Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Cell Extracts and Immunoblot Analysis
2.3. Antibody Array Assay
2.4. Generation of AnxA2 Knockdown in Bladder Cancer Cells
2.5. Cell Proliferation Assay
2.6. Cell Migration Assay
2.7. Transwell Invasion Assay
2.8. Immunoprecipitation
2.9. Elution of Cell Surface AnxA2
2.10. Cell Surface Biotinylation
2.11. Plasmin Generation Assay
2.12. RNA Expression Data for BLCA Patients
2.13. Statistical Analysis
3. Results
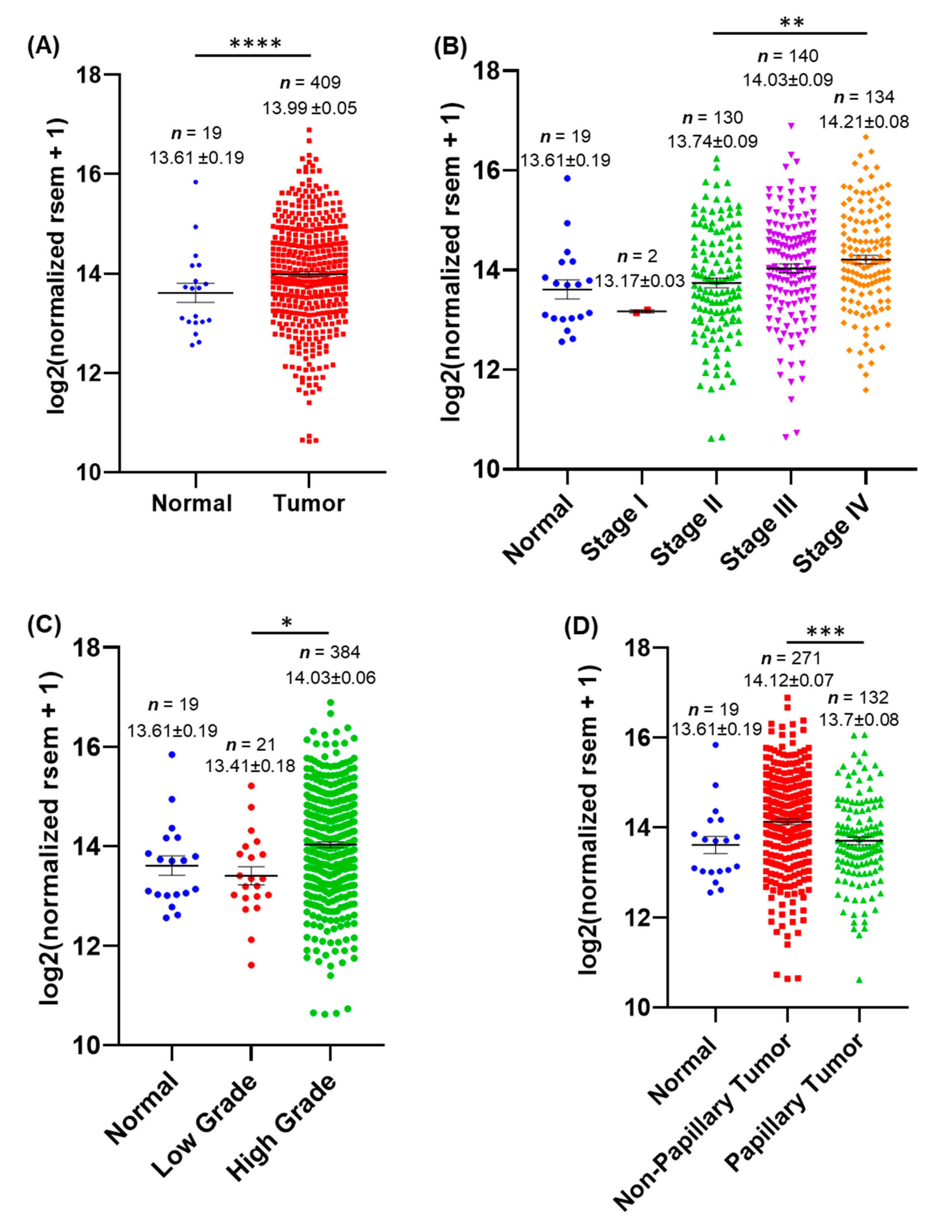
3.1. AnxA2 mRNA Expression Is Associated with BLCA Patients
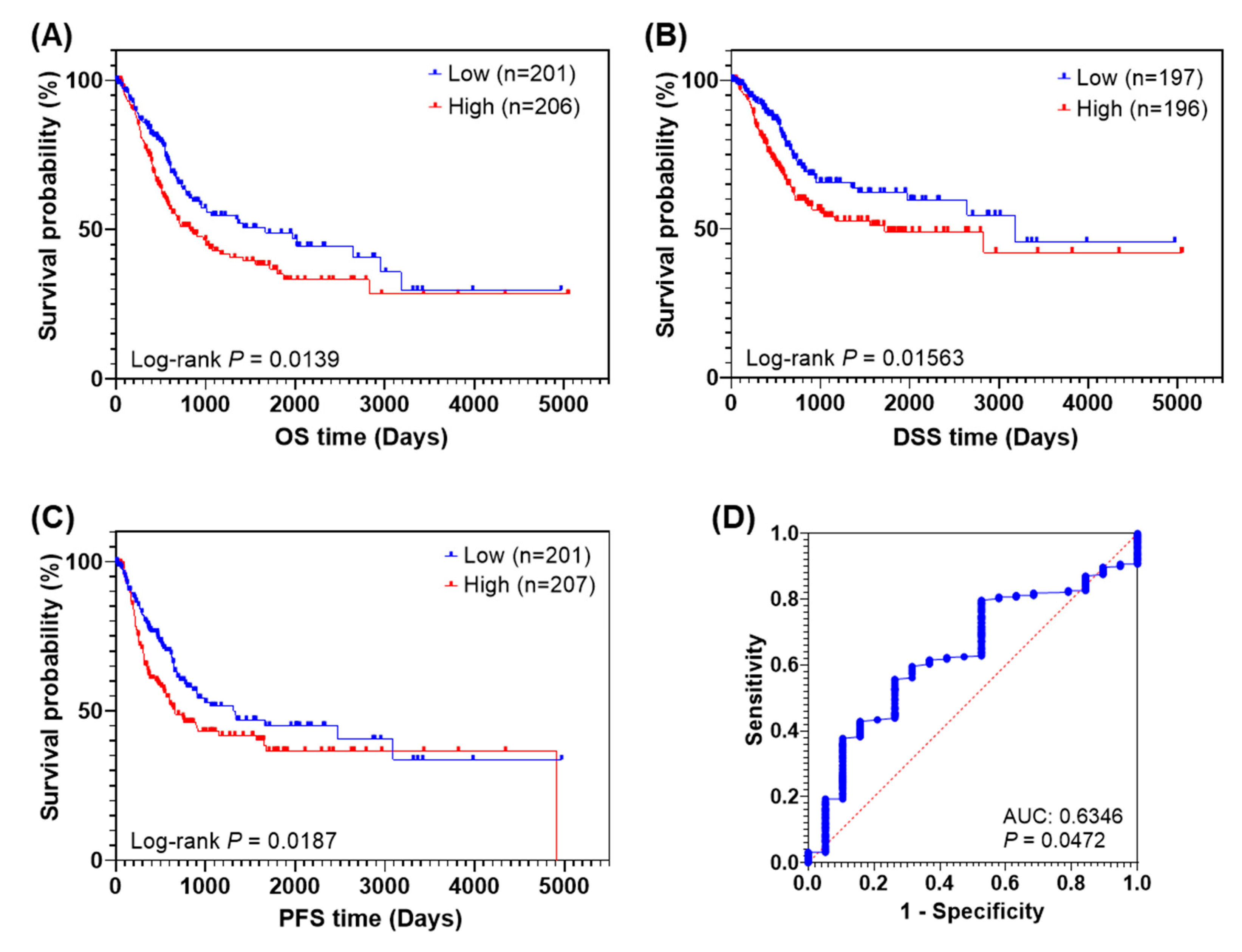
3.2. High Expression of AnxA2 Is Correlated with Poor Prognosis in BLCA Patients
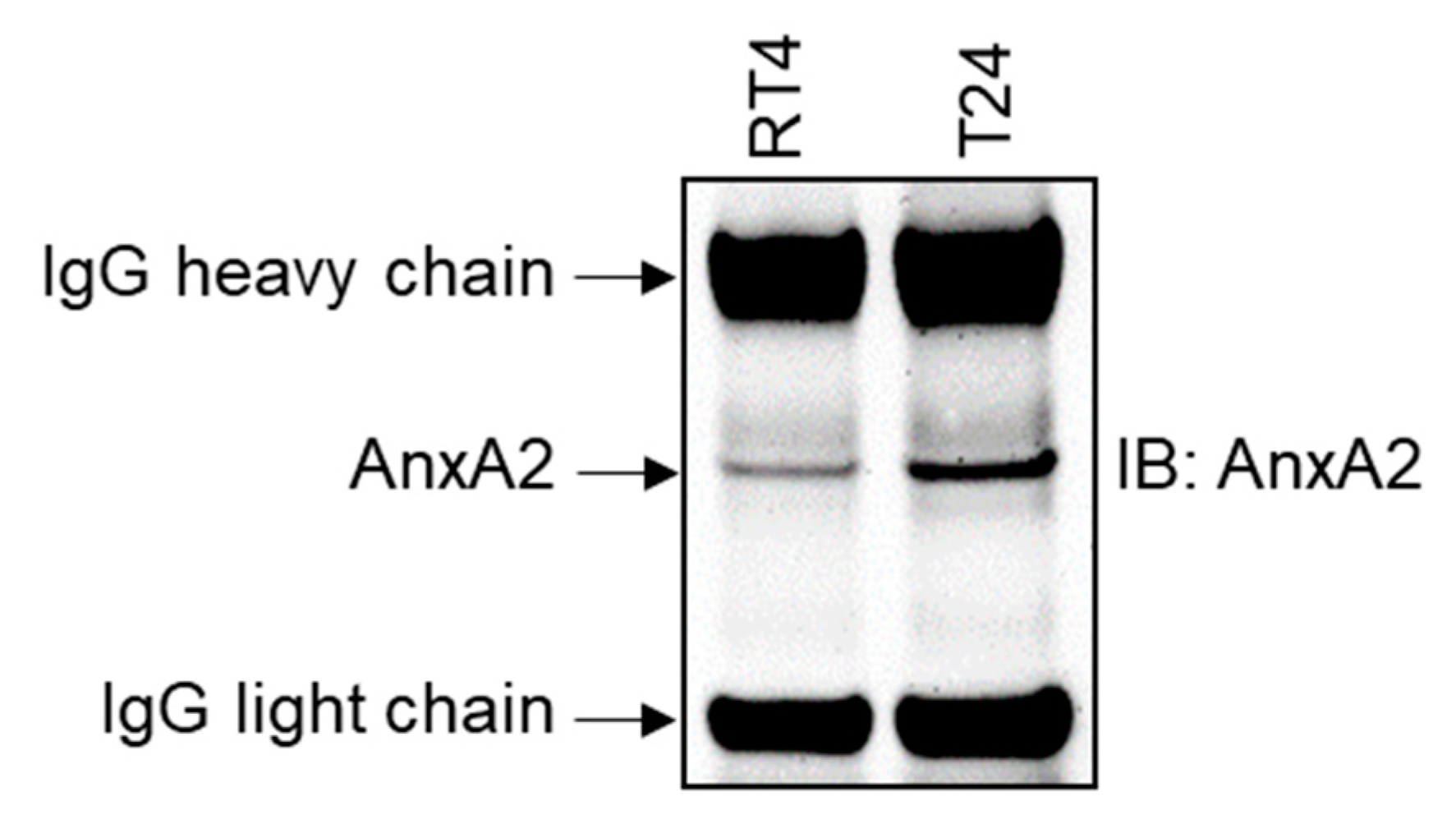
3.3. AnxA2 Expression in Bladder Cancer Cell Lines
3.4. High Expression of AnxA2 at the Cell Surface of Bladder Cancer Cells Promotes Plasmin Generation
3.5. AnxA2 Secretion from Bladder Cancer Cells
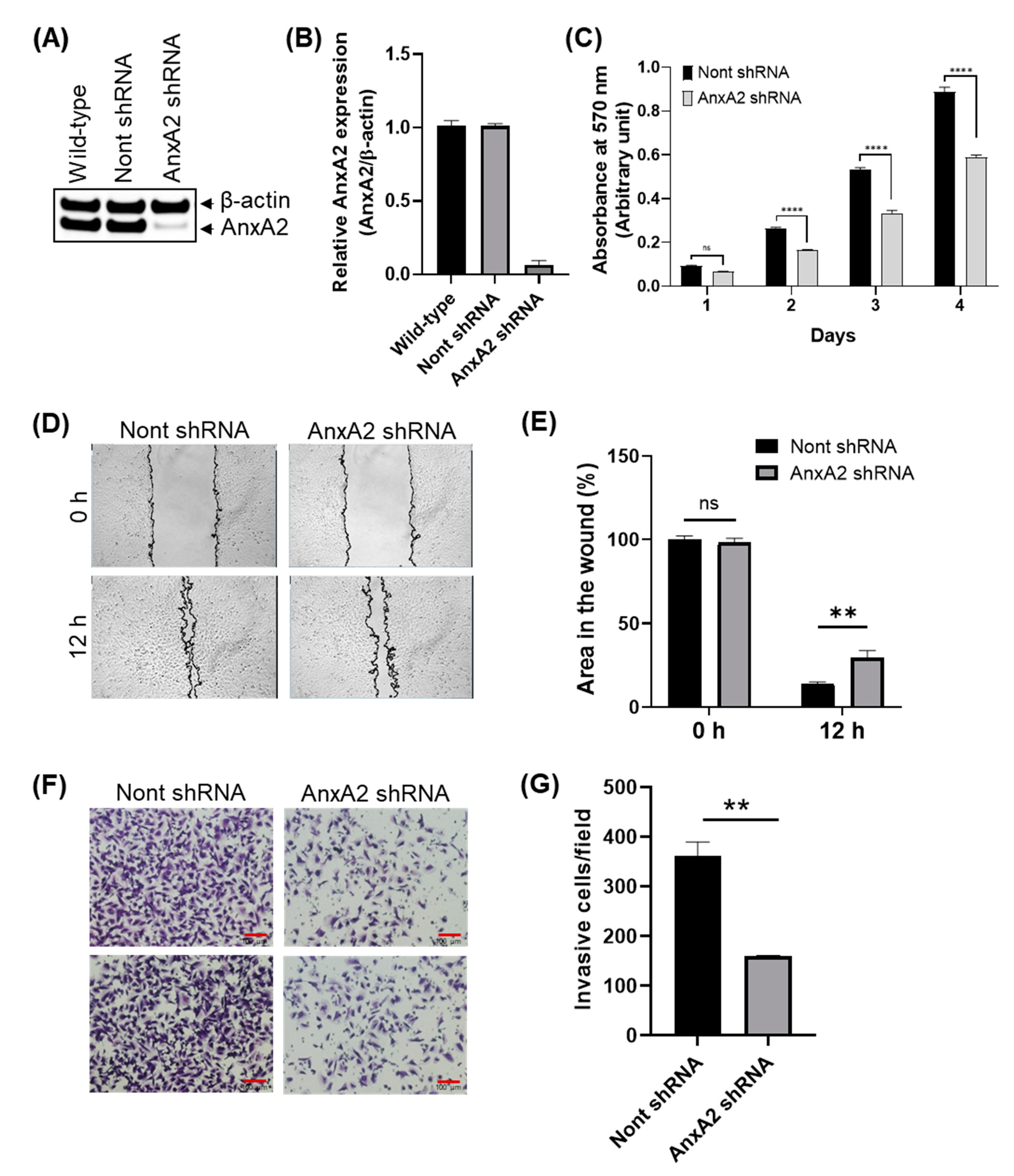
3.6. Depletion of AnxA2 Inhibits the Proliferation, Migration, and Invasion of Bladder Cancer Cells
3.7. Depletion of AnxA2 Downregulates Pro-Angiogenic Factors in Bladder Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER Cancer Stat Facts: Bladder Cancer. National Cancer Institute: Bethesda, MD, USA. 2022. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 1 October 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Byrd, D.; Compton, C.; Fritz, A.; Greene, F.; Trotti, A. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Gray, P.J.; Lin, C.C.; Jemal, A.; Shipley, W.U.; Fedewa, S.A.; Kibel, A.S.; Rosenberg, J.E.; Kamat, A.M.; Virgo, K.S.; Blute, M.L.; et al. Clinical–Pathologic Stage Discrepancy in Bladder Cancer Patients Treated With Radical Cystectomy: Results From the National Cancer Data Base. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.M.; DeCastro, G.J.; Steinberg, G.D. Urothelial carcinoma of the bladder: Definition, treatment and future efforts. Nat. Rev. Urol. 2011, 8, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Botteman, M.F.; Pashos, C.L.; Redaelli, A.; Laskin, B.; Hauser, R. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics 2003, 21, 1315–1330. [Google Scholar] [CrossRef]
- Lozano, F.; Raventos, C.X.; Carrion, A.; Trilla, E.; Morote, J. Current status of genetic urinary biomarkers for surveillance of non-muscle invasive bladder cancer: A systematic review. BMC Urol. 2020, 20, 99. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Abern, M.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chan, K.; Chang, S.; Friedlander, T.; et al. NCCN Guidelines® Insights: Bladder Cancer, Version 2.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 866–878. [Google Scholar] [CrossRef]
- Lokman, N.A.; Ween, M.P.; Oehler, M.K.; Ricciardelli, C. The Role of Annexin A2 in Tumorigenesis and Cancer Progression. Cancer Microenviron. 2011, 4, 199–208. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Bydoun, M.; Holloway, R.; Waisman, D. Annexin A2 Heterotetramer: Structure and Function. Int. J. Mol. Sci. 2013, 14, 6259–6305. [Google Scholar] [CrossRef]
- Rescher, U.; Gerke, V. Annexins—Unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117, 2631–2639. [Google Scholar] [CrossRef]
- Christensen, M.V.; Høgdall, C.K.; Jochumsen, K.M.; Høgdall, E.V.S. Annexin A2 and cancer: A systematic review. Int. J. Oncol. 2018, 52, 5–18. [Google Scholar] [CrossRef] [PubMed]
- de Graauw, M.; Tijdens, I.; Smeets, M.B.; Hensbergen, P.J.; Deelder, A.M.; van de Water, B. Annexin A2 Phosphorylation Mediates Cell Scattering and Branching Morphogenesis via Cofilin Activation. Mol. Cell Biol. 2008, 28, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Grieve, A.G.; Moss, S.E.; Hayes, M.J. Annexin A2 at the Interface of Actin and Membrane Dynamics: A Focus on Its Roles in Endocytosis and Cell Polarization. Int. J. Cell Biol. 2012, 2012, 852430. [Google Scholar] [CrossRef]
- Huang, Y.; Jia, M.; Yang, X.; Han, H.; Hou, G.; Bi, L.; Yang, Y.; Zhang, R.; Zhao, X.; Peng, C.; et al. Annexin A2: The diversity of pathological effects in tumorigenesis and immune response. Int. J. Cancer 2022, 151, 497–509. [Google Scholar] [CrossRef]
- Valapala, M.; Thamake, S.I.; Vishwanatha, J.K. A competitive hexapeptide inhibitor of annexin A2 prevents hypoxia-induced angiogenic events. J. Cell Sci. 2011, 124, 1453–1464. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, J.; Zhang, M. Expression of Annexin A2 and Its Correlation with Drug Resistance and Recurrence of Bladder Cancer. Technol. Cancer Res. Treat. 2016, 15, NP61–NP68. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Z.; Ma, Y.; Wang, H.; Ma, J.; He, X.; Zhang, D. Combined expression of S100A4 and Annexin A2 predicts disease progression and overall survival in patients with urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 798–805. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Lin, C.-F. Annexin A2: Its Molecular Regulation and Cellular Expression in Cancer Development. Dis. Markers 2014, 2014, 308976. [Google Scholar] [CrossRef]
- Gibbs, L.D.; Mansheim, K.; Maji, S.; Nandy, R.; Lewis, C.M.; Vishwanatha, J.K.; Chaudhary, P. Clinical Significance of Annexin A2 Expression in Breast Cancer Patients. Cancers 2020, 13, 2. [Google Scholar] [CrossRef]
- Ma, K.; Chen, X.; Liu, W.; Yang, Y.; Chen, S.; Sun, J.; Ma, C.; Wang, T.; Yang, J. ANXA2 is correlated with the molecular features and clinical prognosis of glioma, and acts as a potential marker of immunosuppression. Sci. Rep. 2021, 11, 20839. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-H.; Young, D.; Chen, Y.; Kuo, H.-C.; Srinivasan, A.; Dobi, A.; Petrovics, G.; Cullen, J.; Mcleod, D.G.; Rosner, I.L.; et al. Prognostic features of Annexin A2 expression in prostate cancer. Pathology 2021, 53, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Gibbs, L.D.; Maji, S.; Lewis, C.M.; Suzuki, S.; Vishwanatha, J.K. Serum exosomal-annexin A2 is associated with African-American triple-negative breast cancer and promotes angiogenesis. Breast Cancer Res. 2020, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xie, C.; Wu, P.; He, J.; Tang, Y.; Xu, J.; Zhao, S. Annexin A2 is an independent prognostic biomarker for evaluating the malignant progression of laryngeal cancer. Exp. Ther. Med. 2017, 14, 6113–6118. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, G.; Cai, J.; Wang, Y.; Qu, X.; He, L.; Liu, F.; Zhang, Y.; Lin, K.; Ma, S.; et al. Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis 2013, 34, 595–604. [Google Scholar] [CrossRef]
- Ohno, Y.; Izumi, M.; Kawamura, T.; Nishimura, T.; Mukai, K.; Tachibana, M. Annexin II represents metastatic potential in clear-cell renal cell carcinoma. Br. J. Cancer 2009, 101, 287–294. [Google Scholar] [CrossRef]
- Yang, S.-F.; Hsu, H.-L.; Chao, T.-K.; Hsiao, C.-J.; Lin, Y.-F.; Cheng, C.-W. Annexin A2 in renal cell carcinoma: Expression, function, and prognostic significance. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 22.e11–22.e21. [Google Scholar] [CrossRef]
- Chaudhary, P.; Thamake, S.I.; Shetty, P.; Vishwanatha, J.K. Inhibition of triple-negative and Herceptin-resistant breast cancer cell proliferation and migration by Annexin A2 antibodies. Br. J. Cancer 2014, 111, 2328–2341. [Google Scholar] [CrossRef]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 2017, 15, 93–105. [Google Scholar] [CrossRef]
- Valapala, M.; Maji, S.; Borejdo, J.; Vishwanatha, J.K. Cell Surface Translocation of Annexin A2 Facilitates Glutamate-induced Extracellular Proteolysis. J. Biol. Chem. 2014, 289, 15915–15926. [Google Scholar] [CrossRef]
- Goldman, M.; Craft, B.; Swatloski, T.; Cline, M.S.; Morozova, O.; Diekhans, M.; Haussler, D.; Zhu, J. The UCSC Cancer Genomics Browser: Update 2015. Nucleic Acids Res. 2015, 43, D812–D817. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Franks, L.; Rigby, C. Letter: Hela cells and rt4 cells. Science 1975, 188, 168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bubenı’k, J.; Baresová, M.; Viklický, V.; Jakoubková, J.; Sainerová, H.; Donner, J. Established cell line of urinary bladder carcinoma (t24) containing tumourspecific antigen. Int. J. Cancer 1973, 11, 765–773. [Google Scholar] [CrossRef]
- Hajjar, K.A.; Mauri, L.; Jacovina, A.T.; Zhong, F.; Mirza, U.A.; Padovan, J.C.; Chait, B.T. Tissue Plasminogen Activator Binding to the Annexin II Tail Domain. Direct modulation by homocysteine. J. Biol. Chem. 1998, 273, 9987–9993. [Google Scholar] [CrossRef]
- Roda, O.; Valero, M.L.; Peiró, S.; Andreu, D.; Real, F.X.; Navarro, P. New insights into the tPA-annexin A2 interaction. Is annexin A2 CYS8 the sole requirement for this association? J. Biol. Chem. 2003, 278, 5702–5709. [Google Scholar] [CrossRef]
- Faure, A.-V.; Migné, C.; Devilliers, G.; Ayala-Sanmartin, J. Annexin 2 “Secretion” Accompanying Exocytosis of Chromaffin Cells: Possible Mechanisms of Annexin Release. Exp. Cell Res. 2002, 276, 79–89. [Google Scholar] [CrossRef]
- Tas, F.; Yasasever, C.T.; Karabulut, S.; Tastekin, D.; Duranyildiz, D. Circulating annexin A2 as a biomarker in gastric cancer patients: Correlation with clinical variables. Biomed. Pharmacother. 2015, 69, 237–241. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, C.; Zhang, S.; Fang, G. Serum Annexin A2 Level Is Associated with Diagnosis and Prognosis in Patients with Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2017, 75, 1081–1087. [Google Scholar] [CrossRef]
- Tufano, A.; Cordua, N.; Nardone, V.; Ranavolo, R.; Flammia, R.S.; D’Antonio, F.; Borea, F.; Anceschi, U.; Leonardo, C.; Morrione, A.; et al. Prognostic Significance of Organ-Specific Metastases in Patients with Metastatic Upper Tract Urothelial Carcinoma. J. Clin. Med. 2022, 11, 5310. [Google Scholar] [CrossRef]
- Li, P.; Li, L.; Li, Z.; Wang, S.; Li, R.; Zhao, W.; Feng, Y.; Huang, S.; Li, L.; Qiu, H.; et al. Annexin A1 promotes the progression of bladder cancer via regulating EGFR signaling pathway. Cancer Cell Int. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-F.; Shen, K.-H.; Huang, L.-C.; Huang, H.-Y.; Wang, Y.-H.; Wu, T.-F. Annexin-I overexpression is associated with tumour progression and independently predicts inferior disease-specific and metastasis-free survival in urinary bladder urothelial carcinoma. Pathology 2010, 42, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.P.; Almal, A.A.; George, B.; Fry, D.W.; Lenehan, P.F.; Pagliarulo, V.; Cote, R.J.; Datar, R.H.; Worzel, W.P. The use of genetic programming in the analysis of quantitative gene expression profiles for identification of nodal status in bladder cancer. BMC Cancer 2006, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.B.; Gu, L.; Chen, L.; Yin, Y.; Fan, B.Y. Annexin A8 regulated by lncRNA-TUG1/miR-140-3p axis promotes bladder cancer progression and metastasis. Mol. Ther.-Oncolytics 2021, 22, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Munksgaard, P.P.; Mansilla, F.; Eskildsen, A.-S.B.; Fristrup, N.; Birkenkamp-Demtröder, K.; Ulhøi, B.P.; Borre, M.; Agerbæk, M.; Hermann, G.G.; Ørntoft, T.F.; et al. Low ANXA10 expression is associated with disease aggressiveness in bladder cancer. Br. J. Cancer 2011, 105, 1379–1387. [Google Scholar] [CrossRef]
- Yao, X.; Qi, X.; Wang, Y.; Zhang, B.; He, T.; Yan, T.; Zhang, L.; Wang, Y.; Zheng, H.; Zhang, G.; et al. Identification and Validation of an Annexin-Related Prognostic Signature and Therapeutic Targets for Bladder Cancer: Integrative Analysis. Biology 2022, 11, 259. [Google Scholar] [CrossRef]
- Spruck, C.H., 3rd; Ohneseit, P.F.; Gonzalez-Zulueta, M.; Esrig, D.; Miyao, N.; Tsai, Y.C.; Lerner, S.P.; Schmütte, C.; Yang, A.S.; Cote, R.; et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994, 54, 784–788. [Google Scholar]
- Cohen, S.; Bryan, G. (Eds.) The Pathology of Bladder Cancer, 1st ed.; CRC Press: Boca Raton, FL, USA, 1983; pp. 1–243. [Google Scholar]
- Kinjo, M.; Oka, K.; Naito, S.; Kohga, S.; Tanaka, K.; Oboshi, S.; Hayata, Y.; Yasumoto, K. Thromboplastic and fibrinolytic activities of cultured human cancer cell lines. Br. J. Cancer 1979, 39, 15–23. [Google Scholar] [CrossRef]
- Janisch, F.; D’Andrea, D.; Iwata, T.; Kimura, S.; Abufaraj, M.; Enikeev, D.; Glybochko, P.V.; Karakiewicz, P.I.; Nyirady, P.; Fajkovic, H.; et al. The prognostic value of the urokinase-plasminogen activator system (uPA) in bladder cancer patients treated with radical cystectomy (RC). Urol. Oncol. Semin. Orig. Investig. 2020, 38, 423–432. [Google Scholar] [CrossRef]
- Lu, C.-M.; Lin, J.-J.; Huang, H.-H.; Ko, Y.-C.; Hsu, J.-L.; Chen, J.-C.; Din, Z.-H.; Wu, Y.-J. A panel of tumor markers, calreticulin, annexin A2, and annexin A3 in upper tract urothelial carcinoma identified by proteomic and immunological analysis. BMC Cancer 2014, 14, 363. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.S.; Yin, X.Q.; Yu, G.; Jia, B.L. Anxa2 gene silencing attenuates obesity-induced insulin resistance by suppressing the NF-κB signaling pathway. Am. J. Physiol.-Cell Physiol. 2019, 316, C223–C234. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M.; Jaffar, J.; Berhan, A.; Langenbach, S.; Harris, T.; Waters, D.; Lee, P.V.S.; Grainge, C.; Westall, G.; Knight, D.; et al. Annexin A2 contributes to lung injury and fibrosis by augmenting factor Xa fibrogenic activity. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L772–L782. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, J.; Narula, N.; Yee, D.S.; Skarecky, D.W.; Lau, A.; Ornstein, D.K.; Tyson, D.R. Annexin A2 positively contributes to the malignant phenotype and secretion of IL-6 in DU145 prostate cancer cells. Int. J. Cancer 2009, 124, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Jäger, T.; Droste, F.; Becker, M.; Kovalszky, I.; Romics, I.; Ergun, S.; Rübben, H. Serum Levels of Angiogenic Factors and their Prognostic Relevance in Bladder Cancer. Pathol. Oncol. Res. 2009, 15, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Jäger, T.; Tötsch, M.; Dorp, F.V.; Kempkensteffen, C.; Kovalszky, I.; Romics, I.; Ergün, S.; Rübben, H. Angiogenic Switch of Angiopietins-Tie2 System and Its Prognostic Value in Bladder Cancer. Clin. Cancer Res. 2008, 14, 8253–8262. [Google Scholar] [CrossRef]
- Szarvas, T.; Jäger, T.; Laszlo, V.; Kramer, G.; Klingler, H.C.; vom Dorp, F.; Romics, I.; Ergün, S.; Rübben, H. Circulating angiostatin, bFGF, and Tie2/TEK levels and their prognostic impact in bladder cancer. Urology 2012, 80, 737.e13–737.e18. [Google Scholar] [CrossRef]
- Fouad, H.; Salem, H.; Ellakwa, D.E.; Abdel-Hamid, M. MMP-2 and MMP-9 as prognostic markers for the early detection of urinary bladder cancer. J. Biochem. Mol. Toxicol. 2019, 33, e22275. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Trivedi, R.; Tripathi, A.K.; Nandy, R.R.; Wagner, D.C.; Narra, K.; Chaudhary, P. Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion. Cancers 2022, 14, 5664. https://doi.org/10.3390/cancers14225664
Guo C, Trivedi R, Tripathi AK, Nandy RR, Wagner DC, Narra K, Chaudhary P. Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion. Cancers. 2022; 14(22):5664. https://doi.org/10.3390/cancers14225664
Chicago/Turabian StyleGuo, Christina, Rucha Trivedi, Amit K. Tripathi, Rajesh R. Nandy, Diana C. Wagner, Kalyani Narra, and Pankaj Chaudhary. 2022. "Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion" Cancers 14, no. 22: 5664. https://doi.org/10.3390/cancers14225664
APA StyleGuo, C., Trivedi, R., Tripathi, A. K., Nandy, R. R., Wagner, D. C., Narra, K., & Chaudhary, P. (2022). Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion. Cancers, 14(22), 5664. https://doi.org/10.3390/cancers14225664







