Circulating Long Non-Coding RNAs Could Be the Potential Prognostic Biomarker for Liquid Biopsy for the Clinical Management of Oral Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Biogenesis of Long Non-Coding RNA
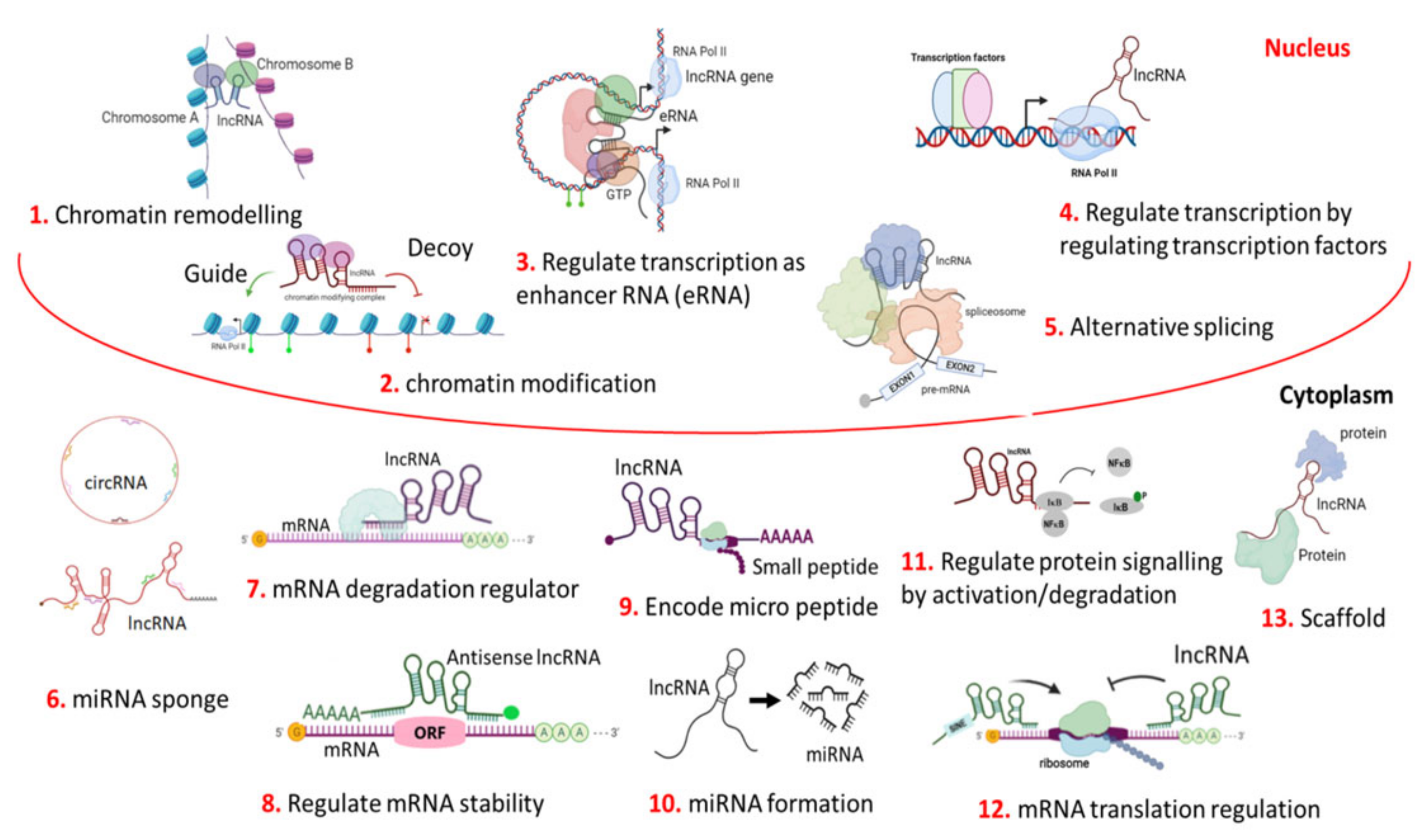
3. Mechanism of Action
3.1. LncRNA as Chromatin Regulators
3.2. LncRNA in Transcriptional Regulation
3.3. LncRNA in Post-Transcriptional Regulation
3.4. Role of lncRNA in Genomic Imprinting
4. Functional Dysregulation of lncRNAs in Oral Squamous Cell Carcinoma
4.1. Dysregulated lncRNAs as Predictive Biomarker for OSCC Disease Management
4.2. Clinical Correlations of Dysregulated LncRNAs in Primary Tumours
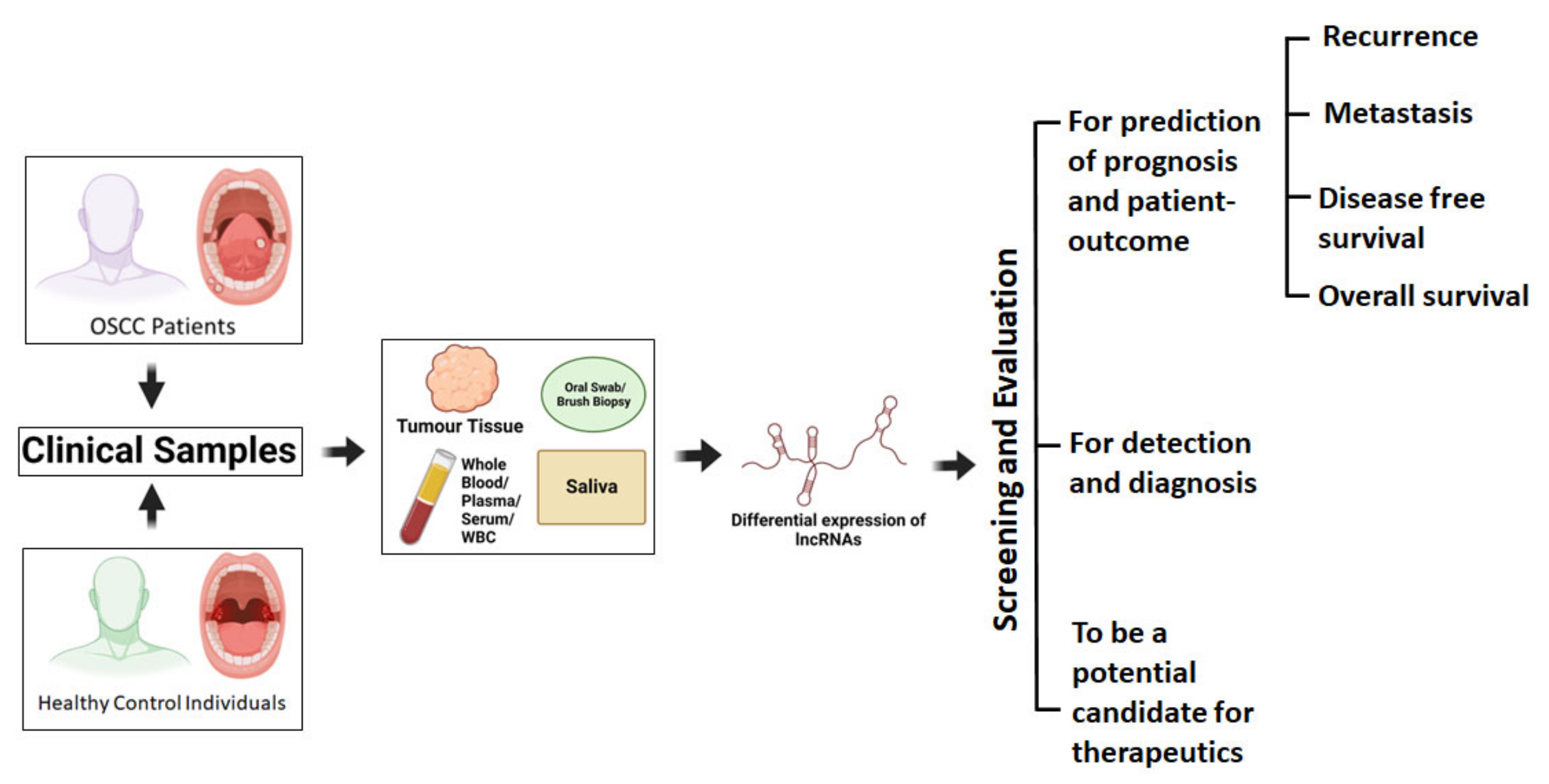
4.3. Clinical Correlations of Dysregulated Circulating LncRNAs in Body Fluids
4.3.1. LncRNA Biomarker in Saliva
4.3.2. LncRNA Biomarker in Plasma
4.3.3. LncRNA Biomarker in Serum
4.3.4. LncRNA Biomarker in Extra-Cellular Vesicles (EVs)
5. Clinical Impact of Dysregulated lncRNAs in OSCC Prognosis
5.1. LncRNA in Lymph Node Metastasis and Distant Metastasis
5.2. LncRNAs in Disease Free Survival (DFS) and Overall Survival (OS)
6. LncRNA as a Therapeutic Target in OSCC
7. Challenges and Future Perspective of Circulating lncRNAs as Prognostic Biomarker for Liquid Biopsy in OSCC
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- SEER Data Surveillance, Epidemiology, and End Results (SEER). Cancer Stat Facts: Oral Cavity and Pharynx Cancer; Surveillance, Epidemiology, and End Results (SEER) Program; National Cancer Institute: Bethesda, MD, USA, 2020.
- International Agency for Research on Cancer; WHO; The Global Cancer Observatory. Lip and Oral Cavity Cancer Fact Sheet; The Global Cancer Observatory: Lyon, France, 2020. [Google Scholar]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Ferris, R.L.; Le, Q.T. Overview of Advances in Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3225–3226. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoir, M.; Schmitz, S.; Suarez, C.; Strojan, P.; Hutcheson, K.A.; Rodrigo, J.P.; Mendenhall, W.M.; Simo, R.; Saba, N.F.; D’Cruz, A.K.; et al. The Current Role of Salvage Surgery in Recurrent Head and Neck Squamous Cell Carcinoma. Cancers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 1990, 10, 28–36. [Google Scholar] [CrossRef]
- Brown, C.J.; Hendrich, B.D.; Rupert, J.L.; Lafreniere, R.G.; Xing, Y.; Lawrence, J.; Willard, H.F. The human XIST gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 1992, 71, 527–542. [Google Scholar] [CrossRef]
- ENCODE. Encyclopedia of DNA Elements (ENCODE) Project; National Human Genome Research Institute NIH: Bethesda, MD, USA, 2016.
- FANTOM5. The FANTOM (Functional ANnoTation of the Mammalian Genome) Project; RIKEN: Wakō, Japan, 2017. [Google Scholar]
- Ghosh, R.D.; Pattatheyil, A.; Roychoudhury, S. Functional Landscape of Dysregulated MicroRNAs in Oral Squamous Cell Carcinoma: Clinical Implications. Front. Oncol. 2020, 10, 619. [Google Scholar] [CrossRef]
- Ghosh, R.D.; Ghuwalewala, S.; Das, P.; Mandloi, S.; Alam, S.K.; Chakraborty, J.; Sarkar, S.; Chakrabarti, S.; Panda, C.K.; Roychoudhury, S. MicroRNA profiling of cisplatin-resistant oral squamous cell carcinoma cell lines enriched with cancer-stem-cell-like and epithelial-mesenchymal transition-type features. Sci. Rep. 2016, 6, 23932. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.D.; Bararia, A.; Majumder, S.G.; Manickam, A.; Bhutia, T.Y.; Jain, P.; Manikantan, K.; Sharan, R.; Arun, P. Cell-free mature miR-100-5p expression in plasma and its correlation with the histopathological markers in oral squamous cell carcinoma. In Biotechnology and Biological Sciences; Taylor & Francis: Abingdon, UK; CRC Press: London, UK, 2019. [Google Scholar]
- Cao, J. The functional role of long non-coding RNAs and epigenetics. Biol. Proced. Online 2014, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Dhanoa, J.K.; Sethi, R.S.; Verma, R.; Arora, J.S.; Mukhopadhyay, C.S. Long non-coding RNA: Its evolutionary relics and biological implications in mammals: A review. J. Anim. Sci. Technol. 2018, 60, 25. [Google Scholar] [CrossRef]
- Leng, F.; Miu, Y.Y.; Zhang, Y.; Luo, H.; Lu, X.L.; Cheng, H.; Zheng, Z.G. A micro-peptide encoded by HOXB-AS3 promotes the proliferation and viability of oral squamous cell carcinoma cell lines by directly binding with IGF2BP2 to stabilize c-Myc. Oncol. Lett. 2021, 22, 697. [Google Scholar] [CrossRef] [PubMed]
- Volders, P.J.; Anckaert, J.; Verheggen, K.; Nuytens, J.; Martens, L.; Mestdagh, P.; Vandesompele, J. LNCipedia 5: Towards a reference set of human long non-coding RNAs. Nucleic Acids Res. 2019, 47, D135–D139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hon, C.C.; Ramilowski, J.A.; Harshbarger, J.; Bertin, N.; Rackham, O.J.; Gough, J.; Denisenko, E.; Schmeier, S.; Poulsen, T.M.; Severin, J.; et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature 2017, 543, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Gutschner, T.; Diederichs, S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Gao, G.; Cao, Y. Long Noncoding RNAs as Novel Biomarkers Have a Promising Future in Cancer Diagnostics. Dis. Markers 2016, 2016, 9085195. [Google Scholar] [CrossRef] [Green Version]
- Freedman, M.L.; Monteiro, A.N.; Gayther, S.A.; Coetzee, G.A.; Risch, A.; Plass, C.; Casey, G.; De Biasi, M.; Carlson, C.; Duggan, D.; et al. Principles for the post-GWAS functional characterization of cancer risk loci. Nat. Genet. 2011, 43, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Sanchez Calle, A.; Kawamura, Y.; Yamamoto, Y.; Takeshita, F.; Ochiya, T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018, 109, 2093–2100. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Liang, L.; Ouyang, K.; Li, Z.; Yi, X. MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/beta-catenin signaling pathway. J. Oral Pathol. Med. 2017, 46, 98–105. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H. Expression of long noncoding RNA-HOX transcript antisense intergenic RNA in oral squamous cell carcinoma and effect on cell growth. Tumour Biol. 2015, 36, 8573–8578. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Zhang, L.; Wang, Y.; Li, H.; Ren, X.; Wei, F.; Yu, W.; Liu, T.; Wang, X.; et al. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int. J. Oncol. 2015, 46, 2586–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Wang, W.; Zhao, J.; Xu, H.; Li, S.; Yang, X. lncRNA MALAT1 promotes cell proliferation and invasion by regulating the miR-101/EZH2 axis in oral squamous cell carcinoma. Oncol. Lett. 2020, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Wang, X.; Yang, M.; Lu, L.; Zhou, Q. Long non-coding RNA TUG1 promotes progression of oral squamous cell carcinoma through upregulating FMNL2 by sponging miR-219. Am. J. Cancer Res. 2017, 7, 1899–1912. [Google Scholar] [PubMed]
- Zhang, H.; Zhao, L.; Wang, Y.X.; Xi, M.; Liu, S.L.; Luo, L.L. Long non-coding RNA HOTTIP is correlated with progression and prognosis in tongue squamous cell carcinoma. Tumour Biol. 2015, 36, 8805–8809. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Cai, G.; Kong, L.; Zhang, T.; Ren, Y.; Wu, Y.; Mei, M.; Zhang, L.; Wang, X. Long Non Coding RNA MALAT1 Promotes Tumor Growth and Metastasis by inducing Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. Sci. Rep. 2015, 5, 15972. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, M.; Pilpel, Y. Genome-wide natural antisense transcription: Coupling its regulation to its different regulatory mechanisms. EMBO Rep. 2006, 7, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, I.; Munita, R.; Agirre, E.; Dittmer, T.A.; Gysling, K.; Misteli, T.; Luco, R.F. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat. Struct. Mol. Biol. 2015, 22, 370–376. [Google Scholar] [CrossRef]
- Xie, J.J.; Jiang, Y.Y.; Jiang, Y.; Li, C.Q.; Lim, M.C.; An, O.; Mayakonda, A.; Ding, L.W.; Long, L.; Sun, C.; et al. Super-Enhancer-Driven Long Non-Coding RNA LINC01503, Regulated by TP63, Is Over-Expressed and Oncogenic in Squamous Cell Carcinoma. Gastroenterology 2018, 154, 2137–2151.e2131. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Jiang, Y.Y.; Xie, J.J.; Mayakonda, A.; Hazawa, M.; Chen, L.; Xiao, J.F.; Li, C.Q.; Huang, M.L.; Ding, L.W.; et al. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat. Commun. 2018, 9, 3619. [Google Scholar] [CrossRef]
- Dempsey, J.L.; Cui, J.Y. Long Non-Coding RNAs: A Novel Paradigm for Toxicology. Toxicol. Sci. 2017, 155, 3–21. [Google Scholar] [CrossRef]
- Romero-Barrios, N.; Legascue, M.F.; Benhamed, M.; Ariel, F.; Crespi, M. Splicing regulation by long noncoding RNAs. Nucleic Acids Res. 2018, 46, 2169–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariner, P.D.; Walters, R.D.; Espinoza, C.A.; Drullinger, L.F.; Wagner, S.D.; Kugel, J.F.; Goodrich, J.A. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol. Cell 2008, 29, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Jayaraj, G.G.; Scaria, V. Integrative transcriptome analysis suggest processing of a subset of long non-coding RNAs to small RNAs. Biol. Direct 2012, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman, M.; Russell, P.; Ingolia, N.T.; Weissman, J.S.; Lander, E.S. Ribosome profiling provides evidence that large noncoding RNAs do not encode proteins. Cell 2013, 154, 240–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Jia, S.; Li, D.; Cai, J.; Tu, J.; Geng, B.; Guan, Y.; Cui, Q.; Yang, J. Silencing of long noncoding RNA AK139328 attenuates ischemia/reperfusion injury in mouse livers. PLoS ONE 2013, 8, e80817. [Google Scholar] [CrossRef] [PubMed]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Bhat, S.A.; Ahmad, S.M.; Mumtaz, P.T.; Malik, A.A.; Dar, M.A.; Urwat, U.; Shah, R.A.; Ganai, N.A. Long non-coding RNAs: Mechanism of action and functional utility. Noncoding RNA Res. 2016, 1, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Kapranov, P.; St Laurent, G.; Raz, T.; Ozsolak, F.; Reynolds, C.P.; Sorensen, P.H.; Reaman, G.; Milos, P.; Arceci, R.J.; Thompson, J.F.; et al. The majority of total nuclear-encoded non-ribosomal RNA in a human cell is ‘dark matter’ un-annotated RNA. BMC Biol. 2010, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Aguilo, F.; Zhou, M.M.; Walsh, M.J. Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Res. 2011, 71, 5365–5369. [Google Scholar] [CrossRef] [Green Version]
- Kotake, Y.; Nakagawa, T.; Kitagawa, K.; Suzuki, S.; Liu, N.; Kitagawa, M.; Xiong, Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 2011, 30, 1956–1962. [Google Scholar] [CrossRef]
- Yang, C.; Chapman, A.G.; Kelsey, A.D.; Minks, J.; Cotton, A.M.; Brown, C.J. X-chromosome inactivation: Molecular mechanisms from the human perspective. Hum. Genet. 2011, 130, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, S.; Murakami, K.; Meguro, M.; Soejima, H.; Higashimoto, K.; Urano, T.; Kugoh, H.; Mukai, T.; Ikeguchi, M.; Oshimura, M. Expression profile of LIT1/KCNQ1OT1 and epigenetic status at the KvDMR1 in colorectal cancers. Cancer Sci. 2006, 97, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.R.; Mondal, T.; Mohammad, F.; Enroth, S.; Redrup, L.; Komorowski, J.; Nagano, T.; Mancini-Dinardo, D.; Kanduri, C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 2008, 32, 232–246. [Google Scholar] [CrossRef]
- Czermin, B.; Melfi, R.; McCabe, D.; Seitz, V.; Imhof, A.; Pirrotta, V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 2002, 111, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Sun, B.K.; Erwin, J.A.; Song, J.J.; Lee, J.T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 2008, 322, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Hemberg, M.; Gray, J.M. Enhancer RNAs: A class of long noncoding RNAs synthesized at enhancers. Cold Spring Harb. Perspect. Biol. 2015, 7, a018622. [Google Scholar] [CrossRef] [Green Version]
- Clemson, C.M.; McNeil, J.A.; Willard, H.F.; Lawrence, J.B. XIST RNA paints the inactive X chromosome at interphase: Evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 1996, 132, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Sado, T.; Hoki, Y.; Sasaki, H. Tsix silences Xist through modification of chromatin structure. Dev. Cell 2005, 9, 159–165. [Google Scholar] [CrossRef]
- Wan, L.B.; Bartolomei, M.S. Regulation of imprinting in clusters: Noncoding RNAs versus insulators. Adv. Genet. 2008, 61, 207–223. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Meng, X.M.; Huang, C.; Wu, B.M.; Zhang, L.; Lv, X.W.; Li, J. Long noncoding RNAs: Novel insights into hepatocelluar carcinoma. Cancer Lett. 2014, 344, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Mondal, T.; Guseva, N.; Pandey, G.K.; Kanduri, C. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development 2010, 137, 2493–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattick, J.S.; Gagen, M.J. The evolution of controlled multitasked gene networks: The role of introns and other noncoding RNAs in the development of complex organisms. Mol. Biol. Evol. 2001, 18, 1611–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Wu, L.; Yang, Q.; Ye, M.; Zhu, X. Functional linc-POU3F3 is overexpressed and contributes to tumorigenesis in glioma. Gene 2015, 554, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Kogo, R.; Shibata, K.; Sawada, G.; Takahashi, Y.; Kurashige, J.; Akiyoshi, S.; Sasaki, S.; Iwaya, T.; Sudo, T.; et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol. Rep. 2013, 29, 946–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zheng, J.; Deng, J.; You, Y.; Wu, H.; Li, N.; Lu, J.; Zhou, Y. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology 2014, 146, 1714–1726.e1715. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Dinger, M.E.; Mercer, T.R.; Mehler, M.F. RNA regulation of epigenetic processes. Bioessays 2009, 31, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kanduri, C. Long noncoding RNAs: Lessons from genomic imprinting. Biochim. Biophys. Acta 2016, 1859, 102–111. [Google Scholar] [CrossRef]
- Thorvaldsen, J.L.; Duran, K.L.; Bartolomei, M.S. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 1998, 12, 3693–3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunkow, M.E.; Tilghman, S.M. Ectopic expression of the H19 gene in mice causes prenatal lethality. Genes Dev. 1991, 5, 1092–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBaun, M.R.; Tucker, M.A. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J. Pediatr. 1998, 132, 398–400. [Google Scholar] [CrossRef] [Green Version]
- Heery, R.; Finn, S.P.; Cuffe, S.; Gray, S.G. Long Non-Coding RNAs: Key Regulators of Epithelial-Mesenchymal Transition, Tumour Drug Resistance and Cancer Stem Cells. Cancers 2017, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Sparago, A.; Cerrato, F.; Vernucci, M.; Ferrero, G.B.; Silengo, M.C.; Riccio, A. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat. Genet. 2004, 36, 958–960. [Google Scholar] [CrossRef]
- Jelinic, P.; Shaw, P. Loss of imprinting and cancer. J. Pathol. 2007, 211, 261–268. [Google Scholar] [CrossRef]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef] [Green Version]
- Prensner, J.R.; Chinnaiyan, A.M. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011, 1, 391–407. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Meng, X.; Zhu, X.W.; Yang, D.C.; Chen, R.; Jiang, Y.; Xu, T. Long non-coding RNAs in Oral squamous cell carcinoma: Biologic function, mechanisms and clinical implications. Mol. Cancer 2019, 18, 102. [Google Scholar] [CrossRef]
- Tang, J.; Fang, X.; Chen, J.; Zhang, H.; Tang, Z. Long Non-Coding RNA (lncRNA) in Oral Squamous Cell Carcinoma: Biological Function and Clinical Application. Cancers 2021, 13, 5944. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.F.; Dias-Oliveira, J.D.; Araujo, T.G.; Marangoni, K.; Goulart, L.R. Prostate cancer antigen 3 (PCA3) RNA detection in blood and tissue samples for prostate cancer diagnosis. Clin. Chem. Lab. Med. 2013, 51, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Badowski, C.; He, B.; Garmire, L.X. Blood-derived lncRNAs as biomarkers for cancer diagnosis: The Good, the Bad and the Beauty. NPJ Precis. Oncol. 2022, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Diez-Fraile, A.; Ceulaer, J.; Derpoorter, C.; Spaas, C.; Backer, T.; Lamoral, P.; Abeloos, J.; Lammens, T. Circulating Non-Coding RNAs in Head and Neck Cancer: Roles in Diagnosis, Prognosis, and Therapy Monitoring. Cells 2020, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, Z.F.; Lou, Q.Y.; Rankine, A.N.; Zheng, W.X.; Zhang, Z.H.; Zhang, L.; Gu, H. Long non-coding RNAs in head and neck squamous cell carcinoma: Diagnostic biomarkers, targeted therapies, and prognostic roles. Eur. J. Pharm. 2021, 902, 174114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.N.; Huang, G.Z.; Wu, Q.Q.; Ye, H.Y.; Zeng, W.S.; Lv, X.Z. NF-kappaB-mediated lncRNA AC007271.3 promotes carcinogenesis of oral squamous cell carcinoma by regulating miR-125b-2-3p/Slug. Cell Death Dis. 2020, 11, 1055. [Google Scholar] [CrossRef]
- Shao, T.; Huang, J.; Zheng, Z.; Wu, Q.; Liu, T.; Lv, X. SCCA, TSGF, and the Long Non-Coding RNA AC007271.3 are Effective Biomarkers for Diagnosing Oral Squamous Cell Carcinoma. Cell. Physiol. Biochem. 2018, 47, 26–38. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Zhang, L.; Li, N.; Zhang, X.; He, J.; He, R.; Shao, M.; Wang, J.; Kang, L.; et al. LncRNAAC132217.4, a KLF8-regulated long non-coding RNA, facilitates oral squamous cell carcinoma metastasis by upregulating IGF2 expression. Cancer Lett. 2017, 407, 45–56. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, Y.; Li, Z.; Zhu, Y.; He, Z.; Zhang, C. Exosome-derived long non-coding RNA ADAMTS9-AS2 suppresses progression of oral submucous fibrosis via AKT signalling pathway. J. Cell. Mol. Med. 2021, 25, 2262–2273. [Google Scholar] [CrossRef]
- Li, M.; Yu, D.; Li, Z.; Zhao, C.; Su, C.; Ning, J. Long noncoding RNA AFAP1AS1 facilitates the growth and invasiveness of oral squamous cell carcinoma by regulating the miR145/HOXA1 axis. Oncol. Rep. 2021, 45, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ning, S.B.; Fu, S.; Mao, Y.; Xiao, M.; Guo, B. Effects of lncRNA ANRIL on proliferation and apoptosis of oral squamous cell carcinoma cells by regulating TGF-beta/Smad pathway. Eur. Rev. Med. Pharm. Sci. 2019, 23, 6194–6201. [Google Scholar] [CrossRef]
- Yao, C.; Kong, F.; Zhang, S.; Wang, G.; She, P.; Zhang, Q. Long non-coding RNA BANCR promotes proliferation and migration in oral squamous cell carcinoma via MAPK signaling pathway. J. Oral Pathol. Med. 2021, 50, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.; Han, P.; Li, J.; Gao, L.; Ji, X.; Dong, F.; Su, Q.; Zhang, Y.; Liu, X. Knockdown of lncRNA BLACAT1 enhances radiosensitivity of head and neck squamous cell carcinoma cells by regulating PSEN1. Br. J. Radiol. 2020, 93, 20190154. [Google Scholar] [CrossRef]
- Tan, D.S.W.; Chong, F.T.; Leong, H.S.; Toh, S.Y.; Lau, D.P.; Kwang, X.L.; Zhang, X.; Sundaram, G.M.; Tan, G.S.; Chang, M.M.; et al. Long noncoding RNA EGFR-AS1 mediates epidermal growth factor receptor addiction and modulates treatment response in squamous cell carcinoma. Nat. Med. 2017, 23, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Ma, L.; Gong, Z.; Xue, L.; Wang, Q. Long non-coding RNA CASC15 promotes tongue squamous carcinoma progression through targeting miR-33a-5p. Env. Sci. Pollut. Res. Int. 2018, 25, 22205–22212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, J.; Zhou, L. Long non-coding RNA CASC2 enhances cisplatin sensitivity in oral squamous cell cancer cells by the miR-31-5p/KANK1 axis. Neoplasma 2020, 67, 1279–1292. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, W. Downregulation of lncRNA CASC2 promotes the postoperative local recurrence of early oral squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2019, 276, 605–610. [Google Scholar] [CrossRef]
- Arunkumar, G.; Murugan, A.K.; Prasanna Srinivasa Rao, H.; Subbiah, S.; Rajaraman, R.; Munirajan, A.K. Long non-coding RNA CCAT1 is overexpressed in oral squamous cell carcinomas and predicts poor prognosis. Biomed. Rep. 2017, 6, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Hu, X.; Shang, C.; Zhong, M.; Guo, Y. Silencing of long non-coding RNA CCAT2 depressed malignancy of oral squamous cell carcinoma via Wnt/beta-catenin pathway. Tumour Biol. 2017, 39, 1010428317717670. [Google Scholar] [CrossRef]
- Ren, Y.; He, W.; Chen, W.; Ma, C.; Li, Y.; Zhao, Z.; Gao, T.; Ni, Q.; Chai, J.; Sun, M. CRNDE promotes cell tongue squamous cell carcinoma cell growth and invasion through suppressing miR-384. J. Cell. Biochem. 2019, 120, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, X.H.; Shi, Y.L.; Ma, Y.; Bao, W.W.; Yang, L.; Li, J.C.; Zhang, F. LncRNA DANCR regulates the growth and metastasis of oral squamous cell carcinoma cells via altering miR-216a-5p expression. Hum. Cell 2020, 33, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Maruyama, R.; Niinuma, T.; Kai, M.; Kitajima, H.; Toyota, M.; Hatanaka, Y.; Igarashi, T.; Kobayashi, J.I.; Ogi, K.; et al. Screening for long noncoding RNAs associated with oral squamous cell carcinoma reveals the potentially oncogenic actions of DLEU1. Cell Death Dis. 2018, 9, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Tang, Z.; Zhang, H.; Quan, H. Long non-coding RNA DNM3OS/miR-204-5p/HIP1 axis modulates oral cancer cell viability and migration. J. Oral Pathol. Med. 2020, 49, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Nakanishi, H.; Steele, R.; Zhang, D.; Varvares, M.A.; Ray, R.B. Long non-coding RNA ELDR enhances oral cancer growth by promoting ILF3-cyclin E1 signaling. EMBO Rep. 2020, 21, e51042. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Sun, Z. Screening and validation of plasma long non-coding RNAs as biomarkers for the early diagnosis and staging of oral squamous cell carcinoma. Oncol. Lett. 2021, 21, 172. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Liu, Y.H.; Ban, J.D.; Wang, W.J.; Han, M.; Kong, P.; Li, B.H. Pathway analysis of a genomewide association study on a long noncoding RNA expression profile in oral squamous cell carcinoma. Oncol. Rep. 2019, 41, 895–907. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zeng, L.; Wang, S.; Wang, R.; Yang, R.; Jin, Z.; Tao, H. LncRNA FER1L4 Promotes Oral Squamous Cell Carcinoma Progression via Targeting miR-133a-5p/Prx1 Axis. Oncotargets Ther. 2021, 14, 795–806. [Google Scholar] [CrossRef]
- Ge, C.; Dong, J.; Chu, Y.; Cao, S.; Zhang, J.; Wei, J. LncRNA FGD5-AS1 promotes tumor growth by regulating MCL1 via sponging miR-153-3p in oral cancer. Aging 2020, 12, 14355–14364. [Google Scholar] [CrossRef]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Hu, Q.; Wang, H.; Song, Y.; Ni, Y.; Hou, Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis 2018, 39, 397–406. [Google Scholar] [CrossRef]
- Kong, X.P.; Yao, J.; Luo, W.; Feng, F.K.; Ma, J.T.; Ren, Y.P.; Wang, D.L.; Bu, R.F. The expression and functional role of a FOXC1 related mRNA-lncRNA pair in oral squamous cell carcinoma. Mol. Cell. Biochem. 2014, 394, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Chen, Z.; Wu, G. FOXD2-AS1 Predicts Dismal Prognosis for Oral Squamous Cell Carcinoma and Regulates Cell Proliferation. Cell Transpl. 2020, 29, 963689720964411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Huang, Z.; Meng, Y.; Jin, T.; Liang, Y.; Zhang, B. Upregulation of long non-coding RNA FOXD2-AS1 promotes progression and predicts poor prognosis in tongue squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z. Long non-coding RNA FTH1P3 facilitates oral squamous cell carcinoma progression by acting as a molecular sponge of miR-224-5p to modulate fizzled 5 expression. Gene 2017, 607, 47–55. [Google Scholar] [CrossRef]
- Zeng, B.; Li, Y.; Jiang, F.; Wei, C.; Chen, G.; Zhang, W.; Zhao, W.; Yu, D. LncRNA GAS5 suppresses proliferation, migration, invasion, and epithelial-mesenchymal transition in oral squamous cell carcinoma by regulating the miR-21/PTEN axis. Exp. Cell Res. 2019, 374, 365–373. [Google Scholar] [CrossRef]
- Cabezas-Camarero, S.; Perez-Segura, P. Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers. Cancers 2022, 14, 2858. [Google Scholar] [CrossRef]
- Fayda, M.; Isin, M.; Tambas, M.; Guveli, M.; Meral, R.; Altun, M.; Sahin, D.; Ozkan, G.; Sanli, Y.; Isin, H.; et al. Do circulating long non-coding RNAs (lncRNAs) (LincRNA-p21, GAS 5, HOTAIR) predict the treatment response in patients with head and neck cancer treated with chemoradiotherapy? Tumour Biol. 2016, 37, 3969–3978. [Google Scholar] [CrossRef]
- Kou, N.; Liu, S.; Li, X.; Li, W.; Zhong, W.; Gui, L.; Chai, S.; Ren, X.; Na, R.; Zeng, T.; et al. H19 Facilitates Tongue Squamous Cell Carcinoma Migration and Invasion via Sponging miR-let-7. Oncol. Res. 2019, 27, 173–182. [Google Scholar] [CrossRef]
- Lee, E.Y.; Song, J.M.; Kim, H.J.; Park, H.R. Hypomethylation of lncRNA H19 as a potential prognostic biomarker for oral squamous cell carcinoma. Arch. Oral Biol. 2021, 129, 105214. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Chen, J.; Wang, Z.; Liang, X.; Wang, X.; Jiang, J.; Lang, J.; Li, L. Long noncoding RNA HAS2-AS1 mediates hypoxia-induced invasiveness of oral squamous cell carcinoma. Mol. Carcinog. 2017, 56, 2210–2222. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Fan, S.; Lin, H.; Lian, W. STAT3-induced upregulation of long noncoding RNA HNF1A-AS1 promotes the progression of oral squamous cell carcinoma via activating Notch signaling pathway. Cancer Biol. Ther. 2019, 20, 444–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Wu, Z.; Zhang, J.; Su, B. Salivary lncRNA as a potential marker for oral squamous cell carcinoma diagnosis. Mol. Med. Rep. 2013, 7, 761–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Cong, B.; Chen, Q.; Liu, L.; Luan, X.; Du, J.; Cao, M. Silencing lncRNA HOXA10-AS decreases cell proliferation of oral cancer and HOXA10-antisense RNA can serve as a novel prognostic predictor. J. Int. Med. Res. 2020, 48, 300060520934254. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, B.; Liu, F.; Fang, Q. LncRNA HOXA11-AS promotes OSCC progression by sponging miR-98-5p to upregulate YBX2 expression. Biomed. Pharm. 2020, 121, 109623. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Q.; Zhang, S.; Liu, L.; Zhang, H.; Zhu, D. HOXC13-AS accelerates cell proliferation and migration in oral squamous cell carcinoma via miR-378g/HOXC13 axis. Oral Oncol. 2020, 111, 104946. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, S.; Lu, N.; Yin, Y.; Liu, Z. LncRNA JPX overexpressed in oral squamous cell carcinoma drives malignancy via miR-944/CDH2 axis. Oral Dis. 2021, 27, 924–933. [Google Scholar] [CrossRef]
- Zhang, C.; Bao, C.; Zhang, X.; Lin, X.; Pan, D.; Chen, Y. Knockdown of lncRNA LEF1-AS1 inhibited the progression of oral squamous cell carcinoma (OSCC) via Hippo signaling pathway. Cancer Biol. Ther. 2019, 20, 1213–1222. [Google Scholar] [CrossRef]
- Li, M.; Ning, J.; Li, Z.; Wang, J.; Zhao, C.; Wang, L. LINC00152 promotes the growth and invasion of oral squamous cell carcinoma by regulating miR-139-5p. Oncotargets Ther. 2018, 11, 6295–6304. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Rui, B.; Cao, Y.; Gong, X.; Li, H. Long non-coding RNA LINC00152 acts as a sponge of miRNA-193b-3p to promote tongue squamous cell carcinoma progression. Oncol. Lett. 2020, 19, 2035–2042. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Wu, F.; Peng, C.; Wang, M. Silencing of LINC00284 inhibits cell proliferation and migration in oral squamous cell carcinoma by the miR-211-3p/MAFG axis and FUS/KAZN axis. Cancer Biol. Ther. 2021, 22, 149–163. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, W.; Wu, K.; Qin, X.; Wang, X.; Li, Y.; Yu, B.; Zhang, Z.; Wang, X.; Yan, M.; et al. LncRNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by facilitating peroxiredoxin-1 into the nucleus. J. Exp. Clin. Cancer Res. 2019, 38, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhang, X.; Ding, X.; Zheng, Y.; Du, H.; Li, H.; Ji, H.; Wang, Z.; Jiao, P.; Song, X.; et al. Long Noncoding RNA LINC00460 Promotes Cell Progression by Sponging miR-4443 in Head and Neck Squamous Cell Carcinoma. Cell Transpl. 2020, 29, 963689720927405. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, Y.; Yuan, C.; Zhang, S.; Peng, W. Long non-coding RNA LINC00662 promotes proliferation and migration in oral squamous cell carcinoma. Oncotargets Ther. 2019, 12, 647–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Z. Long intergenic non-coding RNA 668 regulates VEGFA signaling through inhibition of miR-297 in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2017, 489, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Lin, C.W.; Ju, P.C.; Chang, L.C.; Chuang, C.Y.; Liu, Y.F.; Hsieh, M.J.; Yang, S.F. Association of LINC00673 Genetic Variants with Progression of Oral Cancer. J. Pers. Med. 2021, 11, 468. [Google Scholar] [CrossRef]
- Zhu, K.; Gong, Z.; Li, P.; Jiang, X.; Zeng, Z.; Xiong, W.; Yu, J. A review of linc00673 as a novel lncRNA for tumor regulation. Int. J. Med. Sci. 2021, 18, 398–405. [Google Scholar] [CrossRef]
- Ai, Y.; Wu, S.; Zou, C.; Wei, H. LINC00941 promotes oral squamous cell carcinoma progression via activating CAPRIN2 and canonical WNT/beta-catenin signaling pathway. J. Cell. Mol. Med. 2020, 24, 10512–10524. [Google Scholar] [CrossRef]
- Jia, B.; Dao, J.; Han, J.; Huang, Z.; Sun, X.; Zheng, X.; Xiang, S.; Zhou, H.; Liu, S. LINC00958 promotes the proliferation of TSCC via miR-211-5p/CENPK axis and activating the JAK/STAT3 signaling pathway. Cancer Cell Int. 2021, 21, 147. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Lu, S.; Zhou, C.; Xu, G.; Yan, Z.; Yang, J.; Yu, T.; Chen, W.; Qian, Y.; et al. Circulating Long Noncoding RNAs as Biomarkers for Predicting Head and Neck Squamous Cell Carcinoma. Cell. Physiol. Biochem. 2018, 50, 1429–1440. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, Q.; Qiao, B.; Zhang, L. Silencing of LINC01116 suppresses the development of oral squamous cell carcinoma by up-regulating microRNA-136 to inhibit FN1. Cancer Manag. Res. 2019, 11, 6043–6059. [Google Scholar] [CrossRef]
- Kong, J.; Sun, W.; Zhu, W.; Liu, C.; Zhang, H.; Wang, H. Long noncoding RNA LINC01133 inhibits oral squamous cell carcinoma metastasis through a feedback regulation loop with GDF15. J. Surg. Oncol. 2018, 118, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Che, Y.; Zhang, Z.; Lu, Q. Long Non-Coding RNA LINC01929 Accelerates Progression of Oral Squamous Cell Carcinoma by Targeting the miR-137-3p/FOXC1 Axis. Front. Oncol. 2021, 11, 657876. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, J.; Sun, M.; Qiu, F.; Chen, W.; Qiu, W. Tumor Suppressor LINC02487 Inhibits Oral Squamous Cell Carcinoma Cell Migration and Invasion Through the USP17-SNAI1 Axis. Front. Oncol. 2020, 10, 559808. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, G.; Deva Magendhra Rao, A.K.; Manikandan, M.; Arun, K.; Vinothkumar, V.; Revathidevi, S.; Rajkumar, K.S.; Rajaraman, R.; Munirajan, A.K. Expression profiling of long non-coding RNA identifies linc-RoR as a prognostic biomarker in oral cancer. Tumour Biol. 2017, 39, 1010428317698366. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, X.; Wang, Z.; Hu, Q.; Wu, J.; Li, Y.; Ren, X.; Wu, T.; Tao, X.; Chen, X.; et al. LncRNA-p23154 promotes the invasion-metastasis potential of oral squamous cell carcinoma by regulating Glut1-mediated glycolysis. Cancer Lett. 2018, 434, 172–183. [Google Scholar] [CrossRef]
- Zhang, D.; Song, Y.; Li, D.; Liu, X.; Pan, Y.; Ding, L.; Shi, G.; Wang, Y.; Ni, Y.; Hou, Y. Cancer-associated fibroblasts promote tumor progression by lncRNA-mediated RUNX2/GDF10 signaling in oral squamous cell carcinoma. Mol. Oncol. 2022, 16, 780–794. [Google Scholar] [CrossRef]
- Fan, C.; Wang, J.; Tang, Y.; Zhang, S.; Xiong, F.; Guo, C.; Zhou, Y.; Li, Z.; Li, X.; Li, Y.; et al. Upregulation of long non-coding RNA LOC284454 may serve as a new serum diagnostic biomarker for head and neck cancers. BMC Cancer 2020, 20, 917. [Google Scholar] [CrossRef]
- Liu, W.; Yao, Y.; Shi, L.; Tang, G.; Wu, L. A novel lncRNA LOLA1 may predict malignant progression and promote migration, invasion, and EMT of oral leukoplakia via the AKT/GSK-3beta pathway. J. Cell. Biochem. 2021, 122, 1302–1312. [Google Scholar] [CrossRef]
- Chen, P.Y.; Hsieh, P.L.; Peng, C.Y.; Liao, Y.W.; Yu, C.H.; Yu, C.C. LncRNA MEG3 inhibits self-renewal and invasion abilities of oral cancer stem cells by sponging miR-421. J. Formos. Med. Assoc. 2021, 120, 1137–1142. [Google Scholar] [CrossRef]
- Shih, J.W.; Chiang, W.F.; Wu, A.T.H.; Wu, M.H.; Wang, L.Y.; Yu, Y.L.; Hung, Y.W.; Wang, W.C.; Chu, C.Y.; Hung, C.L.; et al. Long noncoding RNA LncHIFCAR/MIR31HG is a HIF-1alpha co-activator driving oral cancer progression. Nat. Commun. 2017, 8, 15874. [Google Scholar] [CrossRef]
- Le, F.; Ou, Y.; Luo, P.; Zhong, X. LncRNA NCK1-AS1 in plasma distinguishes oral ulcer from early-stage oral squamous cell carcinoma. J. Biol. Res. 2020, 27, 16. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z.; Zheng, H.; Chan, M.T.; Wu, W.K. NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017, 50, e12349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibb, E.A.; Enfield, K.S.; Stewart, G.L.; Lonergan, K.M.; Chari, R.; Ng, R.T.; Zhang, L.; MacAulay, C.E.; Rosin, M.P.; Lam, W.L. Long non-coding RNAs are expressed in oral mucosa and altered in oral premalignant lesions. Oral Oncol. 2011, 47, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhong, T.; Dai, Q. Long Non-Coding RNA NKILA Reduces Oral Squamous Cell Carcinoma Development Through the NF-KappaB Signaling Pathway. Technol. Cancer Res. Treat. 2020, 19, 1533033820960747. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cui, X.; Chen, J.; Feng, Y.; Song, E.; Li, J.; Liu, Y. Long non-coding RNA NKILA inhibits migration and invasion of tongue squamous cell carcinoma cells via suppressing epithelial-mesenchymal transition. Oncotarget 2016, 7, 62520–62532. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Jiang, Y.; Chen, F.; Wei, Z.; Qiu, Y.; Xu, H.; Tian, G.; Gong, W.; Yuan, Y.; Feng, H.; et al. ORAOV1-B Promotes OSCC Metastasis via the NF-kappaB-TNFalpha Loop. J. Dent. Res. 2021, 100, 858–867. [Google Scholar] [CrossRef]
- Sailer, V.; Holmes, E.E.; Gevensleben, H.; Goltz, D.; Droge, F.; de Vos, L.; Franzen, A.; Schrock, F.; Bootz, F.; Kristiansen, G.; et al. PITX2 and PANCR DNA methylation predicts overall survival in patients with head and neck squamous cell carcinoma. Oncotarget 2016, 7, 75827–75838. [Google Scholar] [CrossRef]
- Huang, Z.; Sang, T.; Zheng, Y.; Wu, J. Long non-coding RNA PANDAR overexpression serves as a poor prognostic biomarker in oral squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 2728–2734. [Google Scholar]
- Zhang, P.; Liu, Y.; Li, C.; Zhang, L.; Liu, Q.; Jiang, T. LncRNA PAPAS promotes oral squamous cell carcinoma by upregulating transforming growth factor-beta1. J. Cell. Biochem. 2019, 120, 16120–16127. [Google Scholar] [CrossRef]
- Gao, L.; Ren, W.; Zhang, L.; Li, S.; Kong, X.; Zhang, H.; Dong, J.; Cai, G.; Jin, C.; Zheng, D.; et al. PTENp1, a natural sponge of miR-21, mediates PTEN expression to inhibit the proliferation of oral squamous cell carcinoma. Mol. Carcinog. 2017, 56, 1322–1334. [Google Scholar] [CrossRef]
- Li, X.; Ren, H. Long noncoding RNA PVT1 promotes tumor cell proliferation, invasion, migration and inhibits apoptosis in oral squamous cell carcinoma by regulating miR1505p/GLUT1. Oncol. Rep. 2020, 44, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Jiang, Y.; Zhou, W.; Zhang, B.; Li, Y.; Xie, F.; Zhang, J.; Wang, X.; Yan, M.; Xu, Q.; et al. Long Noncoding RNA RC3H2 Facilitates Cell Proliferation and Invasion by Targeting MicroRNA-101-3p/EZH2 Axis in OSCC. Mol. Ther. Nucleic Acids 2020, 20, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, X.; Lai, W.; Wang, J. Long non-coding RNA SLC16A1-AS1: Its multiple tumorigenesis features and regulatory role in cell cycle in oral squamous cell carcinoma. Cell Cycle 2020, 19, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tan, Y.; Yao, Y.; Lu, N.; Zhang, F. SNHG12/miR-326/E2F1 feedback loop facilitates the progression of oral squamous cell carcinoma. Oral Dis. 2020, 26, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, J.; Xu, P.; Jian, X.; Huang, X.; Liu, D. Silencing of LncRNA SNHG16 Downregulates Cyclin D1 (CCND1) to Abrogate Malignant Phenotypes in Oral Squamous Cell Carcinoma (OSCC) Through Upregulating miR-17-5p. Cancer Manag. Res. 2021, 13, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Qiao, T.; Yang, S.; Liu, L.; Zheng, M. SNHG17/miR-384/ELF1 axis promotes cell growth by transcriptional regulation of CTNNB1 to activate Wnt/beta-catenin pathway in oral squamous cell carcinoma. Cancer Gene Ther. 2022, 29, 122–132. [Google Scholar] [CrossRef]
- Tong, F.; Guo, J.; Miao, Z.; Li, Z. LncRNA SNHG17 promotes the progression of oral squamous cell carcinoma by modulating miR-375/PAX6 axis. Cancer Biomark. 2021, 30, 1–12. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, W.; Wang, Z.; Xiang, X.; Zhang, S.; Liu, L. Long non-coding RNA SNHG20 promotes the tumorigenesis of oral squamous cell carcinoma via targeting miR-197/LIN28 axis. J. Cell. Mol. Med. 2019, 23, 680–688. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, H. Small nucleolar RNA host gene 3 facilitates cell proliferation and migration in oral squamous cell carcinoma via targeting nuclear transcription factor Y subunit gamma. J. Cell. Biochem. 2020, 121, 2150–2158. [Google Scholar] [CrossRef]
- Yang, C.M.; Wang, T.H.; Chen, H.C.; Li, S.C.; Lee, M.C.; Liou, H.H.; Liu, P.F.; Tseng, Y.K.; Shiue, Y.L.; Ger, L.P.; et al. Aberrant DNA hypermethylation-silenced SOX21-AS1 gene expression and its clinical importance in oral cancer. Clin. Epigenet. 2016, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Jin, N.; Jin, N.; Bu, W.; Li, X.; Liu, L.; Wang, Z.; Tong, J.; Li, D. Long non-coding RNA TIRY promotes tumor metastasis by enhancing epithelial-to-mesenchymal transition in oral cancer. Exp. Biol. Med. 2020, 245, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.W.; Zhang, Y.; Li, S.; Shi, Z.Y.; Zhao, J.; He, Q.L. LncRNA TTN-AS1 promotes the progression of oral squamous cell carcinoma via miR-411-3p/NFAT5 axis. Cancer Cell Int. 2020, 20, 415. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, S.; Wang, P.; Yang, C.; Shang, C.; Yang, J.; Wang, J. LncRNA, TUG1 regulates the oral squamous cell carcinoma progression possibly via interacting with Wnt/beta-catenin signaling. Gene 2017, 608, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Li, R.; Zhao, L. Long noncoding RNA UCA1 regulates CCR7 expression to promote tongue squamous cell carcinoma progression by sponging miR-138-5p. Neoplasma 2020, 67, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, N.F.; Manoochehri, H.; Khoei, S.G.; Sheykhhasan, M. The Functional Role of Long Non-coding RNA UCA1 in Human Multiple Cancers: A Review Study. Curr. Mol. Med. 2021, 21, 96–110. [Google Scholar] [CrossRef]
- Zhang, L.M.; Su, L.X.; Hu, J.Z.; Wang, M.; Ju, H.Y.; Li, X.; Han, Y.F.; Xia, W.Y.; Guo, W.; Ren, G.X.; et al. Epigenetic regulation of VENTXP1 suppresses tumor proliferation via miR-205-5p/ANKRD2/NF-kB signaling in head and neck squamous cell carcinoma. Cell Death Dis. 2020, 11, 838. [Google Scholar] [CrossRef]
- Ma, S.Q.; Wang, Y.C.; Li, Y.; Li, X.Y.; Yang, J.; Sheng, Y.M. LncRNA XIST promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by downregulating miR-27b-3p. J. Biol. Regul. Homeost. Agents 2020, 34, 1993–2001. [Google Scholar] [CrossRef]
- Shieh, T.M.; Liu, C.J.; Hsia, S.M.; Ningrum, V.; Liao, C.C.; Lan, W.C.; Shih, Y.H. Lack of Salivary Long Non-Coding RNA XIST Expression Is Associated with Increased Risk of Oral Squamous Cell Carcinoma: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 4622. [Google Scholar] [CrossRef]
- Tao, D.; Zhang, Z.; Liu, X.; Zhang, Z.; Fu, Y.; Zhang, P.; Yuan, H.; Liu, L.; Cheng, J.; Jiang, H. LncRNA HOTAIR promotes the invasion and metastasis of oral squamous cell carcinoma through metastasis-associated gene 2. Mol. Carcinog. 2020, 59, 353–364. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, L.; Wang, L.; Yang, Y.; Meng, Y.; Yang, H. Increased expression of the long non-coding RNA UCA1 in tongue squamous cell carcinomas: A possible correlation with cancer metastasis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 89–95. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, J.; Xie, W.; Sun, Q.; Wang, H.; Qiao, B. LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression. Cancer Med. 2017, 6, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Liu, H.; You, G. Long non-coding RNA C5orf66-AS1 prevents oral squamous cell carcinoma through inhibiting cell growth and metastasis. Int. J. Mol. Med. 2018, 42, 3291–3299. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Houck, J.R.; Lohavanichbutr, P.; Chen, C. Transcriptome analysis reveals differentially expressed lncRNAs between oral squamous cell carcinoma and healthy oral mucosa. Oncotarget 2017, 8, 31521–31531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.F.; Wei, S.B.; Gan, Y.H.; Guo, Y.; Gong, K.; Mitchelson, K.; Cheng, J.; Yu, G.Y. Expression, regulation and roles of miR-26a and MEG3 in tongue squamous cell carcinoma. Int. J. Cancer 2014, 135, 2282–2293. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Taheri, M. The Role of Long Non-coding RNAs in Cancer Metabolism: A Concise Review. Front. Oncol. 2020, 10, 555825. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Adamoski, D.; Genelhould, G.; Zhen, F.; Yamaguto, G.E.; Araujo-Souza, P.S.; Nogueira, M.B.; Raboni, S.M.; Bonatto, A.C.; Gradia, D.F.; et al. NEAT1 and MALAT1 are highly expressed in saliva and nasopharyngeal swab samples of COVID-19 patients. Mol. Oral Microbiol. 2021, 36, 291–294. [Google Scholar] [CrossRef]
- Rossi, R.; Gissi, D.B.; Gabusi, A.; Fabbri, V.P.; Balbi, T.; Tarsitano, A.; Morandi, L. A 13-Gene DNA Methylation Analysis Using Oral Brushing Specimens as an Indicator of Oral Cancer Risk: A Descriptive Case Report. Diagnostics 2022, 12, 284. [Google Scholar] [CrossRef]
- Chiabotto, G.; Gai, C.; Deregibus, M.C.; Camussi, G. Salivary Extracellular Vesicle-Associated exRNA as Cancer Biomarker. Cancers 2019, 11, 891. [Google Scholar] [CrossRef] [Green Version]
- Gomes, C.C.; de Sousa, S.F.; Calin, G.A.; Gomez, R.S. The emerging role of long noncoding RNAs in oral cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 235–241. [Google Scholar] [CrossRef]
- Qi, P.; Zhou, X.Y.; Du, X. Circulating long non-coding RNAs in cancer: Current status and future perspectives. Mol. Cancer 2016, 15, 39. [Google Scholar] [CrossRef] [Green Version]
- Drak Alsibai, K.; Meseure, D. Tumor microenvironment and noncoding RNAs as co-drivers of epithelial-mesenchymal transition and cancer metastasis. Dev. Dyn. 2018, 247, 405–431. [Google Scholar] [CrossRef] [PubMed]
- Meseure, D.; Drak Alsibai, K.; Nicolas, A. Pivotal role of pervasive neoplastic and stromal cells reprogramming in circulating tumor cells dissemination and metastatic colonization. Cancer Microenviron. 2014, 7, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Klibanski, A. MEG3 noncoding RNA: A tumor suppressor. J. Mol. Endocrinol. 2012, 48, R45–R53. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 612393. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Cui, Q. Could circulating miRNAs contribute to cancer therapy? Trends Mol. Med. 2013, 19, 71–73. [Google Scholar] [CrossRef]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [Green Version]
- Schlosser, K.; Hanson, J.; Villeneuve, P.J.; Dimitroulakos, J.; McIntyre, L.; Pilote, L.; Stewart, D.J. Assessment of Circulating LncRNAs Under Physiologic and Pathologic Conditions in Humans Reveals Potential Limitations as Biomarkers. Sci. Rep. 2016, 6, 36596. [Google Scholar] [CrossRef]
| LncRNA ID | Expression Status | Sample Source | Target Gene | Clinical Significance | Reference |
|---|---|---|---|---|---|
| AC007271.3 | up | Tumour, Serum | NFκB, miR-125b-2-3p, Slug | cell proliferation, EMT, Metastasis; | [82,83] |
| AC132217.4 | up | Tumour | KLF8, IGF2 | metastasis, migration, invasion; | [84] |
| ADAMTS9-AS2 | down | Tumour | miR-600, Ezh2, AKT signaling | marker for early diagnosis and metastasis; | [85] |
| AFAP1-AS1 | up | Tumour | miR-145, HOXA1 | cell proliferation, progression; | [86] |
| ANRIL | up | Tumour | TGFβ, Smad, Bcl-2 | proliferation and progression, tumour progression; | [87] |
| BANCR | up | Tumour | MAPK signalling | cell proliferation, progression, migration; | [88] |
| BLACAT1 | up | Tumour | PSEN1 | cell proliferation, progression, radioresistance; | [89] |
| C5orf66-AS1 | down | Tumour | diagnostic marker, treatment response; | [90] | |
| CASC15 | up | Tumour | miR-124, miR-33a-5p | proliferation and invasion, progression, and metastasis; | [91] |
| CASC2 | down | Tumour, Plasma | miR-21, miR-31-5p, KANK1 | local recurrence, cisplatin responsiveness; | [92,93] |
| CCAT1 | up | Tumour | miR-181a, Wnt/β-catenin, DDR2, ERK, AKT, miR-55-5p, let7b-5p | poor therapeutic outcome; | [94] |
| CCAT2 | up | Tumour | Wnt/β-catenin, Ccnd1, Myc, GSK3β | poor prognosis, metastasis, migration, invasion; | [95] |
| CRNDE | up | Tumour | miR-384 | proliferation and invasion, progression and metastasis; | [96] |
| DANCR | up | Tumour | miR-216a-5p | histological grade, clinical staging, lymph node metastasis; | [97] |
| DLEU1 | up | Tumour | miR-149-5p, CDK6, HA, CD44 signalling | disease progression, diagnostic marker; | [98] |
| DNM3OS | up | Tumour | miR-204-5p, HIP1 | disease progression, migration; | [99] |
| ELDR | up | Tumour | ILF3, cyclinE1 signalling | disease progression, cell proliferation; | [100] |
| ENST00000412740 | up | Plasma | biomarker for early diagnosis and staging of OSCC; | [101] | |
| ENST00000527317 | down | Tumour | poor median PFS and OS; | [102] | |
| ENST00000583044 | down | Tumour | poor median PFS and OS; | [102] | |
| ENST00000588803 | up | Plasma | biomarker for early diagnosis and staging of OSCC; | [101] | |
| FER1L4 | up | Tumour | miR133a, Prx1 | disease progression; | [103] |
| FGD5-AS1 | up | Tumour | MCL1, miR-153-3p | proliferation and migration, invasion; | [104] |
| FLJ22447 | up | Tumour | IL-33 | disease progression; | [105] |
| FOXCUT | up | Tumour | FOXC1 | cell proliferation, migration, metastasis, poor survival; | [106] |
| FOXD2-AS1 | up | Tumour | E2F-G2-M checkpoint | migration, pathological grade, poor disease prognosis; | [107,108] |
| FTH1P3 | up | Tumour | miR-224-5p, Fizzled5 | progression, metastasis, high mortality rate, poor overall survival; | [109] |
| GAS5 | down | Tumour, Blood, Plasma | miR-21, PTEN, FoxO1, miR-1297, GSK3β | cell proliferation, migration, EMT, metastasis, treatment responsiveness; | [110,111,112] |
| H19 | up | Tumour | miR-let-7 | tumorigenesis, metastasis, poor prognosis, low disease-free-survival, prognostic biomarker; | [113,114] |
| HAS2-AS1 | up | Tumour | TGFα, HIF-1α, Nfκb | poor prognosis, invasion, EMT | [115] |
| HNF1A-AS1 | up | Tumour | STAT3, Notch signalling | poor prognosis, EMT, migration | [116] |
| HOTAIR | up | Tumour, Saliva, Plasma | Ezh2, E-cadherin | TNM staging, poor prognosis, marker for early detection; | [25,26,111,112,117] |
| HOTTIP | up | Tumour | miR-124-3p, HMGA2, Wnt/β-catenin | migration, invasion, distant metastasis, poor overall survival, poor disease-free-survival; | [29] |
| HOXA10 | up | Tumour | risk factor, tumour grade; | [118] | |
| HOXA11-AS | up | Tumour, Plasma | miR-98-5p, YBX2 | disease progression; | [119] |
| HOXB-AS3 | up | Tumour | cell proliferation and tumour progression; | [16] | |
| HOXC13-AS | up | Tumour | miR-378g, HOXC13 axis | cell proliferation, migration, EMT; | [120] |
| JPX | up | Tumour | miR-944, CDH2 axis | cell proliferation, migration, invasion; | [121] |
| LEF1-AS1 | up | Tumour | LATS1, Hippo signalling | migration, metastasis; | [122] |
| LINC00152 | up | Tumour | miR-139-5p | poor prognosis, metastasis, migration, invasion, EMT; | [123,124] |
| LINC00284 | up | Tumour | miR-211-3p, MAFG axis, FUS, KAZN axis | cell proliferation, migration; | [125] |
| LINC00460 | up | Tumour | Peroxiredoxin-1, miR-4443 | poor prognosis, metastasis, migration, invasion, EMT; | [126,127] |
| LINC00662 | up | Tumour | tumour size, LNM, and TNM staging; | [128] | |
| LINC00668 | up | Tumour | miR-297, VEGFA signaling | poor prognosis; | [129] |
| LINC00673 | up | Tumour | betel nut association, TNM staging, recurrence, migration, invasion, poor overall survival, poor disease-free-survival; | [130,131] | |
| LINC00941 | up | Tumour | CAPRIN2, Wnt/β-catenin | disease progression; | [132] |
| LINC00958 | up | Tumour | miR-211-5p, CENPK axis, JAK, STAT3 signaling | shorter overall survival; | [133] |
| LINC00964 | up | Plasma | marker for early detection; | [134] | |
| LINC01116 | up | Tumour | miR-136, FN1 | disease progression, invasion, and migration; | [135] |
| LINC01133 | up | Tumour | GDF15 | less metastasis, good prognosis; | [136] |
| LINC01929 | up | Tumour | miR-137-3p, FOXC1 axis | tumour progression; | [137] |
| LINC02487 | down | Tumour | USP17, SNAI1 axis | migration, invasion, cancer metastasis; | [138] |
| Linc-ROR | up | Tumour | Oct4, Nanog, Sox4, Klf4, cMyc | cellular migration, invasion, and metastasis; | [139] |
| Lnc-p23154 | up | Tumour | Glut1 | poor prognosis, metastasis, migration, invasion, EMT; | [140] |
| lnc-WRN-10:1 | down | Tumour | poor median PFS and OS; | [102] | |
| LOC100506114 | up | CAFs | RUNX2, GDF10 signaling | proliferation and migration; | [141] |
| LOC284454 | up | Serum | early diagnostic marker; | [142] | |
| LOLA1 | up | Tumour | AKT, GSK3β pathway | tumour progression, migration, invasion, EMT; | [143] |
| MALAT1 | up | Tumour, Plasma, Saliva | Cks1, Wnt/β-catenin, miR-101, Ezh2 axis | EMT, marker for early detection and poor prognosis, metastasis; | [24,27,30,117] |
| MEG3 | down | Tumour | miR-421, Dnmt3B | high mortality rate and poor overall survival, tumour recurrence, metastasis. tumour suppressor; | [144] |
| MIR31HG | up | Tumour | HIF-1α | cellular migration, invasion, and metastasis; | [145] |
| NCK1-AS1 | up | Plasma | miR-100 | marker for early detection and metastasis; | [146] |
| NEAT1 | up | Tumour, Saliva | therapeutic target, marker for early detection; | [147,148] | |
| NKILA | down | Tumour | NFκB signalling | tumour volume, weight, proliferation, invasion, migration, metastasis; | [149,150] |
| NR_038323 | up | Plasma | biomarker for early diagnosis and staging of OSCC; | [101] | |
| NR_104048 | down | Tumour | poor median PFS and OS; | [102] | |
| NR_131012 | up | Plasma | biomarker for early diagnosis and staging of OSCC; | [101] | |
| ORAOV1-B | up | Tumour | NFκB, TNF-α signalling | lymph node metastasis, invasion, migration, metastasis; | [151] |
| PANCR | up | Tumour | Hypermethylation and poor survival; | [152] | |
| PANDAR | up | Tumour | Metastasis, migration, invasion, poor prognosis; | [153] | |
| PAPAS | up | Plasma | TGFβ1 | Biomarker for diagnosis, poor overall survival; | [154] |
| PTENp1 | down | Tumour | miR-21, PTEN | histological differentiation and progression; | [155] |
| PVT1 | up | Tumour | miR-150-5p, GLUT1 | poor prognosis; | [156] |
| RC3H2 | up | Tumour | miR-101-3p, Ezh2 | metastasis, migration, invasion; | [157] |
| SLC16A1-AS1 | up | Tumour | CCND1 | histological grade, overall survival; | [158] |
| SNHG12 | up | Tumour | miR-326, E2F1 | proliferation and migration, invasion, EMT; | [159] |
| SNHG16 | up | Tumour | CCND1 | cell proliferation, viability, migration and EMT; | [160] |
| SNHG17 | up | Tumour | miR-384, ELF1, CTNNB1, Wnt/β-catenin, miR-375, PAX6 axis | disease progression; | [161,162] |
| SNHG20 | up | Tumour | miR-197, LIN28 axis | oncogenesis and tumourigenesis; | [163] |
| SNHG3 | up | Tumour | Wnt/β-catenin, NFYC | proliferation, migration; | [164] |
| SOX21-AS1 | down | Tumour | poor prognosis; | [165] | |
| TIRY | up | Tumour | miR-14, Wnt/β-catenin | proliferation, migration; | [166] |
| TTN-AS1 | up | Tumour | miR-411-3p, NFAT5 | disease progression; | [167] |
| TUG1 | up | Tumour | miR-219, FMNL2, Wnt/β-catenin, cyclin D1, cMyc | tumour promoting, lymph node metastasis; | [28,168] |
| UCA1 | up | Tumour | miR-138-5p, CCR7, Wnt/β-catenin | proliferation, migration, invasion, glycolysis metabolism; | [169,170] |
| VENTXP1 | down | Tumour | miR-205-5p, ANKRD2, NFκB | poor survival; | [171] |
| XIST | up | Tumour, Saliva | miR-27b-3p | cell proliferation, cisplatin resistance. | [172,173] |
| Name | Symbol | Simple Somatic Mutation Affected (%) | Number of Mutations | Mutation Details (Changes in DNA) |
|---|---|---|---|---|
| X inactive specific transcript | XIST | 7/37 (18.92%) | 7 | chrX:g.73841444C>T chrX:g.73844493T>C chrX:g.73845294A>G chrX:g.73844293G>C chrX:g.73843798G>T chrX:g.73842529A>G chrX:g.73844433G>C |
| AL109984.1 | AL109984.1 | 3/37 (8.11%) | 3 | chr20:g.52088576G>A chr20:g.52084589C>T chr20:g.52085000C>G |
| family with sequence similarity E5 | FAM27E5 | 3/37 (8.11%) | 3 | chr17:g.22299660G>C chr17:g.22298919G>A chr17:g.22298929C>A |
| chromosome 8 open reading frame 31 | C8orf31 | 3/37 (8.11%) | 3 | chr8:g.143043244G>A chr8:g.143043089T>C chr8:g.143043028C>G |
| RNF217 antisense RNA 1 (head to head) | RNF217-AS1 | 2/37 (5.41%) | 2 | chr6:g.124910865G>A chr6:g.124910984G>C |
| spermatogenesis associated 8 | SPATA8 | 2/37 (5.41%) | 2 | chr15:g.96783707G>A chr15:g.96784195C>T |
| long intergenic non-protein coding RNA 482 | LINC00482 | 2/37 (5.41%) | 2 | chr17:g.81304684C>A chr17:g.81305023G>A |
| AC092718.9 | AC092718.9 | 2/37 (5.41%) | 3 | chr16:g.81149700C>G chr16:g.81147473C>T chr16:g.81147475T>A |
| long intergenic non-protein coding RNA 1588 | LINC01588 | 2/37 (5.41%) | 2 | chr14:g.50005654C>T chr14:g.49992302T>C |
| SUGT1P4-STRA6LP readthrough | SUGT1P4-STRA6LP | 1/37 (2.70%) | 1 | chr9:g.97294104G>A |
| chromosome 14 putative open reading frame 177 | C14orf177 | 1/37 (2.70%) | 1 | chr14:g.98716342delAG |
| long intergenic non-protein coding RNA 1559 | LINC01559 | 1/37 (2.70%) | 1 | chr12:g.13376270C>T |
| chromosome 8 open reading frame 86 | C8orf86 | 1/37 (2.70%) | 1 | chr8:g.38528390C>T |
| MIR1-1HG antisense RNA 1 | MIR1-1HG-AS1 | 1/37 (2.70%) | 1 | chr20:g.62546276C>T |
| HLA complex group 27 | HCG27 | 1/37 (2.70%) | 1 | chr6:g.31202764C>T |
| long intergenic non-protein coding RNA 1600 | LINC01600 | 1/37 (2.70%) | 1 | chr6:g.2623640G>A |
| long intergenic non-protein coding RNA 1098 | LINC01098 | 1/37 (2.70%) | 1 | chr4:g.177975927delTT |
| long intergenic non-protein coding RNA 173 | LINC00173 | 1/37 (2.70%) | 1 | chr12:g.116534694C>T |
| mir-99a-let-7c cluster host gene | MIR99AHG | 1/37 (2.70%) | 1 | chr21:g.16391630G>A |
| chromosome 9 putative open reading frame 106 | C9orf106 | 1/37 (2.70%) | 1 | chr9:g.129322444delCCAGTTCT… |
| long intergenic non-protein coding RNA 2877 | LINC02877 | 1/37 (2.70%) | 1 | chr3:g.153484635C>G |
| chromosome 20 putative open reading frame 197 | C20orf197 | 1/37 (2.70%) | 1 | chr20:g.60070819G>A |
| long intergenic non-protein coding RNA 2870 | LINC02870 | 1/37 (2.70%) | 1 | chr10:g.132448053G>A |
| long intergenic non-protein coding RNA 1565 | LINC01565 | 1/37 (2.70%) | 1 | chr3:g.128573545delC |
| myelodysplastic syndrome 2 translocation associated | MDS2 | 1/37 (2.70%) | 1 | chr1:g.23627122C>A |
| chromosome 11 putative open reading frame 40 | C11orf40 | 1/37 (2.70%) | 1 | chr11:g.4573322G>A |
| family with sequence similarity 153 member C | FAM153CP | 1/37 (2.70%) | 1 | chr5:g.178055978G>A |
| BACH1-IT1 | BACH1-IT1 | 1/37 (2.70%) | 1 | chr21:g.29351669C>G |
| Biotype | Circulating lncRNAs | Suggested Clinical Implications | References |
|---|---|---|---|
| PLASMA | CASC2 | Prognosis, treatment response | [93] |
| ENST00000412740, | Diagnosis and prognosis | [101] | |
| ENST00000588803, | Diagnosis and prognosis | [101] | |
| GAS5 | Prognosis, treatment response | [112] | |
| HOXA11-AS | Diagnosis and prognosis | [134] | |
| LINC00964 | Diagnosis and prognosis | [134] | |
| MALAT1 | Diagnosis and prognosis | [134] | |
| NCK1-AS1 | Diagnosis, prognosis | [146] | |
| NR_131012 | Diagnosis and prognosis | [101] | |
| NR_038323 | Diagnosis and prognosis | [101] | |
| PAPAS | Prognosis | [154] | |
| SERUM | AC007271.3 | Prognosis | [82,83] |
| LOC284454 | Diagnosis | [142] | |
| SALIVA | HOTAIR | Prognosis | [117] |
| MALAT1 | Prognosis | [117] | |
| NEAT-1 | Prognosis | [148] | |
| XIST | Prognosis, treatment response | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey Ghosh, R.; Guha Majumder, S. Circulating Long Non-Coding RNAs Could Be the Potential Prognostic Biomarker for Liquid Biopsy for the Clinical Management of Oral Squamous Cell Carcinoma. Cancers 2022, 14, 5590. https://doi.org/10.3390/cancers14225590
Dey Ghosh R, Guha Majumder S. Circulating Long Non-Coding RNAs Could Be the Potential Prognostic Biomarker for Liquid Biopsy for the Clinical Management of Oral Squamous Cell Carcinoma. Cancers. 2022; 14(22):5590. https://doi.org/10.3390/cancers14225590
Chicago/Turabian StyleDey Ghosh, Ruma, and Sudhriti Guha Majumder. 2022. "Circulating Long Non-Coding RNAs Could Be the Potential Prognostic Biomarker for Liquid Biopsy for the Clinical Management of Oral Squamous Cell Carcinoma" Cancers 14, no. 22: 5590. https://doi.org/10.3390/cancers14225590







