Tumor Location and a Tumor Volume over 2.8 cc Predict the Prognosis for Japanese Localized Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Patients
2.3. Variables
2.4. Tumor Volume and Location Estimation Method
2.4.1. Measurement of Tumor Volume
2.4.2. Tumor Localization
2.5. Statistical Methods
3. Results
3.1. Participants
3.2. Predictive Factors for Progression-Free Survival (PFS)
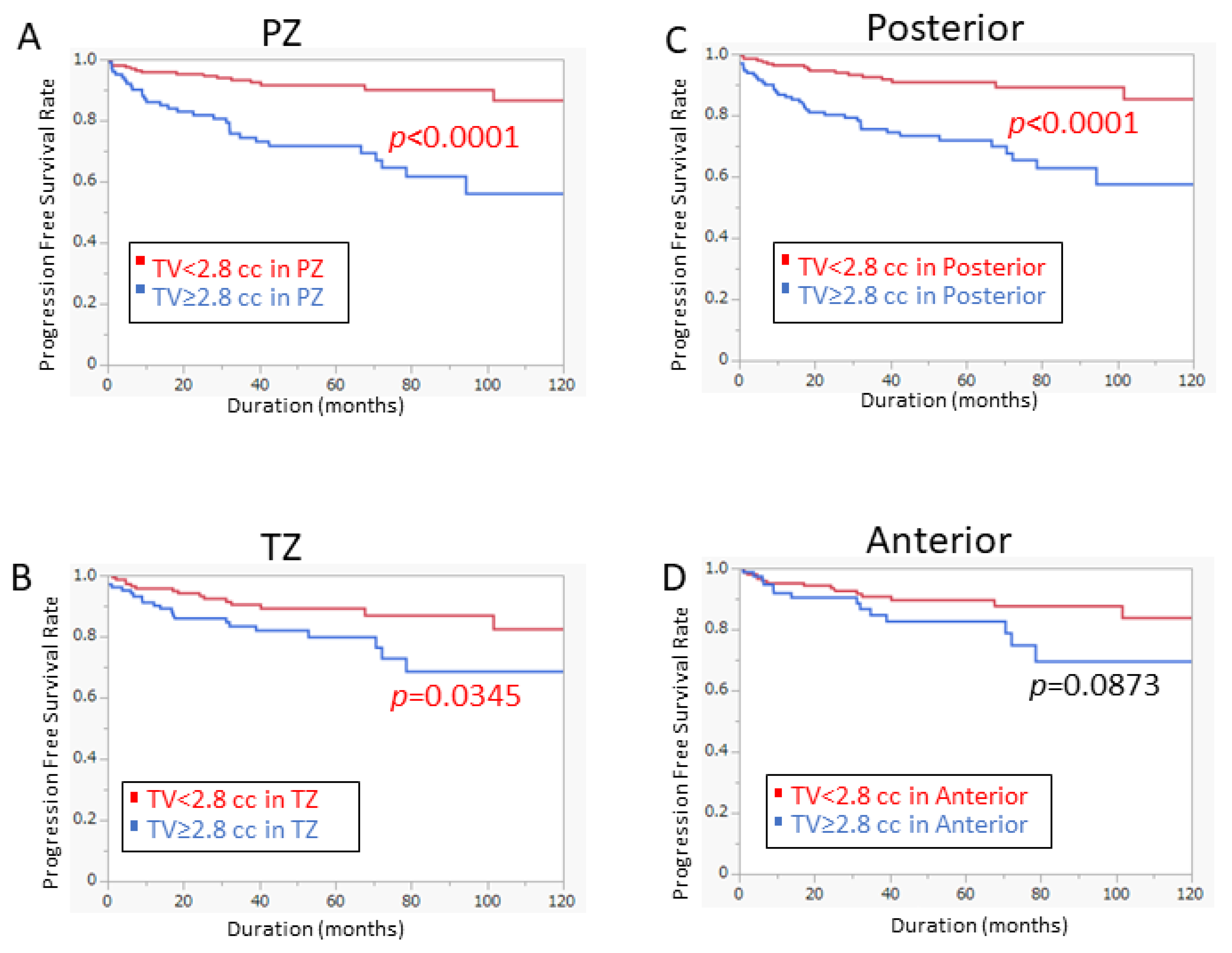
3.3. Model for Predicting PFS by Tumor Volume at Specific Location
3.4. Risk Model to Stratify Patient Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Pca | prostate cancer |
| RP | radical prostatectomy |
| BCR | biochemical recurrence |
| CRPC | castration-resistant prostate cancer |
| PSA | prostate-specific antigen |
| PZ | peripheral zone |
| TZ | transition zone |
| CZ | central zone |
| TV | tumor volume |
| GS | Gleason score |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| PFS | Progression-Free Survival |
| ACS | American Cancer Society |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Costello, A.J. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat. Rev. Urol. 2020, 17, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Partin, A.W.; Pound, C.R.; Epstein, J.I.; Walsh, P.C. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol. Clin. N. Am. 2001, 28, 555–565. [Google Scholar] [CrossRef]
- Everist, M.M.; Howard, L.E.; Aronson, W.J.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Terris, M.K.; Freedland, S.J. Socioeconomic status, race, and long-term outcomes after radical prostatectomy in an equal access health system: Results from the SEARCH database. Urol. Oncol. 2019, 37, 289.e11–289.e17. [Google Scholar] [CrossRef] [PubMed]
- Pagliarulo, V. Androgen Deprivation Therapy for Prostate Cancer. Adv. Exp. Med. Biol. 2018, 1096, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [PubMed]
- Teeter, A.E.; Griffin, K.; Howard, L.E.; Aronson, W.J.; Terris, M.K.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Freedland, S.J. Does Early Prostate Specific Antigen Doubling Time after Radical Prostatectomy, Calculated Prior to Prostate Specific Antigen Recurrence, Correlate with Prostate Cancer Outcomes? A Report from the SEARCH Database Group. J. Urol. 2018, 199, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, X.; Li, Y.; Tao, X.; Wang, B.; Yin, G. Identification of Potential Predictor of Biochemical Recurrence in Prostate Cancer. Int. J. Gen. Med. 2022, 15, 4897–4905. [Google Scholar] [CrossRef]
- Stamey, T.A.; Freiha, F.S.; McNeal, J.E.; Redwine, E.A.; Whittemore, A.S.; Schmid, H.P. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993, 71, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Walsh, P.C.; Carmichael, M.; Brendler, C.B. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage t1 c) prostate cancer. JAMA 1994, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Wolters, T.; Roobol, M.J.; van Leeuwen, P.J.; van den Bergh, R.C.; Hoedemaeker, R.F.; van Leenders, G.J.; Schröder, F.H.; van der Kwast, T.H. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J. Urol. 2011, 185, 121–125. [Google Scholar] [CrossRef]
- Ploussard, G.; Epstein, J.I.; Montironi, R.; Carroll, P.R.; Wirth, M.; Grimm, M.-O.; Bjartell, A.S.; Montorsi, F.; Freedland, S.J.; Erbersdobler, A. The contemporary concept of significant versus insignificant prostate cancer. Eur. Urol. 2011, 60, 291–303. [Google Scholar] [CrossRef]
- Ito, Y.; Udo, K.; Vertosick, E.A.; Sjoberg, D.D.; Vickers, A.J.; Al-Ahmadie, H.A.; Chen, Y.B.; Gopalan, A.; Sirintrapun, S.J.; Tickoo, S.K.; et al. Clinical Usefulness of Prostate and Tumor Volume Related Parameters following Radical Prostatectomy for Localized Prostate Cancer. J. Urol. 2019, 201, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ting, F.; van Leeuwen, P.J.; Delprado, W.; Haynes, A.M.; Brenner, P.; Stricker, P.D. Tumor volume in insignificant prostate cancer: Increasing the threshold is a safe approach to reduce over-treatment. Prostate 2015, 75, 1768–1773. [Google Scholar] [CrossRef]
- Fugini, A.V.; Antonelli, A.; Giovanessi, L.; Gardini, V.C.; Abuhilal, M.; Zambolin, T.; Tardanico, R.; Simeone, C.; Cunico, S.C. Insignificant Prostate Cancer: Charateristics and Predictive Factors. Urol. J. 2011, 78, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Vismara Fugini, A.; Tardanico, R.; Giovanessi, L.; Zambolin, T.; Simeone, C. The percentage of core involved by cancer is the best predictor of insignificant prostate cancer, according to an updated definition (tumor volume up to 2.5 cm3): Analysis of a cohort of 210 consecutive patients with low-risk disease. Urology 2014, 83, 28–32. [Google Scholar] [CrossRef]
- Yamada, Y.; Sakamoto, S.; Sazuka, T.; Goto, Y.; Kawamura, K.; Imamoto, T.; Nihei, N.; Suzuki, H.; Akakura, K.; Ichikawa, T. Validation of active surveillance criteria for pathologically insignificant prostate cancer in Asian men. Int. J. Urol. 2016, 23, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Frankcombe, D.E.; Li, J.; Cohen, R.J. Redefining the Concept of Clinically Insignificant Prostate Cancer. Urology 2020, 136, 176–179. [Google Scholar] [CrossRef]
- Schiffmann, J.; Connan, J.; Salomon, G.; Boehm, K.; Beyer, B.; Schlomm, T.; Tennstedt, P.; Sauter, G.; Karakiewicz, P.I.; Graefen, M.; et al. Tumor volume in insignificant prostate cancer: Increasing threshold gains increasing risk. Prostate 2015, 75, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wender, R.C.; Etzioni, R.B.; Thompson, I.M.; D’Amico, A.V.; Volk, R.J.; Brooks, D.D.; Dash, C.; Guessous, I.; Andrews, K.; et al. American Cancer Society guideline for the early detection of prostate cancer: Update 2010. CA Cancer J. Clin. 2010, 60, 70–98. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Dev, H.S.; Skarecky, D.; Ahlering, T. The importance of surgical margins in prostate cancer. J. Surg. Oncol. 2016, 113, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Matti, B.; Reeves, F.; Prouse, M.; Zargar-Shoshtari, K. The impact of the extent and location of positive surgical margins on the risk of biochemical recurrence following radical prostatectomy in men with Gleason 7 prostate cancers. Prostate 2021, 81, 1428–1434. [Google Scholar] [CrossRef]
- Ploussard, G.; Drouin, S.J.; Rode, J.; Allory, Y.; Vordos, D.; Hoznek, A.; Abbou, C.C.; de la Taille, A.; Salomon, L. Location, extent, and multifocality of positive surgical margins for biochemical recurrence prediction after radical prostatectomy. World J. Urol. 2014, 32, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.J.; Eastham, J.A. Radical prostatectomy: Positive surgical margins matter. Urol. Oncol. 2013, 31, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, H.; Yang, Y.; Ian, L.H.; Pun, W.H.; Ho, S.F. Risk factors of positive surgical margin and biochemical recurrence of patients treated with radical prostatectomy: A single-center 10-year report. Chin. Med. J. 2011, 124, 1001–1005. [Google Scholar] [PubMed]
- Sammon, J.D.; Trinh, Q.D.; Sukumar, S.; Ravi, P.; Friedman, A.; Sun, M.; Schmitges, J.; Jeldres, C.; Jeong, W.; Mander, N.; et al. Risk factors for biochemical recurrence following radical perineal prostatectomy in a large contemporary series: A detailed assessment of margin extent and location. Urol. Oncol. 2013, 31, 1470–1476. [Google Scholar] [CrossRef]
- Wu, S.; Lin, S.X.; Wirth, G.J.; Lu, M.; Lu, J.; Subtelny, A.O.; Wang, Z.; Dahl, D.M.; Olumi, A.F.; Wu, C.L. Impact of Multifocality and Multilocation of Positive Surgical Margin After Radical Prostatectomy on Predicting Oncological Outcome. Clin. Genitourin. Cancer 2019, 17, e44–e52. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Kuroiwa, K.; Ohori, M.; Serio, A.M.; Gorbonos, A.; Maru, N.; Vickers, A.J.; Slawin, K.M.; Wheeler, T.M.; Reuter, V.E.; et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology 2007, 70, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.; Tsuzuki, T.; Hernandez, D.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Positive proximal (bladder neck) margin at radical prostatectomy confers greater risk of biochemical progression. Urology 2004, 64, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Sooriakumaran, P.; Ploumidis, A.; Nyberg, T.; Olsson, M.; Akre, O.; Haendler, L.; Egevad, L.; Nilsson, A.; Carlsson, S.; Jonsson, M.; et al. The impact of length and location of positive margins in predicting biochemical recurrence after robot-assisted radical prostatectomy with a minimum follow-up of 5 years. BJU Int. 2015, 115, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Shikanov, S.; Song, J.; Royce, C.; Al-Ahmadie, H.; Zorn, K.; Steinberg, G.; Zagaja, G.; Shalhav, A.; Eggener, S. Length of positive surgical margin after radical prostatectomy as a predictor of biochemical recurrence. J. Urol. 2009, 182, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Abalajon, M.J.; Jang, W.S.; Kwon, J.K.; Yoon, C.Y.; Lee, J.Y.; Cho, K.S.; Ham, W.S.; Choi, Y.D. Association of Anterior and Lateral Extraprostatic Extensions with Base-Positive Resection Margins in Prostate Cancer. PLoS ONE 2016, 11, e0158922. [Google Scholar] [CrossRef] [PubMed]
- Vrang, M.L.; Røder, M.A.; Vainer, B.; Christensen, I.J.; Gruschy, L.; Brasso, K.; Iversen, P. First Danish single-institution experience with radical prostatectomy: Impact of surgical margins on biochemical outcome. Scand. J. Urol. Nephrol. 2012, 46, 172–179. [Google Scholar] [CrossRef]
- You, D.; Jeong, I.G.; Song, C.; Cho, Y.M.; Hong, J.H.; Kim, C.S.; Ahn, H. High percent tumor volume predicts biochemical recurrence after radical prostatectomy in pathological stage T3a prostate cancer with a negative surgical margin. Int. J. Urol. 2014, 21, 484–489. [Google Scholar] [CrossRef]
- De La Roca, R.L.; Da Cunha, I.W.; Bezerra, S.M.; Da Fonseca, F.P. Radical prostatectomy and positive surgical margins: Relationship with prostate cancer outcome. Int. Braz. J. Urol. 2014, 40, 306–315. [Google Scholar] [CrossRef][Green Version]
- Hashine, K.; Ueno, Y.; Shinomori, K.; Ninomiya, I.; Teramoto, N.; Yamashita, N. Correlation between cancer location and oncological outcome after radical prostatectomy. Int. J. Urol. 2012, 19, 855–860. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, L.M.; Walsh, S.; Cohen, R.J.; Lee, S. Prostate carcinoma with positive margins at radical prostatectomy: Role of tumour zonal origin in biochemical recurrence. BJU Int. 2015, 116 (Suppl. 3), 42–48. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Kang, T.; Yoo, S.; Jeong, I.G.; Ro, J.Y.; Hong, J.H.; Kim, C.S.; Ahn, H. Tumor volume, surgical margin, and the risk of biochemical recurrence in men with organ-confined prostate cancer. Urol. Oncol. 2013, 31, 168–174. [Google Scholar] [CrossRef]
- Shannon, B.A.; McNeal, J.E.; Cohen, R.J. Transition zone carcinoma of the prostate gland: A common indolent tumour type that occasionally manifests aggressive behaviour. Pathology 2003, 35, 467–471. [Google Scholar] [CrossRef]
- Augustin, H.; Hammerer, P.G.; Blonski, J.; Graefen, M.; Palisaar, J.; Daghofer, F.; Huland, H.; Erbersdobler, A. Zonal location of prostate cancer: Significance for disease-free survival after radical prostatectomy? Urology 2003, 62, 79–85. [Google Scholar] [CrossRef]
- Ali, A.; Du Feu, A.; Oliveira, P.; Choudhury, A.; Bristow, R.G.; Baena, E. Prostate zones and cancer: Lost in transition? Nat. Rev. Urol. 2022, 19, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Magheli, A.; Rais-Bahrami, S.; Peck, H.J.; Walsh, P.C.; Epstein, J.I.; Trock, B.J.; Gonzalgo, M.L. Importance of tumor location in patients with high preoperative prostate specific antigen levels (greater than 20 ng/mL) treated with radical prostatectomy. J. Urol. 2007, 178, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Hayee, A.; Lugo, I.; Iakymenko, O.A.; Kwon, D.; Briski, L.M.; Zhao, W.; Nemov, I.; Punnen, S.; Ritch, C.R.; Pollack, A.; et al. Anterior or Posterior Prostate Cancer Tumor Nodule Location Predicts Likelihood of Certain Adverse Outcomes at Radical Prostatectomy. Arch. Pathol. Lab. Med. 2022, 146, 833–839. [Google Scholar] [CrossRef]
- Mygatt, J.G.; Cullen, J.; Streicher, S.A.; Kuo, H.C.; Chen, Y.; Young, D.; Gesztes, W.; Williams, G.; Conti, G.; Porter, C.; et al. Race, tumor location, and disease progression among low-risk prostate cancer patients. Cancer Med. 2020, 9, 2235–2242. [Google Scholar] [CrossRef]
- Augustin, H.; Erbersdobler, A.; Graefen, M.; Fernandez, S.; Palisaar, J.; Huland, H.; Hammerer, P. Biochemical recurrence following radical prostatectomy: A comparison between prostate cancers located in different anatomical zones. Prostate 2003, 55, 48–54. [Google Scholar] [CrossRef]
- Meng, Y.; Li, H.; Xu, P.; Wang, J. Do tumor volume, percent tumor volume predict biochemical recurrence after radical prostatectomy? A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 22319–22327. [Google Scholar]
- Kim, K.H.; Lim, S.K.; Shin, T.Y.; Kang, D.R.; Han, W.K.; Chung, B.H.; Rha, K.H.; Hong, S.J. Tumor volume adds prognostic value in patients with organ-confined prostate cancer. Ann. Surg. Oncol. 2013, 20, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M., III; Salem, S.; Chang, S.S.; Clark, P.E.; Davis, R.; Herrell, S.D.; Kordan, Y.; Baumgartner, R.; Phillips, S.; Smith, J.A., Jr.; et al. Tumor volume as a predictor of adverse pathologic features and biochemical recurrence (BCR) in radical prostatectomy specimens: A tale of two methods. World J. Urol. 2011, 29, 15–20. [Google Scholar] [CrossRef]
- Yuk, H.D.; Byun, S.S.; Hong, S.K.; Lee, H. The tumor volume after radical prostatectomy and its clinical impact on the prognosis of patients with localized prostate cancer. Sci. Rep. 2022, 12, 6003. [Google Scholar] [CrossRef]
- Ates, M.; Teber, D.; Gözen, A.S.; Tefekli, A.; Sugiono, M.; Hruza, M.; Rassweiler, J. Do tumor volume, tumor volume ratio, type of nerve sparing and surgical experience affect prostate specific antigen recurrence after laparoscopic radical prostatectomy? A matched pair analysis. J. Urol. 2007, 177, 1771–1775; discussion 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Okamoto, A.; Imai, A.; Yoneyama, T.; Hatakeyama, S.; Yoneyama, T.; Koie, T.; Kaminura, N.; Ohyama, C. Biochemical outcome of small-volume or insignificant prostate cancer treated with radical prostatectomy in Japanese population. Int. J. Clin. Oncol. 2012, 17, 119–123. [Google Scholar] [CrossRef]
- Furusato, B.; Rosner, I.L.; Osborn, D.; Ali, A.; Srivastava, S.; Davis, C.J.; Sesterhenn, I.A.; McLeod, D.G. Do patients with low volume prostate cancer have prostate specific antigen recurrence following radical prostatectomy? J. Clin. Pathol. 2008, 61, 1038–1040. [Google Scholar] [CrossRef]
- Lee, D.H.; Koo, K.C.; Lee, S.H.; Rha, K.H.; Choi, Y.D.; Hong, S.J.; Chung, B.H. Analysis of different tumor volume thresholds of insignificant prostate cancer and their implications for active surveillance patient selection and monitoring. Prostate Int. 2014, 2, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.I.; Tarin, T.V.; Ferrari, M.; Brooks, J.D. Comparison of prostate cancer tumor volume and percent cancer in prediction of biochemical recurrence and cancer specific survival. Urol. Oncol. 2011, 29, 314–318. [Google Scholar] [CrossRef]
- Friedersdorff, F.; Groß, B.; Maxeiner, A.; Jung, K.; Miller, K.; Stephan, C.; Busch, J.; Kilic, E. Does the Prostate Health Index Depend on Tumor Volume?-A Study on 196 Patients after Radical Prostatectomy. Int. J. Mol. Sci. 2017, 18, 488. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Park, C.K.; Park, S.Y.; Jang, W.S.; Lee, J.Y.; Choi, Y.D.; Cho, N.H. Total intraglandular and index tumor volumes predict biochemical recurrence in prostate cancer. Virchows Arch. 2016, 469, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Sakai, I.; Harada, K.; Takechi, Y.; Hara, I.; Eto, H. Prognostic significance of the tumor volume in radical prostatectomy specimens after neoadjuvant hormonal therapy. Urol. Int. 2005, 74, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Raison, N.; Servian, P.; Patel, A.; Santhirasekaram, A.; Smith, A.; Yeung, M.; Lloyd, J.; Mannion, E.; Rockall, A.; Ahmed, H.; et al. Is tumour volume an independent predictor of outcome after radical prostatectomy for high-risk prostate cancer? Prostate Cancer Prostatic Dis. 2021, 1–5. [Google Scholar] [CrossRef]
- Salomon, L.; Levrel, O.; Anastasiadis, A.G.; Irani, J.; De La Taille, A.; Saint, F.; Vordos, D.; Cicco, A.; Hoznek, A.; Chopin, D.; et al. Prognostic significance of tumor volume after radical prostatectomy: A multivariate analysis of pathological prognostic factors. Eur. Urol. 2003, 43, 39–44. [Google Scholar] [CrossRef]
- Akaza, H.; Onozawa, M.; Hinotsu, S. Prostate cancer trends in Asia. World J. Urol. 2017, 35, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Fuletra, J.G.; Kamenko, A.; Ramsey, F.; Eun, D.D.; Reese, A.C. African-American men with prostate cancer have larger tumor volume than Caucasian men despite no difference in serum prostate specific antigen. Can. J. Urol. 2018, 25, 9193–9198. [Google Scholar]
- Gupta, K.; Mehrotra, V.; Fu, P.; Scarberry, K.; MacLennan, G.T.; Gupta, S. Racial disparities in biochemical recurrence of prostate cancer. Am. J. Clin. Exp. Urol. 2022, 10, 266–270. [Google Scholar] [PubMed]
- Reinhardt, D.; Helfand, B.T.; Cooper, P.R.; Roehl, K.A.; Catalona, W.J.; Loeb, S. Prostate cancer risk alleles are associated with prostate cancer volume and prostate size. J. Urol. 2014, 191, 1733–1736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Helfand, B.T.; Paterakos, M.; Wang, C.H.; Talaty, P.; Abran, J.; Bennett, J.; Hall, D.W.; Lehman, A.; Aboushwareb, T. The 17-gene Genomic Prostate Score assay as a predictor of biochemical recurrence in men with intermediate and high-risk prostate cancer. PLoS ONE 2022, 17, e0273782. [Google Scholar] [CrossRef]
- Santos, A.; Mattiolli, A.; Carvalheira, J.B.; Ferreira, U.; Camacho, M.; Silva, C.; Costa, F.; Matheus, W.; Lima, M.; Etchebehere, E. PSMA whole-body tumor burden in primary staging and biochemical recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 493–500. [Google Scholar] [CrossRef]
- Pinckaers, H.; van Ipenburg, J.; Melamed, J.; De Marzo, A.; Platz, E.A.; van Ginneken, B.; van der Laak, J.; Litjens, G. Predicting biochemical recurrence of prostate cancer with artificial intelligence. Commun. Med. 2022, 2, 64. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | |
|---|---|
| Number of patients | 557 |
| Median age at operation (range), years | 67 (46–77) |
| Median follow-up time (range), months | 45.3 (12–161.5) |
| Median initial PSA (range) (ng/mL) | 7.71 (2.15–87.16) |
| Gleason score sum, n (%) | |
| ≤7 | 444 (79.7) |
| 8 | 48 (8.6) |
| ≥9 | 61 (11.0) |
| T stage, n (%) | |
| ≤2b | 195 (35.0) |
| ≥2c | 361 (64.8) |
| Risk Group; Low/Intermediate/High, n (%) | 77 (13.8)/279 (50.1)/201 (36.1) |
| Tumor Volume (range), cc | 2.12 (0.02–57) |
| Tumor Location, n (%) | |
| apex | 355 (63.7) |
| middle | 353 (63.4) |
| bladder neck | 119 (21.4) |
| Tumor Location, n (%) | |
| anterior | 268 (48.1) |
| posterior | 292 (52.4) |
| Tumor Location, n (%) | |
| PZ | 374 (67.1) |
| TZ | 208 (37.3) |
| N stage, n (%) | |
| positive | 8 (1.4) |
| Seminal Vesicle Invasion, n, (%) | 48 (8.6) |
| Extracapsular Extension, n, (%) | 138 (24.8) |
| Resection Margins, n, (%) | 169 (30.3) |
| PSA Recurrence, n, (%) | 66 (11.8) |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Cut Off | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | ≥67 | 0.96 | 0.59–1.57 | 0.8842 | |||
| initial PSA | ≥7.71 ng/mL | 1.65 | 1.00–2.73 | 0.0505 | |||
| PSAD | ≥0.26 | 2.06 | 1.21–3.53 | 0.0082 | 1.51 | 0.73–3.09 | 0.2643 |
| GS | ≥7 | 1.15 | 0.46–2.88 | 0.7593 | |||
| T stage | ≥T3 | 4.66 | 2.81–7.73 | <0.0001 | 1.69 | 0.77–3.71 | 0.1894 |
| RM | positive | 4.18 | 2.46–7.10 | <0.0001 | 1.99 | 0.94–4.20 | 0.0712 |
| Tumor location | |||||||
| Apex | 1.45 | 0.70–3.02 | 0.3166 | ||||
| PZ | 3.28 | 1.01–10.60 | 0.0472 | 2.21 | 0.49–10.05 | 0.3030 | |
| posterior | 2.24 | 1.07–4.65 | 0.0314 | 1.72 | 0.72–4.12 | 0.2193 | |
| TV | |||||||
| ≥0.5 cc | 1.61 | 0.73–3.53 | 0.2344 | ||||
| ≥1.0 cc | 2.18 | 1.11–4.27 | 0.0240 | ||||
| ≥2.0 cc | 2.74 | 1.55–4.82 | 0.0005 | ||||
| ≥2.8 cc ** | 3.10 | 1.86–5.17 | <0.0001 | 2.47 | 1.14–5.36 | 0.0225 * | |
| ≥3.0 cc | 2.96 | 1.80–4.88 | <0.0001 | ||||
| ≥3.5 cc | 2.80 | 1.72–4.58 | <0.0001 | ||||
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Cut Off | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | ≥67 | 0.96 | 0.59–1.57 | 0.8842 | - | - | - |
| initial PSA | ≥7.71 ng/mL | 1.65 | 1.00–2.73 | 0.0505 | - | - | - |
| PSAD | ≥0.26 | 2.06 | 1.21–3.53 | 0.0082 | 1.55 | 0.76–3.15 | 0.2307 |
| GS | ≥7 | 1.15 | 0.46–2.88 | 0.7593 | - | - | - |
| T stage | ≥T3 | 4.66 | 2.81–7.73 | <0.0001 | 1.64 | 0.74–3.65 | 0.2261 |
| RM | positive | 4.18 | 2.46–7.10 | <0.0001 | 2.09 | 0.99–4.42 | 0.0548 |
| Unfavorable Risk | PZ + Post + TV2.8 cc | 4.74 | 2.60–8.65 | <0.0001 | 3.16 | 1.52–6.56 | 0.0020 * |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Cut Off | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | ≥67 | 0.76 | 0.40–1.47 | 0.4167 | - | - | - |
| initial PSA | ≥7.71 ng/mL | 1.04 | 0.52–2.08 | 0.9097 | - | - | - |
| PSAD | ≥0.26 | 1.9 | 0.82–4.40 | 0.1326 | - | - | - |
| GS | ≥7 | 1.29 | 0.18–9.46 | 0.7991 | - | - | - |
| T stage | ≥T3 | 4.38 | 2.11–9.10 | <0.0001 | 1.98 | 0.75–5.25 | 0.1701 |
| RM | positive | 4.65 | 2.16–10.02 | <0.0001 | 2.37 | 0.95–5.91 | 0.0649 |
| Unfavorable Risk | PZ + Post + TV2.8 cc | 3.5 | 1.64–7.47 | 0.0012 | 1.87 | 0.77–4.53 | 0.1653 |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Cut Off | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | ≥67 | 1.07 | 0.51–2.25 | 0.8546 | - | - | - |
| initial PSA | ≥7.71 ng/mL | 1.56 | 0.74–3.28 | 0.2458 | - | - | - |
| PSAD | ≥0.26 | 1.52 | 0.72–3.19 | 0.2716 | - | - | - |
| GS | ≥7 | 0.74 | 0.26–2.15 | 0.5855 | - | - | - |
| T stage | ≥T3 | 3.34 | 1.59–7.01 | 0.0015 | 0.97 | 0.28–3.38 | 0.961 |
| RM | positive | 3.03 | 1.42–6.47 | 0.0043 | 1.38 | 0.43–4.41 | 0.5904 |
| Unfavorable Risk | PZ + Post + TV2.8 cc | 4.71 | 1.75–12.69 | 0.0022 | 4.43 | 1.51–13.01 | 0.0068 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba, H.; Sakamoto, S.; Zhao, X.; Yamada, Y.; Rii, J.; Fujimoto, A.; Kanesaka, M.; Takeuchi, N.; Sazuka, T.; Imamura, Y.; et al. Tumor Location and a Tumor Volume over 2.8 cc Predict the Prognosis for Japanese Localized Prostate Cancer. Cancers 2022, 14, 5823. https://doi.org/10.3390/cancers14235823
Baba H, Sakamoto S, Zhao X, Yamada Y, Rii J, Fujimoto A, Kanesaka M, Takeuchi N, Sazuka T, Imamura Y, et al. Tumor Location and a Tumor Volume over 2.8 cc Predict the Prognosis for Japanese Localized Prostate Cancer. Cancers. 2022; 14(23):5823. https://doi.org/10.3390/cancers14235823
Chicago/Turabian StyleBaba, Haruki, Shinichi Sakamoto, Xue Zhao, Yasutaka Yamada, Junryo Rii, Ayumi Fujimoto, Manato Kanesaka, Nobuyoshi Takeuchi, Tomokazu Sazuka, Yusuke Imamura, and et al. 2022. "Tumor Location and a Tumor Volume over 2.8 cc Predict the Prognosis for Japanese Localized Prostate Cancer" Cancers 14, no. 23: 5823. https://doi.org/10.3390/cancers14235823
APA StyleBaba, H., Sakamoto, S., Zhao, X., Yamada, Y., Rii, J., Fujimoto, A., Kanesaka, M., Takeuchi, N., Sazuka, T., Imamura, Y., Akakura, K., & Ichikawa, T. (2022). Tumor Location and a Tumor Volume over 2.8 cc Predict the Prognosis for Japanese Localized Prostate Cancer. Cancers, 14(23), 5823. https://doi.org/10.3390/cancers14235823





