The Expression of Cell Cycle-Related Genes in USP8-Mutated Corticotroph Neuroendocrine Pituitary Tumors and Their Possible Role in Cell Cycle-Targeting Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Characteristics
2.2. Quantitative Real-Time RT-PCR
2.3. Immunohistochemistry
2.4. Cell Line Culture, Transfection and Cell Cycle Inhibition
2.5. Cell Viability Assay
2.6. Cell Proliferation Assay
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. USP8 and USP48 Mutations in Corticotroph PitNETs
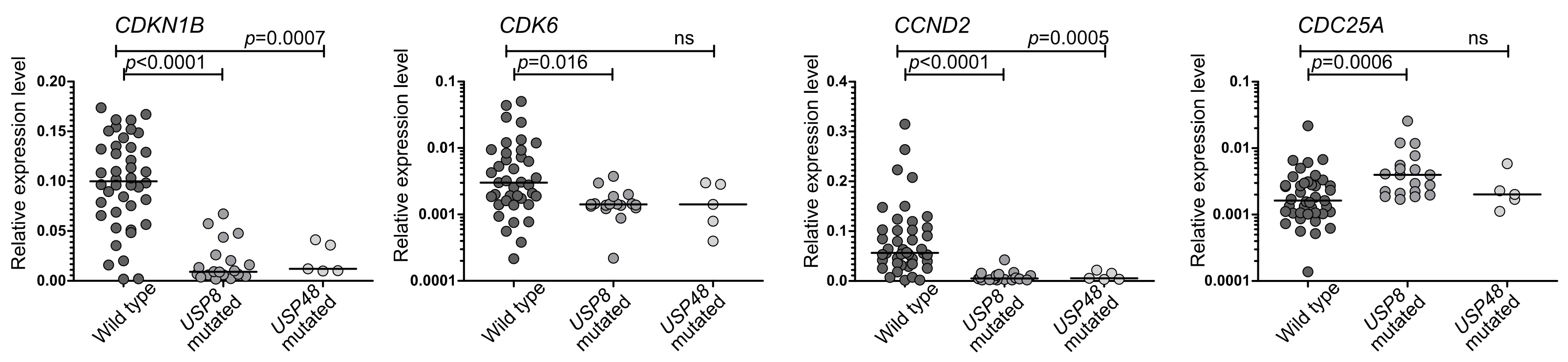
3.2. The Expression of Genes Encoding for Cell Cycle Regulators in Corticotrph PitNETs Stratified According to USP8 and USP48 Status
3.3. The Expression of Cell-Cycle Related Proteins in Corticotroph PitNETs
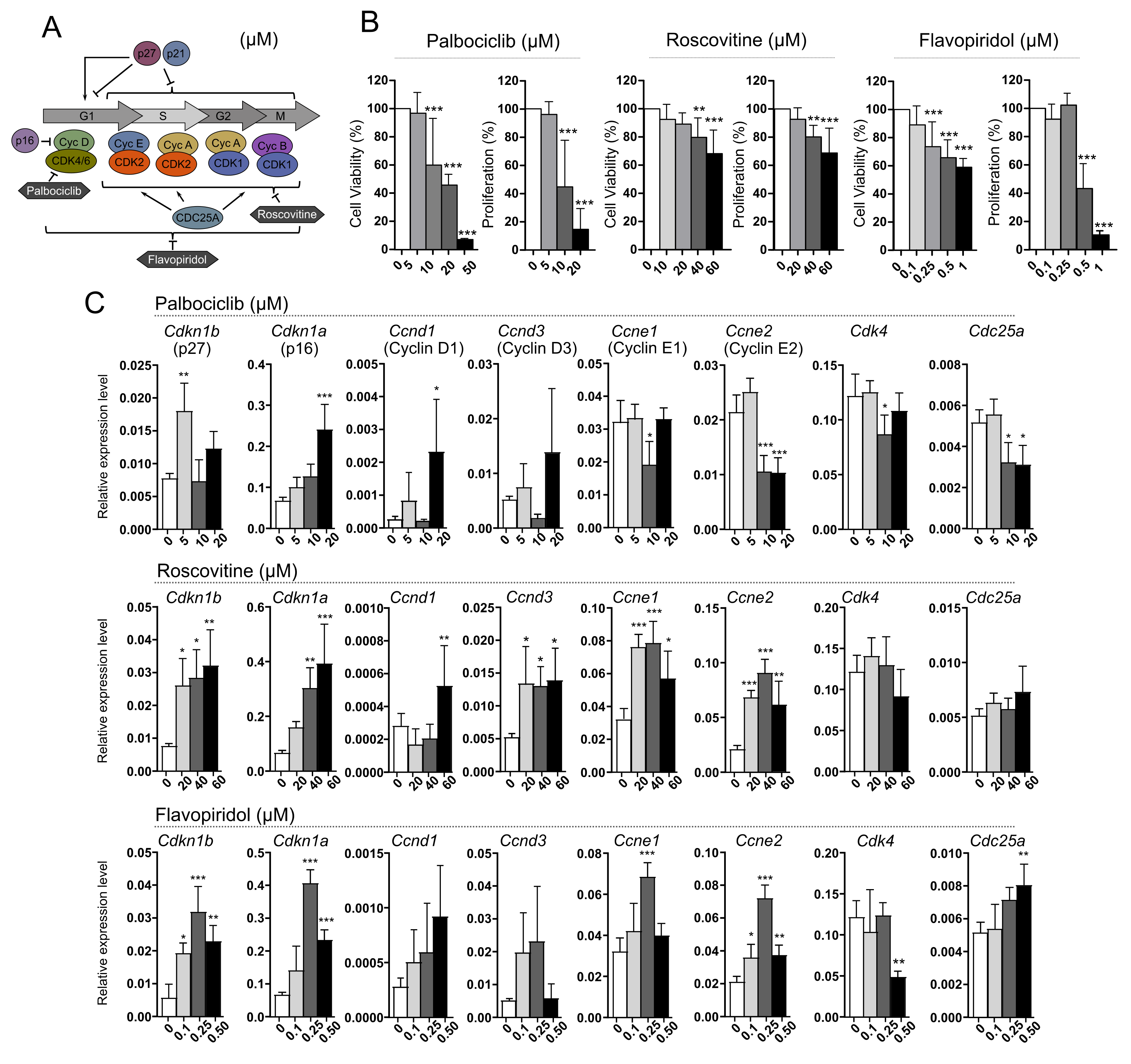
3.4. Treatment of AtT-20/D16v-F2 Cells with Cell Cycle Inhibitors Palbociclib, Roscovitine and Flavopiridol
3.5. Treatment of AtT-20/D16v-F2 Cells Harboring Usp8 Mutation with Cell Cycle Inhibitors
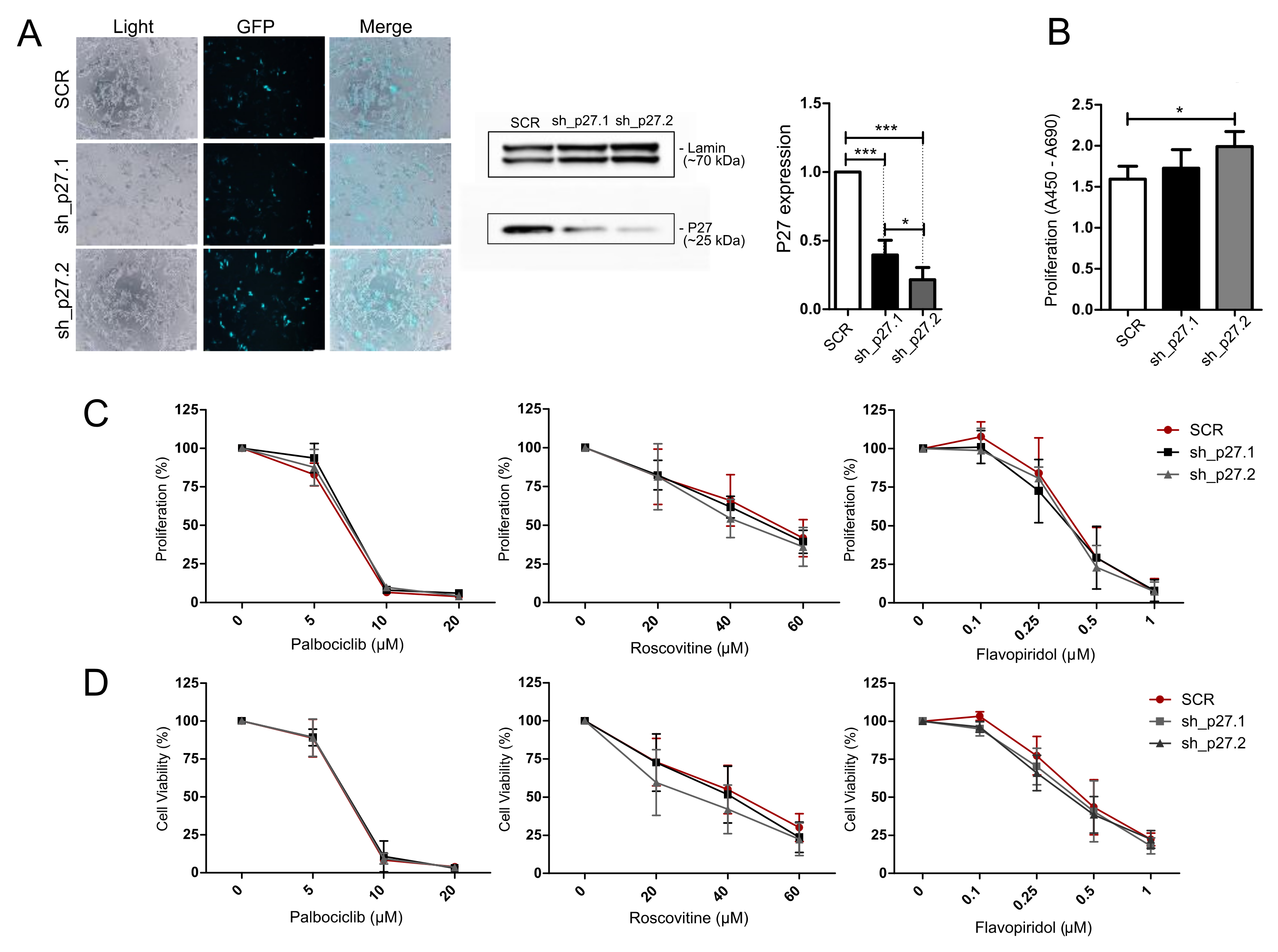
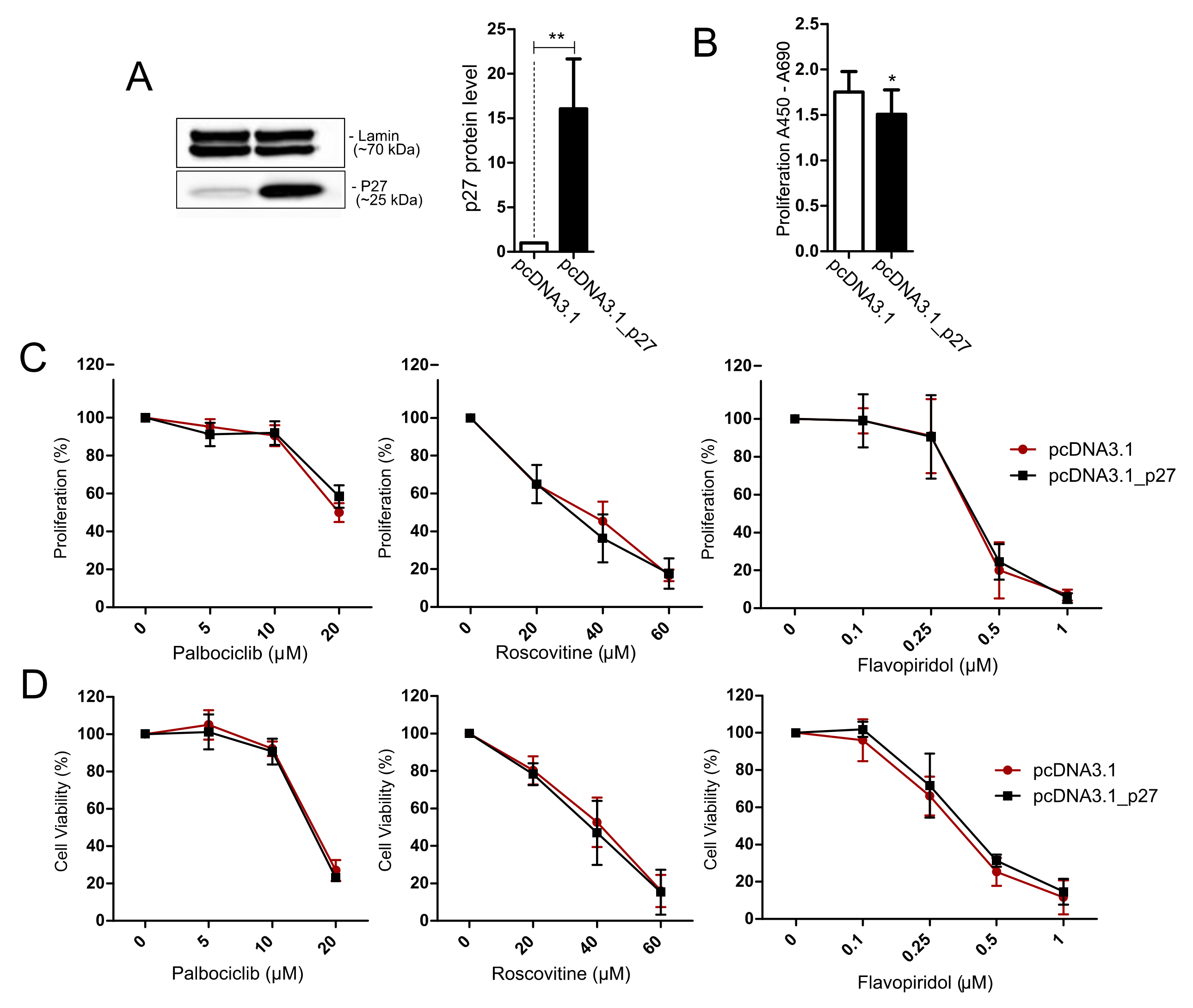
3.6. Treatment of AtT-20/D16v-F2 Cells with Modified p27 Expression Level with Cell Cycle Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inomoto, C.; Tahara, S.; Oyama, K.; Kimura, M.; Matsuno, A.; Teramoto, A.; Osamura, R.Y. Molecular, Functional, and Histopathological Classification of the Pituitary Neuroendocrine Neoplasms. Brain Tumor Pathol. 2021, 38, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Bujko, M.; Kober, P.; Boresowicz, J.; Rusetska, N.; Paziewska, A.; Daąbrowska, M.; Piaścik, A.; Pȩkul, M.; Zieliński, G.; Kunicki, J.; et al. USP8 Mutations in Corticotroph Adenomas Determine a Distinct Gene Expression Profile Irrespective of Functional Tumour Status. Eur. J. Endocrinol. 2019, 181, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Neou, M.; Villa, C.; Armignacco, R.; Jouinot, A.; Raffin-Sanson, M.L.; Septier, A.; Letourneur, F.; Diry, S.; Diedisheim, M.; Izac, B.; et al. Pangenomic Classification of Pituitary Neuroendocrine Tumors. Cancer Cell 2020, 37, 123–134.e5. [Google Scholar] [CrossRef] [PubMed]
- Reincke, M.; Sbiera, S.; Hayakawa, A.; Theodoropoulou, M.; Osswald, A.; Beuschlein, F.; Meitinger, T.; Mizuno-Yamasaki, E.; Kawaguchi, K.; Saeki, Y.; et al. Mutations in the Deubiquitinase Gene USP8 Cause Cushing’s Disease. Nat. Genet. 2015, 47, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Song, Z.-J.; Chen, J.-H.; Wang, Y.-F.; Li, S.-Q.; Zhou, L.-F.; Mao, Y.; Li, Y.-M.; Hu, R.-G.; Zhang, Z.-Y.; et al. Recurrent Gain-of-Function USP8 Mutations in Cushing’s Disease. Cell Res. 2015, 25, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rivas, L.G.; Theodoropoulou, M.; Ferraù, F.; Nusser, C.; Kawaguchi, K.; Stratakis, C.A.; Rueda Faucz, F.; Wildemberg, L.E.; Assié, G.; Beschorner, R.; et al. The Gene of the Ubiquitin-Specific Protease 8 Is Frequently Mutated in Adenomas Causing Cushing’s Disease. J. Clin. Endocrinol. Metab. 2015, 100, E997–E1004. [Google Scholar] [CrossRef]
- Albani, A.; Theodoropoulou, M.; Reincke, M. Genetics of Cushing’s Disease. Clin. Endocrinol. 2018, 88, 3–12. [Google Scholar] [CrossRef]
- Centorrino, F.; Ballone, A.; Wolter, M.; Ottmann, C. Biophysical and Structural Insight into the USP8/14-3-3 Interaction. FEBS Lett. 2018, 592, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Sbiera, S.; Perez-Rivas, L.G.; Taranets, L.; Weigand, I.; Flitsch, J.; Graf, E.; Monoranu, C.M.; Saeger, W.; Hagel, C.; Honegger, J.; et al. Driver Mutations in USP8 Wild-Type Cushing’s Disease. Neuro-Oncology 2019, 21, 1273–1283. [Google Scholar] [CrossRef]
- Hinojosa-Amaya, J.M.; Lam-Chung, C.E.; Cuevas-Ramos, D. Recent Understanding and Future Directions of Recurrent Corticotroph Tumors. Front. Endocrinol. 2021, 12, 657382. [Google Scholar] [CrossRef]
- Liu, N.A.; Jiang, H.; Ben-Shlomo, A.; Wawrowsky, K.; Fan, X.M.; Lin, S.; Melmed, S. Targeting Zebrafish and Murine Pituitary Corticotroph Tumors with a Cyclin-Dependent Kinase (CDK) Inhibitor. Proc. Natl. Acad. Sci. USA 2011, 108, 8414–8419. [Google Scholar] [CrossRef] [PubMed]
- Guiley, K.Z.; Stevenson, J.W.; Lou, K.; Barkovich, K.J.; Kumarasamy, V.; Wijeratne, T.U.; Bunch, K.L.; Tripathi, S.; Knudsen, E.S.; Witkiewicz, A.K.; et al. P27 Allosterically Activates Cyclin-Dependent Kinase 4 and Antagonizes Palbociclib Inhibition. Science 2019, 366, 6417. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zheng, L.; Sun, Z.; Li, J. CDK4/6 Inhibitor Resistance Mechanisms and Treatment Strategies. Int. J. Mol. Med. 2022, 50, 128. [Google Scholar] [CrossRef] [PubMed]
- Langlois, F.; Lim, D.S.T.; Yedinak, C.G.; Cetas, I.; McCartney, S.; Cetas, J.; Dogan, A.; Fleseriu, M. Predictors of Silent Corticotroph Adenoma Recurrence; a Large Retrospective Single Center Study and Systematic Literature Review. Pituitary 2017, 21, 32–40. [Google Scholar] [CrossRef]
- Bujko, M.; Kober, P.; Boresowicz, J.; Rusetska, N.; Zeber-Lubecka, N.; Paziewska, A.; Pekul, M.; Zielinski, G.; Styk, A.; Kunicki, J.; et al. Differential MicroRNA Expression in USP8-Mutated and Wild-Type Corticotroph Pituitary Tumors Reflect the Difference in Protein Ubiquitination Processes. J. Clin. Med. 2021, 10, 375. [Google Scholar] [CrossRef]
- McCarty, K.S.; Szabo, E.; Flowers, J.L.; Cox, E.B.; Leight, G.S.; Miller, L.; Konrath, J.; Soper, J.T.; Budwit, D.A.; Creasman, W.T. Use of a Monoclonal Anti-Estrogen Receptor Antibody in the Immunohistochemical Evaluation of Human Tumors. Cancer Res. 1986, 46, 4244–4249. [Google Scholar]
- Hayashi, K.; Inoshita, N.; Kawaguchi, K.; Ardisasmita, A.I.; Suzuki, H.; Fukuhara, N.; Okada, M.; Nishioka, H.; Takeuchi, Y.; Komada, M.; et al. The USP8 Mutational Status May Predict Drug Susceptibility in Corticotroph Adenomas of Cushing’s Disease. Eur. J. Endocrinol. 2016, 174, 213–226. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on Diagnosis and Management of Cushing’s Disease: A Guideline Update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Castellnou, S.; Vasiljevic, A.; Lapras, V.; Raverot, V.; Alix, E.; Borson-Chazot, F.; Jouanneau, E.; Raverot, G.; Lasolle, H. Sst5 Expression and Usp8 Mutation in Functioning and Silent Corticotroph Pituitary Tumors. Endocr. Connect. 2020, 9, 243–253. [Google Scholar] [CrossRef]
- Albani, A.; Perez-Rivas, L.G.; Tang, S.; Simon, J.; Lucia, K.E.; Colón-Bolea, P.; Schopohl, J.; Roeber, S.; Buchfelder, M.; Rotermund, R.; et al. Improved Pasireotide Response in USP8 Mutant Corticotroph Tumours in Vitro. Endocr. Relat. Cancer 2022, 29, 503–511. [Google Scholar] [CrossRef]
- Fleseriu, M.; Laws, E.R.; Witek, P.; Pivonello, R.; Ferrigno, R.; de Martino, M.C.; Simeoli, C.; di Paola, N.; Pivonello, C.; Barba, L.; et al. Medical Treatment of Cushing’s Disease: An Overview of the Current and Recent Clinical Trials. Front. Endocrinol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Elbelt, U.; Schlaffer, S.M.; Buchfelder, M.; Knappe, U.J.; Vila, G.; Micko, A.; Deutschbein, T.; Unger, N.; Lammert, A.; Topuzoglu-Müller, T.; et al. Efficacy of Temozolomide Therapy in Patients With Aggressive Pituitary Adenomas and Carcinomas—A German Survey. J. Clin. Endocrinol. Metab. 2020, 105, dgz211. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.D.; Vasiljevic, A.; Jouanneau, E.; Raverot, G. Immunotherapy in Aggressive Pituitary Tumors and Carcinomas: A Systematic Review. Endocr. Relat. Cancer 2022, 29, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Ettl, T.; Schulz, D.; Bauer, R.J. The Renaissance of Cyclin Dependent Kinase Inhibitors. Cancers 2022, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.A.; Araki, T.; Cuevas-Ramos, D.; Hong, J.; Ben-Shlomo, A.; Tone, Y.; Tone, M.; Melmed, S. Cyclin E-Mediated Human Proopiomelanocortin Regulation as a Therapeutic Target for Cushing Disease. J. Clin. Endocrinol. Metab. 2015, 100, 2557–2564. [Google Scholar] [CrossRef]
- Lidhar, K. Low Expression of the Cell Cycle Inhibitor P27Kip1 in Normal Corticotroph Cells, Corticotroph Tumors, and Malignant Pituitary Tumors. J. Clin. Endocrinol. Metab. 1999, 84, 3823–3830. [Google Scholar] [CrossRef]
- Weigand, I.; Knobloch, L.; Flitsch, J.; Saeger, W.; Monoranu, C.M.; Höfner, K.; Herterich, S.; Rotermund, R.; Ronchi, C.L.; Buchfelder, M.; et al. Impact of USP8 Gene Mutations on Protein Deregulation in Cushing Disease. J. Clin. Endocrinol. Metab. 2019, 104, 2535–2546. [Google Scholar] [CrossRef]
- Martins, C.S.; Camargo, R.C.; Coeli-Lacchini, F.B.; Saggioro, F.P.; Moreira, A.C.; de Castro, M. USP8 Mutations and Cell Cycle Regulation in Corticotroph Adenomas. Horm. Metab. Res. 2020, 52, 117–123. [Google Scholar] [CrossRef]
- Sotillo, R.; Renner, O.; Dubus, P.; Ruiz-Cabello, J.; Martín-Caballero, J.; Barbacid, M.; Carnero, A.; Malumbres, M. Cooperation between Cdk4 and P27kip1 in Tumor Development: A Preclinical Model to Evaluate Cell Cycle Inhibitors with Therapeutic Activity. Cancer Res. 2005, 65, 3846–3852. [Google Scholar] [CrossRef]


| Clinical Feature | Cushing’s Disease | Silent Corticotroph Tumors |
|---|---|---|
| Number of patients | n = 47 | n = 23 |
| Sex (ratio females/males) | 38/9 | 12/11 |
| Age at surgery (years; median (range) | 44 (20–78) | 54 (23–77) |
| Cortisol 08:00 h (µg/dL; median (range) | 25.65 (11.54–49.7) | 16.6 (6.8–50.8) |
| Cortisol 24:00 h (µg/dL; median (range) | 19.35 (6.83–36.5) | 0.9 (0.3–14.1) |
| ACTH 08:00 h (pg/dL; median (range) | 76.05 (35.6–207) | 47.7 (14.7–96.1) |
| UFC (μg/24 h; median (range) | 490 (163.9–961.8) | 94.7 (13.7–139) |
| Tumor volume (mm3; median (range) | 873.3 (13.5–12,000) | 3465 (900–11,088) |
| Invasive tumor growth (Knosp grade 0, 1, 2/3, 4) | 33/14 | 19/4 |
| Pathomorphology (sparsely/densely granulated) | 14/33 | 14/9 |
| Antibody | Incubation Conditions |
|---|---|
| CDC25A (MA513794; Invitrogen) | Dilution 1:100; overnight incubation in 4 °C |
| CDK6 (PA5-87490; Invitrogen) | Dilution 1:100; 1 h |
| P27 (PA5-32530; Invitrogen) | Dilution 1:100; 10 min |
| Cycl D2 (PA5-95922; Invitrogen) | Dilution 1:100; 1 h |
| T-PIT (AMAB91409; Sigma-Aldrich) | Dilution 1:2000; 30 min |
| Experiment | Plasmids |
|---|---|
| Overexpression of USP8 and USP8 with P720R mutation | pcDNA3.1 pcDNA3.1_USP8 pcDNA3.1_MMUT6 |
| Knockdown of p27 expression | plKO1gfp_SCR plKO1gfp_p27.2 plKO1gfp_p27.3 |
| Overexpression of p27 | pcDNA3.1 pcDNA3.1_p27 |
| Inhibitor | Target | Concentrations (μM) | Supplier |
|---|---|---|---|
| Palbociclib | CDK4, CDK6 | 5, 10, 20 | Sigma-Aldrich (no. PZ0199) |
| Roscovitine | CDK1, CDK2 | 20, 40, 60 | Sigma-Aldrich (no. 557364) |
| Flavopiridol | Multiple CDKs, CDC25 | 0.1, 0.25, 0.5 | Sigma-Aldrich (no. F3055) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mossakowska, B.J.; Rusetska, N.; Konopinski, R.; Kober, P.; Maksymowicz, M.; Pekul, M.; Zieliński, G.; Styk, A.; Kunicki, J.; Bujko, M. The Expression of Cell Cycle-Related Genes in USP8-Mutated Corticotroph Neuroendocrine Pituitary Tumors and Their Possible Role in Cell Cycle-Targeting Treatment. Cancers 2022, 14, 5594. https://doi.org/10.3390/cancers14225594
Mossakowska BJ, Rusetska N, Konopinski R, Kober P, Maksymowicz M, Pekul M, Zieliński G, Styk A, Kunicki J, Bujko M. The Expression of Cell Cycle-Related Genes in USP8-Mutated Corticotroph Neuroendocrine Pituitary Tumors and Their Possible Role in Cell Cycle-Targeting Treatment. Cancers. 2022; 14(22):5594. https://doi.org/10.3390/cancers14225594
Chicago/Turabian StyleMossakowska, Beata Joanna, Natalia Rusetska, Ryszard Konopinski, Paulina Kober, Maria Maksymowicz, Monika Pekul, Grzegorz Zieliński, Andrzej Styk, Jacek Kunicki, and Mateusz Bujko. 2022. "The Expression of Cell Cycle-Related Genes in USP8-Mutated Corticotroph Neuroendocrine Pituitary Tumors and Their Possible Role in Cell Cycle-Targeting Treatment" Cancers 14, no. 22: 5594. https://doi.org/10.3390/cancers14225594
APA StyleMossakowska, B. J., Rusetska, N., Konopinski, R., Kober, P., Maksymowicz, M., Pekul, M., Zieliński, G., Styk, A., Kunicki, J., & Bujko, M. (2022). The Expression of Cell Cycle-Related Genes in USP8-Mutated Corticotroph Neuroendocrine Pituitary Tumors and Their Possible Role in Cell Cycle-Targeting Treatment. Cancers, 14(22), 5594. https://doi.org/10.3390/cancers14225594






