Safety and Efficacy of Irreversible Electroporation in Locally Advanced Pancreatic Cancer: An Evaluation from a Surgeon’s Perspective
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Data Collection and Definition
2.4. Perioperative Management
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Patients
3.2. Early Clinical Response
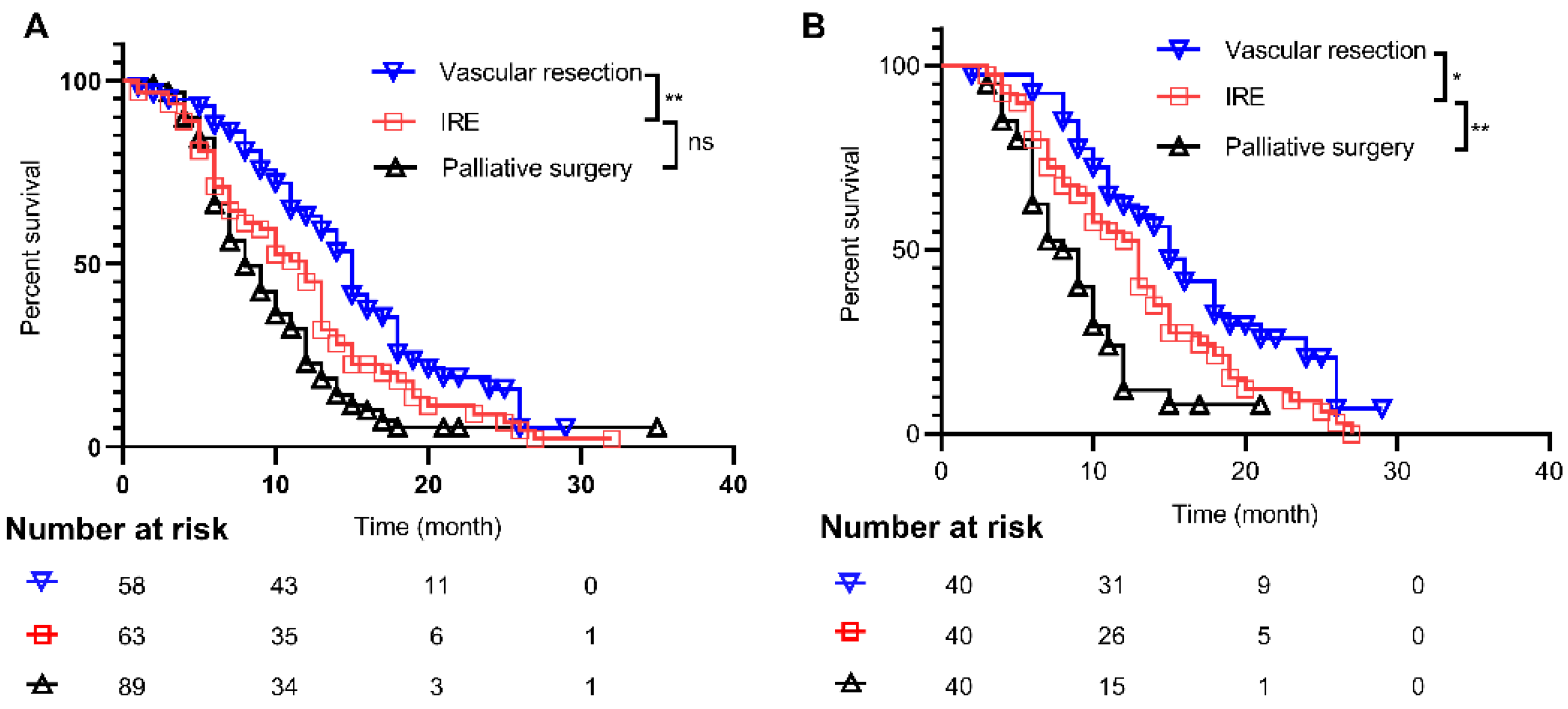
3.3. Long-Term Prognosis of Patients
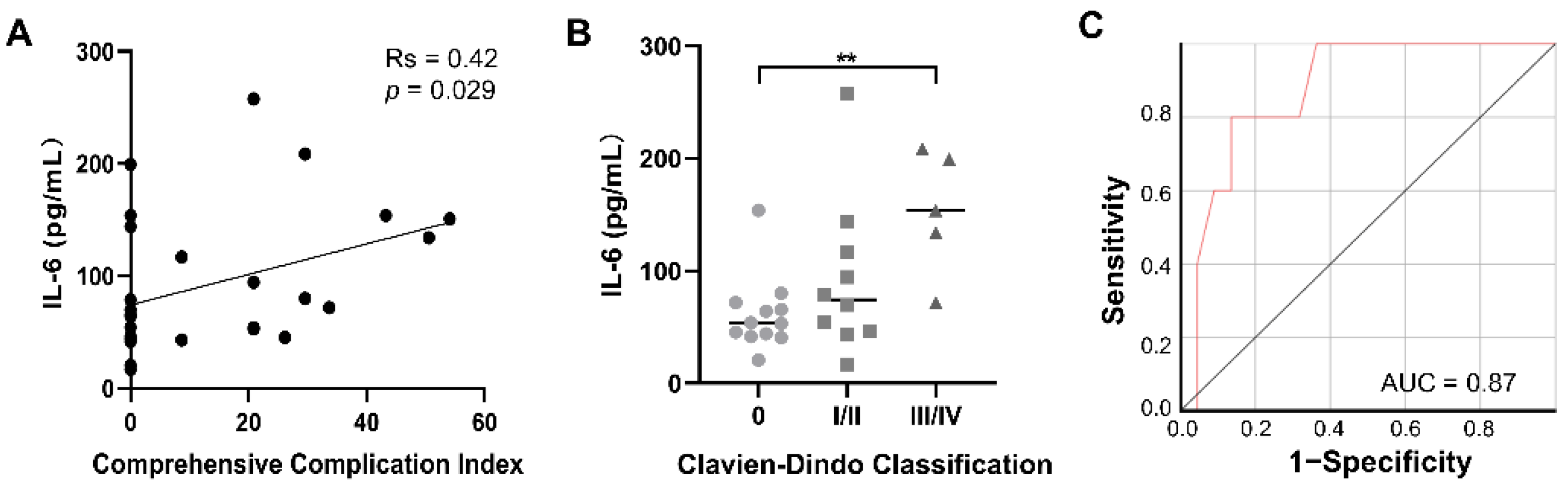
3.4. Peripheral Immune Responses after IRE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Complications | Definition |
|---|---|
| Pancreatic fistula | Drain amylase level more than 3 times the upper limit of normal serum amylase level on or after POD 3 associated with a clinically relevant change in management |
| Intra-abdominal hemorrhage | Blood loss that needs for blood transfusion, invasive intervention (such as re-operation or interventional angiography) |
| Delayed gastric emptying | Nasogastric tube intubation lasting more than 3 days or inability to tolerate a standard diet by the end of the first postoperative week |
| Re-intervention | Various interventions that were performed under local anesthesia, including endoscopy, intervention, or minor surgeries |
| Re-operation | Unplanned operation performed under general anesthesia due to an operative complication |
| Infection | Diagnosed per clinical features or microbiologic confirmation |
| Overall survival | The time from surgery to the last date of follow-up or the date of death, whichever occurred first |
| censored | Patients who were still alive at the last moment of follow-up |
| Mortality | Death happened within 30 days of surgery |
| Re-admission | Unplanned return to hospital within 90 days of surgery |
| The prognostic nutritional index | A widely used marker that reflects the patient’s physiological state, calculated as follows: serum albumin (g/L) + 0.005 × total lymphocyte count (per mm3) |
| Variables | Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| IRE Group (n = 63) | Vascular Resection Group (n = 58) | Palliative Surgery Group (n = 89) | p | IRE Group (n = 40) | Vascular Resection Group (n = 40) | Palliative Surgery (n = 40) | p | |
| Age (years) | 60.0 ± 9.1 | 59.4 ± 8.7 | 61.0 ± 9.3 | 0.782 | 61.0 ± 9.1 | 58.6 ± 9.5 | 61.5 ± 9.1 | 0.630 |
| BMI (kg/m2) | 21.8 ± 2.6 | 22.3 ± 2.3 | 21.2 ± 2.8 | 0.022 | 21.7 ± 2.3 | 22.3 ± 1.9 | 21.9 ± 2.8 | 0.607 |
| Sex | 0.892 | 0.967 | ||||||
| Female | 26 (41.3%) | 25 (43.1%) | 40 (45.0%) | 20 (50.0%) | 20 (50.0%) | 19 (47.5%) | ||
| Male | 37 (58.7%) | 33 (56.9%) | 49 (55.0%) | 20 (50.0%) | 20 (50.0%) | 21 (52.5%) | ||
| Smoking | 0.763 | 0.546 | ||||||
| Yes | 14 (22.3%) | 16 (27.6%) | 24 (27.0%) | 7 (17.5%) | 11 (27.5%) | 10 (25.0%) | ||
| No | 49 (77.8%) | 42 (72.3%) | 65 (73.0%) | 33 (82.5%) | 29 (72.5%) | 30 (75.0%) | ||
| Drinking | 0.479 | 0.360 | ||||||
| Yes | 15 (23.8%) | 12 (20.7%) | 14 (15.7%) | 8 (20.0%) | 10 (25.0%) | 5 (12.5%) | ||
| No | 48 (76.2%) | 46 (79.3%) | 75 (84.3%) | 32 (80.0%) | 30 (75.0%) | 35 (87.5%) | ||
| Diabetes | 0.249 | 0.298 | ||||||
| Yes | 15 (23.8%) | 7 (12.1%) | 17 (19.1%) | 10 (25.0%) | 5 (12.5%) | 6 (15.0%) | ||
| No | 48 (76.2%) | 51 (87.9%) | 72 (80.9%) | 30 (75.0%) | 35 (87.5%) | 34 (85.0%) | ||
| Hypertension | 0.712 | 0.670 | ||||||
| Yes | 15 (23.8%) | 15 (25.86%) | 18 (20.22%) | 11 (27.50%) | 8 (20.00%) | 11 (27.50%) | ||
| No | 48 (76.2%) | 43 (74.14%) | 71 (79.18%) | 29 (72.5%) | 32 (80.0%) | 29 (72.5%) | ||
| Tumor size (cm) | 4.1 ± 1.3 | 3.7 ± 1.4 | 4.2 ± 1.6 | 0.027 | 4.0 (3.2–5.0) | 3.5 (3.0–4.0) | 4.0 (3.5–5.0) | 0.286 |
| Length of interface (cm) | 2.7 (2.4–3.0) | 2.2 (2.0–2.5) | 2.5 (2.0–3.0) | 0.001 | 2.5 (2.1–3.0) | 2.5 (2.0–3.0) | 2.5 (2.0–3.0) | 0.783 |
| CA 19-9 (U/L) | 188.4 (34.2–808.3) | 321.6 (35.5–1200.0) | 645.6 (75.8–1200.0) | 0.055 | 288.2 (81.0–1196.3) | 251.0 (37.7–1200.0) | 397.2 (84.2–1200.0) | 0.979 |
| CA 125 (U/L) | 28.10 (15.4–52.1) | 24.10 (11.4–47.3) | 29.10 (16.9–56.1) | 0.16 | 33.1 (15.6–54.7) | 22.2 (11.7–43.1) | 28.6 (17.2–55.4) | 0.101 |
| PNI | 47.1 ± 6.3 | 46.3 ± 8.1 | 44.1 ± 7.5 | 0.002 | 45.9 ± 6.5 | 47.2 ± 9.3 | 43.9 ± 6.1 | 0.138 |
| Preoperative albumin | 39.6 ± 5.2 | 38.9 ± 6.6 | 37.9 ± 5.00 | 0.024 | 38.7 ± 5.6 | 39.6 ± 7.4 | 37.7 ± 4.7 | 0.304 |
| Total bilirubin (μmol/L) | 17.4 (11.5–70.9) | 20.8 (12.7–87.6) | 49.6 (14.5–121.3) | 0.028 | 17.6 (11.6–98.5) | 16.9 (11.8–43.7) | 48.5 (13.3–101.4) | 0.380 |
| ASA score | 0.753 | 0.207 | ||||||
| I–II | 46 (73.0%) | 40 (69.0%) | 60 (67.4%) | 26 (65.0%) | 29 (72.5%) | 33 (82.5%) | ||
| III–IV | 17 (27.0%) | 18 (31.0%) | 29 (32.6%) | 14 (35.0%) | 11 (27.5%) | 7 (17.5%) | ||
| Adjuvant chemotherapy | 0.176 | 0.236 | ||||||
| Yes | 16 (25.4%) | 11 (19.0%) | 12 (13.5%) | 13 (32.5%) | 8 (20.0%) | 7 (17.5%) | ||
| No | 47 (74.6%) | 47 (81.0%) | 77 (86.5%) | 27 (67.5%) | 32 (80.0%) | 33 (82.5%) | ||
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaPelusa, M.; Shen, C.; Arhin, N.D.; Cardin, D.; Tan, M.; Idrees, K.; Geevarghese, S.; Chakravarthy, B.; Berlin, J.; Eng, C. Trends in the Incidence and Treatment of Early-Onset Pancreatic Cancer. Cancers 2022, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Leen, E.; Picard, J.; Stebbing, J.; Abel, M.; Dhillon, T.; Wasan, H. Percutaneous irreversible electroporation with systemic treatment for locally advanced pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2018, 9, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Flak, R.V.; Stender, M.T.; Jensen, T.M.; Andersen, K.L.; Henriksen, S.D.; Mortensen, P.B.; Sall, M.; Thorlacius-Ussing, O. Treatment of locally advanced pancreatic cancer with irreversible electroporation—A Danish single center study of safety and feasibility. Scand. J. Gastroenterol. 2019, 54, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.; Moris, D.; Paul Tabet, P.; Wensley Richards, B.D.; Kourounis, G. Ablation of the locally advanced pancreatic cancer: An introduction and brief summary of techniques. Off. J. Balk. Union Oncol. 2016, 21, 650–658. [Google Scholar]
- Martin, R.C., II; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: Safety and efficacy. Ann. Surg. 2015, 262, 486–494. [Google Scholar] [CrossRef]
- Ruarus, A.H.; Vroomen, L.; Geboers, B.; van Veldhuisen, E.; Puijk, R.S.; Nieuwenhuizen, S.; Besselink, M.G.; Zonderhuis, B.M.; Kazemier, G.; de Gruijl, T.D.; et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology 2020, 294, 212–220. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Palaia, R.; Belli, A.; Miele, V.; Brunese, L.; Grassi, R.; Petrillo, A.; et al. Assessment of Ablation Therapy in Pancreatic Cancer: The Radiologist’s Challenge. Front. Oncol. 2020, 10, 560952. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Fusco, R.; D’Alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Tafuto, S.; et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy. J. Clin. Med. 2021, 10, 1305. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Tafuto, S.; von Arx, C.; De Divitiis, C.; Maura, C.T.; Palaia, R.; Albino, V.; Fusco, R.; Membrini, M.; Petrillo, A.; Granata, V.; et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 2015, 21 (Suppl. S1), S78–S82. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Piccirillo, M.; Palaia, R.; Petrillo, A.; Lastoria, S.; Izzo, F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Rudno-Rudzinska, J.; Kielan, W.; Guzinski, M.; Plochocki, M.; Antonczyk, A.; Kulbacka, J. New therapeutic strategy: Personalization of pancreatic cancer treatment-irreversible electroporation (IRE), electrochemotherapy (ECT) and calcium electroporation (CaEP)—A pilot preclinical study. Surg. Oncol. 2021, 38, 101634. [Google Scholar] [CrossRef] [PubMed]
- Al Efishat, M.; Wolfgang, C.L.; Weiss, M.J. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ 2015, 350, h521. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Kristoffersson, S.; Andersson, R.; Bergenfeldt, M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: A systematic review of safety and efficacy. Scand. J. Gastroenterol. 2017, 52, 1165–1171. [Google Scholar] [CrossRef]
- Martin, R.C., II; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J. Am. Coll. Surg. 2012, 215, 361–369. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; van Tilborg, A.A.; Vieveen, J.M.; Bouwman, A.R.; Meijer, S.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: A systematic review of safety and efficacy. J. Vasc. Interv. Radiol. 2014, 25, 997–1011. [Google Scholar] [CrossRef]
- He, C.; Huang, X.; Zhang, Y.; Lin, X.; Li, S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin. Transl. Med. 2020, 10, e39. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Stam AG, M.; Geboers, B.; Vroomen, L.; Ruarus, A.; de Bruijn, B.; van den Tol, M.P.; Kazemier, G.; Meijerink, M.R.; de Gruijl, T.D. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology 2019, 8, 1652532. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, B.; Krol, R.; Mrowiec, S. Vascular Resection in Pancreatectomy-Is It Safe and Useful for Patients with Advanced Pancreatic Cancer? Cancers 2022, 14, 1193. [Google Scholar] [CrossRef] [PubMed]
- Rettig, T.C.; Verwijmeren, L.; Dijkstra, I.M.; Boerma, D.; van de Garde, E.M.; Noordzij, P.G. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann. Surg. 2016, 263, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Pandit, H.; Hong, Y.K.; Li, Y.; Rostas, J.; Pulliam, Z.; Li, S.P.; Martin, R.C. Evaluating the Regulatory Immunomodulation Effect of Irreversible Electroporation (IRE) in Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 800–806. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef] [Green Version]
- El-Mesery, M.; Shaker, M.E.; Elgaml, A. The SMAC mimetic BV6 induces cell death and sensitizes different cell lines to TNF-alpha and TRAIL-induced apoptosis. Exp. Biol. Med. 2016, 241, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 899. [Google Scholar] [CrossRef]

| Variables | Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| IRE Group (n = 63) | Vascular Resection Group (n = 58) | Palliative Surgery Group (n = 89) | p | IRE Group (n = 58) | Vascular Resection Group (n = 58) | Palliative Surgery (n = 58) | p | |
| Age (years) | 60.0 ± 9.1 | 59.4 ± 8.7 | 61.0 ± 9.3 | 0.782 | 60.7 ± 9.0 | 59.4 ± 8.7 | 60.5 ± 9.1 | 0.969 |
| BMI (kg/m2) | 21.8 ± 2.5 | 22.3 ± 2.3 | 21.2 ± 2.9 | 0.022 | 21.7 ± 2.4 | 22.3 ± 2.3 | 21.4 ± 2.5 | 0.129 |
| Sex | 0.892 | 0.999 | ||||||
| Female | 26 (41.2%) | 25 (43.1%) | 40 (45.0%) | 25 (43.1%) | 25 (43.1%) | 25 (43.1%) | ||
| Male | 37 (58.8%) | 33 (56.9%) | 49 (55.0%) | 33 (56.9%) | 33 (56.9%) | 33 (56.9%) | ||
| Smoking | 0.763 | 0.718 | ||||||
| Yes | 14 (22.2%) | 16 (27.6%) | 24 (27.0%) | 12 (20.7%) | 16 (27.6%) | 14 (24.1%) | ||
| No | 49 (77.8%) | 42 (72.4%) | 65 (73.0%) | 46 (79.3%) | 42 (72.4%) | 44 (75.9%) | ||
| Drinking | 0.479 | 0.718 | ||||||
| Yes | 15 (23.8%) | 12 (20.7%) | 14 (15.7%) | 15 (25.9%) | 12 (20.7%) | 11 (19.0%) | ||
| No | 48 (76.2%) | 46 (79.3%) | 75 (84.3%) | 43 (74.1%) | 46 (79.3%) | 47 (81.0%) | ||
| Diabetes | 0.249 | 0.268 | ||||||
| Yes | 15 (23.8%) | 7 (12.1%) | 17 (19.1%) | 14 (24.1%) | 7 (12.1%) | 11 (19.0%) | ||
| No | 48 (76.2%) | 51 (87.9%) | 72 (80.9%) | 44 (75.9%) | 51 (87.9%) | 47 (81.0%) | ||
| Hypertension | 0.712 | 0.487 | ||||||
| Yes | 15 (23.8) | 15 (25.9%) | 18 (20.2%) | 15 (25.9%) | 15 (25.9%) | 10 (17.2%) | ||
| No | 48 (76.2) | 43 (74.1%) | 71 (79.8%) | 43 (74.1%) | 43 (74.1%) | 48 (82.8%) | ||
| Tumor size (cm) | 4.1 ± 1.3 | 3.7 ± 1.4 | 4.2 ± 1.6 | 0.027 | 3.9 ± 1. | 3.7 ± 1.4 | 3.8 ± 0.9 | 0.125 |
| CA 19-9 (U/L) | 188.4 (34.2–808.3) | 321.6 (35.5–1200.0) | 645.6 (75.8–1200.0) | 0.055 | 272.9 (37.1–1200.0) | 331.1 (33.3–1200.0) | 700.0 (84.2–1200.0) | 0.152 |
| CA 125 (U/L) | 28.1 (15.4–52.1) | 24.1 (11.4–47.3) | 29.1 (16.9–56.1) | 0.160 | 29.7 (15.4–50.6) | 25.4 (13.9–44.8) | 27.4 (16.6–47.1) | 0.346 |
| PNI | 47.1 ± 6.3 | 46.3 ± 8.1 | 44.1 ± 7.5 | 0.002 | 47.0 ± 5.6 | 46.3 ± 8.1 | 45.4 ± 5.5 | 0.130 |
| Pre-operative albumin | 39.6 ± 5.3 | 38.9 ± 6.6 | 37.9 ± 5.0 | 0.024 | 39.6 ± 4.9 | 38.9 ± 6.6 | 39.1 ± 4.1 | 0.203 |
| Total bilirubin (μmol/L) | 17.4 (11.5–70.9) | 20.8 (12.7–87.6) | 49.6 (14.5–121.3) | 0.028 | ||||
| ASA | 0.753 | 0.634 | ||||||
| I–II | 46 (73.0%) | 40 (69.0%) | 60 (67.4%) | 42 (72.4%) | 40 (69.0%) | 37 (63.8%) | ||
| III–IV | 17 (27.0%) | 18 (31.0%) | 29 (32.6%) | 16 (27.6%) | 18 (31.0%) | 21 (36.2%) | ||
| Adjuvant chemotherapy | 0.187 | 0.268 | ||||||
| Yes | 16 (25.4%) | 11 (19.0%) | 12 (13.5%) | 14 (24.1%) | 11 (19.0%) | 7 (12.1%) | ||
| No | 47 (74.6%) | 47 (81.0%) | 77 (86.5%) | 44 (75.9%) | 47 (81.0%) | 51 (87.9%) | ||
| Complications | Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| IRE Group (n = 63) | Vascular Resection Group (n = 58) | Palliative Surgery Group (n = 89) | p | IRE Group (n = 58) | Vascular Resection Group (n = 58) | Palliative Surgery Group (n = 58) | p | |
| Pancreatic fistula | <0.001 | 0.001 | ||||||
| Yes | 1 (1.6%) | 9 (15.5%) † | 0 (0.0%) | 1 (1.7%) | 9 (15.5%) † | 0 (0.0%) | ||
| No | 62 (98.4%) | 49 (84.5%) | 89 (100.0%) | 57 (98.3%) | 49 (84.5%) | 58 (100.0%) | ||
| Intra-abdominal hemorrhage | 0.604 | 0.081 | ||||||
| Yes | 5 (7.9%) | 5 (8.6%) | 4 (4.5%) | 5 (8.6%) | 5 (8.6%) | 0 (0.0%) † | ||
| No | 58 (92.1%) | 53 (91.4%) | 85 (95.5%) | 53 (91.4%) | 53 (91.4%) | 58 (100%) | ||
| Intra-abdominal infection | 0.025 | 0.035 | ||||||
| Yes | 2 (3.2%) | 9 (15.5%) † | 5 (5.6%) | 2 (3. 5%) | 9 (15.5%) † | 3 (5.2%) | ||
| No | 61 (96.9%) | 49 (84.5%) | 84 (94.4%) | 56 (96.5%) | 49 (84.5%) | 55 (94.8%) | ||
| Delayed gastric emptying | 0.408 | 0.569 | ||||||
| Yes | 8 (12.7%) | 7 (12.1%) | 17 (19.1%) | 5 (8.6%) | 7 (12.1%) | 9 (15.5%) | ||
| No | 55 (87.3%) | 51 (87.9%) | 72 (80.9%) | 53 (91.4%) | 51 (87.9%) | 49 (84.5%) | ||
| Pancreatitis | 0.474 | 0.999 | ||||||
| Yes | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 1 (1.7%) | 1 (1.7%) | 0 (0.0%) | ||
| No | 62 (98.4%) | 57 (98.3%) | 89 (100.0%) | 57 (98.3%) | 57 (98.3%) | 58 (100%) | ||
| Re-intervention | 0.008 | 0.059 | ||||||
| Yes | 8 (12.7%) | 7 (12.1%) | 1 (1.1%) † | 8 (13.8%) | 7 (12.1%) | 1 (1.7%) † | ||
| No | 55 (87.3%) | 51 (87.9%) | 88 (98.9%) | 50 (86.2%) | 51 (87.9%) | 57 (98.3%) | ||
| Re-operation | 0.264 | 0.999 | ||||||
| Yes | 2 (3.2%) | 1 (1.7%) | 0 (0.0%) | 1 (1.7%) | 1 (1.7%) | 0 (0.0%) | ||
| No | 61 (96.9%) | 57 (98.3%) | 89 (100.0%) | 57 (98.3%) | 57 (98.3%) | 58 (100%) | ||
| 30-day re-admission | 0.652 | 0.301 | ||||||
| Yes | 5 (7.9%) | 3 (5.2%) | 4 (4.5%) | 5 (8.6%) | 3 (5.2%) | 1 (1.7%) | ||
| No | 58 (92.1%) | 55 (94.8%) | 85 (95.5%) | 53 (91.4%) | 55 (94.8%) | 57 (98.3%) | ||
| Clavien–Dindo classification | 0.002 | 0.020 | ||||||
| lower than grade III | 52 (82.5%) | 46 (79.3%) | 86 (96.6%) | 48 (82.8%) | 46 (79.3%) | 56 (96.6%) | ||
| Grade III and higher | 11 (17.5%) | 12 (20.7%) | 3 (3.4%)† | 10 (17.2%) | 12 (20.7%) | 2 (3.4%) † | ||
| Death | 0.264 | 0.774 | ||||||
| Yes | 2 (3.2%) | 1 (1.7%) | (0.0%) | 2 (3.5%) | 1 (1.7%) | 0 (0.0%) | ||
| No | 61 (96.9%) | 57 (98.3%) | 89 (100%) | 56 (96.5%) | 57 (98.3%) | 58 (100.0%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Pan, P.; Hu, X.; Zhao, J.; Wu, H. Safety and Efficacy of Irreversible Electroporation in Locally Advanced Pancreatic Cancer: An Evaluation from a Surgeon’s Perspective. Cancers 2022, 14, 5677. https://doi.org/10.3390/cancers14225677
Shen J, Pan P, Hu X, Zhao J, Wu H. Safety and Efficacy of Irreversible Electroporation in Locally Advanced Pancreatic Cancer: An Evaluation from a Surgeon’s Perspective. Cancers. 2022; 14(22):5677. https://doi.org/10.3390/cancers14225677
Chicago/Turabian StyleShen, Jian, Penglin Pan, Xiaoli Hu, Jun Zhao, and Heshui Wu. 2022. "Safety and Efficacy of Irreversible Electroporation in Locally Advanced Pancreatic Cancer: An Evaluation from a Surgeon’s Perspective" Cancers 14, no. 22: 5677. https://doi.org/10.3390/cancers14225677
APA StyleShen, J., Pan, P., Hu, X., Zhao, J., & Wu, H. (2022). Safety and Efficacy of Irreversible Electroporation in Locally Advanced Pancreatic Cancer: An Evaluation from a Surgeon’s Perspective. Cancers, 14(22), 5677. https://doi.org/10.3390/cancers14225677





