Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Statistical Analysis
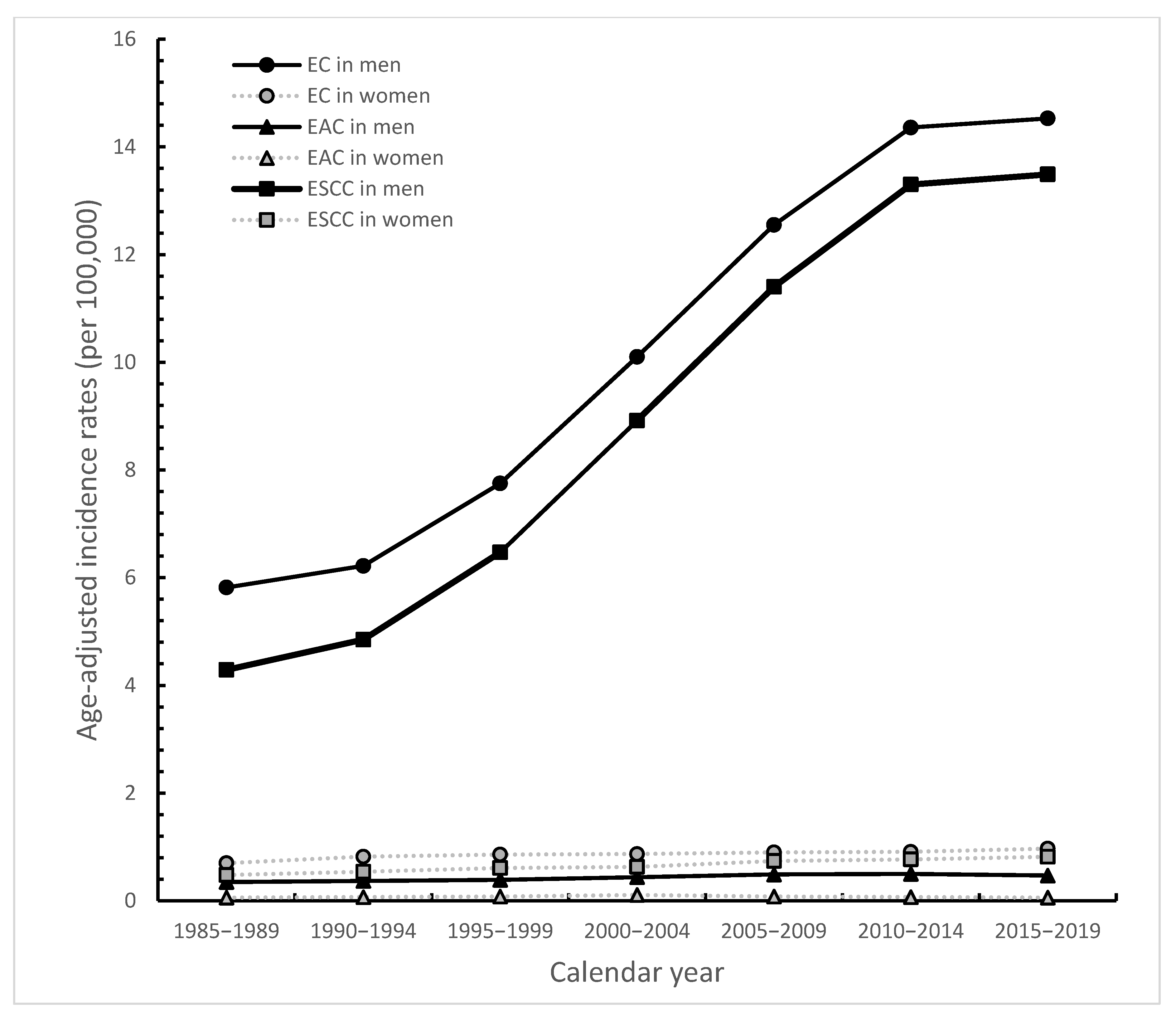
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Koulaouzidis, A.; Marlicz, W.; Lok, V.; Chu, C.; Ngai, C.; Zhang, L.; Chen, P.; Wang, S.; Yuan, J.; et al. Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries. Cancers 2021, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fang, Y.; Wu, M.; Chen, C.; Chen, Y.; Yu, C.; Kuo, C.; Chiu, M.; Hu, W.; Tsai, M.; et al. Application of Helicobacter pylori stool antigen test to survey the updated prevalence of Helicobacter pylori infection in Taiwan. J. Gastroenterol. Hepatol. 2020, 35, 233–240. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Huang, Y.-T.; Tsai, Y.-W.; Huang, S.-M.; Kuo, K.N.; McKee, M.; Nolte, E. The impact of universal National Health Insurance on population health: The experience of Taiwan. BMC Health Serv. Res. 2010, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.-L.; Yu, S.-J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef]
- Lu, C.-L.; Lang, H.-C.; Luo, J.-C.; Liu, C.-C.; Lin, H.-C.; Chang, F.-Y.; Lee, S.-D. Increasing trend of the incidence of esophageal squamous cell carcinoma, but not adenocarcinoma, in Taiwan. Cancer Causes Control 2010, 21, 269–274. [Google Scholar] [CrossRef]
- Thrift, A.P. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 432–443. [Google Scholar] [CrossRef]
- Xie, F.-J.; Zhang, Y.-P.; Zheng, Q.-Q.; Jin, H.-C.; Wang, F.-L.; Chen, M.; Shao, L.; Zou, D.-H.; Yu, X.-M.; Mao, W.-M. Helicobacter pylori infection and esophageal cancer risk: An updated meta-analysis. World J. Gastroenterol. 2013, 19, 6098–6107. [Google Scholar] [CrossRef]
- Taiwan Tobacco and Liquor Corporation. Available online: https://www.ttl.com.tw (accessed on 10 July 2022).
- The Health Promotion Administration, Ministry of Health and Welfare. Available online: https://www.hpa.gov.tw/EngPages/Index.aspx (accessed on 27 November 2022).
- Chiang, C.J.; Wang, Y.W.; Lee, W.C. Taiwan’s Nationwide Cancer Registry System of 40 years: Past, present, and future. J. Formos. Med. Assoc. 2019, 118, 856–858. [Google Scholar] [CrossRef]
- Chiang, C.-J.; You, S.-L.; Chen, C.-J.; Yang, Y.-W.; Lo, W.-C.; Lai, M.-S. Quality assessment and im-provement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015, 45, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Cancer Registry. Taiwan Cancer Registry Reporting Manual. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=14913 (accessed on 10 November 2022).
- National Cancer Institute. Joinpoint Regression Program, Version 4.9.1.0. Available online: https://surveillance.cancer.gov/joinpoint (accessed on 10 November 2022).
- Rosenberg, P.S.; Check, D.P.; Anderson, W.F. A Web Tool for Age–Period–Cohort Analysis of Cancer Incidence and Mortality Rates. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2296–2302. Available online: https://analysistools.cancer.gov/apc/ (accessed on 27 November 2022). [CrossRef] [PubMed] [Green Version]
- Eng, M.Y.; Luczak, S.E.; Wall, T.L. ALDH2, ADH1B, and ADH1C Genotypes in Asians: A Literature Review. Alcohol Res. Heal. J. Natl. Inst. Alcohol Abus. Alcohol. 2007, 30, 22–27. [Google Scholar]
- Tai, S.-Y.; Wu, I.-C.; Wu, D.-C.; Su, H.-J.; Huang, J.-L.; Tsai, H.-J.; Lu, C.-Y.; Lee, J.-M.; Wu, M.-T. Cigarette smoking and alcohol drinking and esophageal cancer risk in Taiwanese women. World J. Gastroenterol. 2010, 16, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lee, J.-M.; Wu, D.-C.; Goan, Y.-G.; Chou, S.-H.; Wu, I.-C.; Kao, E.-L.; Chan, T.-F.; Huang, M.-C.; Chen, P.-S.; et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int. J. Cancer 2008, 122, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Edgren, G.; Adami, H.-O.; Weiderpass, E.; Nyrén, O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013, 62, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.H.; Lagergren, J. Time trends in the incidence of oesophageal cancer in Asia: Variations across populations and histo-logical types. Cancer Epidemiol. 2016, 44, 71. [Google Scholar] [CrossRef] [Green Version]
- Castro, C.; Bosetti, C.; Malvezzi, M.; Bertuccio, P.; Levi, F.; Negri, E.; La Vecchia, C.; Lunet, N. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Ann. Oncol. 2014, 25, 283–290. [Google Scholar] [CrossRef]
- GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [Google Scholar] [CrossRef]
- Ohashi, S.; Miyamoto, S.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent Advances from Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology 2015, 149, 1700–1715. [Google Scholar] [CrossRef] [Green Version]
- Toh, Y.; Oki, E.; Ohgaki, K.; Sakamoto, Y.; Ito, S.; Egashira, A.; Saeki, H.; Kakeji, Y.; Morita, M.; Sakaguchi, Y.; et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: Molecular mechanisms of carcinogenesis. Int. J. Clin. Oncol. 2010, 15, 135–144. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: A meta-analysis of case–control studies. Cancer Causes Control 2013, 24, 257–265. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, J.-M.; Wu, D.-C.; Hsu, H.-K.; Kao, E.-L.; Huang, H.-L.; Wang, T.-N.; Huang, M.-C.; Wu, M.-T. Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. Int. J. Cancer 2004, 113, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Levy, D.T.; Cheng, T.Y.; Hsu, C.C.; Tsai, S.P. Smoking behaviour in Taiwan, 2001. Tob. Control 2005, 14 (Suppl. S1), i51–i55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef]
- Tramacere, I.; La Vecchia, C.; Negri, E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: A meta-analysis. Epidemiology 2011, 22, 344–349. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lee, Y.-C.; Chiu, H.-M.; Wu, M.-S.; Wang, H.-P.; Lin, J.-T. Time trends of endoscopic and pathological diagnoses related to gastroesophageal reflux disease in a Chinese population: Eight years single institution experience. Dis. Esophagus 2010, 23, 201–207. [Google Scholar] [CrossRef]
- Hung, L.-J.; Hsu, P.-I.; Yang, C.-Y.; Wang, E.-M.; Lai, K.-H. Prevalence of gastroesophageal reflux disease in a general population in Taiwan. J. Gastroenterol. Hepatol. 2011, 26, 1164–1168. [Google Scholar] [CrossRef]
- Chu, N.-F. Prevalence of obesity in Taiwan. Obes. Rev. 2005, 6, 271–274. [Google Scholar] [CrossRef]
- Lin, J.T.; Chang, C.Y.; Lee, Y.C.; Lee, C.T.; Wang, W.L.; Tai, C.M.; Wu, M.S.; Wang, H.P. Endoscopic surveillance, diagnosis, and treatment of esophageal cancer in Taiwan. Gastroenterol. Endosc. 2009, 51 (Suppl. S1), 630–631. [Google Scholar]
- Yang, C.S.; Chen, X.L. Research on esophageal cancer: With personal perspectives from studies in China and Kenya. Int. J. Cancer 2021, 149, 264–276. [Google Scholar] [CrossRef]
- Grille, V.J.; Campbell, S.; Gibbs, J.F.; Bauer, T.L. Esophageal cancer: The rise of adenocarcinoma over squamous cell carcinoma in the Asian belt. J. Gastrointest. Oncol. 2021, 12 (Suppl. S2), S339–S349. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, J.; Zheng, Y.; Gao, Y.; He, S.; Li, H.; Zou, K.; Li, N.; Tian, J.; Chen, W.; et al. Esophageal cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2021, 33, 535–547. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, W.Q.; Li, Z.S.; Li, N.; Ren, J.S.; Tian, J.H.; Tian, W.J.; Hu, F.L.; Peng, J.; Expert Group of China Guideline for the Screening, Early Detection and Early Treatment of Esophageal Cancer. [China guideline for the screening, early detection and early treatment of esophageal cancer (2022, Beijing)]. Zhonghua Zhong Liu Za Zhi 2022, 44, 491–522. (In Chinese) [Google Scholar] [PubMed]
- Nezu, Y.; Manabe, N.; Yoda, Y.; Haruma, K. Effectiveness of screening endoscopy for esophageal squamous cell carcinoma in Japanese males. United Eur. Gastroenterol. J. 2022, 10, 868–873. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Joinpoint Segment Year Start | Joinpoint Segment Year End | APC (95% CI) | p-Value | AAPC (95% CI) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC | ||||||||||
| men | 1985 | 2009 | 4.4 | (1.8~7.2) | 0.02 | 3.5 | (2.3~4.6) | <0.001 | ||
| 2010 | 2019 | 1.6 | (−3.6~7.1) | 0.33 | ||||||
| women | 1985 | 2019 | 0.8 | (0.4~1.2) | <0.01 | |||||
| ESCC | ||||||||||
| men | 1985 | 2009 | 5.5 | (2.9~8.3) | 0.01 | 4.2 | (3.1~5.4) | <0.001 | ||
| 2010 | 2019 | 1.7 | (−3.1~6.7) | 0.27 | ||||||
| women | 1985 | 2019 | 1.7 | (1.4~2.1) | <0.001 | |||||
| EAC | ||||||||||
| men | 1985 | 2009 | 2.0 | (1.3~2.6) | 0.01 | 1.2 | (0.9~1.5) | <0.001 | ||
| 2010 | 2019 | −0.3 | (−1.9~1.4) | 0.58 | ||||||
| women | 1985 | 2004 | 4.0 | (1.1~7.0) | 0.03 | 0.2 | (−0.6~1.1) | 0.56 | ||
| 2005 | 2019 | −3.4 | (−5.5~−1.2) | 0.02 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-C.; Chou, Y.-C.; Lee, Y.-K.; Hsu, W.-L.; Tang, C.-S.; Chen, S.-Y.; Huang, S.-P.; Chen, Y.-C.; Lee, J.-M. Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis. Cancers 2022, 14, 5844. https://doi.org/10.3390/cancers14235844
Tsai M-C, Chou Y-C, Lee Y-K, Hsu W-L, Tang C-S, Chen S-Y, Huang S-P, Chen Y-C, Lee J-M. Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis. Cancers. 2022; 14(23):5844. https://doi.org/10.3390/cancers14235844
Chicago/Turabian StyleTsai, Min-Chen, Yu-Ching Chou, Yu-Kwang Lee, Wan-Lun Hsu, Chin-Sheng Tang, Shiow-Ying Chen, Shih-Pei Huang, Yong-Chen Chen, and Jang-Ming Lee. 2022. "Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis" Cancers 14, no. 23: 5844. https://doi.org/10.3390/cancers14235844
APA StyleTsai, M.-C., Chou, Y.-C., Lee, Y.-K., Hsu, W.-L., Tang, C.-S., Chen, S.-Y., Huang, S.-P., Chen, Y.-C., & Lee, J.-M. (2022). Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis. Cancers, 14(23), 5844. https://doi.org/10.3390/cancers14235844





