Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
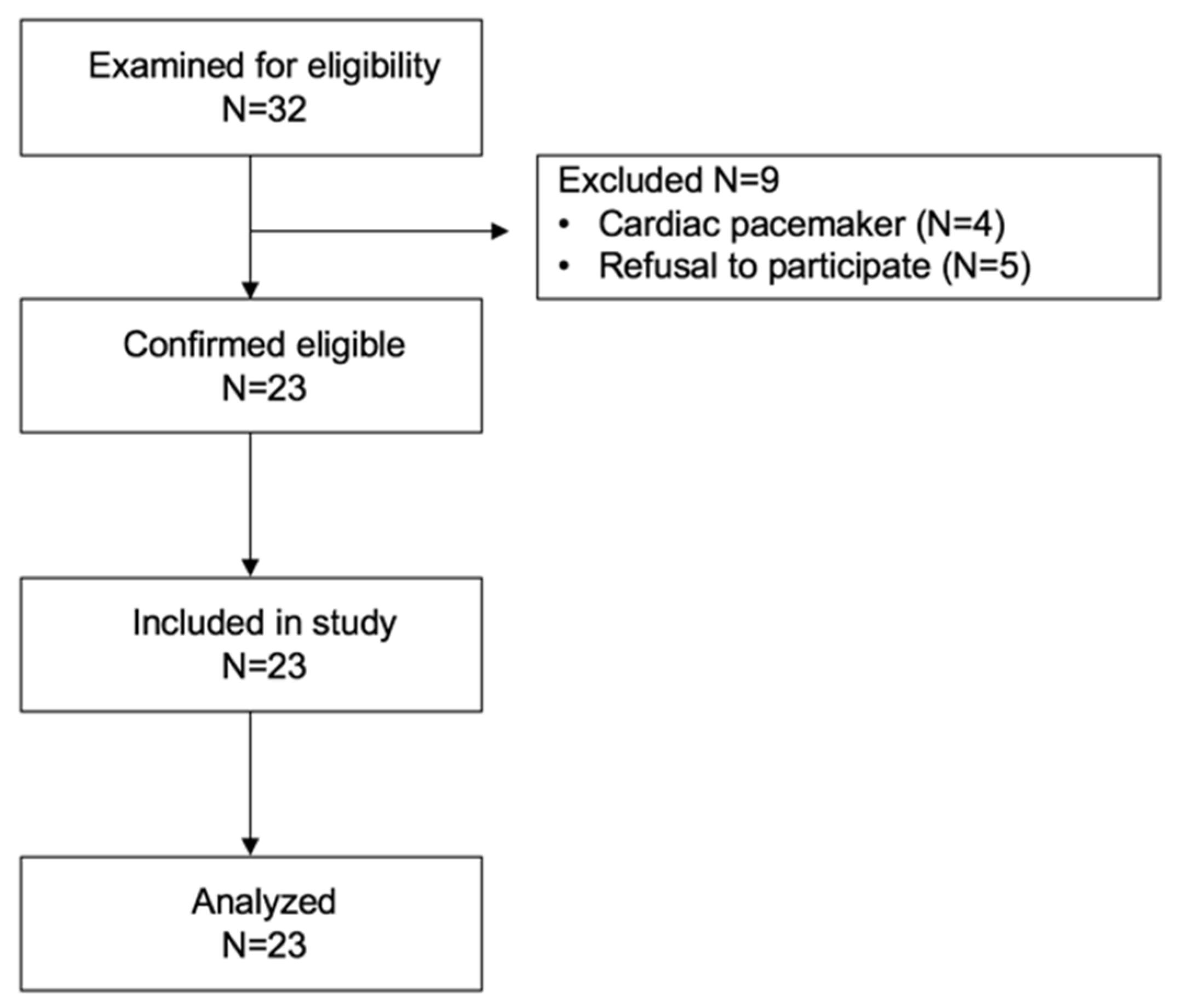
2.1. Study Design and Patient Cohort
2.2. Clinical Data
2.3. Data Acquisition
2.4. Data Reconstruction
2.5. T2w Imaging
2.6. Image Analysis
2.7. Qualitative Image Analysis
2.8. Quantitative Image Analysis
2.9. Statistical Analysis
3. Results
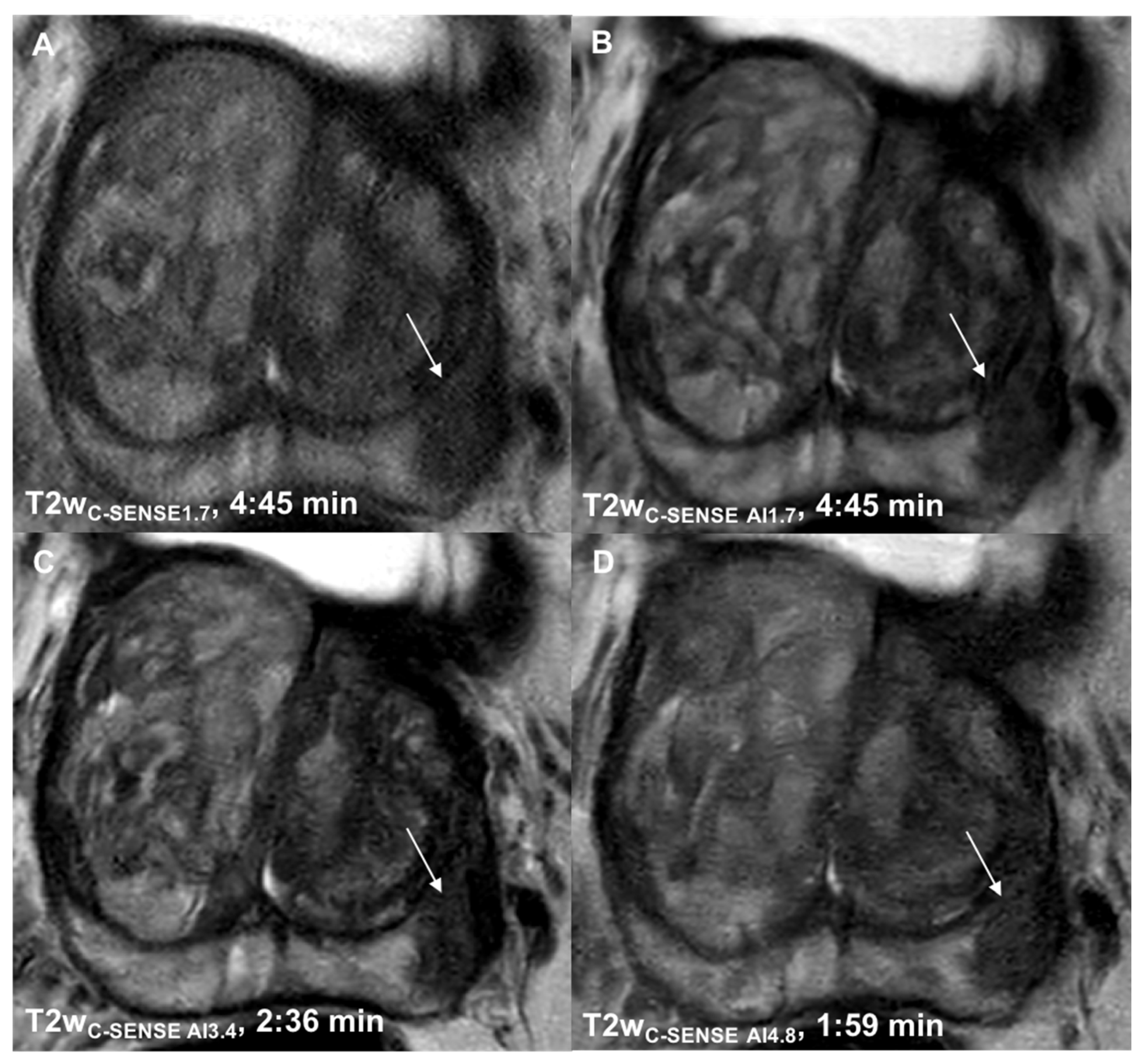
3.1. Determination of Suitable Acceleration Factors
3.2. Qualitative Analysis
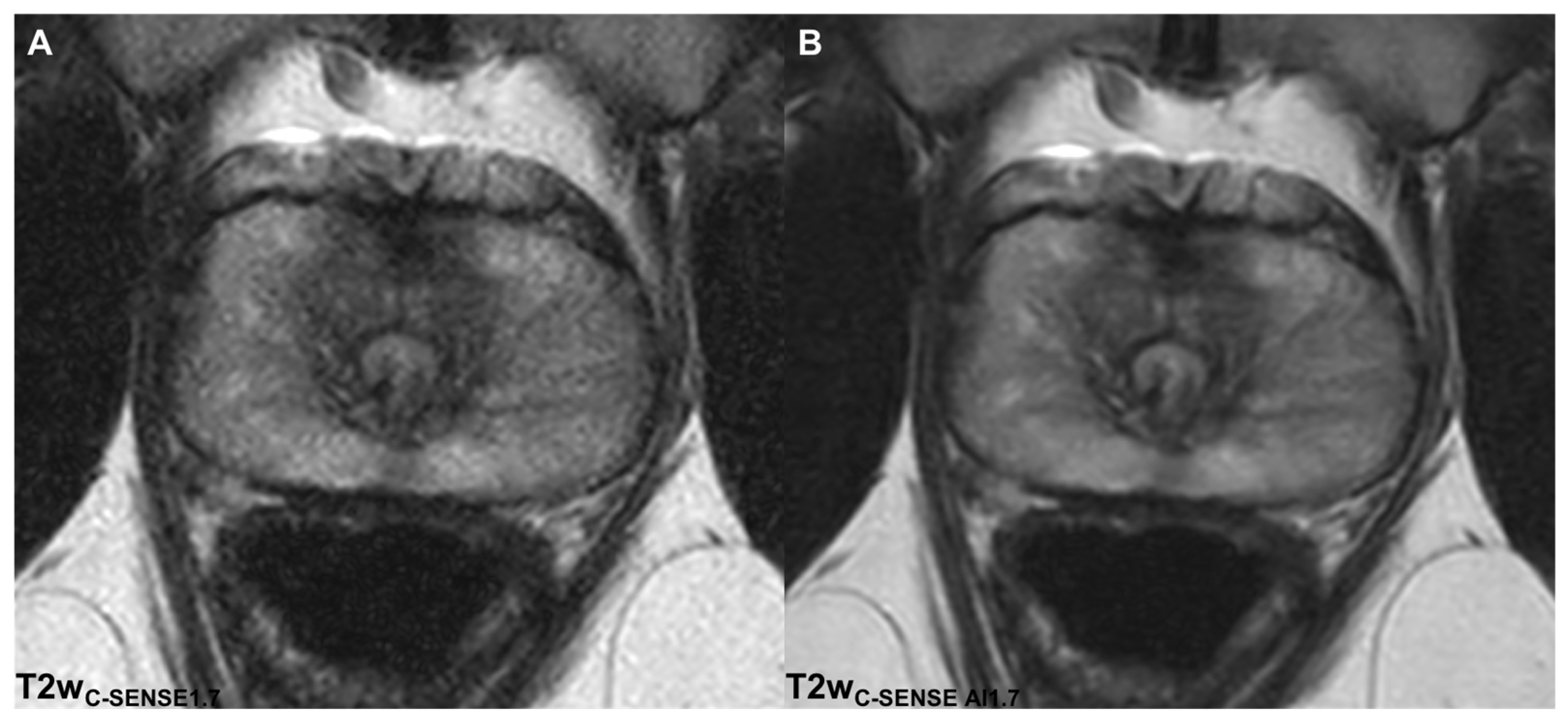
3.2.1. Image Quality
3.2.2. Noise
3.2.3. Motion Artifacts
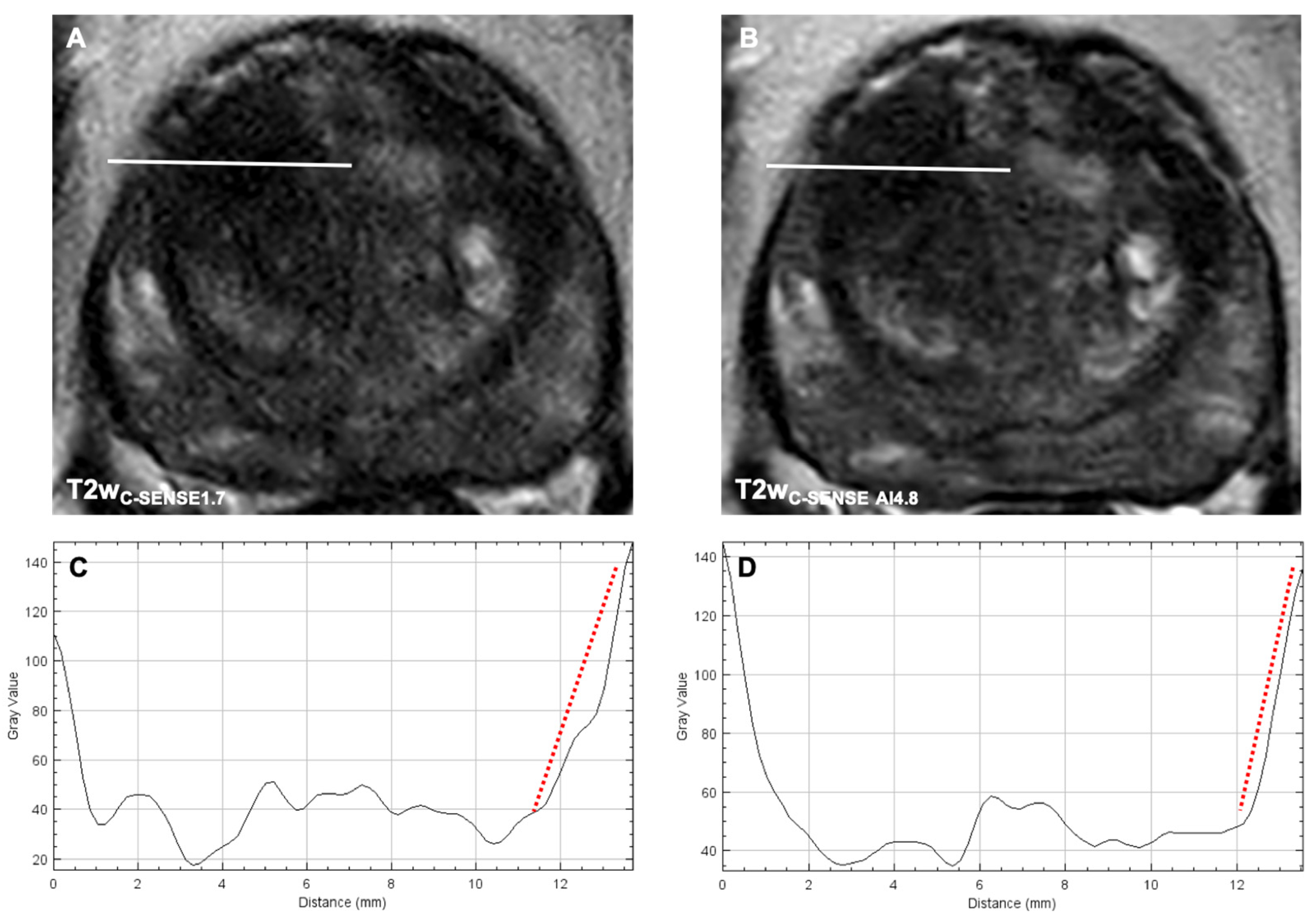
3.2.4. Image Sharpness
3.2.5. Lesion Detection and Diagnostic Certainty
3.2.6. T2 and PI-RADS Scores
3.3. Quantitative Analysis
3.3.1. aSNR
3.3.2. aCNR
3.3.3. Image Sharpness
3.3.4. Lesion Size
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A

References
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, C.M.; Barentsz, J.O.; Hambrock, T.; Yakar, D.; Somford, D.M.; Heijmink, S.W.T.P.J.; Scheenen, T.W.J.; Vos, P.C.; Huisman, H.; Van Oort, I.M.; et al. Prostate cancer: Multiparametric MR imaging for detection, localization, and staging. Radiology 2011, 261, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.C.; Litjens, G.J.S.; Wright, A.J.; Attenberger, U.I.; Haider, M.A.; Helbich, T.H.; Kiefer, B.; Macura, K.J.; Margolis, D.J.; Padhani, A.; et al. A single-arm, multicenter validation study of prostate cancer localization and aggressiveness with a quantitative multiparametric magnetic resonance imaging approach. Investig. Radiol. 2019, 54, 437–447. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Ocak, I.; Bernardo, M.; Metzger, G.; Barrett, T.; Pinto, P.; Albert, P.S.; Choyke, P.L. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: A study of pharmacokinetic parameters. AJR Am. J. Roentgenol. 2007, 189, 849. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.E.; Martirosian, P.; Schraml, C.; Taron, J.; Weiss, J.; Bier, G.; Schwentner, C.; Nickel, D.; Bamberg, F.; Kramer, U.; et al. Feasibility of CAIPIRINHA-Dixon-TWIST-VIBE for dynamic contrast-enhanced MRI of the prostate. Eur. J. Radiol. 2015, 84, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Geppert, C.; Grimm, R.; Block, T.K.; Glielmi, C.; Feng, L.; Otazo, R.; Ream, J.M.; Rt, M.M.R.; Taneja, S.S.; et al. Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: Preliminary experience. J. Magn. Reson. Imaging 2015, 41, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Pinto, P.A.; Mani, H.; Bernardo, M.; Pang, Y.; McKinney, Y.L.; Khurana, K.; Ravizzini, G.C.; Albert, P.S.; Merino, M.J.; et al. Prostate cancer: Value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology 2010, 255, 89–99. [Google Scholar] [CrossRef]
- Winkel, D.J.; Breit, H.C.; Shi, B.; Boll, D.T.; Seifert, H.H.; Wetterauer, C. Predicting clinically significant prostate cancer from quantitative image features including compressed sensing radial MRI of prostate perfusion using machine learning: Comparison with PI-RADS v2 assessment scores. Quant. Imaging Med. Surg. 2020, 10, 808–823. [Google Scholar] [CrossRef]
- Winkel, D.J.; Heye, T.J.; Benz, M.R.; Glessgen, C.G.; Wetterauer, C.; Bubendorf, L.; Block, T.K.; Boll, D.T. Compressed sensing radial sampling MRI of prostate perfusion: Utility for detection of prostate cancer. Radiology 2019, 290, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Donoho, D.L. Compressed sensing. IEEE Trans. Inf. Theor. 2006, 52, 1289–1306. [Google Scholar] [CrossRef]
- Feng, L.; Benkert, T.; Block, K.T.; Sodickson, D.K.; Otazo, R.; Chandarana, H. Compressed sensing for body MRI. J. Magn. Reson. Imaging 2017, 45, 966–987. [Google Scholar] [CrossRef] [PubMed]
- Jaspan, O.N.; Fleysher, R.; Lipton, M.L. Compressed sensing MRI: A review of the clinical literature. Br. J. Radiol. 2015, 88, 20150487. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Taviani, V.; Malkiel, I.; Cheng, J.Y.; Tamir, J.I.; Shaikh, J.; Chang, S.T.; Hardy, C.J.; Pauly, J.M.; Vasanawala, S.S. Variable-density single-shot fast spin-echo MRI with deep learning reconstruction by using variational networks. Radiology 2018, 289, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hammernik, K.; Klatzer, T.; Kobler, E.; Recht, M.P.; Sodickson, D.; Pock, T.; Knoll, F. Learning a variational network for reconstruction of accelerated MRI data. Magn. Reson. Med. 2018, 79, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Gassenmaier, S.; Nickel, D.; Arberet, S.; Afat, S.; Lingg, A.; Kündel, M.; Othman, A.E. Diagnostic confidence and feasibility of a deep learning accelerated HASTE sequence of the abdomen in a single breath-hold. Investig. Radiol. 2021, 56, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Recht, M.P.; Zbontar, J.; Sodickson, D.K.; Knoll, F.; Yakubova, N.; Sriram, A.; Murrell, T.; Defazio, A.; Rabbat, M.; Rybak, L.; et al. Using deep learning to accelerate knee MRI at 3 T: Results of an interchangeability study. AJR Am. J. Roentgenol. 2020, 215, 1421–1429. [Google Scholar] [CrossRef]
- Johnson, P.M.; Tong, A.; Donthireddy, A.; Melamud, K.; Petrocelli, R.; Smereka, P.; Qian, K.; Keerthivasan, M.B.; Chandarana, H.; Knoll, F. Deep learning reconstruction enables highly accelerated biparametric MR imaging of the prostate. J. Magn. Reson. Imaging 2021, 56, 184–195. [Google Scholar] [CrossRef]
- Kaye, E.A.; Aherne, E.A.; Duzgol, C.; Häggström, I.; Kobler, E.; Mazaheri, Y.; Fung, M.M.; Zhang, Z.; Otazo, R.; Vargas, H.A.; et al. Accelerating prostate diffusion-weighted MRI using a guided denoising convolutional neural network: Retrospective feasibility study. Radiol. Artif. Intell. 2020, 2, e200007. [Google Scholar] [CrossRef]
- Pezzotti, N.; Yousefi, S.; Elmahdy, M.S.; Van Gemert, J.H.F.; Schuelke, C.; Doneva, M.; Nielsen, T.; Kastryulin, S.; Lelieveldt, B.P.F.; Van Osch, M.J.P.; et al. An adaptive intelligence algorithm for undersampled knee MRI reconstruction. IEEE Access 2020, 8, 204825–204838. [Google Scholar] [CrossRef]
- Zhang, J.; Ghanem, B. ISTA-Net: Interpretable optimization-inspired deep network for image compressive sensing. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018; pp. 1828–1837. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, D.T.; Casalino, D.D.; Miller, F.H.; Meeks, J.J. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom. Radiol. 2017, 42, 1255–1258. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef]
- Otazo, R.; Kim, D.; Axel, L.; Sodickson, D.K. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn. Reson. Med. 2010, 64, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Eun D-i Jang, R.; Ha, W.S.; Lee, H.; Jung, S.C.; Kim, N. Deep-learning-based image quality enhancement of compressed sensing magnetic resonance imaging of vessel wall: Comparison of self-supervised and unsupervised approaches. Sci. Rep. 2020, 10, 13950. [Google Scholar]
- Padhani, A.R.; Turkbey, B. Detecting prostate cancer with deep learning for MRI: A small step forward. Radiology 2019, 293, 618–619. [Google Scholar] [CrossRef]
- Schelb, P.; Kohl, S.; Radtke, J.P.; Wiesenfarth, M.; Kickingereder, P.V.; Bickelhaupt, S.; Kuder, T.A.; Stenzinger, A.; Hohenfellner, M.; Schlemmer, H.-P.; et al. Classification of cancer at prostate MRI: Deep learning versus clinical PI-RADS assessment. Radiology 2019, 293, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Gujrathi, I.; Haider, M.A.; Khalvati, F. Prostate cancer detection using deep convolutional neural networks. Sci. Rep. 2019, 9, 19518. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Afat, S.; Nickel, D.; Mostapha, M.; Herrmann, J.; Othman, A.E. Deep learning-accelerated T2-weighted imaging of the prostate: Reduction of acquisition time and improvement of image quality. Eur. J. Radiol. 2021, 137, 109600. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Afat, S.; Nickel, M.D.; Mostapha, M.; Herrmann, J.; Almansour, H.; Nikolaou, K.; Othman, A. Accelerated T2-weighted TSE imaging of the prostate using deep learning image reconstruction: A prospective comparison with standard T2-weighted TSE imaging. Cancers 2021, 13, 3593. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Choi, M.H.; Lee, Y.J.; Han, D.; Mostapha, M.; Nickel, D. Deep learning-accelerated T2-weighted imaging of the prostate: Impact of further acceleration with lower spatial resolution on image quality. Eur. J. Radiol. 2021, 145, 110012. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, T.; Quentin, M.; Schmaltz, A.K.; Arsov, C.; Rubbert, C.; Blondin, D.; Rabenalt, R.; Albers, P.; Antoch, G.; Schimmöller, L. Hyoscine butylbromide significantly decreases motion artefacts and allows better delineation of anatomic structures in mp-MRI of the prostate. Eur. Radiol. 2018, 28, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Roethke, M.C.; Kuru, T.H.; Radbruch, A.; Hadaschik, B.; Schlemmer, H.P. Prostate magnetic resonance imaging at 3 Tesla: Is administration of hyoscine-N-butyl-bromide mandatory? World J. Radiol. 2013, 5, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.M.; Grant, A.K.; Madhuranthakam, A.J.; Lattanzi, R.; Sodickson, D.K.; McKenzie, C.A. Comprehensive quantification of signal-to-noise ratio and g-factor for image-based and k-space-based parallel imaging reconstructions. Magn. Reson. Med. 2008, 60, 895–907. [Google Scholar] [CrossRef]
- Yoon, J.H.; Nickel, M.D.; Peeters, J.M.; Lee, J.M. Rapid imaging: Recent advances in abdominal MRI for reducing acquisition time and its clinical applications. Korean J. Radiol. 2019, 20, 1597–1615. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Guo, W.; Narayan, S.; Wilson, D.L. A simple application of compressed sensing to further accelerate partially parallel imaging. Magn. Reson. Imaging 2013, 31, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Barth, B.K.; De Visschere, P.J.L.; Cornelius, A.; Nicolau, C.; Vargas, H.A.; Eberli, D.; Donati, O.F. Detection of clinically significant prostate cancer: Short dual-pulse sequence versus standard multiparametric MR imaging—A multireader study. Radiology 2017, 284, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Martirosian, P.; Notohamiprodjo, M.; Kaufmann, S.; Othman, A.E.; Grosse, U.; Nikolaou, K.; Gatidis, S. Implementation of a 5-min magnetic resonance imaging screening protocol for prostate cancer in men with elevated prostate-specific antigen before biopsy. Investig. Radiol. 2018, 53, 186–190. [Google Scholar] [CrossRef]

| Acquisition Parameters | ||
|---|---|---|
| TE/TR ms | 120/4600 | 82/3000 (b1500: 86/3000) |
| FOV mm3 | 150 × 150 × 90 | 160 × 160 × 90 |
| Voxel size mm3 | 0.46 × 0.5 × 3 | 2 × 2 × 3 |
| Slices | 30 | 30 |
| Bandwith (Hz) | 232 | 1854.2 |
| Acceleration factor | 1.7/3.4/4.8 | - |
| Parallel imaging factor (SENSE) | - | 3 |
| B values (averages) s/mm2 | - | 50 (3), 500 (2), 1000 (12), 1500 (12) |
| Scan time (min) | 4:45/2:36/1:59 | 5:12 (b50-1000) 3:48 (b1500) |
| Parameter | Variable | Value |
|---|---|---|
| Age (years) | Mean ± SD | 64.4 ± 6.2 |
| Number of lesions | 1 | 12 (52%) |
| 2 | 8 (39%) | |
| 3 | 2 (9%) | |
| Lesion location | PZpl | 8 (35%) |
| PZpm | 4 (17%) | |
| PZa | 6 (26%) | |
| TZa | 5 (22%) | |
| PIRADS score | 3 | 3 (13%) |
| 4 | 14 (61%) | |
| 5 | 6 (26%) | |
| Gleason score | 6 | 1 (4%) |
| 7a | 11 (48%) | |
| 7b | 9 (39%) | |
| 9 | 2 (9%) | |
| Tumor size | pT2a | 1 (4%) |
| pT2c | 13 (57%) | |
| pT3a | 4 (17%) | |
| pT3b | 5 (22%) | |
| Nodal status | pN0 | 22 (96%) |
| pN1 | 1 (4%) | |
| Metastasis | cM0 | 23 (100%) |
| PSA (ng/mL) | Mean ± SD | 14.4 ± 18.4 |
| Category | T2w C-SENSE1.7 | T2w C-SENSE AI1.7 | p | T2w C-SENSE AI3.4 | p | T2w C-SENSE AI4.8 | p |
|---|---|---|---|---|---|---|---|
| Image quality | 3.05 ± 0.76 (2–5) | 5.06 ± 0.79 (3–6) | <0.00001 | 5.34 ± 0.69 (3–6) | <0.00001 | 4.28 ± 0.51 (4–6) | <0.00001 |
| Noise | 3.19 ± 0.68 (2–5) | 4.63 ± 0.86 (3–6) | <0.00001 | 4.69 ± 0.81 (2–6) | <0.00001 | 4.09 ± 0.68 (3–6) | <0.00001 |
| Motion Artifacts | 3.53 ± 0.87 (2–5) | 3.59 ± 0.91 (2–5) | 0.39 | 4.91 ± 0.67 (3–6) | <0.00001 | 5.06 ± 0.68 (4–6) | <0.00001 |
| Image sharpness | 3.13 ± 0.72 (2–5) | 4.74 ± 0.61 (3–6) | <0.00001 | 4.72 ± 0.62 (3–6) | <0.00001 | 4.66 ± 0.53 (4–6) | <0.00001 |
| Lesion detection | 3.7 ± 0.62 (3–5) | 4.73 ± 0.99 (3–6) | 0.000065 | 5.09 ± 0.78 (3–6) | <0.00001 | 4.91 ± 0.72 (4–6) | <0.00001 |
| Diagnostic certainty | 3.57 ± 1.01 (2–5) | 4.6 ± 1.17 (3–6) | 0.0014 | 4.96 ± 0.85 (3–6) | <0.00001 | 4.74 ± 0.89 (3–6) | 0.000096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harder, F.N.; Weiss, K.; Amiel, T.; Peeters, J.M.; Tauber, R.; Ziegelmayer, S.; Burian, E.; Makowski, M.R.; Sauter, A.P.; Gschwend, J.E.; et al. Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer. Cancers 2022, 14, 5741. https://doi.org/10.3390/cancers14235741
Harder FN, Weiss K, Amiel T, Peeters JM, Tauber R, Ziegelmayer S, Burian E, Makowski MR, Sauter AP, Gschwend JE, et al. Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer. Cancers. 2022; 14(23):5741. https://doi.org/10.3390/cancers14235741
Chicago/Turabian StyleHarder, Felix N., Kilian Weiss, Thomas Amiel, Johannes M. Peeters, Robert Tauber, Sebastian Ziegelmayer, Egon Burian, Marcus R. Makowski, Andreas P. Sauter, Jürgen E. Gschwend, and et al. 2022. "Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer" Cancers 14, no. 23: 5741. https://doi.org/10.3390/cancers14235741
APA StyleHarder, F. N., Weiss, K., Amiel, T., Peeters, J. M., Tauber, R., Ziegelmayer, S., Burian, E., Makowski, M. R., Sauter, A. P., Gschwend, J. E., Karampinos, D. C., & Braren, R. F. (2022). Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer. Cancers, 14(23), 5741. https://doi.org/10.3390/cancers14235741







