Elevated Levels of Circulating Hsp70 and an Increased Prevalence of CD94+/CD69+ NK Cells Is Predictive for Advanced Stage Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
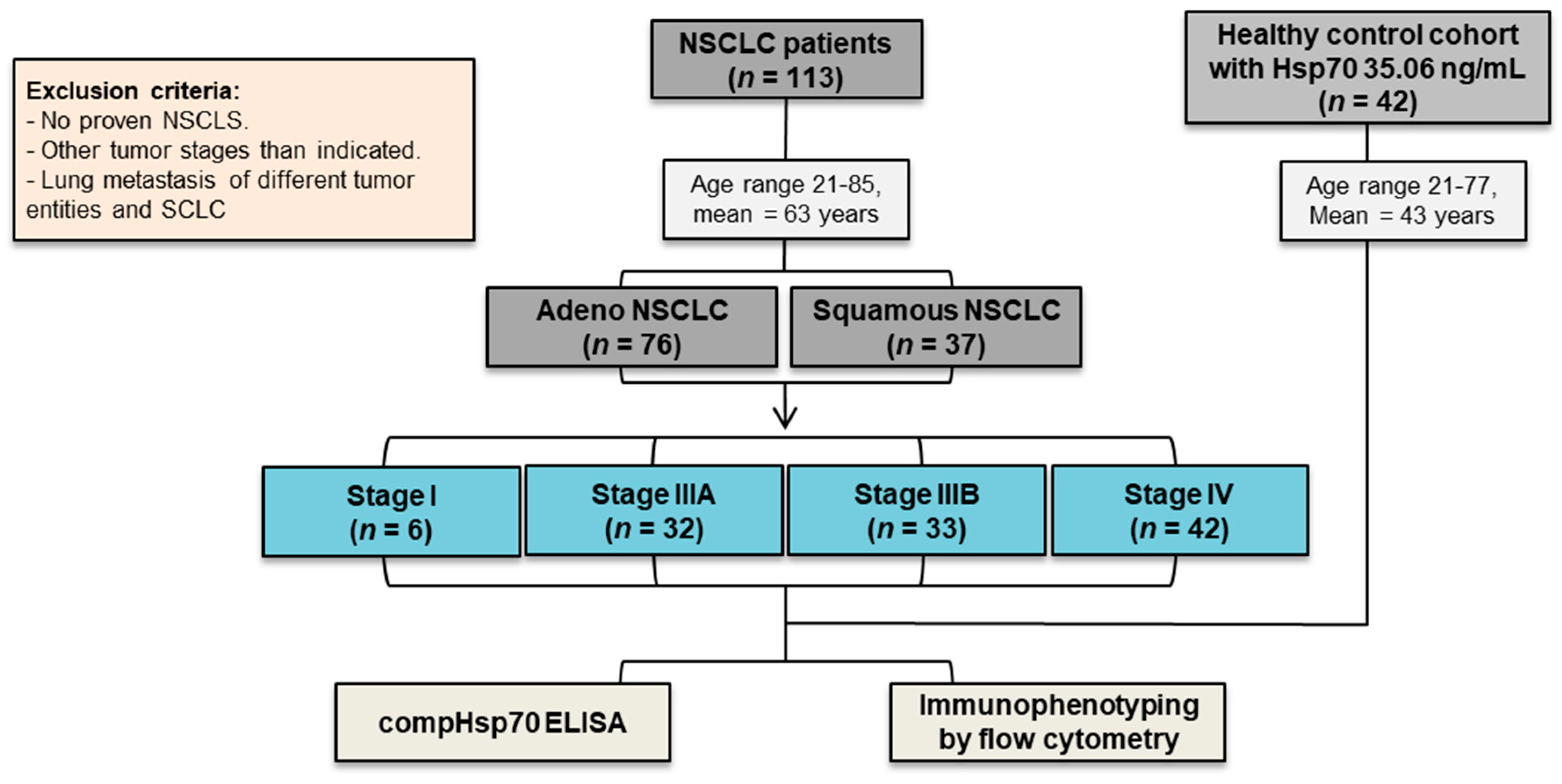
2.1. Study Participants
2.2. Measurement of Free and Exosomal Hsp70 in Serum and Plasma Using the compHsp70 ELISA
2.3. Immunophenotyping of Lymphocyte Subpopulations by Multiparameter Flow Cytometry
2.4. Multiplex Cytokine Analysis
2.5. Statistical Analysis
3. Results
3.1. NSCLC Patients in Different UICC Stages and Healthy Control Cohorts
3.2. Comparison of Circulating Hsp70 Levels in NSCLC Patients in Different UICC Stages
3.3. Immunophenotype in the Peripheral Blood of NSCLC Patients in Different UICC Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.M.; De Ruysscher, D.; Hoffmann, H.; Reu, S.; Tufman, A. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur. Respir. Rev. 2019, 28, 190024. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Oberije, C.; De Ruysscher, D.; Houben, R.; van de Heuvel, M.; Uyterlinde, W.; Deasy, J.O.; Belderbos, J.; Dingemans, A.M.; Rimner, A.; Din, S.; et al. A Validated Prediction Model for Overall Survival from Stage III Non-Small Cell Lung Cancer: Toward Survival Prediction for Individual Patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Malusecka, E.; Zborek, A.; Krzyzowska-Gruca, S.; Krawczyk, Z. Expression of heat shock proteins HSP70 and HSP27 in primary non-small cell lung carcinomas. An immunohistochemical study. Anticancer Res. 2001, 21, 1015–1021. [Google Scholar] [PubMed]
- Hwang, T.S.; Han, H.S.; Choi, H.K.; Lee, Y.J.; Kim, Y.J.; Han, M.Y.; Park, Y.M. Differential, stage-dependent expression of Hsp70, Hsp110 and Bcl-2 in colorectal cancer. J. Gastroenterol. Hepatol. 2003, 18, 690–700. [Google Scholar] [CrossRef]
- Abe, M.; Manola, J.B.; Oh, W.K.; Parslow, D.L.; George, D.J.; Austin, C.L.; Kantoff, P.W. Plasma levels of heat shock protein 70 in patients with prostate cancer: A potential biomarker for prostate cancer. Clin. Prostate Cancer 2004, 3, 49–53. [Google Scholar] [CrossRef]
- Lobinger, D.; Gempt, J.; Sievert, W.; Barz, M.; Schmitt, S.; Nguyen, H.T.; Stangl, S.; Werner, C.; Wang, F.; Wu, Z.; et al. Potential Role of Hsp70 and Activated NK Cells for Prediction of Prognosis in Glioblastoma Patients. Front. Mol. Biosci. 2021, 8, 669366. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. The Warburg Effect: Historical Dogma Versus Current Rationale. Adv. Exp. Med. Biol. 2021, 1269, 169–177. [Google Scholar] [CrossRef]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Muller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef]
- Murakami, N.; Kuhnel, A.; Schmid, T.E.; Ilicic, K.; Stangl, S.; Braun, I.S.; Gehrmann, M.; Molls, M.; Itami, J.; Multhoff, G. Role of membrane Hsp70 in radiation sensitivity of tumor cells. Radiat. Oncol. 2015, 10, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Multhoff, G.; Pockley, A.G.; Schmid, T.E.; Schilling, D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015, 368, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botzler, C.; Schmidt, J.; Luz, A.; Jennen, L.; Issels, R.; Multhoff, G. Differential Hsp70 plasma-membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int. J. Cancer 1998, 77, 942–948. [Google Scholar] [CrossRef]
- Gehrmann, M.; Marienhagen, J.; Eichholtz-Wirth, H.; Fritz, E.; Ellwart, J.; Jaattela, M.; Zilch, T.; Multhoff, G. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: Protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 2005, 12, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Bashiri Dezfouli, A.; Yazdi, M.; Benmebarek, M.-R.; Schwab, M.; Michaelides, S.; Miccichè, A.; Geerts, D.; Stangl, S.; Klapproth, S.; Wagner, E.; et al. CAR T Cells Targeting Membrane-Bound Hsp70 on Tumor Cells Mimic Hsp70-Primed NK Cells. Front. Immunol. 2022, 13, 883694. [Google Scholar] [CrossRef]
- Krause, S.W.; Gastpar, R.; Andreesen, R.; Gross, C.; Ullrich, H.; Thonigs, G.; Pfister, K.; Multhoff, G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: A clinical phase i trial. Clin. Cancer Res. 2004, 10, 3699–3707. [Google Scholar] [CrossRef] [Green Version]
- Specht, H.M.; Ahrens, N.; Blankenstein, C.; Duell, T.; Fietkau, R.; Gaipl, U.S.; Gunther, C.; Gunther, S.; Habl, G.; Hautmann, H.; et al. Heat shock protein 70 (Hsp70) peptide activated natural killer (NK) cells for the treatment of patients with non-small Cell lung cancer (NSCLC) after radiochemotherapy (RCTx)—From preclinical studies to a clinical Phase II trial. Front. Immunol. 2015, 6, 162. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.; Koelch, W.; DeMaio, A.; Arispe, N.; Multhoff, G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J. Biol. Chem. 2003, 278, 41173–41181. [Google Scholar] [CrossRef] [Green Version]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef] [Green Version]
- Breuninger, S.; Ertl, J.; Bayer, C.; Knape, C.; Gunther, S.; Regel, I.; Rodel, F.; Gaipl, U.; Thorsteinsdottir, J.; Giannitrapani, L.; et al. Quantitative analysis of liposomal heat shock protein 70 in the blood of tumor patients using a novel lipHsp70 ELISA. J. Clin. Cell. Immunol. 2015, 5, 4–10. [Google Scholar] [CrossRef]
- Werner, C.; Stangl, S.; Salvermoser, L.; Schwab, M.; Shevtsov, M.; Xanthopoulos, A.; Wang, F.; Dezfouli, A.B.; Tholke, D.; Ostheimer, C.; et al. Hsp70 in Liquid Biopsies-A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer. Cancers 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Bayer, C.; Liebhardt, M.E.; Schmid, T.E.; Trajkovic-Arsic, M.; Hube, K.; Specht, H.M.; Schilling, D.; Gehrmann, M.; Stangl, S.; Siveke, J.T.; et al. Validation of heat shock protein 70 as a tumor-specific biomarker for monitoring the outcome of radiation therapy in tumor mouse models. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S.; Ostheimer, C.; Stangl, S.; Specht, H.M.; Mozes, P.; Jesinghaus, M.; Vordermark, D.; Combs, S.E.; Peltz, F.; Jung, M.P.; et al. Correlation of Hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front. Immunol. 2015, 6, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Multhoff, G.; Seier, S.; Stangl, S.; Sievert, W.; Shevtsov, M.; Werner, C.; Pockley, A.G.; Blankenstein, C.; Hildebrandt, M.; Offner, R.; et al. Targeted Natural Killer Cell-Based Adoptive Immunotherapy for the Treatment of Patients with NSCLC after Radiochemotherapy: A Randomized Phase II Clinical Trial. Clin. Cancer. Res. 2020, 26, 5368–5379. [Google Scholar] [CrossRef]
- Multhoff, G. Heat shock proteins in immunity. In Handbook of Experimental Pharmacology; Huebert, M., Ed.; Molecular chaperones in health and disease; Springer: Berlin/Heidelberg, Germany, 2006; Volume 172, pp. 279–304. [Google Scholar]
- Kokowski, K.; Stangl, S.; Seier, S.; Hildebrandt, M.; Vaupel, P.; Multhoff, G. Radiochemotherapy combined with NK cell transfer followed by second-line PD-1 inhibition in a patient with NSCLC stage IIIb inducing long-term tumor control: A case study. Strahlenther. Onkol. 2019, 195, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Stangl, S.; Gehrmann, M.; Riegger, J.; Kuhs, K.; Riederer, I.; Sievert, W.; Hube, K.; Mocikat, R.; Dressel, R.; Kremmer, E.; et al. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Nickl, S.; Lambers, C.; Hacker, S.; Mitterbauer, A.; Hoetzenecker, K.; Rozsas, A.; Ostoros, G.; Laszlo, V.; Hofbauer, H.; et al. Discrimination of clinical stages in non-small cell lung cancer patients by serum HSP27 and HSP70: A multi-institutional case-control study. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 413, 1115–1120. [Google Scholar] [CrossRef] [Green Version]
- Bashiri Dezfouli, A.; Yazdi, M.; Pockley, A.G.; Khosravi, M.; Kobold, S.; Wagner, E.; Multhoff, G. NK cells armed with chimeric antigen receptors (CAR): Roadblocks to successful development. Cells 2021, 10, 3390. [Google Scholar] [CrossRef]
- Ramirez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Perez, A.; Onate, C.; Andres-Tovar, A.; Garzon-Tituana, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228. [Google Scholar] [CrossRef]
- Multhoff, G.; Pfister, K.; Gehrmann, M.; Hantschel, M.; Gross, C.; Hafner, M.; Hiddemann, W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress. Chaperones. 2001, 6, 337–344. [Google Scholar] [CrossRef]
- Gross, C.; Schmidt-Wolf, I.G.; Nagaraj, S.; Gastpar, R.; Ellwart, J.; Kunz-Schughart, L.A.; Multhoff, G. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones 2003, 8, 348–360. [Google Scholar] [CrossRef]
- Koyama-Nasu, R.; Wang, Y.; Hasegawa, I.; Endo, Y.; Nakayama, T.; Kimura, M.Y. The cellular and molecular basis of CD69 function in anti-tumor immunity. Int. Immunol. 2022, 34, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiringhelli, F.; Menard, C.; Martin, F.; Zitvogel, L. The role of regulatory T cells in the control of natural killer cells: Relevance during tumor progression. Immunol. Rev. 2006, 214, 229–238. [Google Scholar] [CrossRef]
- Miggelbrink, A.M.; Jackson, J.D.; Lorrey, S.J.; Srinivasan, E.S.; Waibl-Polania, J.; Wilkinson, D.S.; Fecci, P.E. CD4 T-Cell Exhaustion: Does It Exist and What Are Its Roles in Cancer? Clin. Cancer Res. 2021, 27, 5742–5752. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seier, S.; Bashiri Dezfouli, A.; Lennartz, P.; Pockley, A.G.; Klein, H.; Multhoff, G. Elevated Levels of Circulating Hsp70 and an Increased Prevalence of CD94+/CD69+ NK Cells Is Predictive for Advanced Stage Non-Small Cell Lung Cancer. Cancers 2022, 14, 5701. https://doi.org/10.3390/cancers14225701
Seier S, Bashiri Dezfouli A, Lennartz P, Pockley AG, Klein H, Multhoff G. Elevated Levels of Circulating Hsp70 and an Increased Prevalence of CD94+/CD69+ NK Cells Is Predictive for Advanced Stage Non-Small Cell Lung Cancer. Cancers. 2022; 14(22):5701. https://doi.org/10.3390/cancers14225701
Chicago/Turabian StyleSeier, Sophie, Ali Bashiri Dezfouli, Philipp Lennartz, Alan Graham Pockley, Henriette Klein, and Gabriele Multhoff. 2022. "Elevated Levels of Circulating Hsp70 and an Increased Prevalence of CD94+/CD69+ NK Cells Is Predictive for Advanced Stage Non-Small Cell Lung Cancer" Cancers 14, no. 22: 5701. https://doi.org/10.3390/cancers14225701
APA StyleSeier, S., Bashiri Dezfouli, A., Lennartz, P., Pockley, A. G., Klein, H., & Multhoff, G. (2022). Elevated Levels of Circulating Hsp70 and an Increased Prevalence of CD94+/CD69+ NK Cells Is Predictive for Advanced Stage Non-Small Cell Lung Cancer. Cancers, 14(22), 5701. https://doi.org/10.3390/cancers14225701







