A Narrative Review of the Usefulness of Indocyanine Green Fluorescence Angiography for Perfusion Assessment in Colorectal Surgery
Abstract
Simple Summary
Abstract
1. Introduction
2. Assessment of Tissue Perfusion at the Anastomotic Site
2.1. Anastomotic Leak in Colorectal Surgery
2.2. Perfusion Assessment in Colonic Stumps
3. Subjective Assessment of Colon Perfusion Using ICG-Fluorescence Angiography
3.1. Non-Randomized Studies
3.2. Randomized Controlled Trials
| First Author | Year of Publication (Study Interval) | Country | Study Design | N (ICG/Control) | Age (ICG/Control) | Sex, Male (%) (ICG/Control) | Patient Type of Surgery | ICG Dose | NIR System | Transection Line Change (%) | AL (%) (ICG/Control) | p Value * for AL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kudszus S [39] | 2010 (1998–2008) | Germany | Retrospective, Single-center, Case-matched | 402 (201/201) | 67.8/69.0 | 42.2/42.2 | Colorectal cancer (Rt, Lt, AR) | 0.2–0.5 mg/kg | IC-View (Pulsion) | 13.9 | 3.5/7.5 | NA |
| Jafari MD [40] | 2015 (2012–2013) | USA | Prospective, Multi-center (PILLAR-II) | 139 (139/-) | 58/- | 53.2/- | Colorectal cancer Benign disease (Lap-Lt, AR) | 3.75–7.5 mg/body | PINPOINT (Novadaq) | 8 | 1.4/- | - |
| Kin C [41] | 2015 (2005–2012) | USA | Retrospective, Single-center, Case-matched | 346 (173/173) | 58.1/58.2 | 54/54 | Colorectal cancer Benign disease (Lt, AR) | 7.5 mg/body | SPY Elite (Novadaq) | 5 | 7.5/6.4 | 0.67 |
| Boni L [48] | 2017 (2012–2015) | Italy | Retrospective, Single-center | 80 (42/38) | 69/67 | 66.7/57.9 | Colorectal cancer (Lap-LAR) | 0.2 mg/kg | IMAGE1 (Karl Storz) | 4.7 | 0/5 | NS |
| Kim JC [42] | 2017 (2010–2016) | Korea | Retrospective, Single-center | 657 (310/347) | 58/57 | 58.9/62.2 | Rectal cancer (Rob-LAR, ISR) | 10 mg/body | Da Vinci firefly | N.A. | 0.6/5.2 | <0.001 |
| Brescia A [49] | 2018 (2014–2017) | Italy | Prospective, Single-center | 182 (75/107) | 67.1/65.7 | 57.3/58.9 | Colorectal cancer Benign disease (Lap-Rt, Lt, AR) | 0.25 mg/kg | SPIES (Karl Storz) | 6.6 | 0/5.6 | 0.03 |
| Ris F [43] | 2018 (2013–2016) | Switzerland Ireland UK | Prospective, Multi-center | 1677 (504/1173) | 64/NA | 55.4/NA | Colorectal cancer Benign disease (Rt, AR) | 7.5 mg/body | PINPOINT | 5.8 | 2.6/5.8 | 0.009 |
| Dinallo AM [44] | 2019 (2010–2016) | USA | Retrospective, Single-center | 554 (234/320) | 61.5/62.5 | 45.2/43.1 | Colorectal resection (Rt, Lt, LAR, total,) | 5 mg/body | SPY Elite | 5.6 | 1.3/1.3 | >0.05 |
| Shapera E [50] | 2019 (2012–2018) | USA | Retrospective, Single-center | 104 (74/30) | 58/60 | 56.8/56.7 | Colorectal cancer Benign disease (Rob-Lt, AR) | 25 mg/body | Da Vinci firefly | 5.4 | 0/3.3 | 0.289 |
| Wada T [51] | 2019 (2009–2016) | Japan | Retrospective, Single-center, PSM | 68 (34/34) | 67.5/66.5 | 58.8/70.6 | Rectal cancer (Lap-LAR) | 5 mg/body | PDE-neo (Hamamatsu Photonics) | 27.1 | 8.8/14.7 | 0.71 |
| De Nardi P [61] | 2020 (2016–2017) | Italy | RCT, Multi-center | 240 (118/122) | 66.1/65.1 | 50.8/54.1 | Colorectal cancer Benign disease (Lap-AR) | 0.3 mg/kg | D-light P (Karl Storz) | 11 | 5/9 | 0.2 |
| Alekseev M [62] | 2020 (2018–2019) | Russia | RCT (FLAG trial), Single-center | 377 (187/190) | 63/63 | 49.2/48.4 | Colorectal cancer Benign disease (S and AR) | 0.2 mg/kg | D-light P | 19.3 | 9.1/16.3 | 0.04 |
| Foo CC [45] | 2020 (2013–2018) | China | Retrospective, Single-center, PSM | 506 (253/253) | 66.6/67.2 | 65.6/64.4 | Rectal cancer Benign disease (Lap-AR) | 5–7.5 mg/body | PINPOINT SPY Elite DaVinci firefly | 20.9 | 3.6/7.9 | 0.035 |
| Hasegawa H [46] | 2020 (2007–2017) | Japan | Retrospective, Single-center | 420 (141/279) | 63/63 | 70.2/72.8 | Malignant rectal tumor (Lap-LAR) | 5 mg/body | IMAGE1 1588 AIM Hyper Eye | 17.0 | 2.8/13.6 | 0.001 |
| Impellizzeri HG [52] | 2020 (2014–2019) | Italy | Retrospective, Single-center | 196 (98/98) | 66/71 | 55/58 | Colorectal cancer Benign disease (Lt, AR, LAR) | 12.5 mg/body | D-light P | 8 | 0/6 | 0.029 |
| Ishii M [53] | 2020 (2014–2018) | Japan | Retrospective, Single-center, PSM | 174 (87/87) | 64/65 | 56.3/57.5 | Rectal cancer (Lap/Rob-LAR, ISR) | 5 mg/body | N.A. | 3.1 | 3.4/11.5 | 0.044 |
| Skrovina M [54] | 2020 (2015–2017) | Czech Republic | Retrospective, Single-center | 100 (50/50) | 64/66 | 68/58 | Rectal cancer (Lap/Rob-LAR, ISR) | 0.2 mg/kg | SPIES Da Vinci Firefly | 12 | 10/18 | 0.163 |
| Tsang YP [55] | 2020 (2018–2019) | China | Prospective, Single-center | 131 (62/69) | 69.8/67.7 | 62.9/68.1 | Colorectal cancer Benign disease (Lt, AR, LAR) | 10 mg/body | CLV-S200-IR (Olympus) Da Vinci Firefly | 1.6 | 3.23/4.35 | 1.000 |
| Watanabe J [47] | 2020 (2014–2017) | Japan | Retrospective, Multi-center, PSM | 422 (211/211) | 66/66 | 60.7/62.1 | Rectal cancer (Lap-LAR) | 0.25 mg/kg | 1588 AIM (Stryker) D-light P | 5.7 | 4.7/10.4 | 0.042 |
| Wojcik M [56] | 2020 (2017–2018) | France | Prospective, Case-matched | 84 (42/42) | 67/69 | 69/69 | Colorectal cancer (Lap-AR, LAR) | 0.1 mg/kg | PINPOINT | 10.9 | 2.4/16.7 | 0.026 |
| Jafari MD [63] | 2021 (2015–2017) | USA | RCT (PILLAR-III), Multi-center | 347 (178/167) | 57.2/57.0 | 61.2/58.6 | Rectal cancer (AR) | 5–10 mg/body | PINPOINT SPY Elite | N.A. | 9.0/9.6 | 0.37 |
| T Yanagita [57] | 2021 (2011–2018) | Japan | Retrospective, Single-center, PSM | 186 (93/93) | NA/NA | NA/NA | Colorectal cancer (Lt, AR) | 0.1 mg/kg | Hyper Eye (Mizuho) IMAGE1 | 9.1 | 3.2/10.8 | 0.046 |
| Otero-Pineiro AM [58] | 2021 (2011–2018) | Spain | Retrospective, Single-center | 284 (80/204) | 68.0/66.6 | 63.7/60.3 | Rectal cancer (taTME-AR) | N.A. | PINPOINT | 28.7 | 2.5/11.3 | 0.020 |
| Hasegawa H [59] | 2022 (2010–2016) | Japan | Retrospective, Single-center, PSM | 169 (66/103) | NA/NA | 75.8/78.6 | Rectal cancer (Lap-ISR) | 5 mg/body | IMAGE1 SPY Hyper Eye | 30.0 | 0/14.6 | 0.001 |
| Neddermeyer M [60] | 2022 (2017–2020) | Germany | Retrospective, Single-center | 132 (70/62) | 66.5/59.5 | 68.6/62.9 | Colorectal cancer (S, AR) | 25 mg/body | PINPOINT | 12.9 | 1.4/14.5 | 0.006 |
3.3. Discussion of Clinical Studies with Subjective Assessment of ICG-Fluorescence Angiography
4. Quantitative Assessment of Colon Perfusion Using ICG-Fluorescence Angiography
4.1. Limitations of Subjective Assessment
4.2. Methods for Quantitative Assessment of ICG-Fluorescence Angiography
4.3. Clinical Studies with Quantitative Assessment of ICG-Fluorescence Angiography
4.4. Discussion of Clinical Studies with Quantitative Assessment of ICG-Fluorescence Angiography
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—Technical notes and outcome. Color. Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef]
- Gouvas, N.; Agalianos, C.; Papaparaskeva, K.; Perrakis, A.; Hohenberger, W.; Xynos, E. Surgery along the embryological planes for colon cancer: A systematic review of complete mesocolic excision. Int. J. Colorectal Dis. 2016, 31, 1577–1594. [Google Scholar] [CrossRef]
- Heald, R.J.; Husband, E.M.; Ryall, R.D. The mesorectum in rectal cancer surgery—The clue to pelvic recurrence? Br. J. Surg. 1982, 69, 613–616. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Putter, H.; van de Velde, C.J.H. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br. J. Surg. 2002, 89, 1142–1149. [Google Scholar] [CrossRef]
- Nelson, H.; Sargent, D.J.; Wieand, H.S.; Fleshman, J.; Anvari, M.; Stryker, S.J.; Beart, R.W., Jr.; Hellinger, M.; Flanagan, R.; Peters, W.; et al. Laparoscopically assisted colectomy is as safe and effective as open colectomy in people with colon cancer. N. Engl. J. Med. 2004, 350, 2050–2059. [Google Scholar] [CrossRef]
- Veldkamp, R.; Kuhry, E.; Hop, W.C.; Jeekel, J.; Kazemier, G.; Bonjer, H.J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Laparoscopic surgery versus open surgery for colon cancer: Short-term outcomes of a randomised trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef]
- Buunen, M.; Veldkamp, R.; Hop, W.C.; Kuhry, E.; Jeekel, J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; Morino, M.; et al. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef]
- Soriano, C.; Bahnson, H.T.; Kaplan, J.A.; Lin, B.; Moonka, R.; Pham, H.T.; Kennecke, H.F.; Simianu, V. Contemporary, national patterns of surgery after preoperative therapy for stage II/III rectal adenocarcinoma. World J. Gastrointest. Oncol. 2022, 14, 1148–1161. [Google Scholar] [CrossRef]
- Lee, G.C.; Bordeianou, L.G.; Francone, T.D.; Blaszkowsky, L.S.; Goldstone, R.N.; Ricciardi, R.; Kunitake, H.; Qadan, M. Superior pathologic and clinical outcomes after minimally invasive rectal cancer resection, compared to open resection. Surg. Endosc. 2020, 34, 3435–3448. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse Reactions due to Indocyanine Green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef]
- Guyer, D.R.; Puliafito, C.A.; Monés, J.M.; Friedman, E.; Chang, W.; Verdooner, S.R. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology 1992, 99, 287–291. [Google Scholar] [CrossRef]
- Namikawa, T.; Sato, T.; Hanazaki, K. Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green. Surg. Today 2015, 45, 1467–1474. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Mieog, J.S.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef]
- Watanabe, J.; Ota, M.; Suwa, Y.; Ishibe, A.; Masui, H.; Nagahori, K. Real-Time Indocyanine Green Fluorescence Imaging-Guided Complete Mesocolic Excision in Laparoscopic Flexural Colon Cancer Surgery. Dis. Colon Rectum 2016, 59, 701–705. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.S.; Kim, H.J.; Woo, I.T.; Park, I.K.; Choi, G.S. Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Surgery Could Achieve Radical D3 Dissection in Patients With Advanced Right-Sided Colon Cancer. Dis. Colon Rectum 2020, 63, 441–449. [Google Scholar] [CrossRef]
- Zhou, S.C.; Tian, Y.T.; Wang, X.W.; Zhao, C.D.; Ma, S.; Jiang, J.; Li, E.N.; Zhou, H.T.; Liu, Q.; Liang, J.W.; et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J. Gastroenterol. 2019, 25, 4502–4511. [Google Scholar] [CrossRef]
- Nagata, J.; Fukunaga, Y.; Akiyoshi, T.; Konishi, T.; Fujimoto, Y.; Nagayama, S.; Yamamoto, N.; Ueno, M. Colonic Marking With Near-Infrared, Light-Emitting, Diode-Activated Indocyanine Green for Laparoscopic Colorectal Surgery. Dis. Colon Rectum 2016, 59, e14–e18. [Google Scholar] [CrossRef]
- Miyoshi, N.; Ohue, M.; Noura, S.; Yano, M.; Sasaki, Y.; Kishi, K.; Yamada, T.; Miyashiro, I.; Ohigashi, H.; Iishi, H.; et al. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg. Endosc. 2009, 23, 347–351. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, H.S.; Kwon, I.S.; Yun, G.Y.; Lee, S.H.; Park, D.H.; Kim, J.S.; Kang, S.H.; Lee, E.S.; Kim, S.H.; et al. Usefulness of colonic tattooing using indocyanine green in patients with colorectal tumors. World J. Clin. Cases 2018, 6, 632–640. [Google Scholar] [CrossRef]
- Barnes, T.G.; Penna, M.; Hompes, R.; Cunningham, C. Fluorescence to highlight the urethra: A human cadaveric study. Tech. Coloproctol. 2017, 21, 439–444. [Google Scholar] [CrossRef]
- Mandovra, P.; Kalikar, V.; Patankar, R.V. Real-Time Visualization of Ureters Using Indocyanine Green During Laparoscopic Surgeries: Can We Make Surgery Safer? Surg. Innov. 2019, 26, 464–468. [Google Scholar] [CrossRef]
- Frasson, M.; Flor-Lorente, B.; Rodríguez, J.L.; Granero-Castro, P.; Hervás, D.; Alvarez Rico, M.A.; Brao, M.J.; Sánchez González, J.M.; Garcia-Granero, E. Risk Factors for Anastomotic Leak After Colon Resection for Cancer: Multivariate Analysis and Nomogram from a Multicentric, Prospective, National Study With 3193 Patients. Ann. Surg. 2015, 262, 321–330. [Google Scholar] [CrossRef]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef]
- Shogan, B.D.; Carlisle, E.M.; Alverdy, J.C.; Umanskiy, K. Do we really know why colorectal anastomoses leak? J. Gastrointest. Surg. 2013, 17, 1698–1707. [Google Scholar] [CrossRef]
- Snijders, H.S.; Wouters, M.W.; van Leersum, N.J.; Kolfschoten, N.E.; Henneman, D.; de Vries, A.C.; Tollenaar, R.A.; Bonsing, B.A. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur. J. Surg. Oncol. 2012, 38, 1013–1019. [Google Scholar] [CrossRef]
- Hammond, J.; Lim, S.; Wan, Y.; Gao, X.; Patkar, A. The burden of gastrointestinal anastomotic leaks: An evaluation of clinical and economic outcomes. J. Gastrointest. Surg. 2014, 18, 1176–1185. [Google Scholar] [CrossRef]
- La Regina, D.; Di Giuseppe, M.; Lucchelli, M.; Saporito, A.; Boni, L.; Efthymiou, C.; Cafarotti, S.; Marengo, M.; Mongelli, F. Financial Impact of Anastomotic Leakage in Colorectal Surgery. J. Gastrointest. Surg. 2019, 23, 580–586. [Google Scholar] [CrossRef]
- Mirnezami, A.; Mirnezami, R.; Chandrakumaran, K.; Sasapu, K.; Sagar, P.; Finan, P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: Systematic review and meta-analysis. Ann. Surg. 2011, 253, 890–899. [Google Scholar] [CrossRef]
- Karim, A.; Cubas, V.; Zaman, S.; Khan, S.; Patel, H.; Waterland, P. Anastomotic leak and cancer-specific outcomes after curative rectal cancer surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2020, 24, 513–525. [Google Scholar] [CrossRef]
- Ha, G.W.; Kim, J.H.; Lee, M.R. Oncologic Impact of Anastomotic Leakage Following Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 3289–3299. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Karliczek, A.; Harlaar, N.J.; Zeebregts, C.J.; Wiggers, T.; Baas, P.C.; van Dam, G.M. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int. J. Color. Dis. 2009, 24, 569–576. [Google Scholar] [CrossRef]
- Kudszus, S.; Roesel, C.; Schachtrupp, A.; Höer, J.J. Intraoperative laser fluorescence angiography in colorectal surgery: A noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s Arch. Surg. 2010, 395, 1025–1030. [Google Scholar] [CrossRef]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): A multi-institutional study. J. Am. Coll. Surg. 2015, 220, 82–89. [Google Scholar] [CrossRef]
- Kin, C.; Vo, H.; Welton, L.; Welton, M. Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis. Colon Rectum 2015, 58, 582–587. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, J.L.; Park, S.H. Interpretative Guidelines and Possible Indications for Indocyanine Green Fluorescence Imaging in Robot-Assisted Sphincter-Saving Operations. Dis. Colon Rectum 2017, 60, 376–384. [Google Scholar] [CrossRef]
- Ris, F.; Liot, E.; Buchs, N.C.; Kraus, R.; Ismael, G.; Belfontali, V.; Douissard, J.; Cunningham, C.; Lindsey, I.; Guy, R.; et al. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br. J. Surg. 2018, 105, 1359–1367. [Google Scholar] [CrossRef]
- Dinallo, A.M.; Kolarsick, P.; Boyan, W.P.; Protyniak, B.; James, A.; Dressner, R.M.; Arvanitis, M.L. Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks? A retrospective cohort analysis. Am. J. Surg. 2019, 218, 136–139. [Google Scholar] [CrossRef]
- Foo, C.C.; Ng, K.K.; Tsang, J.; Wei, R.; Chow, F.; Chan, T.Y.; Lo, O.; Law, W.L. Colonic perfusion assessment with indocyanine-green fluorescence imaging in anterior resections: A propensity score-matched analysis. Tech. Coloproctol. 2020, 24, 935–942. [Google Scholar] [CrossRef]
- Hasegawa, H.; Tsukada, Y.; Wakabayashi, M.; Nomura, S.; Sasaki, T.; Nishizawa, Y.; Ikeda, K.; Akimoto, T.; Ito, M. Impact of intraoperative indocyanine green fluorescence angiography on anastomotic leakage after laparoscopic sphincter-sparing surgery for malignant rectal tumors. Int. J. Color. Dis. 2020, 35, 471–480. [Google Scholar] [CrossRef]
- Watanabe, J.; Ishibe, A.; Suwa, Y.; Suwa, H.; Ota, M.; Kunisaki, C.; Endo, I. Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: A propensity score-matched cohort study. Surg. Endosc. 2020, 34, 202–208. [Google Scholar] [CrossRef]
- Boni, L.; Fingerhut, A.; Marzorati, A.; Rausei, S.; Dionigi, G.; Cassinotti, E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: Results of a case-matched study. Surg. Endosc. 2017, 31, 1836–1840. [Google Scholar] [CrossRef]
- Brescia, A.; Pezzatini, M.; Romeo, G.; Cinquepalmi, M.; Pindozzi, F.; Dall’Oglio, A.; Gasparrini, M.; Lazar, F. Indocyanine green fluorescence angiography: A new ERAS item. Updates Surg. 2018, 70, 427–432. [Google Scholar] [CrossRef]
- Shapera, E.; Hsiung, R.W. Assessment of Anastomotic Perfusion in Left-Sided Robotic Assisted Colorectal Resection by Indocyanine Green Fluorescence Angiography. Minim. Invasive Surg. 2019, 2019, 3267217. [Google Scholar] [CrossRef]
- Wada, T.; Kawada, K.; Hoshino, N.; Inamoto, S.; Yoshitomi, M.; Hida, K.; Sakai, Y. The effects of intraoperative ICG fluorescence angiography in laparoscopic low anterior resection: A propensity score-matched study. Int. J. Clin. Oncol. 2019, 24, 394–402. [Google Scholar] [CrossRef]
- Impellizzeri, H.G.; Pulvirenti, A.; Inama, M.; Bacchion, M.; Marrano, E.; Creciun, M.; Casaril, A.; Moretto, G. Near-infrared fluorescence angiography for colorectal surgery is associated with a reduction of anastomotic leak rate. Updates Surg. 2020, 72, 991–998. [Google Scholar] [CrossRef]
- Ishii, M.; Hamabe, A.; Okita, K.; Nishidate, T.; Okuya, K.; Usui, A.; Akizuki, E.; Satoyoshi, T.; Takemasa, I. Efficacy of indocyanine green fluorescence angiography in preventing anastomotic leakage after laparoscopic colorectal cancer surgery. Int. J. Colorectal Dis. 2020, 35, 269–275. [Google Scholar] [CrossRef]
- Skrovina, M.; Bencurik, V.; Martinek, L.; Machackova, M.; Bartos, J.; Andel, P.; Stepanova, E.; Bunakova, M.; Vomackova, K. The significance of intraoperative fluorescence angiography in miniinvasive low rectal resections. Videosurgery Other Miniinvasive Tech. 2020, 15, 43–48. [Google Scholar] [CrossRef]
- Tsang, Y.P.; Leung, L.A.; Lau, C.W.; Tang, C.N. Indocyanine green fluorescence angiography to evaluate anastomotic perfusion in colorectal surgery. Int. J. Color. Dis. 2020, 35, 1133–1139. [Google Scholar] [CrossRef]
- Wojcik, M.; Doussot, A.; Manfredelli, S.; Duclos, C.; Paquette, B.; Turco, C.; Heyd, B.; Lakkis, Z. Intra-operative fluorescence angiography is reproducible and reduces the rate of anastomotic leak after colorectal resection for cancer: A prospective case-matched study. Color. Dis. 2020, 22, 1263–1270. [Google Scholar] [CrossRef]
- Yanagita, T.; Hara, M.; Osaga, S.; Nakai, N.; Maeda, Y.; Shiga, K.; Hirokawa, T.; Matsuo, Y.; Takahashi, H.; Takiguchi, S. Efficacy of intraoperative ICG fluorescence imaging evaluation for preventing anastomotic leakage after left-sided colon or rectal cancer surgery: A propensity score-matched analysis. Surg. Endosc. 2021, 35, 2373–2385. [Google Scholar] [CrossRef]
- Otero-Piñeiro, A.M.; de Lacy, F.B.; Van Laarhoven, J.J.; Martín-Perez, B.; Valverde, S.; Bravo, R.; Lacy, A.M. The impact of fluorescence angiography on anastomotic leak rate following transanal total mesorectal excision for rectal cancer: A comparative study. Surg. Endosc. 2021, 35, 754–762. [Google Scholar] [CrossRef]
- Hasegawa, H.; Tsukada, Y.; Wakabayashi, M.; Nomura, S.; Sasaki, T.; Nishizawa, Y.; Ikeda, K.; Takeshita, N.; Teramura, K.; Ito, M. Impact of near-infrared fluorescence imaging with indocyanine green on structural sequelae of anastomotic leakage after laparoscopic intersphincteric resection of malignant rectal tumors. Tech. Coloproctol. 2022, 26, 561–570. [Google Scholar] [CrossRef]
- Neddermeyer, M.; Kanngießer, V.; Maurer, E.; Bartsch, D.K. Indocyanine Green Near-Infrared Fluoroangiography Is a Useful Tool in Reducing the Risk of Anastomotic Leakage Following Left Colectomy. Front. Surg. 2022, 9, 850256. [Google Scholar] [CrossRef]
- De Nardi, P.; Elmore, U.; Maggi, G.; Maggiore, R.; Boni, L.; Cassinotti, E.; Fumagalli, U.; Gardani, M.; De Pascale, S.; Parise, P.; et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: Results of a multicenter randomized controlled trial. Surg. Endosc. 2020, 34, 53–60. [Google Scholar] [CrossRef]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Color. Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef]
- Jafari, M.D.; Pigazzi, A.; McLemore, E.C.; Mutch, M.G.; Haas, E.; Rasheid, S.H.; Wait, A.D.; Paquette, I.M.; Bardakcioglu, O.; Safar, B.; et al. Perfusion Assessment in Left-Sided/Low Anterior Resection (PILLAR III): A Randomized, Controlled, Parallel, Multicenter Study Assessing Perfusion Outcomes With PINPOINT Near-Infrared Fluorescence Imaging in Low Anterior Resection. Dis. Colon Rectum 2021, 64, 995–1002. [Google Scholar] [CrossRef]
- Takemasa, I.; Watanabe, J.; Kotake, M.; Noura, S.; Ikeda, M.; Suwa, H.; Tei, M.; Takano, Y.; Munakata, K.; Matoba, S.; et al. Randomized Phase III Trial Evaluating the Efficacy of ICG Fluorescence Imaging on Anastomotic Leakage in Laparoscopic Surgery for Rectal Cancer (EssentiAL study). In Proceedings of the 30th International Congress of the European Association for Endoscopic Surgery, Krakow, Poland, 5–8 July 2022. [Google Scholar]
- Meijer, R.P.J.; Faber, R.A.; Bijlstra, O.D.; Braak, J.P.B.M.; Meershoek-Klein Kranenbarg, E.; Putter, H.; Mieog, J.S.D.; Burggraaf, K.; Vahrmeijer, A.L.; Hilling, D.E. AVOID; a phase III, randomised controlled trial using indocyanine green for the prevention of anastomotic leakage in colorectal surgery. BMJ Open 2022, 12, e051144. [Google Scholar] [CrossRef]
- Armstrong, G.; Croft, J.; Corrigan, N.; Brown, J.M.; Goh, V.; Quirke, P.; Hulme, C.; Tolan, D.; Kirby, A.; Cahill, R.; et al. IntAct: Intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: A randomized controlled trial. Color. Dis. 2018, 20, O226–O234. [Google Scholar] [CrossRef]
- Hardy, N.P.; Dalli, J.; Khan, M.F.; Andrejevic, P.; Neary, P.M.; Cahill, R.A. Inter-user variation in the interpretation of near infrared perfusion imaging using indocyanine green in colorectal surgery. Surg. Endosc. 2021, 35, 7074–7081. [Google Scholar] [CrossRef]
- Wada, T.; Kawada, K.; Takahashi, R.; Yoshitomi, M.; Hida, K.; Hasegawa, S.; Sakai, Y. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg. Endosc. 2017, 31, 4184–4193. [Google Scholar] [CrossRef]
- Son, G.M.; Kwon, M.S.; Kim, Y.; Kim, J.; Kim, S.H.; Lee, J.W. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg. Endosc. 2019, 33, 1640–1649. [Google Scholar] [CrossRef]
- Hayami, S.; Matsuda, K.; Iwamoto, H.; Ueno, M.; Kawai, M.; Hirono, S.; Okada, K.; Miyazawa, M.; Tamura, K.; Mitani, Y.; et al. Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech. Coloproctol. 2019, 23, 973–980. [Google Scholar] [CrossRef]
- Iwamoto, H.; Matsuda, K.; Hayami, S.; Tamura, K.; Mitani, Y.; Mizumoto, Y.; Nakamura, Y.; Murakami, D.; Ueno, M.; Yokoyama, S.; et al. Quantitative Indocyanine Green Fluorescence Imaging Used to Predict Anastomotic Leakage Focused on Rectal Stump During Laparoscopic Anterior Resection. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 542–546. [Google Scholar] [CrossRef]
- Amagai, H.; Miyauchi, H.; Muto, Y.; Uesato, M.; Ohira, G.; Imanishi, S.; Maruyama, T.; Tochigi, T.; Okada, K.; Maruyama, M.; et al. Clinical utility of transanal indocyanine green near-infrared fluorescence imaging for evaluation of colorectal anastomotic perfusion. Surg. Endosc. 2020, 34, 5283–5293. [Google Scholar] [CrossRef]
- Gomez-Rosado, J.C.; Valdes-Hernandez, J.; Cintas-Catena, J.; Cano-Matias, A.; Perez-Sanchez, A.; Del Rio-Lafuente, F.J.; Torres-Arcos, C.; Lara-Fernandez, Y.; Capitan-Morales, L.C.; Oliva-Mompean, F. Feasibility of quantitative analysis of colonic perfusion using indocyanine green to prevent anastomotic leak in colorectal surgery. Surg. Endosc. 2022, 36, 1688–1695. [Google Scholar] [CrossRef]
- Soares, A.S.; Bano, S.; Clancy, N.T.; Stoyanov, D.; Lovat, L.B.; Chand, M. Multisensor perfusion assessment cohort study: Preliminary evidence toward a standardized assessment of indocyanine green fluorescence in colorectal surgery. Surgery 2022, 172, 69–73. [Google Scholar] [CrossRef]
- Serra-Aracil, X.; Lucas-Guerrero, V.; Garcia-Nalda, A.; Mora-López, L.; Pallisera-Lloveras, A.; Serracant, A.; Navarro-Soto, S. When should indocyanine green be assessed in colorectal surgery, and at what distance from the tissue? Quantitative measurement using the SERGREEN program. Surg. Endosc. 2022, 36, 8943–8949. [Google Scholar] [CrossRef]
- Ahn, H.M.; Son, G.M.; Lee, I.Y.; Park, S.H.; Kim, N.S.; Baek, K.R. Optimization of indocyanine green angiography for colon perfusion during laparoscopic colorectal surgery. Color. Dis. 2021, 23, 1848–1859. [Google Scholar] [CrossRef]
- Spota, A.; Al-Taher, M.; Felli, E.; Morales Conde, S.; Dal Dosso, I.; Moretto, G.; Spinoglio, G.; Baiocchi, G.; Vilallonga, R.; Impellizzeri, H.; et al. Fluorescence-based bowel anastomosis perfusion evaluation: Results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg. Endosc. 2021, 35, 7142–7153. [Google Scholar] [CrossRef]
- Nwaiwu, C.A.; Buharin, V.E.; Mach, A.; Grandl, R.; King, M.L.; Dechert, A.F.; O’Shea, L.; Schwaitzberg, S.D.; Kim, P.C.W. Feasibility and comparison of laparoscopic laser speckle contrast imaging to near-infrared display of indocyanine green in intraoperative tissue blood flow/tissue perfusion in preclinical porcine models. Surg. Endosc. 2022, 233, S78–S79. [Google Scholar] [CrossRef]
- Protyniak, B.; Dinallo, A.M.; Boyan, W.P., Jr.; Dressner, R.M.; Arvanitis, M.L. Intraoperative Indocyanine Green Fluorescence Angiography—An Objective Evaluation of Anastomotic Perfusion in Colorectal Surgery. Am. Surg. 2015, 81, 580–584. [Google Scholar] [CrossRef]
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef]
- Arpaia, P.; Bracale, U.; Corcione, F.; De Benedetto, E.; Di Bernardo, A.; Di Capua, V.; Duraccio, L.; Peltrini, R.; Prevete, R. Assessment of blood perfusion quality in laparoscopic colorectal surgery by means of Machine Learning. Sci. Rep. 2022, 12, 14682. [Google Scholar] [CrossRef]
- Park, S.H.; Park, H.M.; Baek, K.R.; Ahn, H.M.; Lee, I.Y.; Son, G.M. Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery. World J. Gastroenterol. 2020, 26, 6945–6962. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Shah, S.K.; Sanders, C.M.; Nwaiwu, C.A.; Dechert, A.F.; Mehrotra, S.; Schwaitzberg, S.D.; Kim, P.C.W.; Wilson, E.B. Utility and usability of laser speckle contrast imaging (LSCI) for displaying real-time tissue perfusion/blood flow in robot-assisted surgery (RAS): Comparison to indocyanine green (ICG) and use in laparoscopic surgery. Surg. Endosc. ahead of print. 2022. [Google Scholar] [CrossRef]

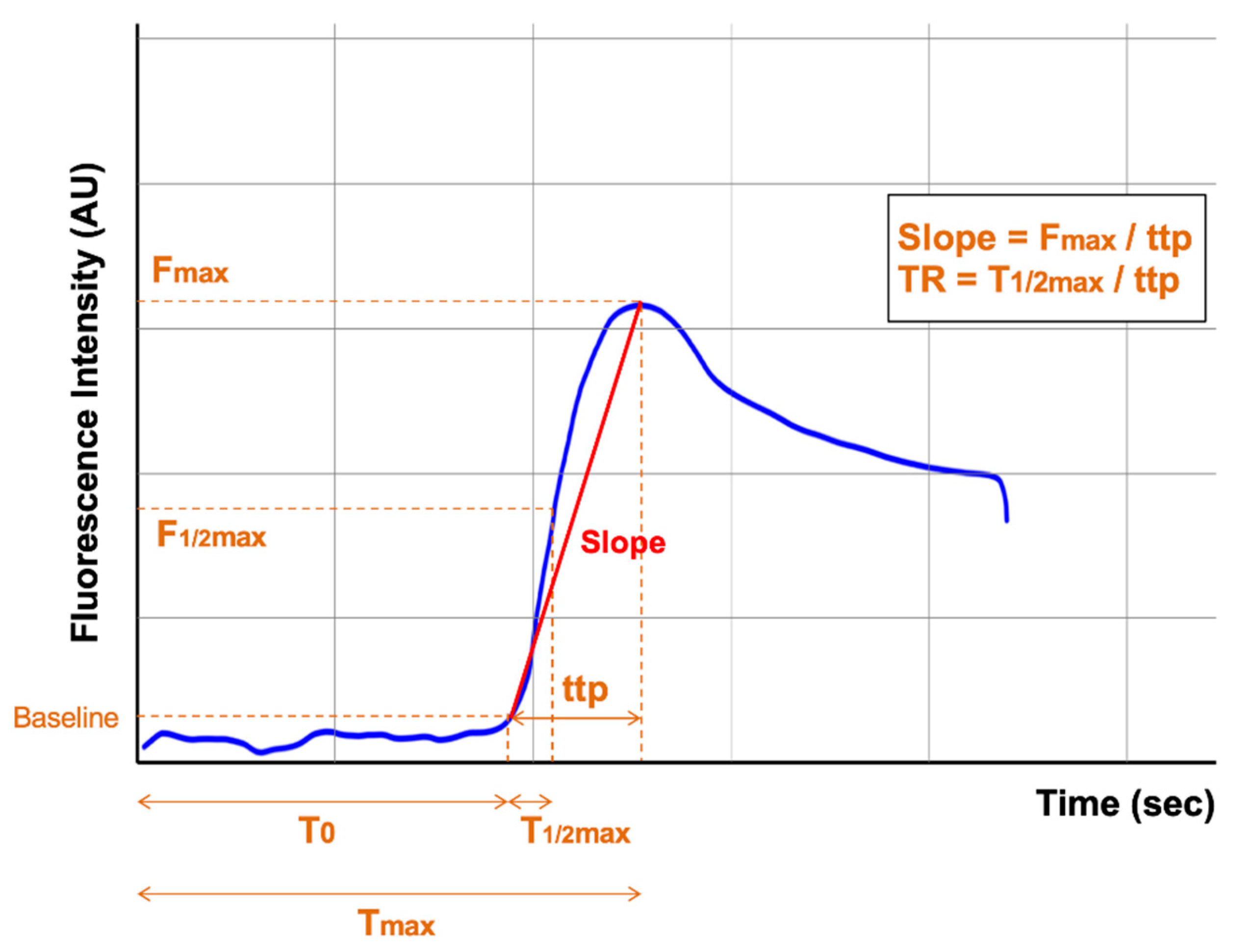
| First Author | Year | ICG Dose | Imaging System (Analysis Software) | Intensity Parameter | AL | Non-AL- | p Value | Inflow Parameter | AL | Non-AL- | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gomez-Rosado JC [73] | 2021 | 7.5 mg /body | ElevisionTM IR Platform (Not shown) | Fmax (AU) | 151 (±13.1) | 169 (±24.0) | 0.03 | Tmax (s) Slope (AU/s) T0 (s) | 47.6 (±20.7) 10.8 (±6.2) 19.9 (±14.3) | 35.5 (±16.6) 17.4 (±7.4) 19.8 (±11.8) | 0.10 0.03 0.98 |
| Amagai H [72] | 2020 | 0.2 mg /kg | Olympus (Image J) | Fmax (AU) | Not shown | Not shown | 0.380 | ttp (s) Tmax | 52.8 (±30.3) 74.3 (±42.3) | 30.0 (±15.0) 45.4 (±21.4) | 0.040 0.015 |
| Iwamoto H [71] | 2020 | 7.5 mg /body | PINPOINT (ROIs) | - | - | - | - | ttp (s) T0 (s) | 11.5 (±7.3) 37.5 (±17.1) | 12.0 (±9.3) 11.0 (±13.1) | 0.85 0.03 |
| Hayami S [70] | 2019 | 5 mg /body | D-light P (ROIs) | Fmax (AU) | 79.9 (±28.5) | 87.6 (±33.2) | 0.42 | ttp (s) T1/2max (s) Slope (AU/s) T0 (s) | 26.4 (±8.4) 13.3 (±4.9) 3.4 (±2.0) 64.3 (±27.6) | 18.6 (±6.2) 7.8 (±2.9) 5.5 (±2.8) 18.2 (±6.6) | 0.09 0.12 0.27 0.0022 |
| Son GM [69] | 2019 | 0.25 mg /kg | IMAGE1 STM (Tracker 4.97) | Fmax (AU) | 34.9 (±7.4) | 58.0 (±3.4) | 0.074 | ttp (s) T1/2max (s) Slope (AU/s) TR | 64.0 (±11.7) 40.37 (±7.8) 0.7 (±0.2) 0.6 (±0.0) | 30.3 (±2.3) 11.7 (±0.8) 2.5 (±0.2) 0.4 (±0.0) | <0.001 <0.001 <0.001 <0.001 |
| Wada T [68] | 2017 | 5 mg /body | PDE-neo (ROIs) | Fmax (AU) | 38.1 (±11.4) | 91.4 (±31.9) | NA | ttp (s) T1/2max (s) Slope (AU/s) | 52.1 (±28.5) 26.1 (±18.9) 0.98 (±0.7) | 32.8 (±15.9) 12.5 (±7.6) 3.6 (±2.2) | NA NA NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, M.; Ueda, K.; Kawamura, J. A Narrative Review of the Usefulness of Indocyanine Green Fluorescence Angiography for Perfusion Assessment in Colorectal Surgery. Cancers 2022, 14, 5623. https://doi.org/10.3390/cancers14225623
Iwamoto M, Ueda K, Kawamura J. A Narrative Review of the Usefulness of Indocyanine Green Fluorescence Angiography for Perfusion Assessment in Colorectal Surgery. Cancers. 2022; 14(22):5623. https://doi.org/10.3390/cancers14225623
Chicago/Turabian StyleIwamoto, Masayoshi, Kazuki Ueda, and Junichiro Kawamura. 2022. "A Narrative Review of the Usefulness of Indocyanine Green Fluorescence Angiography for Perfusion Assessment in Colorectal Surgery" Cancers 14, no. 22: 5623. https://doi.org/10.3390/cancers14225623
APA StyleIwamoto, M., Ueda, K., & Kawamura, J. (2022). A Narrative Review of the Usefulness of Indocyanine Green Fluorescence Angiography for Perfusion Assessment in Colorectal Surgery. Cancers, 14(22), 5623. https://doi.org/10.3390/cancers14225623





