Melanoma of the Upper Limb and Shoulder: A Surveillance, Epidemiology, and End Results Analysis of Epidemiology and Survival 2000–2019
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Variable Selection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Facts & Figures. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (accessed on 15 September 2022).
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. In Vivo 2014, 28, 1005–1011. [Google Scholar] [PubMed]
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Derm.-Venereol. 2020, 100, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Rosner, B.A.; Colditz, G.A. Risk Factors for Melanoma by Body Site. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1241–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteman, D.C.; Stickley, M.; Watt, P.; Hughes, M.C.; Davis, M.B.; Green, A.C. Anatomic Site, Sun Exposure, and Risk of Cutaneous Melanoma. J. Clin. Oncol. 2006, 24, 3172–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.N.; Shin, D.B.; Troxel, A.B.; Khan, S.; Sober, A.J.; Ming, M.E. Association between the Anatomic Distribution of Melanoma and Sex. J. Am. Acad. Dermatol. 2007, 56, 768–773. [Google Scholar] [CrossRef]
- Wu, S.; Cho, E.; Li, W.-Q.; Weinstock, M.A.; Han, J.; Qureshi, A.A. History of Severe Sunburn and Risk of Skin Cancer among Women and Men in 2 Prospective Cohort Studies. Am. J. Epidemiol. 2016, 183, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Vuong, K.; McGeechan, K.; Armstrong, B.K.; Cust, A.E. Occupational Sun Exposure and Risk of Melanoma According to Anatomical Site. Int. J. Cancer 2014, 134, 2735–2741. [Google Scholar] [CrossRef] [Green Version]
- Ghiasvand, R.; Robsahm, T.E.; Green, A.C.; Rueegg, C.S.; Weiderpass, E.; Lund, E.; Veierød, M.B. Association of Phenotypic Characteristics and UV Radiation Exposure with Risk of Melanoma on Different Body Sites. JAMA Dermatol. 2019, 155, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Wallingford, S.C.; Alston, R.D.; Birch, J.M.; Green, A.C. Increases in Invasive Melanoma in England, 1979–2006, by Anatomical Site. Br. J. Dermatol. 2011, 165, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Bobos, M. Histopathologic Classification and Prognostic Factors of Melanoma: A 2021 Update. Ital. J. Dermatol. Venerol. 2021, 156, 300–321. [Google Scholar] [CrossRef]
- SEER. SEER* Stat Databases: November 2021 Submission. Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2021/index.html (accessed on 18 October 2022).
- Buettner, P.G.; Leiter, U.; Eigentler, T.K.; Garbe, C. Development of Prognostic Factors and Survival in Cutaneous Melanoma over 25 Years: An Analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer 2005, 103, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.E.; Swetter, S.M.; Fu, T.; Geller, A.C. Screening, Early Detection, Education, and Trends for Melanoma: Current Status (2007–2013) and Future Directions: Part II. Screening, Education, and Future Directions. J. Am. Acad. Dermatol. 2014, 71, e1–e611. [Google Scholar] [CrossRef]
- Johansson, M.; Brodersen, J.; Gøtzsche, P.C.; Jørgensen, K.J. Screening for Reducing Morbidity and Mortality in Malignant Melanoma. Cochrane Database Syst. Rev. 2019, 2019, CD012352. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Thompson, J.F.; Pandeya, N.; Whiteman, D.C. Evaluation of Sex-Specific Incidence of Melanoma. JAMA Dermatol. 2020, 156, 553–560. [Google Scholar] [CrossRef]
- Bulliard, J.L.; Cox, B. Cutaneous Malignant Melanoma in New Zealand: Trends by Anatomical Site, 1969–1993. Int. J. Epidemiol. 2000, 29, 416–423. [Google Scholar]
- Bulliard, J.-L.; Cox, B.; Semenciw, R. Trends by anatomic site in the incidence of cutaneous malignant melanoma in Canada, 1969–1993. Cancer Causes Control 1999, 10, 407–416. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Guy, G.P.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Centers for Disease Control and Prevention (CDC). Vital Signs: Melanoma Incidence and Mortality Trends and Projections—United States, 1982–2030. Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
- Gall Myrick, J.; Noar, S.M.; Sontag, J.M.; Kelley, D. Connections between Sources of Health and Beauty Information and Indoor Tanning Behavior among College Women. J. Am. Coll. Health 2020, 68, 163–168. [Google Scholar] [CrossRef]
- Phan, M.N.; Kohn, J.; Dao, H. Skin Cancer Risk and Tanning in Pageant Contestants. Bayl. Univ. Med. Cent. Proc. 2020, 33, 557–559. [Google Scholar] [CrossRef]
- Gardner, L.J.; Strunck, J.L.; Wu, Y.P.; Grossman, D. Current Controversies in Early-Stage Melanoma: Questions on Incidence, Screening, and Histologic Regression. J. Am. Acad. Dermatol. 2019, 80, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carli, P.; De Giorgi, V.; Palli, D.; Maurichi, A.; Mulas, P.; Orlandi, C.; Imberti, G.; Stanganelli, I.; Soma, P.; Dioguardi, D.; et al. Patterns of Detection of Superficial Spreading and Nodular-Type Melanoma: A Multicenter Italian Study. Dermatol. Surg. 2004, 30, 1371–1375. [Google Scholar] [CrossRef]
- McPherson, M.; Elwood, M.; English, D.R.; Baade, P.D.; Youl, P.H.; Aitken, J.F. Presentation and Detection of Invasive Melanoma in a High-Risk Population. J. Am. Acad. Dermatol. 2006, 54, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Robsahm, T.E.; Helsing, P.; Svendsen, H.L.; Veierød, M.B. Clinical Suspicion Sensitivity of Nodular and Superficial Spreading Melanoma. Acta Derm. Venereol. 2021, 101, adv00427. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, W.R.; Xiong, M.; Weinstock, M.A. The Contribution of Nodular Subtype to Melanoma Mortality in the United States, 1978 to 2007. Arch. Dermatol. 2012, 148, 30–36. [Google Scholar] [CrossRef]
- Huismans, A.M.; Haydu, L.E.; Shannon, K.F.; Quinn, M.J.; Saw, R.P.M.; Spillane, A.J.; Stretch, J.R.; Thompson, J.F. Primary Melanoma Location on the Scalp Is an Important Risk Factor for Brain Metastasis: A Study of 1687 Patients with Cutaneous Head and Neck Melanomas. Ann. Surg. Oncol. 2014, 21, 3985–3991. [Google Scholar] [CrossRef]
- Cabrera, C.I.; Li, S.; Conic, R.; Gastman, B.R. The National Cancer Database: Survival between Head and Neck Melanoma and Melanoma of Other Regions. Otolaryngol. Head Neck Surg. 2022, 167, 286–297. [Google Scholar] [CrossRef]
- Radiation Therapy for Melanoma| Melanoma Skin Cancer Radiation. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/treating/radiation-therapy.html (accessed on 18 September 2022).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Melanoma: Cutaneous. 2022. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjO26fftrP7AhU3gv0HHccVAFoQFnoECBIQAQ&url=https%3A%2F%2Fwww.nccn.org%2Fprofessionals%2Fphysician_gls%2Fpdf%2Fcutaneous_melanoma.pdf&usg=AOvVaw3CB2HpBeSO1AEeTY54FeCZ (accessed on 20 September 2022).
- Adkinson, J.M.; Chung, K.C. Flap Reconstruction of the Elbow and Forearm: A Case-Based Approach. Hand Clin. 2014, 30, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Jordan, S.W.; Wayne, J.D.; Dumanian, G.A. The Pedicled Lateral Arm Flap for Oncologic Reconstruction Near the Shoulder. Ann. Plast. Surg. 2015, 74, 30–33. [Google Scholar] [CrossRef]
- Melanoma Survival Rates|Melanoma Survival Statistics. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html (accessed on 18 September 2022).
- Treatment Options|Melanoma Skin Cancer|Cancer Research UK. Available online: https://www.cancerresearchuk.org/about-cancer/melanoma/treatment/treatment-decisions (accessed on 19 October 2022).
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization for Research and Treatment of Cancer (EORTC). European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef]
- Hartman, R.I.; Lin, J.Y. Cutaneous Melanoma-A Review in Detection, Staging, and Management. Hematol. Oncol. Clin. N. Am. 2019, 33, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Melanoma by Stage. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/treating/by-stage.html (accessed on 18 September 2022).
- Tas, F.; Erturk, K. Relapse Patterns in Patients with Local and Regional Cutaneous Melanoma. Clin. Transl. Oncol. 2019, 21, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Erturk, K. Major Histotypes in Skin Melanoma: Nodular and Acral Lentiginous Melanomas Are Poor Prognostic Factors for Relapse and Survival. Am. J. Dermatopathol. 2022, 44, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-L.; Liao, Y.-H.; Liau, J.-Y.; Sheen, Y.-S. Risk Factors of Recurrence and Distant Metastasis in Primary Cutaneous Melanoma in Taiwan. Sci. Rep. 2021, 11, 21012. [Google Scholar] [CrossRef]
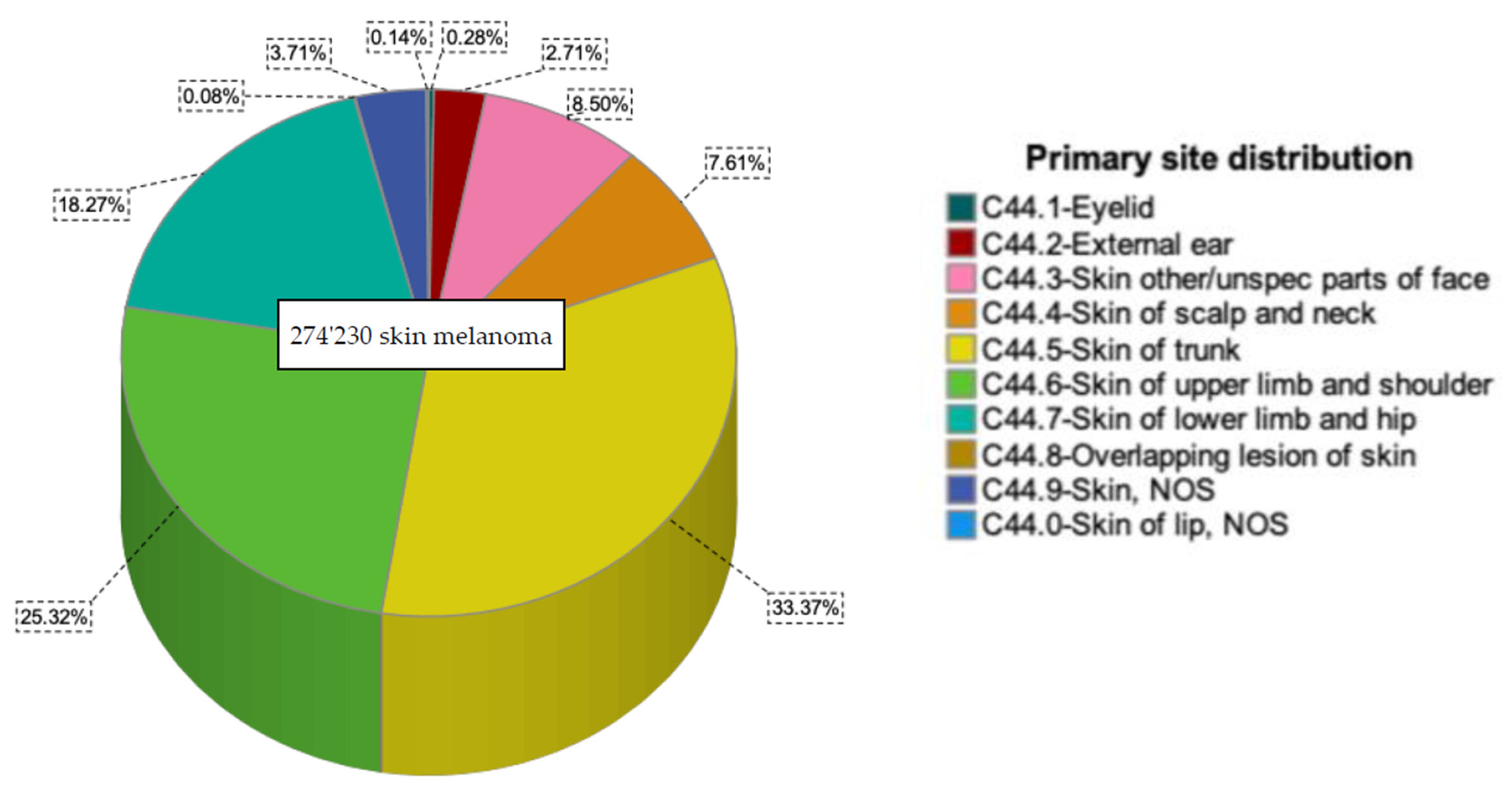
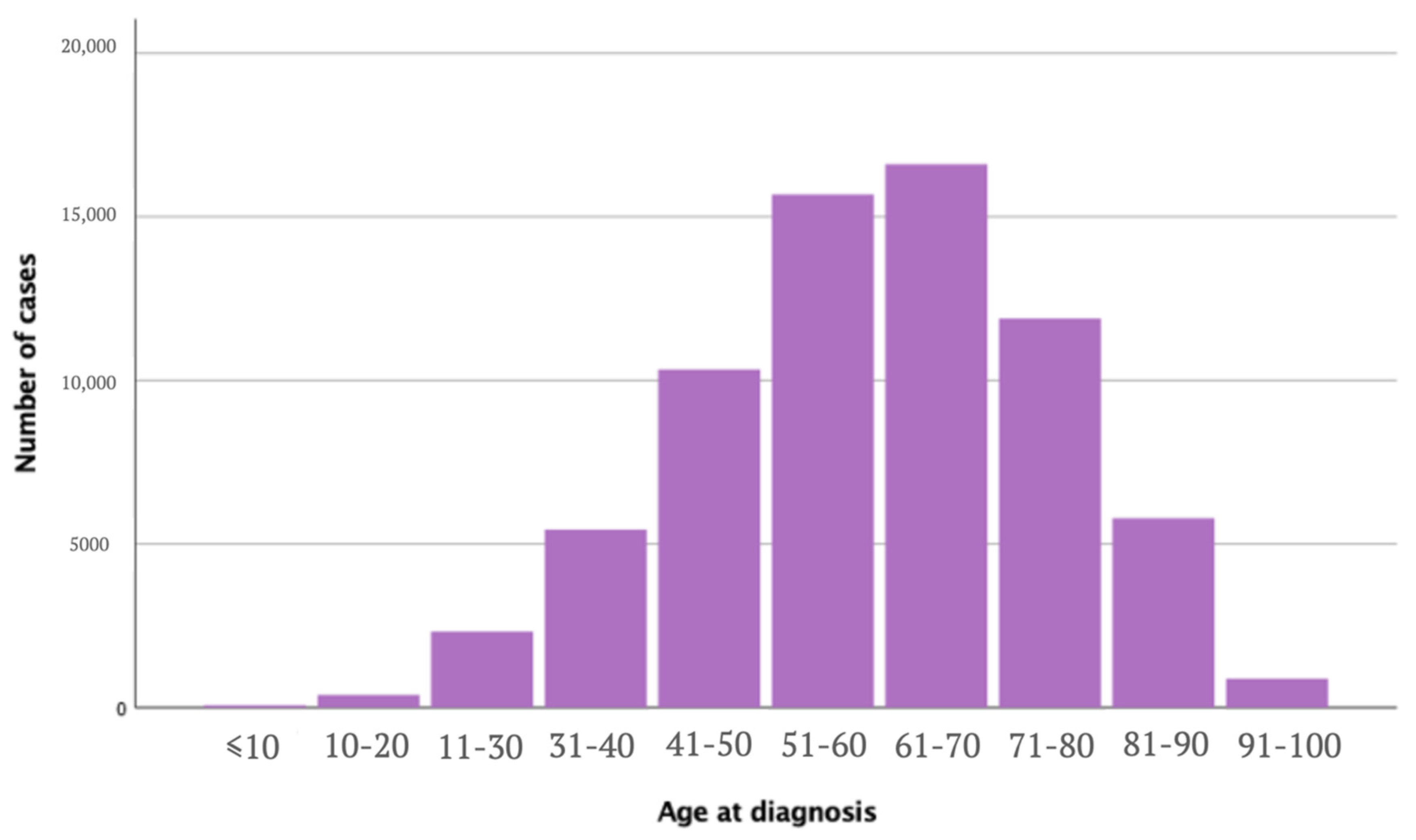

| Upper Limb and Shoulder n (%) | Other Sites n (%) | p-Value | Total n (%) | |
|---|---|---|---|---|
| Total | 69,436 (100) | 204,794 (100) | 274,230 (100) | |
| Age at diagnosis [years] | <0.005 | |||
| Mean (SD) | 60.00 (15.7) | 58.86 (16.9) | ||
| Median | 61 | 60 | ||
| Age group | <0.005 | |||
| ≤50 | 18,563 (26.7) | 63,946 (31.2) | 82,509 (30.1) | |
| 51–61 | 17,484 (25.2) | 49,863 (24.3) | 67,347 (24.6) | |
| 62–71 | 16,311 (23.5) | 43,435 (21.2) | 59,746 (21.8) | |
| ≥72 | 17,078 (24.6) | 47,550 (23.2) | 64,628 (23.6) | |
| Gender | <0.005 | |||
| Male | 35,267 (50.8) | 118,654 (57.9) | 153,921 (56.1) | |
| Female | 34,169 (49.2) | 86,140 (42.1) | 120,309 (43.9) | |
| Ethnicity | <0.005 | |||
| White | 65,287 (94) | 192,416 (94) | 257,703 (94) | |
| Black | 167 (0.2) | 1024 (0.5) | 1191 (0.4) | |
| Asian or Pacific Islander | 333 (0.5) | 1513 (0.7) | 1846 (0.7) | |
| American Indian/Alaska Native | 164 (0.2) | 522 (0.3) | 686 (0.3) | |
| Unknown | 3485 (5.0) | 9319 (4.6) | 12,804 (4.7) | |
| Year of Diagnosis (%) | <0.005 | |||
| 2000–2004 | 13,613 (19.6) | 42,737 (20.9) | (20.6) | |
| 2005–2009 | 16,546 (23.8) | 49,278 (24.2) | 65,824 (24) | |
| 2010–2014 | 18,650 (26.9) | 53,957 (26.4) | 72,607 (26.5) | |
| 2015–2019 | 20,627 (29.7) | 58,822 (28.7) | 79,449 (29) | |
| Histologic Subtypes (%) | <0.005 | |||
| 8720/3: Malignant Melanoma, NOS | 33,643 (48.5) | 103,084 (50.3) | 136,727 (49.9) | |
| 8743/3: Superficial Spreading Melanoma | 23,479 (33.8) | 64,158 (31.3) | 87,637 (32) | |
| 8721/3: Nodular Melanoma | 5361 (7.7) | 1363 (6.7) | 18,999 (6.9) | |
| 8742/3: Lentigo Maligna Melanoma | 3787 (5.5) | 13,289 (6.5) | 17,076 (6.2) | |
| 8772/3: Spindle cell melanoma, NOS | 724 (1) | 2076 (1) | 2800 (1) | |
| 8745/3: Desmoplastic melanoma, malignant | 689 (1) | 2173 (1.1) | 2862 (1) | |
| 8744/3: Acral lentiginous melanoma, malignant | 471 (0.7) | 2270 (1.1) | 2741 (1) | |
| 8730/3: Amelanotic melanoma | 303 (0.4) | 707 (0.4) | 1010 (0.4) | |
| 8723/3: Malignant melanoma, regressing | 299 (0.4) | 890 (0.4) | 1189 (0.4) | |
| 8761/3: Malignant melanoma in giant pigmented nevus | 204 (0.3) | 727 (0.4) | 931 (0.3) | |
| 8740/3: Malignant melanoma in junctional nevus | 194 (0.3) | 677 (0.3) | 871 (0.3) | |
| 8771/3: Epithelioid cell melanoma | 135 (0.2) | 506 (0.2) | 641 (0.2) | |
| 8770/3: Mixed epithelioid and spindle cell melanoma | 113 (0.2) | 470 (0.2) | 583 (0.2) | |
| 8722/3: Balloon Cell Melanoma | 18 (0.03) | 67 (0.03) | 85 (0.03) | |
| 8780/3: Blue nevus, malignant | 10 (0.01) | 27 (0.01) | 37 (0.01) | |
| 8741/3: Malignant melanoma in precancerous melanosis | 4 (0.01) | 12 (0.01) | 16 (0.01) | |
| 8727/3: Dysplastic nevus, malignant | 1 (0.00) | 0 (0.00) | 1 (0.0) | |
| 8773/3: Spindle cell melanoma, type A | 1 (0.00) | 7 (0.00) | 1 (0.0) | |
| Stage (%) | <0.005 | |||
| Localized | 61,135 (88.02) | 166,042 (81.1) | 227,177 (82.8) | |
| Regional | 4725 (6.8) | 19,343 (9.5) | 24,068 (8.8) | |
| Distant | 767 (1.1) | 9900 (4.8) | 10,667 (3.9) | |
| Other/not specified | 2809 (4.1) | 9509 (4.6) | 12,318 (4.5) | |
| Type of Surgery (%) | <0.005 | |||
| No Surgery or Radiation | 3425 (4.9) | 16,265 (7.9) | 19,690 (7.2) | |
| Radiation Only | 399 (0.6) | 1183 (0.6) | 1582 (0.6) | |
| Surgery with Radiation | 739 (1.1) | 2403 (1.2) | 3142 (1.1) | |
| Surgery without Radiation | 64,617 (93.1) | 184,329 (90.0) | 248,946 (90.8) | |
| Other/Not specified | 256 (0.4) | 614 (0.3) | 870 (0.3) | |
| Reason for no surgery (%) | <0.005 | |||
| Not recommended | 2341 (3.4) | 14,886 (7.3) | 17,227 (6.3) | |
| Recommended but not performed; patient refused | 39 (0.1) | 217 (0.1) | 217 (0.1) | |
| Recommended but not performed, unknown reason | 474 (0.7) | 1879 (0.9) | 1879 (0.9) | |
| Not performed; patient died prior to recommended surgery | 7 (0.01) | 31 (0.02) | 38 (0.01) | |
| Recommended, unknown if performed | 185 (0.3) | 575 (0.3) | 760 (0.3) | |
| Not recommended, contraindicated due to other cond; autopsy only (1973–2002) | 10 (0.01) | 183 (0.1) | 193 (0.1) | |
| Surgery performed | 66,208 (95.4) | 186,428 (91.0) | 252,636 (92.1) | |
| Unknown; death certificate; or autopsy only (2003+) | 172 (0.3) | 595 (0.3) | 767 (0.3) | |
| Lymph Node Removal | <0.005 | |||
| No Regional Lymph Nodes Removed | 59,877 (86.2) | 174,517 (85.2) | 234,394 (85.5) | |
| Regional Lymph Nodes Removed | 4244 (6.1) | 14,939 (5.4) | 19,183 (7.0) | |
| Sentinel Nodes Removed | 4653 (6.7) | 12,692 (4.6) | 17,345 (6.3) | |
| Unknown | 662 (1.0) | 2646 (1.0) | 3308 (1.2) | |
| Ulceration 1 | <0.005 | |||
| Ulceration not identified | 32,548 (46.87) | 88,395 (43.16) | 120,943 (44.10) | |
| Ulceration present | 4686 (6.71) | 13,853 (6.76) | 18,509 (6.75) | |
| Not documented | 32,323 (46.42) | 102,546 (50.07) | 134,778 (49.15) | |
| Breslow Thickness (mm) 1 | <0.005 | |||
| <0.8 mm | 21,773 (59.0) | 58,615 (58.5) | 80,388 (58.6) | |
| 0.8 to 1 mm | 4212 (11,4) | 11,603 (11.0) | 15,275 (11.1) | |
| >1 to 2 mm | 5398 (14.6) | 14,265 (14.2) | 19,663 (14.3) | |
| >2 to 4 mm | 3113 (8.4) | 8845 (8.8) | 11,958 (8.7) | |
| >4 mm | 2384 (6.5) | 7465 (7.4) | 9849 (7.2) | |
| No mass found | 29 (0.1) | 2601 (2.5) | 2630 (1.9) |
| Upper Limbs & Shoulders | Other Sites | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤50 | 51–61 | 62–71 | ≥72 | ≤50 | 51–61 | 62–71 | ≥72 | |
| Gender (%) | ||||||||
| Female | 11,280 (33.0) | 8372 (24.5) | 6954 (20.4) | 7563 (22.1) | 35,211 (40.9) | 19,449 (22.6) | 14,537 (16.9) | 16,943 (19.7) |
| Male | 7283 (20.7) | 9112 (25.8) | 9357 (26.5) | 9515 (27.9) | 28,735 (24.2) | 30,414 (25.6) | 28,898 (24.4) | 30,607 (25.8) |
| Histological Subtypes (%) | ||||||||
| Malignant Melanoma | 9252 (27.5) | 8529 (25.4) | 7810 (23.2) | 8052 (23.9) | 32,145 (31.2) | 23,393 (24.6) | 21,872 (21.2) | 23,674 (23.0) |
| Superficial Spreading Melanoma | 266 (7.0) | 765 (20.2) | 1205 (31.8) | 1551 (41.0) | 1208 (9.1) | 2544 (19.1) | 2963 (21.7) | 4285 (31.4) |
| Nodular Melanoma | 1029 (19.2) | 1184 (22.1) | 1181 (22.0) | 1967 (36.7) | 3239 (23.7) | 3151 (23.1) | 2963 (21.7) | 4285 (31.4) |
| Lentigo Melanoma | 0 (0.0) | 1 (25.0) | 1 (25.0) | 2 (50.0) | 5 (41.7) | 2 (16.7) | 2 (16.7) | 3 (25.0) |
| Spindle Cell Melanoma | 43 (38.1) | 15 (13.3) | 27 (23.9) | 28 (24.8) | 183 (38.9) | 96 (20.4) | 78 (16.6) | 113 (24.0) |
| Cause of death (%) | ||||||||
| Dead attributable to causes other than melanoma | 437 (4.4) | 1001 (10.0) | 2095 (20.9) | 6468 (64.7) | 1400 (5.2) | 2908 (10.8) | 5450 (20.2) | 17,260 (63.9) |
| Dead attributable to melanoma | 843 (19.2) | 880 (20.0) | 1010 (23.0) | 1665 (37.9) | 4761 (20.7) | 5240 (22.8) | 5116 (22.3) | 7863 (34.2) |
| Other (alive/unknown) | 17,283 (31.4) | 15,603 (28.4) | 13,206 (24.0) | 8945 (16.3) | 57,785 (37.3) | 41,715 (26.9) | 32,869 (21.2) | 22,427 (14.5) |
| 95%CI for Exp(B) | |||||
|---|---|---|---|---|---|
| B | Sig | Exp (B) | Lower | Upper | |
| Age at diagnosis a | |||||
| ≤50 | |||||
| 51–61 | 0.611 | <0.005 | 1.822 | 1.788 | 1.898 |
| 62–71 | 1.197 | <0.005 | 3.310 | 3.217 | 3.405 |
| ≥72 | 2.265 | <0.005 | 9.629 | 9.386 | 9.879 |
| Gender b | |||||
| Female | |||||
| Male | 0.322 | <0.005 | 1.380 | 1.357 | 1.402 |
| Main histological Subtypes c | |||||
| 8720/3: Malignant Melanoma | |||||
| 8743/3: Superficial Spreading Melanoma | −0.320 | <0.005 | 0.726 | 0.704 | 0.749 |
| 8721/3: Nodular Melanoma | 0.648 | <0.005 | 1.912 | 1.867 | 1.959 |
| 8742/3: Lentigo Maligna Melanoma | 0.319 | 0.435 | 1.375 | 0.618 | 3.061 |
| 8772/3: Spindle Cell Melanoma | 0.379 | <0.005 | 1.460 | 1.272 | 1.677 |
| Other | −0.290 | <0.005 | 0.748 | 0.735 | 0.762 |
| Stage d | |||||
| Localized | |||||
| Regional | 0.581 | <0.005 | 1.789 | 1.690 | 1.893 |
| Distant | 1.622 | <0.005 | 5.063 | 4.655 | 5.506 |
| Treatment sequence e | |||||
| Surgery with radiotherapy | |||||
| Surgery without radiotherapy | −0.461 | <0.005 | 0.630 | 0.591 | 0.672 |
| No surgery with radiotherapy | 0.500 | <0.005 | 1.649 | 1.531 | 1.776 |
| No surgery without radiotherapy | −0.125 | <0.005 | 0.883 | 0.827 | 0.942 |
| Breslow f | |||||
| <0.8 mm | |||||
| 0.8 to 1 mm | 0.224 | <0.005 | 1.251 | 1.162 | 1.348 |
| >1 to 2 mm | 0.656 | <0.005 | 1.927 | 1.823 | 2.037 |
| >2 to 4 mm | 1.085 | <0.005 | 2.960 | 2.799 | 3.131 |
| >4 mm | 1.722 | <0.005 | 5.594 | 5.295 | 5.911 |
| Ulceration f | |||||
| No | |||||
| Yes | 1.010 | <0.005 | 2.747 | 2.638 | 2.859 |
| Mitotic Rate f | |||||
| 2 or less | |||||
| 3 to 10 | 0.885 | <0.005 | 2.422 | 2.305 | 2.546 |
| 11 or more | 1.222 | <0.005 | 3.394 | 3.170 | 3.634 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walz, S.N.; Martineau, J.; Scampa, M.; Kalbermatten, D.F.; Oranges, C.M. Melanoma of the Upper Limb and Shoulder: A Surveillance, Epidemiology, and End Results Analysis of Epidemiology and Survival 2000–2019. Cancers 2022, 14, 5672. https://doi.org/10.3390/cancers14225672
Walz SN, Martineau J, Scampa M, Kalbermatten DF, Oranges CM. Melanoma of the Upper Limb and Shoulder: A Surveillance, Epidemiology, and End Results Analysis of Epidemiology and Survival 2000–2019. Cancers. 2022; 14(22):5672. https://doi.org/10.3390/cancers14225672
Chicago/Turabian StyleWalz, Solange N., Jérôme Martineau, Matteo Scampa, Daniel F. Kalbermatten, and Carlo M. Oranges. 2022. "Melanoma of the Upper Limb and Shoulder: A Surveillance, Epidemiology, and End Results Analysis of Epidemiology and Survival 2000–2019" Cancers 14, no. 22: 5672. https://doi.org/10.3390/cancers14225672
APA StyleWalz, S. N., Martineau, J., Scampa, M., Kalbermatten, D. F., & Oranges, C. M. (2022). Melanoma of the Upper Limb and Shoulder: A Surveillance, Epidemiology, and End Results Analysis of Epidemiology and Survival 2000–2019. Cancers, 14(22), 5672. https://doi.org/10.3390/cancers14225672







