinoma: Radiologic Findings and Assessment of Response to Targeted Antiangiogenic Therapy by Using Multidetector CT. Radiographics 2013, 33, 1691–1716. [Google Scholar] [CrossRef]
- Voss, J.; Drake, T.; Matthews, H.; Jenkins, J.; Tang, S.; Doherty, J.; Chan, K.; Dawe, H.; Thomas, T.; Kearley, S.; et al. Chest computed tomography for staging renal tumours: Validation and simplification of a risk prediction model from a large contemporary retrospective cohort. BJU Int. 2020, 125, 561–567. [Google Scholar] [CrossRef]
- Kim, H.L.; Belldegrun, A.S.; Freitas, D.G.; Bui, M.H.; Han, K.R.; Dorey, F.J.; Figlin, R.A. Paraneoplastic signs and symptoms of renal cell carcinoma: Implications for prognosis. J. Urol. 2003, 170, 1742–1746. [Google Scholar] [CrossRef]
- Suh, B.; Park, S.; Shin, D.W.; Yun, J.M.; Keam, B.; Yang, H.K.; Ahn, E.; Lee, H.; Park, J.H.; Cho, B. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann. Oncol. 2014, 25, 2260–2266. [Google Scholar] [CrossRef]
- Du, X.J.; Tang, L.L.; Mao, Y.P.; Sun, Y.; Zeng, M.S.; Kang, T.B.; Jia, W.H.; Lin, A.H.; Ma, J. The Pretreatment Albumin to Globulin Ratio Has Predictive Value for Long-Term Mortality in Nasopharyngeal Carcinoma. PLoS ONE 2014, 9, e94473. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, M.; Yuan, D.; Gu, X.; Liu, H.; Song, Y. Elevated pretreatment serum globulin albumin ratio predicts poor prognosis for advanced non-small cell lung cancer patients. J. Thorac. Dis. 2014, 6, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Gorgel, S.N.; Kose, O.; Koc, E.M.; Ates, E.; Akin, Y.; Yilmaz, Y. The prognostic significance of preoperatively assessed AST/ALT (De Ritis) ratio on survival in patients underwent radical cystectomy. Int. Urol. Nephrol. 2017, 49, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, C.J.; Tang, Q.; Zhang, L.; Yang, K.W.; Yu, X.T.; Gong, Y.; Li, X.S.; He, Z.S.; Zhou, L.Q. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. 2018, 18, 20. [Google Scholar] [CrossRef]
- Chen, Z.; Shao, Y.; Yao, H.; Zhuang, Q.; Wang, K.; Xing, Z.; Xu, X.; He, X.; Xu, R. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget 2017, 8, 48291–48302. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Lee, S.E.; Byun, S.S.; Kim, H.H.; Kwak, C.; Hong, S.K. De Ritis ratio (aspartate transaminase/alanine transaminase ratio) as a significant prognostic factor after surgical treatment in patients with clear-cell localized renal cell carcinoma: A propensity score-matched study. BJU Int. 2017, 119, 261–267. [Google Scholar] [CrossRef]
- Chung, J.W.; Park, D.J.; Chun, S.Y.; Choi, S.H.; Lee, J.N.; Kim, B.S.; Kim, H.T.; Kim, T.H.; Yoo, E.S.; Byun, S.S.; et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with non-metastatic renal cell carcinoma undergoing partial or radical nephrectomy. Sci. Rep. 2020, 10, 11999. [Google Scholar] [CrossRef]
- Mejean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Bedke, J.; Albiges, L.; Capitanio, U.; Giles, R.H.; Hora, M.; Lam, T.B.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A.; et al. The 2021 Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Immune Checkpoint Inhibitor-based Combination Therapies for Treatment-naive Metastatic Clear-cell Renal Cell Carcinoma Are Standard of Care. Eur. Urol. 2021, 80, 393–397. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Boyle, S.; Carlo, M.I.; Manley, B.; Agarwal, N.; Alva, A.; Beckermann, K.; Choueiri, T.K.; Costello, B.A.; et al. NCCN Guidelines Insights: Kidney Cancer, Version 1.2021. J. Natl. Compr. Canc. Netw. 2020, 18, 1160–1170. [Google Scholar] [CrossRef]



| Variable | Overall | Negative Chest CT Scan Prior to Surgery | Positive Chest CT Scan Prior to Surgery | p Value |
|---|---|---|---|---|
| Population, n (%) | 890 (100.00) | 863 (96.97) | 27 (3.03) | |
| Positive chest CT scan after surgery | <0.001 | |||
| No | 772 (86.74) | 772 (89.46) | 0 (0.0) | |
| Yes | 118 (13.26) | 91 (10.54) | 27 (100.00) | |
| Age, years | 60.29 ± 11.91 | 60.28 ± 11.91 | 60.81 ± 12.12 | 0.818 |
| Sex, n (%) | 0.320 | |||
| Male | 597 (67.1) | 576 (66.74) | 21 (77.78) | |
| Female | 293 (32.9) | 287 (33.26) | 6 (22.22) | |
| BMI, kg/m2 | 24.58 ± 3.61 | 24.63 ± 3.59 | 23.05 ± 3.97 | 0.025 |
| CCI, n (%) | 0.022 | |||
| 0–1 | 185 (20.79) | 183 (21.21) | 2 (7.41) | |
| 2–3 | 363 (40.79) | 355 (41.14) | 8 (29.63) | |
| ≥4 | 342 (38.43) | 325 (37.66) | 17 (62.96) | |
| Systemic symptoms, n (%) | 0.999 | |||
| Absent | 387 (43.5) | 375 (43.45) | 12 (44.44) | |
| Present | 503 (56.5) | 488 (56.55) | 15 (55.56) | |
| Clinical size, mm | 47.77 ± 29.73 | 46.59 ± 28.40 | 85.70 ± 43.85 | <0.001 |
| Clinical T stage, n (%) | <0.001 | |||
| cT1a | 424 (47.6) | 423 (49.02) | 1 (3.70) | |
| cT1b | 251 (28.2) | 247 (28.62) | 4 (14.81) | |
| cT2 | 98 (11.0) | 95 (11.01) | 3 (11.11) | |
| cT3–cT4 | 117 (13.1) | 98 (11.36) | 19 (70.37) | |
| Clinical N stage, n (%) | 0.001 | |||
| cN0 | 848 (95.3) | 827 (95.83) | 21 (77.78) | |
| cN1 | 42 (4.7) | 36 (4.17) | 6 (22.22) | |
| Preoperative PLT, 109/L | 259.40 ± 82.02 | 257.01 ± 78.31 | 335.74 ± 143.09 | 0.008 |
| Preoperative Hb, g/dL | 13.62 ± 1.95 | 13.66 ± 1.90 | 12.40 ± 2.85 | 0.031 |
| PLT/Hb ratio | 19.96 ± 10.75 | 19.65 ± 10.29 | 29.79 ± 18.35 | 0.008 |
| Serum albumin (g/L) | 4.34 ± 0.40 | 4.35 ± 0.39 | 3.99 ± 0.51 | 0.001 |
| Serum globulin (g/L) | 3.03 ± 1.17 | 3.02 ± 1.18 | 3.52 ± 0.55 | <0.001 |
| AGR | 1.49 ± 0.30 | 1.50 ± 0.30 | 1.17 ± 0.24 | <0.001 |
| AST | 26.93 ± 19.17 | 26.98 ± 19.34 | 25.41 ± 12.36 | 0.528 |
| ALT | 24.64 ± 17.25 | 24.69 ± 17.33 | 23.00 ± 14.75 | 0.616 |
| De Ritis Ratio | 1.27 ± 0.67 | 1.27 ± 0.67 | 1.25 ± 0.46 | 0.801 |
| Time to diagnosis of lung metastasis (months) | 12.68 ± 17.50 | 16.49 ± 18.32 (only 91 patients) | 0.00 ± 0.00 | <0.001 |
| Follow-up period (months) | 44.80 ± 30.19 | 45.56 ± 30.00 | 20.48 ± 26.44 | <0.001 |
| Variable | Overall | cT1a | cT1b | p Value |
|---|---|---|---|---|
| Population, n (%) | 675 (100.00) | 424 (62.81) | 251 (37.19) | |
| Positive chest CT scan prior to surgery | 0.066 | |||
| No | 670 (99.26) | 423 (99.76) | 247 (98.41) | |
| Yes | 5 (0.74) | 1 (0.24) | 4 (1.59) | |
| Positive chest CT scan after surgery | 0.001 | |||
| No | 629 (93.19%) | 406 (95.75) | 223 (88.84) | |
| Yes | 46 (6.81%) | 18 (4.25) | 28 (11.16) | |
| Age, years | 60.39 ± 11.62 | 60.74 ± 12.00 | 59.81 ± 10.96 | 0.318 |
| Sex, n (%) | 0.283 | |||
| Male | 454 (67.26%) | 292 (68.87) | 162 (64.54) | |
| Female | 221 (32.74%) | 132 (31.13) | 89 (35.46) | |
| BMI, kg/m2 | 24.68 ± 3.64 | 24.54 ± 3.66 | 24.91 ± 3.61 | 0.207 |
| CCI, n (%) | 0.514 | |||
| 0–1 | 142 (21.04) | 92 (21.70) | 50 (19.92) | |
| 2–3 | 282 (41.78) | 170 (40.09) | 112 (44.62) | |
| ≥ 4 | 251 (37.19) | 162 (38.21) | 89 (35.46) | |
| Systemic symptoms, n (%) | 0.883 | |||
| Absent | 292 (43.26%) | 182 (42.92) | 110 (43.82) | |
| Present | 383 (56.74%) | 242 (57.08) | 141 (56.18) | |
| Clinical size, mm | 35.90 ± 15.96 | 26.14 ± 10.03 | 52.37 ± 9.07 | <0.001 |
| Clinical N stage, n (%) | <0.001 | |||
| cN0 | 661 (97.93%) | 422 (99.53) | 239 (95.22) | |
| cN1 | 14 (2.07%) | 2 (0.47) | 12 (4.78) | |
| Preoperative PLT, 109/L | 250.54 ± 72.62 | 243.22 ± 70.70 | 262.92 ± 74.25 | 0.001 |
| Preoperative Hb, g/dL | 13.80 ± 1.79 | 13.88 ± 1.77 | 13.66 ± 1.82 | 0.131 |
| PLT/Hb ratio | 18.89 ± 10.03 | 18.27 ± 10.90 | 19.92 ± 8.28 | 0.027 |
| Serum albumin (g/L) | 4.39 ± 0.36 | 4.42 ± 0.34 | 4.35 ± 0.39 | 0.014 |
| Serum globulin (g/L) | 2.91 ± 0.48 | 2.88 ± 0.45 | 2.98 ± 0.52 | 0.011 |
| AGR | 1.54 ± 0.28 | 1.56 ± 0.27 | 1.50 ± 0.30 | 0.003 |
| AST | 27.80 ± 20.97 | 27.73 ± 20.27 | 27.92 ± 22.16 | 0.910 |
| ALT | 25.65 ± 17.98 | 26.14 ± 18.12 | 24.82 ± 17.74 | 0.354 |
| De Ritis Ratio | 1.25 ± 0.70 | 1.25 ± 0.77 | 1.26 ± 0.58 | 0.835 |
| Time to diagnosis of lung metastasis (months) | 17.20 ± 20.18 | 15.06 ± 16.94 | 18.50 ± 22.11 | 0.585 |
| Follow-up period (months) | 45.13 ± 30.04 | 43.43 ± 27.98 | 48.00 ± 33.08 | 0.068 |
| Predictor | Multivariable Analysis | |
|---|---|---|
| OR (95% CI) | p Value | |
| BMI | 0.970 (0.893–1.054) | 0.472 |
| CCI, n (%) | ||
| 0–1 | 1.000 (reference) | |
| 2–3 | 1.618 (0.791–3.308) | 0.188 |
| ≥4 | 2.874 (1.437–5.747) | 0.003 |
| Clinical T stage, n (%) | ||
| cT1a | 1.000 (reference) | |
| cT1b | 2.636 (1.412–4.921) | 0.002 |
| cT2 | 4.103 (1.947–8.647) | <0.001 |
| cT3–cT4 | 13.847 (7.302–26.259) | <0.001 |
| Clinical N stage, n (%) | ||
| cN0 | 1.000 (reference) | |
| cN1 | 1.868 (0.871–4.004) | 0.108 |
| PLT/Hb ratio | 1.013 (0.993–1.034) | 0.207 |
| AGR | 0.431 (0.197–0.941) | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, J.-W.; Kang, J.-K.; Jang, S.W.; Lee, E.H.; Chun, S.Y.; Choi, S.H.; Lee, J.N.; Kim, B.S.; Kim, H.T.; Kim, S.H.; et al. Should We Always Perform Preoperative Chest Computed Tomography in Patients with cT1a Renal Cell Carcinoma? Cancers 2022, 14, 5558. https://doi.org/10.3390/cancers14225558
Chung J-W, Kang J-K, Jang SW, Lee EH, Chun SY, Choi SH, Lee JN, Kim BS, Kim HT, Kim SH, et al. Should We Always Perform Preoperative Chest Computed Tomography in Patients with cT1a Renal Cell Carcinoma? Cancers. 2022; 14(22):5558. https://doi.org/10.3390/cancers14225558
Chicago/Turabian StyleChung, Jae-Wook, Jun-Koo Kang, Se Won Jang, Eun Hye Lee, So Young Chun, Seock Hwan Choi, Jun Nyung Lee, Bum Soo Kim, Hyun Tae Kim, See Hyung Kim, and et al. 2022. "Should We Always Perform Preoperative Chest Computed Tomography in Patients with cT1a Renal Cell Carcinoma?" Cancers 14, no. 22: 5558. https://doi.org/10.3390/cancers14225558
APA StyleChung, J.-W., Kang, J.-K., Jang, S. W., Lee, E. H., Chun, S. Y., Choi, S. H., Lee, J. N., Kim, B. S., Kim, H. T., Kim, S. H., Kim, T.-H., Yoo, E. S., Kwon, T. G., Park, D. J., & Ha, Y.-S. (2022). Should We Always Perform Preoperative Chest Computed Tomography in Patients with cT1a Renal Cell Carcinoma? Cancers, 14(22), 5558. https://doi.org/10.3390/cancers14225558