Impact of Blood–Brain Barrier to Delivering a Vascular-Disrupting Agent: Predictive Role of Multiparametric MRI in Rodent Craniofacial Metastasis Models
Abstract
:Simple Summary
Abstract
1. Introduction
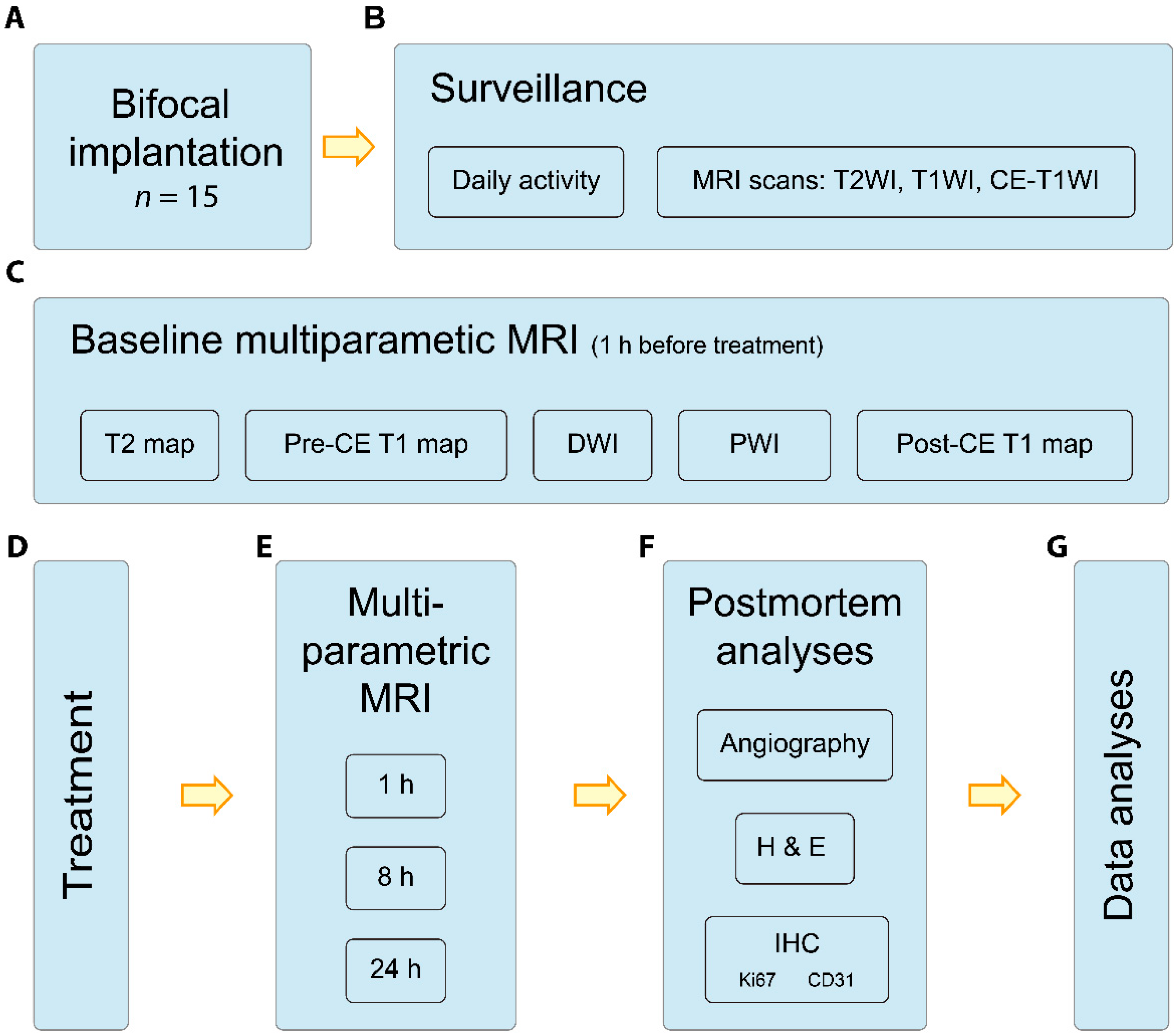
2. Materials and Methods
2.1. Study Design
2.2. Simultaneous Intracranial and Extracranial Tumor Model
2.3. VDA Treatment
2.4. MRI Acquisitions
2.5. Postmortem NanoCT Scan and Pathology
2.6. Image Analyses
2.7. Statistical Analyses
3. Results
3.1. General Aspects of the Study
3.2. Pre-Treatment MRI Characterization of Tumors
3.3. Differential Responses towards VDA Treatment
3.4. Postmortem Angiography and Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Feng, Y.; Chen, L.; Yu, J.; Van Ongeval, C.; Bormans, G.; Li, Y.; Ni, Y. Towards updated understanding of brain metastasis. Am. J. Cancer Res. 2022, 12, 4290–4311. [Google Scholar]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro. Oncol. 2021, 23, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, L.; Feng, Y.; Swinnen, J.V.; Jonscher, C.; Van Ongeval, C.; Ni, Y. Heterogeneity of Synchronous Lung Metastasis Calls for Risk Stratification and Prognostic Classification: Evidence from a Population-Based Database. Cancers 2022, 14, 1608. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Y.; Swinnen, J.; Oyen, R.; Li, Y.; Ni, Y. Incidence and prognosis of liver metastasis at diagnosis: A pan-cancer population-based study. Am. J. Cancer Res. 2020, 10, 1477–1517. [Google Scholar]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro. Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood–brain barrier to treat glioblastoma. Neuro-Oncology 2015, 18, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers 2019, 11, 1782. [Google Scholar] [CrossRef] [Green Version]
- Valiente, M.; Ahluwalia, M.S.; Boire, A.; Brastianos, P.K.; Goldberg, S.B.; Lee, E.Q.; Le Rhun, E.; Preusser, M.; Winkler, F.; Soffietti, R. The Evolving Landscape of Brain Metastasis. Trends. Cancer 2018, 4, 176–196. [Google Scholar] [CrossRef] [Green Version]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of brain metastases according to molecular subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef]
- Shah, N.; Liu, Z.; Tallman, R.M.; Mohammad, A.; Sprowls, S.A.; Saralkar, P.A.; Vickers, S.D.; Pinti, M.V.; Gao, W.; Lockman, P.R. Drug resistance occurred in a newly characterized preclinical model of lung cancer brain metastasis. BMC Cancer 2020, 20, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steeg, P.S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., 2nd; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, S.; Zhao, X.; Feng, Y.; Bormans, G.; Swinnen, J.; Oyen, R.; Huang, G.; Ni, Y.; Li, Y. Predicting Clinical Efficacy of Vascular Disrupting Agents in Rodent Models of Primary and Secondary Liver Cancers: An Overview with Imaging-Histopathology Correlation. Diagnostics 2020, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cona, M.M.; Chen, F.; Feng, Y.; Zhou, L.; Zhang, G.; Nuyts, J.; de Witte, P.; Zhang, J.; Yu, J.; et al. Sequential systemic administrations of combretastatin A4 Phosphate and radioiodinated hypericin exert synergistic targeted theranostic effects with prolonged survival on SCID mice carrying bifocal tumor xenografts. Theranostics 2013, 3, 127–137. [Google Scholar] [CrossRef]
- Shi, C.; Liu, D.; Xiao, Z.; Zhang, D.; Liu, G.; Liu, G.; Chen, H.; Luo, L. Monitoring Tumor Response to Antivascular Therapy Using Non-Contrast Intravoxel Incoherent Motion Diffusion-Weighted MRI. Cancer Res. 2017, 77, 3491. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, M.; Ciesielski, M.J. MRI-based characterization of vascular disruption by 5,6-dimethylxanthenone-acetic acid in gliomas. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2009, 29, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, S.; Jiang, X.; Wang, X.; Zhou, X.; Wan, L.; Zhao, H.; Zhou, Z.; Gao, L.; Huang, G.; et al. Preparation and validation of cyclodextrin-based excipients for radioiodinated hypericin applied in a targeted cancer radiotherapy. Int. J. Pharm. 2021, 599, 120393. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhao, Y.; Saiyin, H.; He, X.; Zhao, J.; Li, L.; Talebi, A.; Huang, G.; Ni, Y. A Model In Vitro Study Using Hypericin: Tumor-Versus Necrosis-Targeting Property and Possible Mechanisms. Biology 2020, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sun, Z.; Zhang, J.; Shao, H.; Cona, M.M.; Wang, H.; Marysael, T.; Chen, F.; Prinsen, K.; Zhou, L.; et al. A Dual-targeting Anticancer Approach: Soil and Seed Principle. Radiology 2011, 260, 799–807. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Feng, Y.; Yin, T.; Yu, J.; De Keyzer, F.; Peeters, R.; Van Ongeval, C.; Bormans, G.; Swinnen, J.; et al. Development and characterization of a rat brain metastatic tumor model by multiparametric magnetic resonance imaging and histomorphology. Clin. Exp. Metastasis 2022, 39, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Heye, A.K.; Culling, R.D.; Valdés Hernández Mdel, C.; Thrippleton, M.J.; Wardlaw, J.M. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin. 2014, 6, 262–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Peeters, R.; Feng, Y.; Liu, Y.; Yu, J.; Dymarkowski, S.; Himmelreich, U.; Oyen, R.; Ni, Y. Characterization of a rat orthotopic pancreatic head tumor model using three-dimensional and quantitative multi-parametric MRI. NMR Biomed. 2017, 30, e3676. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Yung, R.; Seyfoddin, V.; Guise, C.; Tijono, S.; McGregor, A.; Connor, B.; Ching, L.M. Efficacy against subcutaneous or intracranial murine GL261 gliomas in relation to the concentration of the vascular-disrupting agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), in the brain and plasma. Cancer Chemother. Pharmacol. 2014, 73, 639–649. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Jackson, A.; Parker, G.J.M.; Jayson, G.C. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br. J. Cancer 2007, 96, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, Y.; Peeters, R.; Feng, Y.; Yu, J.; Himmelreich, U.; Oyen, R.; Ni, Y. Vascular disrupting agent in pancreatic and hepatic tumour allografts: Observations of location-dependent efficacy by MRI, microangiography and histomorphology. Br. J. Cancer 2017, 117, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, M.; Bellnier, D.A.; Cheney, R.T. Assessment of the Early Effects of 5,6-Dimethylxanthenone-4-Acetic Acid Using Macromolecular Contrast Media–Enhanced Magnetic Resonance Imaging: Ectopic Versus Orthotopic Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bähr, O.; Gross, S.; Harter, P.N.; Kirches, E.; Mawrin, C.; Steinbach, J.P.; Mittelbronn, M. ASA404, a vascular disrupting agent, as an experimental treatment approach for brain tumors. Oncol. Lett. 2017, 14, 5443–5451. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Stockmann, C.; Schadendorf, D.; Klose, R.; Helfrich, I. The impact of the immune system on tumor: Angiogenesis and vascular remodeling. Front. Oncol. 2014, 4, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechmann, C.M.; Woenne, E.C.; Brix, G.; Radzwill, N.; Ilg, M.; Bachert, P.; Peschke, P.; Kirsch, S.; Kauczor, H.U.; Delorme, S.; et al. Impact of stroma on the growth, microcirculation, and metabolism of experimental prostate tumors. Neoplasia 2007, 9, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef] [Green Version]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Thomas, F.C.; Taskar, K.; Rudraraju, V.; Goda, S.; Thorsheim, H.R.; Gaasch, J.A.; Mittapalli, R.K.; Palmieri, D.; Steeg, P.S.; Lockman, P.R.; et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 2009, 26, 2486–2494. [Google Scholar] [CrossRef] [Green Version]
- Prados, M.D.; Schold, S.C., Jr.; Fine, H.A.; Jaeckle, K.; Hochberg, F.; Mechtler, L.; Fetell, M.R.; Phuphanich, S.; Feun, L.; Janus, T.J.; et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003, 5, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Lei, X.; Lin, Z.; Zhao, J.; Wu, F.; Yang, Z.; Pu, J.; Liu, Z. Preparation and evaluation of sustained-release solid dispersions co-loading gastrodin with borneol as an oral brain-targeting enhancer. Acta Pharm. Sin. B 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.M.; Jansen, J.F.A.; Zhang, C.E.; Staals, J.; Hofman, P.A.M.; van Oostenbrugge, R.J.; Jeukens, C.; Backes, W.H. Measuring subtle leakage of the blood-brain barrier in cerebrovascular disease with DCE-MRI: Test-retest reproducibility and its influencing factors. J. Magn. Reson. Imaging 2017, 46, 159–166. [Google Scholar] [CrossRef] [PubMed]
- de Gooijer, M.C.; Kemper, E.M.; Buil, L.C.M.; Çitirikkaya, C.H.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. ATP-binding cassette transporters restrict drug delivery and efficacy against brain tumors even when blood-brain barrier integrity is lost. Cell Rep. Med. 2021, 2, 100184. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Feng, Y.; Chen, L.; Yu, J.; Li, Y.; Ni, Y. Impact of Blood–Brain Barrier to Delivering a Vascular-Disrupting Agent: Predictive Role of Multiparametric MRI in Rodent Craniofacial Metastasis Models. Cancers 2022, 14, 5826. https://doi.org/10.3390/cancers14235826
Wang S, Feng Y, Chen L, Yu J, Li Y, Ni Y. Impact of Blood–Brain Barrier to Delivering a Vascular-Disrupting Agent: Predictive Role of Multiparametric MRI in Rodent Craniofacial Metastasis Models. Cancers. 2022; 14(23):5826. https://doi.org/10.3390/cancers14235826
Chicago/Turabian StyleWang, Shuncong, Yuanbo Feng, Lei Chen, Jie Yu, Yue Li, and Yicheng Ni. 2022. "Impact of Blood–Brain Barrier to Delivering a Vascular-Disrupting Agent: Predictive Role of Multiparametric MRI in Rodent Craniofacial Metastasis Models" Cancers 14, no. 23: 5826. https://doi.org/10.3390/cancers14235826
APA StyleWang, S., Feng, Y., Chen, L., Yu, J., Li, Y., & Ni, Y. (2022). Impact of Blood–Brain Barrier to Delivering a Vascular-Disrupting Agent: Predictive Role of Multiparametric MRI in Rodent Craniofacial Metastasis Models. Cancers, 14(23), 5826. https://doi.org/10.3390/cancers14235826








