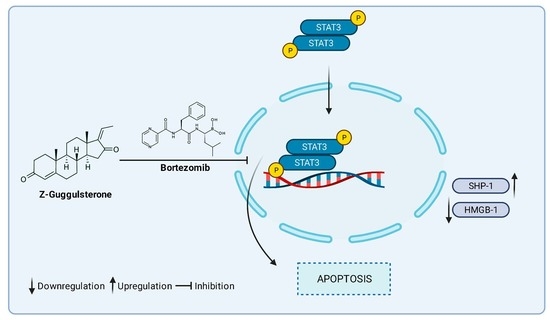
Guggulsterone Induces Apoptosis in Multiple Myeloma Cells by Targeting High Mobility Group Box 1 via Janus Activated Kinase/Signal Transducer and Activator of Transcription Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
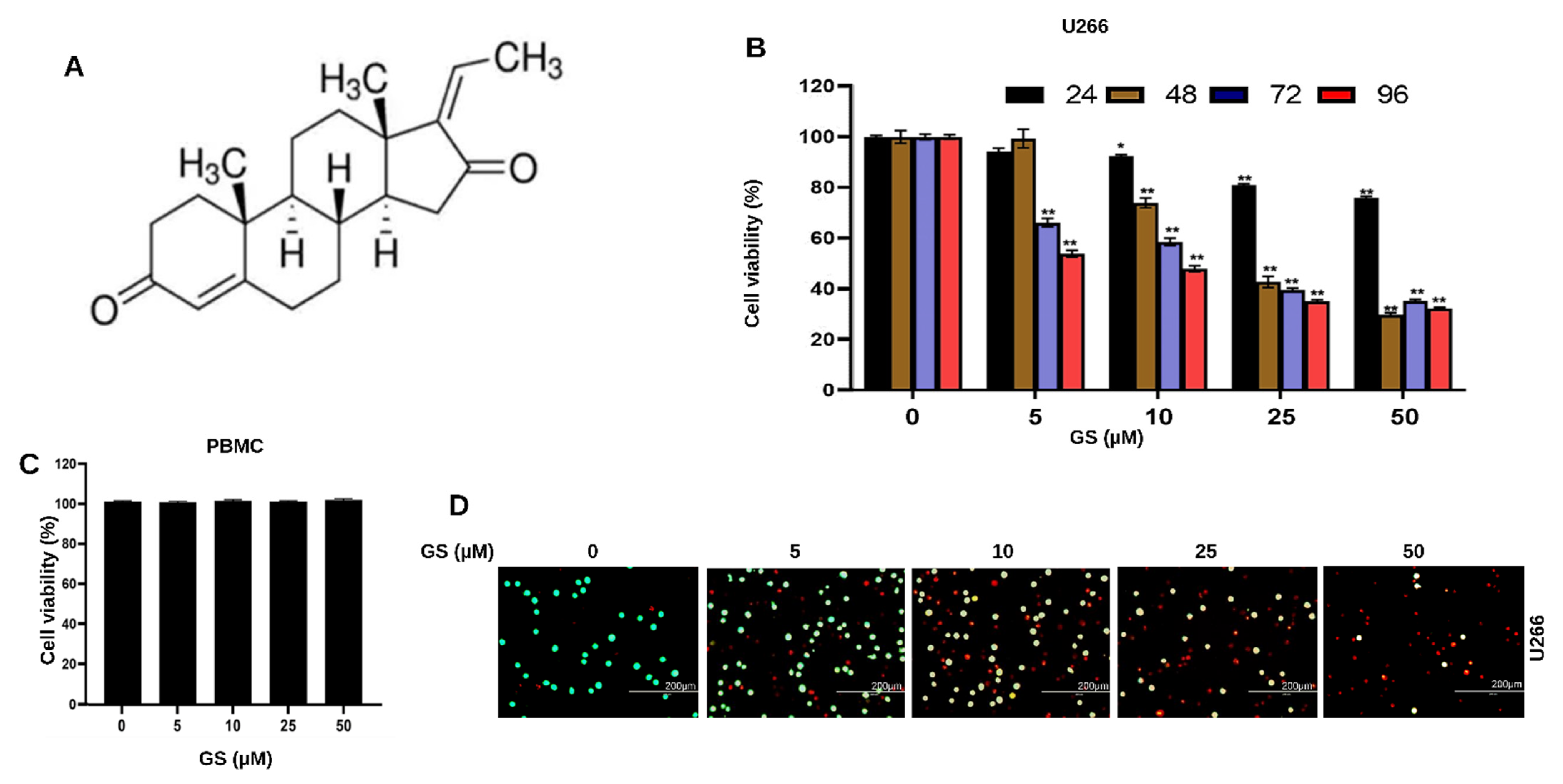
2.3. Cell Viability Assay
2.4. Live/Dead Assay
2.5. Caspase-3/Caspase-7 Green Apoptosis Assay
2.6. Cell Lysis and Immunoblotting
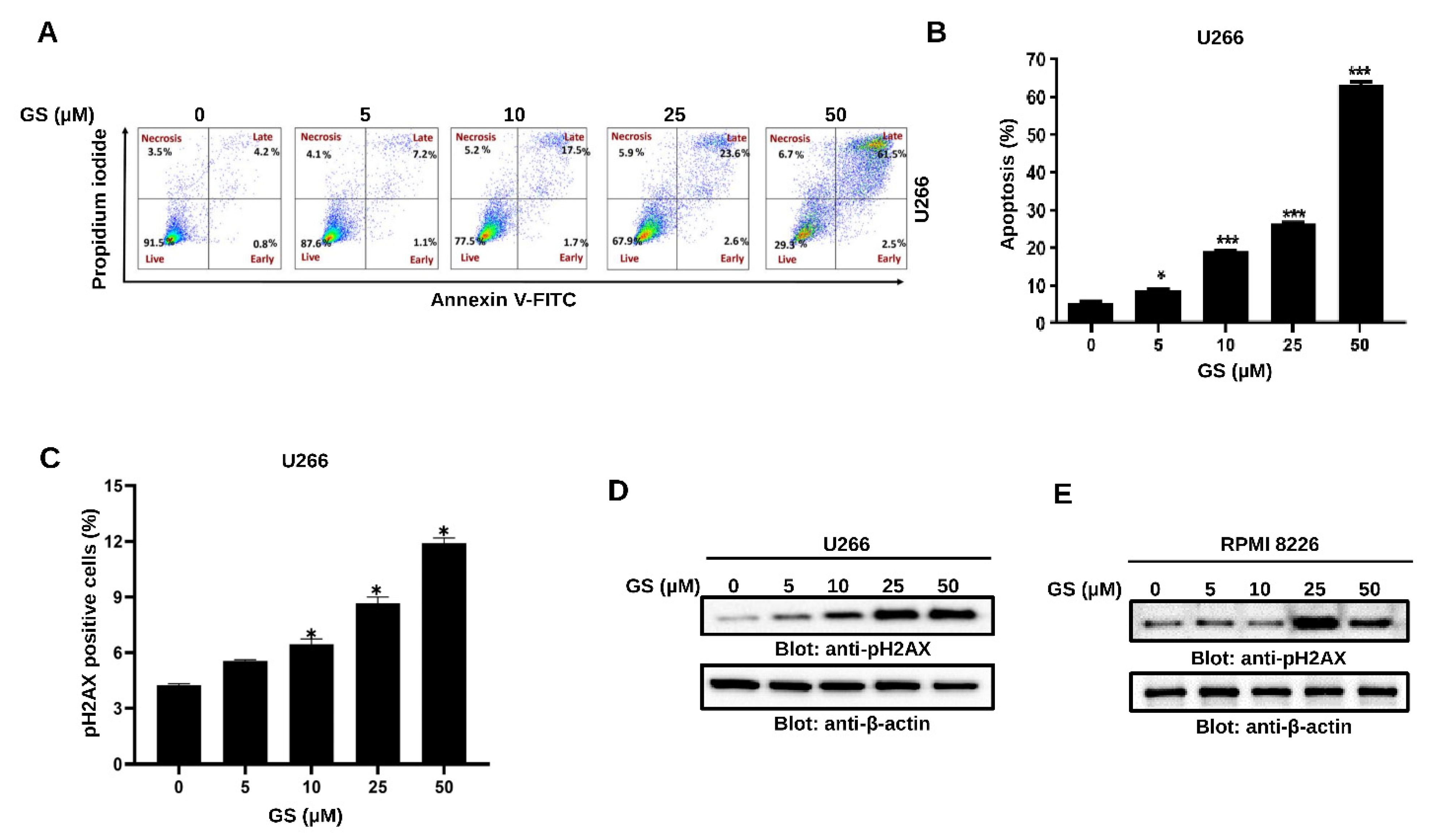
2.7. AnnexinV/Propidium Iodide (PI) Dual Staining
2.8. Measurement of DNA Double-Strand Breaks
2.9. Measurement of Mitochondrial Membrane Potential
2.10. Flow Cytometric Analysis of Activated Caspase-3 and Cleaved PARP
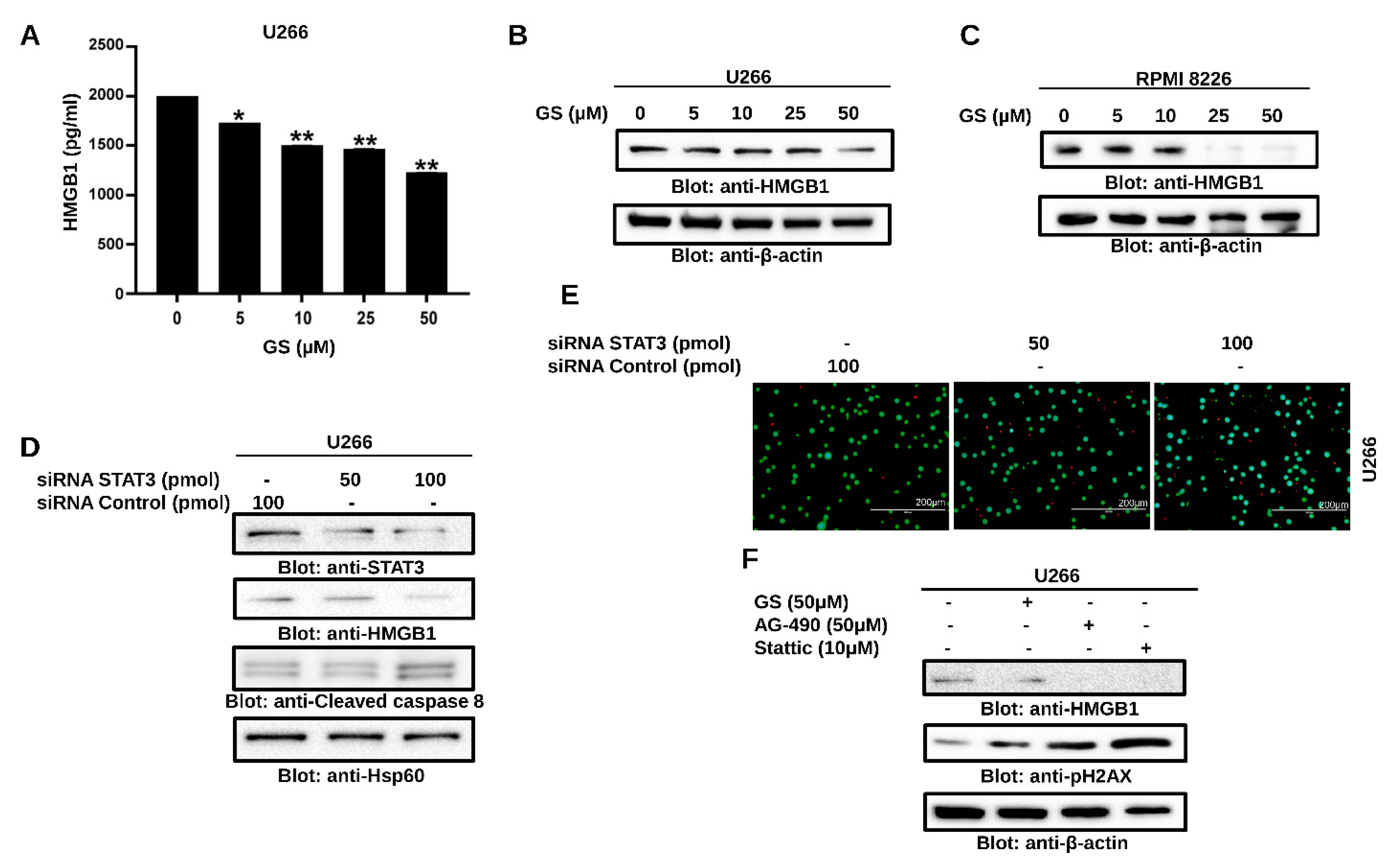
2.11. HMGB1 ELISA
2.12. Gene Silencing Using siRNA
2.13. Multiplex Cytokine Analysis
2.14. Statistical Analysis
3. Results
3.1. GS Treatment Reduces the Viability of MM Cells
3.2. GS Induces Apoptosis and Mediates Cellular DNA Damage in MM Cells
3.3. GS Activates the Intrinsic and Extrinsic Apoptotic Pathways in MM Cells
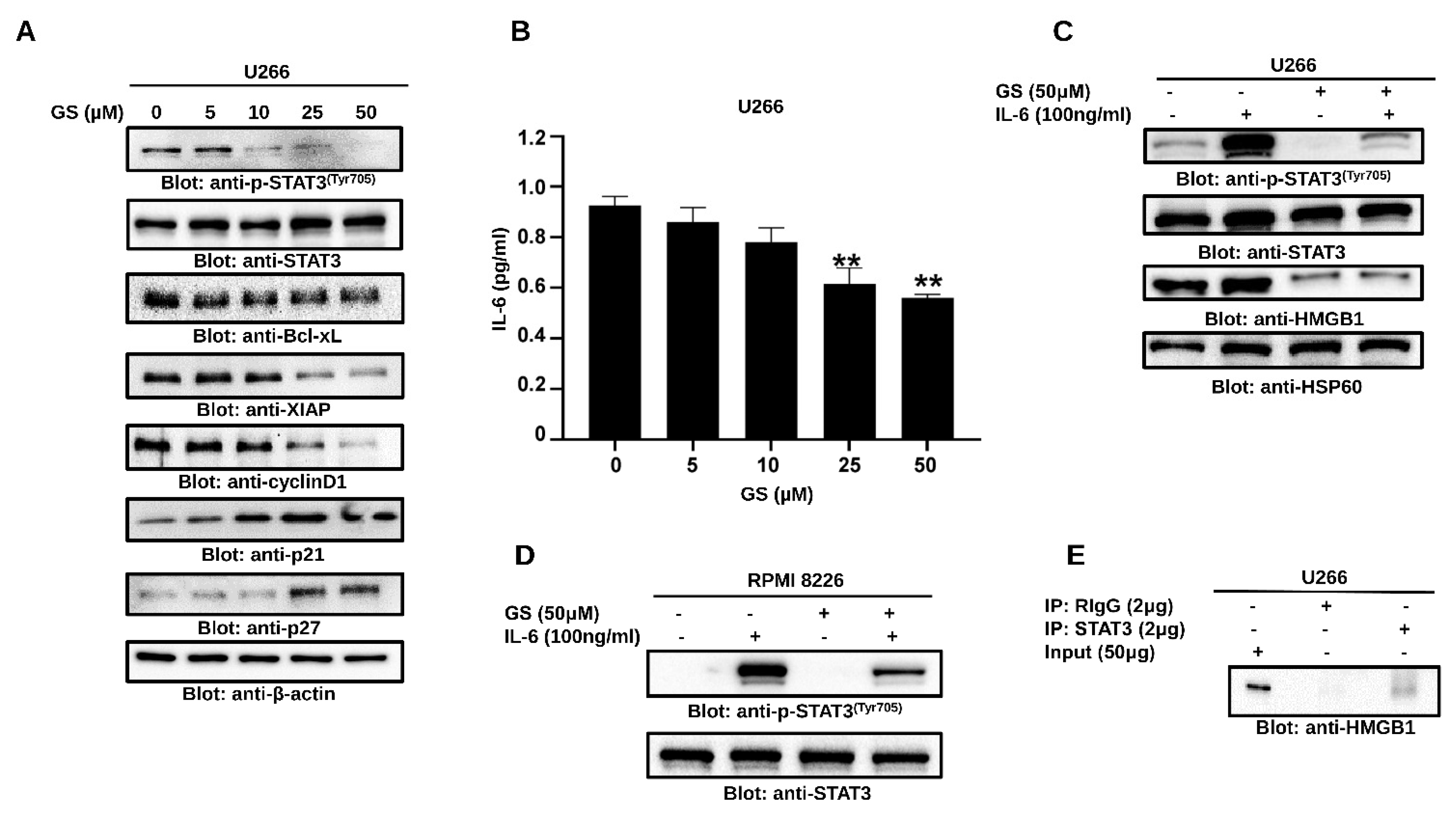
3.4. GS Treatment Downregulates the Expression of HMGB1 via the JAK/STAT Pathway
3.5. GS Suppresses Constitutively Active and IL-6-Induced STAT3 Activation in MM Cells
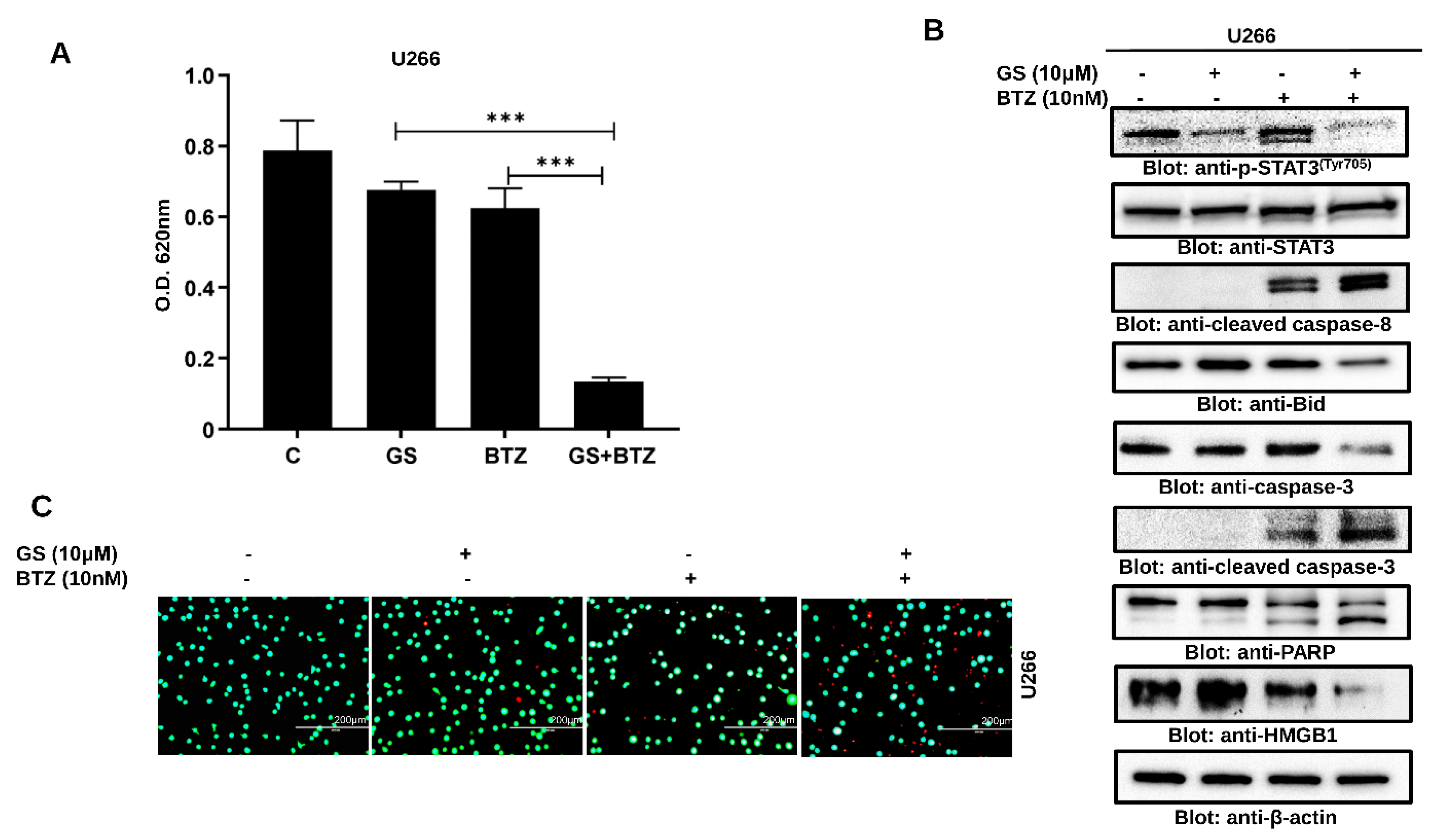
3.6. Synergistic Activity of GS and BTZ in U266 Cells
3.7. GS sensitizes U266 Cells to BTZ
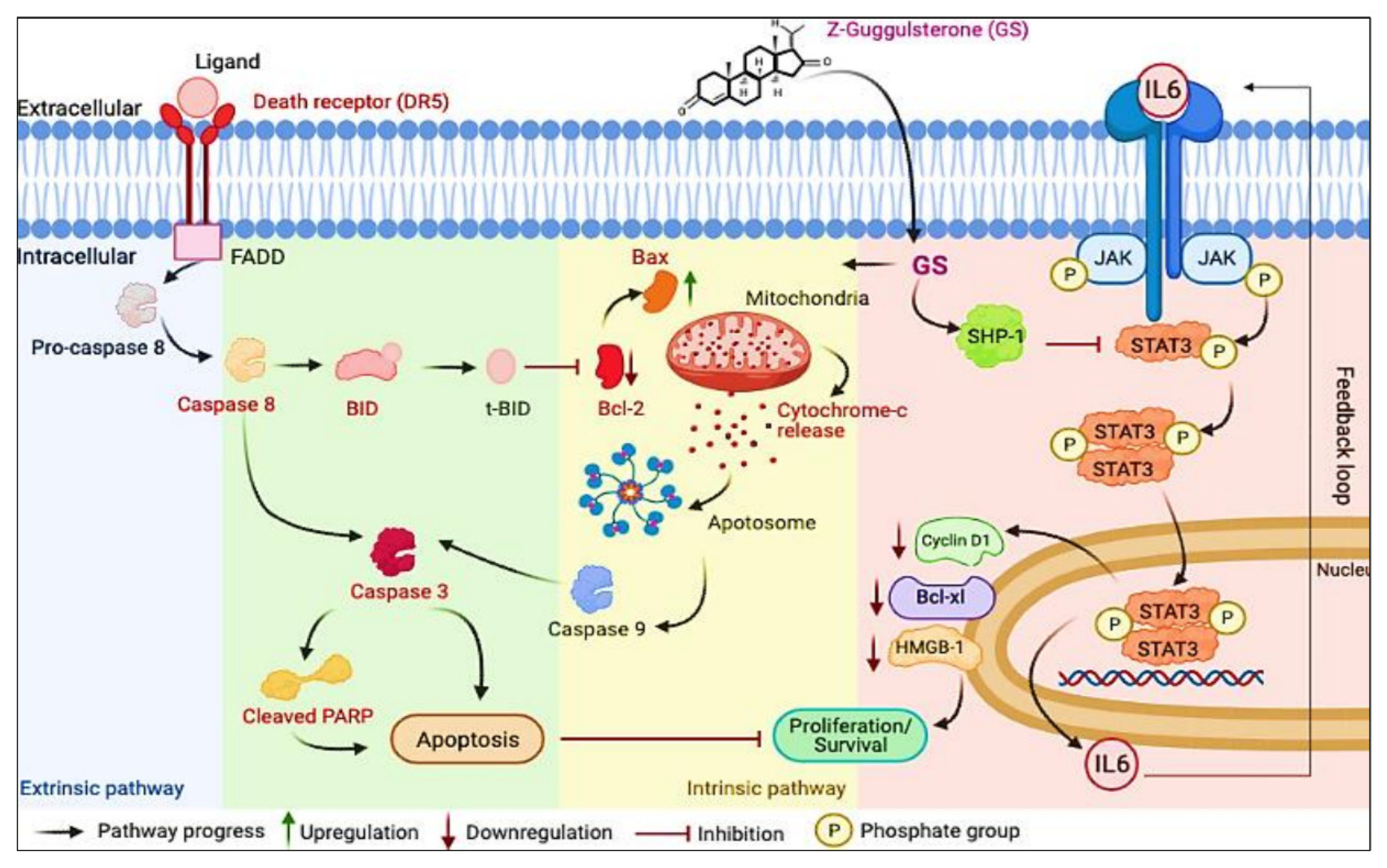
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Gao, Q.; Foltz, S.M.; Fowles, J.S.; Yao, L.; Wang, J.T.; Cao, S.; Sun, H.; Wendl, M.C.; Sethuraman, S.; et al. Co-evolution of tumor and immune cells during progression of multiple myeloma. Nat. Commun. 2021, 12, 2559. [Google Scholar] [CrossRef] [PubMed]
- Firth, J. Haematology: Multiple myeloma. Clin. Med. 2019, 19, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cao, W.; Que, Y.; Wang, Q.; Xiao, Y.; Gu, C.; Wang, D.; Wang, J.; Jiang, L.; Xu, H.; et al. A phase I study of anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma and plasma cell leukemia. Clin. Transl. Med. 2021, 11, e346. [Google Scholar] [CrossRef]
- Hammarén, H.M.; Virtanen, A.T.; Raivola, J.; Silvennoinen, O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine 2019, 118, 48–63. [Google Scholar] [CrossRef]
- Rah, B.; Rather, R.A.; Bhat, G.R.; Baba, A.B.; Mushtaq, I.; Farooq, M.; Yousuf, T.; Dar, S.B.; Parveen, S.; Hassan, R.; et al. JAK/STAT Signaling: Molecular Targets, Therapeutic Opportunities, and Limitations of Targeted Inhibitions in Solid Malignancies. Front. Pharmacol. 2022, 13, 821344. [Google Scholar] [CrossRef]
- Brooks, A.J.; Putoczki, T. JAK-STAT Signalling Pathway in Cancer. Cancers 2020, 12, 1971. [Google Scholar] [CrossRef]
- Chong, P.S.Y.; Chng, W.J.; de Mel, S. STAT3: A Promising Therapeutic Target in Multiple Myeloma. Cancers 2019, 11, 731. [Google Scholar] [CrossRef] [Green Version]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2019, 9, 788. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Antoine, D.J.; Kwan, K.; Lundback, P.; Wahamaa, H.; Schierbeck, H.; Robinson, M.; Van Zoelen, M.A.; Yang, H.; Li, J.; et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc. Natl. Acad. Sci. USA 2014, 111, 3068–3073. [Google Scholar] [CrossRef] [Green Version]
- Venereau, E.; De Leo, F.; Mezzapelle, R.; Careccia, G.; Musco, G.; Bianchi, M.E. HMGB1 as biomarker and drug target. Pharmacol. Res. 2016, 111, 534–544. [Google Scholar] [CrossRef]
- Martinotti, S.; Patrone, M.; Ranzato, E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015, 4, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; He, D.; Zhang, E.; Chen, J.; Chen, Q.; Li, Y.; Yang, L.; Yang, Y.; Zhao, Y.; Wang, G.; et al. HMGB1 knockdown increases MM cell vulnerability by regulating autophagy and DNA damage repair. J. Exp. Clin. Cancer Res. CR 2018, 37, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, J.; Yang, R.; Wang, H.; Cui, L. HMGB1 enhances chemotherapy resistance in multiple myeloma cells by activating the nuclear factor-κB pathway. Exp. Ther. Med. 2021, 22, 705. [Google Scholar] [CrossRef] [PubMed]
- Sarup, P.; Bala, S.; Kamboj, S. Pharmacology and Phytochemistry of Oleo-Gum Resin of Commiphora wightii (Guggulu). Scientifica 2015, 2015, 138039. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Sugimoto, K. Guggulsterone and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 329–361. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Banik, K.; Bordoloi, D.; Harsha, C.; Sailo, B.L.; Padmavathi, G.; Roy, N.K.; Gupta, S.C.; Aggarwal, B.B. Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Front. Pharmacol. 2018, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.R.; Kim, J.; Lee, J.E.; Lee, E.J.; Yoon, J.S. Therapeutic Effect of Guggulsterone in Primary Cultured Orbital Fibroblasts Obtained From Patients with Graves’ Orbitopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef] [Green Version]
- Deng, R. Therapeutic effects of guggul and its constituent guggulsterone: Cardiovascular benefits. Cardiovasc. Drug Rev. 2007, 25, 375–390. [Google Scholar] [CrossRef]
- Malhotra, S.C.; Ahuja, M.M.; Sundaram, K.R. Long term clinical studies on the hypolipidaemic effect of Commiphora mukul (Guggulu) and clofibrate. Indian J. Med. Res. 1977, 65, 390–395. [Google Scholar]
- Szapary, P.O.; Wolfe, M.L.; Bloedon, L.T.; Cucchiara, A.J.; DerMarderosian, A.H.; Cirigliano, M.D.; Rader, D.J. Guggulipid for the treatment of hypercholesterolemia: A randomized controlled trial. JAMA 2003, 290, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Nityanand, S.; Srivastava, J.S.; Asthana, O.P. Clinical trials with gugulipid. A new hypolipidaemic agent. J. Assoc. Phys. India 1989, 37, 323–328. [Google Scholar]
- Kuppurajan, K.; Rajagopalan, S.S.; Rao, T.K.; Sitaraman, R. Effect of guggulu (Commiphora mukul--Engl.) on serum lipids in obese, hypercholesterolemic and hyperlipemic cases. J. Assoc. Phys. India 1978, 26, 367–373. [Google Scholar]
- Sook, S.H.; Lee, H.J.; Kim, J.H.; Sohn, E.J.; Jung, J.H.; Kim, B.; Kim, J.H.; Jeong, S.J.; Kim, S.H. Reactive oxygen species-mediated activation of AMP-activated protein kinase and c-Jun N-terminal kinase plays a critical role in beta-sitosterol-induced apoptosis in multiple myeloma U266 cells. Phytother. Res. PTR 2014, 28, 387–394. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, K.S.; Jeong, S.J.; Kwon, T.R.; Jung, J.H.; Yun, S.M.; Han, I.; Lee, S.G.; Kim, D.K.; Kang, M.; et al. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur. J. Pharmacol. 2011, 654, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Ahn, K.S.; Jeong, S.J.; Jung, J.H.; Kwon, T.R.; Rhee, Y.H.; Kim, S.H.; Kim, S.Y.; Yoon, H.J.; Zhu, S.; et al. Signal transducer and activator of transcription 3 pathway mediates genipin-induced apoptosis in U266 multiple myeloma cells. J. Cell. Biochem. 2011, 112, 1552–1562. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.J.; Jeong, S.J.; Song, H.S.; Kim, M.; Lee, H.J.; Lee, E.O.; Kim, D.H.; Ahn, K.S.; Kim, S.H. Inhibition of JAK1/STAT3 signaling mediates compound K-induced apoptosis in human multiple myeloma U266 cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1367–1372. [Google Scholar] [CrossRef]
- Shi, J.J.; Jia, X.L.; Li, M.; Yang, N.; Li, Y.P.; Zhang, X.; Gao, N.; Dang, S.S. Guggulsterone induces apoptosis of human hepatocellular carcinoma cells through intrinsic mitochondrial pathway. World J. Gastroenterol. 2015, 21, 13277–13287. [Google Scholar] [CrossRef]
- Bhat, A.A.; Prabhu, K.S.; Kuttikrishnan, S.; Krishnankutty, R.; Babu, J.; Mohammad, R.M.; Uddin, S. Potential therapeutic targets of Guggulsterone in cancer. Nutr. Metab. 2017, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.S.; Sethi, G.; Sung, B.; Goel, A.; Ralhan, R.; Aggarwal, B.B. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008, 68, 4406–4415. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, K.S.; Siveen, K.S.; Kuttikrishnan, S.; Iskandarani, A.N.; Khan, A.Q.; Merhi, M.; Omri, H.E.; Dermime, S.; El-Elimat, T.; Oberlies, N.H.; et al. Greensporone C, a Freshwater Fungal Secondary Metabolite Induces Mitochondrial-Mediated Apoptotic Cell Death in Leukemic Cell Lines. Front. Pharmacol. 2018, 9, 720. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.R.; Al-Jomah, N.A.; Siraj, A.K.; Manogaran, P.; Al-Hussein, K.; Abubaker, J.; Platanias, L.C.; Al-Kuraya, K.S.; Uddin, S. Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells. Cancer Res. 2007, 67, 3888–3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, K.S.; Siveen, K.S.; Kuttikrishnan, S.; Iskandarani, A.; Tsakou, M.; Achkar, I.W.; Therachiyil, L.; Krishnankutty, R.; Parray, A.; Kulinski, M.; et al. Targeting of X-linked inhibitor of apoptosis protein and PI3-kinase/AKT signaling by embelin suppresses growth of leukemic cells. PLoS ONE 2017, 12, e0180895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX phosphorylation: A marker for DNA damage. Methods Mol. Biol. 2012, 920, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Green, D.R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008, 18, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, J.L.; Grandis, J.R.; Bauman, J.E. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral Oncol. 2016, 56, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpos, E.; Dimopoulos, M.A. Myeloma bone disease: Pathophysiology and management. Ann. Oncol. 2005, 16, 1223–1231. [Google Scholar] [CrossRef]
- Wang, Y.; van Boxel-Dezaire, A.H.; Cheon, H.; Yang, J.; Stark, G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16975–16980. [Google Scholar] [CrossRef] [Green Version]
- Iskandarani, A.; Bhat, A.A.; Siveen, K.S.; Prabhu, K.S.; Kuttikrishnan, S.; Khan, M.A.; Krishnankutty, R.; Kulinski, M.; Nasr, R.R.; Mohammad, R.M.; et al. Bortezomib-mediated downregulation of S-phase kinase protein-2 (SKP2) causes apoptotic cell death in chronic myelogenous leukemia cells. J. Transl. Med. 2016, 14, 69. [Google Scholar] [CrossRef] [Green Version]
- Zaal, E.A.; Wu, W.; Jansen, G.; Zweegman, S.; Cloos, J.; Berkers, C.R. Bortezomib resistance in multiple myeloma is associated with increased serine synthesis. Cancer Metab. 2017, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Murray, M.Y.; Auger, M.J.; Bowles, K.M. Overcoming bortezomib resistance in multiple myeloma. Biochem. Soc. Trans. 2014, 42, 804–808. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.; Fathima, H.; Prabhu, K.S.; Siveen, K.S.; Kulinski, M.; Azizi, F.; Dermime, S.; Ahmad, A.; et al. Curcumin-Mediated Apoptotic Cell Death in Papillary Thyroid Cancer and Cancer Stem-Like Cells through Targeting of the JAK/STAT3 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, V.; Bergantim, R.; Caires, H.R.; Seca, H.; Guimaraes, J.E.; Vasconcelos, M.H. Multiple Myeloma: Available Therapies and Causes of Drug Resistance. Cancers 2020, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Zhu, M.; Chen, K.; Xie, H.; Bai, H.; Chen, Q. Z-Guggulsterone Induces Apoptosis in Gastric Cancer Cells through the Intrinsic Mitochondria-Dependent Pathway. Sci. World J. 2021, 2021, 3152304. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 2007, 74, 118–130. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Kang, R.; Zeh, H.J.; Lotze, M.T. High-mobility group box 1 and cancer. Biochim. Biophys. Acta 2010, 1799, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.; Achkar, I.W.; Siveen, K.S.; Kuttikrishnan, S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Sahir, F.; Jerobin, J.; Raza, A.; et al. Sanguinarine Induces Apoptosis Pathway in Multiple Myeloma Cell Lines via Inhibition of the JaK2/STAT3 Signaling. Front. Oncol. 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Yan, L.; Liu, G.Y.; Chen, W.J.; Gong, W.H.; Yu, J.M. Inhibition of janus kinase 2 by compound AG490 suppresses the proliferation of MDA-MB-231 cells via up-regulating SARI (suppressor of AP-1, regulated by IFN). Iran J. Basic Med. Sci. 2015, 18, 599–603. [Google Scholar]
- Valentino, L.; Pierre, J. JAK/STAT signal transduction: Regulators and implication in hematological malignancies. Biochem. Pharmacol. 2006, 71, 713–721. [Google Scholar] [CrossRef]
- Li, F.; Rajendran, P.; Sethi, G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br. J. Pharmacol. 2010, 161, 541–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeman-Neill, R.J.; Wheeler, S.E.; Singh, S.V.; Thomas, S.M.; Seethala, R.R.; Neill, D.B.; Panahandeh, M.C.; Hahm, E.R.; Joyce, S.C.; Sen, M.; et al. Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis 2009, 30, 1848–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zang, Y.; Sen, M.; Leeman-Neill, R.J.; Man, D.S.; Grandis, J.R.; Johnson, D.E. Bortezomib up-regulates activated signal transducer and activator of transcription-3 and synergizes with inhibitors of signal transducer and activator of transcription-3 to promote head and neck squamous cell carcinoma cell death. Mol. Cancer Ther. 2009, 8, 2211–2220. [Google Scholar] [CrossRef]
- Zhong, F.; Yang, J.; Tong, Z.T.; Chen, L.L.; Fan, L.L.; Wang, F.; Zha, X.L.; Li, J. Guggulsterone inhibits human cholangiocarcinoma Sk-ChA-1 and Mz-ChA-1 cell growth by inducing caspase-dependent apoptosis and downregulation of survivin and Bcl-2 expression. Oncol. Lett. 2015, 10, 1416–1422. [Google Scholar] [CrossRef]



| GS (µM) | Bortezomib (nM) | Fractional Effect (Fa) | Combination Index (CI) | Concentration Reduction Index (DRI) GS (µM) | Concentration Reduction Index (DRI) Bortezomib (nM) |
|---|---|---|---|---|---|
| 5 | 5 | 0.096 | 2.04474 | 7.404 | 0.523 |
| 10 | 10 | 0.524 | 0.46825 | 5.115 | 3.666 |
| 25 | 25 | 0.633 | 0.87011 | 2.177 | 2.434 |
| 50 | 50 | 0.556 | 2.13938 | 1.041 | 0.848 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, S.; Zarif, L.; Kuttikrishnan, S.; Prabhu, K.S.; Patil, K.; Nisar, S.; Abou-Saleh, H.; Merhi, M.; Dermime, S.; Bhat, A.A.; et al. Guggulsterone Induces Apoptosis in Multiple Myeloma Cells by Targeting High Mobility Group Box 1 via Janus Activated Kinase/Signal Transducer and Activator of Transcription Pathway. Cancers 2022, 14, 5621. https://doi.org/10.3390/cancers14225621
Akhtar S, Zarif L, Kuttikrishnan S, Prabhu KS, Patil K, Nisar S, Abou-Saleh H, Merhi M, Dermime S, Bhat AA, et al. Guggulsterone Induces Apoptosis in Multiple Myeloma Cells by Targeting High Mobility Group Box 1 via Janus Activated Kinase/Signal Transducer and Activator of Transcription Pathway. Cancers. 2022; 14(22):5621. https://doi.org/10.3390/cancers14225621
Chicago/Turabian StyleAkhtar, Sabah, Lubna Zarif, Shilpa Kuttikrishnan, Kirti S. Prabhu, Kalyani Patil, Sabah Nisar, Haissam Abou-Saleh, Maysaloun Merhi, Said Dermime, Ajaz A. Bhat, and et al. 2022. "Guggulsterone Induces Apoptosis in Multiple Myeloma Cells by Targeting High Mobility Group Box 1 via Janus Activated Kinase/Signal Transducer and Activator of Transcription Pathway" Cancers 14, no. 22: 5621. https://doi.org/10.3390/cancers14225621
APA StyleAkhtar, S., Zarif, L., Kuttikrishnan, S., Prabhu, K. S., Patil, K., Nisar, S., Abou-Saleh, H., Merhi, M., Dermime, S., Bhat, A. A., & Uddin, S. (2022). Guggulsterone Induces Apoptosis in Multiple Myeloma Cells by Targeting High Mobility Group Box 1 via Janus Activated Kinase/Signal Transducer and Activator of Transcription Pathway. Cancers, 14(22), 5621. https://doi.org/10.3390/cancers14225621